Abstract
Background
Cerium oxide (CeO2) nanoparticles have been posited to have both beneficial and toxic effects on biological systems. Herein, we examine if a single intratracheal instillation of CeO2 nanoparticles is associated with systemic toxicity in male Sprague-Dawley rats.
Methods and results
Compared with control animals, CeO2 nanoparticle exposure was associated with increased liver ceria levels, elevations in serum alanine transaminase levels, reduced albumin levels, a diminished sodium-potassium ratio, and decreased serum triglyceride levels (P < 0.05). Consistent with these data, rats exposed to CeO2 nanoparticles also exhibited reductions in liver weight (P < 0.05) and dose-dependent hydropic degeneration, hepatocyte enlargement, sinusoidal dilatation, and accumulation of granular material. No histopathological alterations were observed in the kidney, spleen, and heart. Analysis of serum biomarkers suggested an elevation of acute phase reactants and markers of hepatocyte injury in the rats exposed to CeO2 nanoparticles.
Conclusion
Taken together, these data suggest that intratracheal instillation of CeO2 nanoparticles can result in liver damage.
Keywords: cerium oxide nanoparticles, systemic toxicity, hepatic toxicity, hydropic degeneration
Introduction
Cerium is a rare earth lanthanide metal and a strong oxidizing agent. Cerium exists both in the trivalent state (Ce3+, cerous) and very stable tetravalent state (Ce4+, ceric) as cerium oxide (CeO2).1 CeO2 is widely used as a polishing agent for glass mirrors, television tubes, and ophthalmic lenses.2 In addition, CeO2 can also act as a catalyst because it can both accept and donate oxygen.3 This latter property has led to the widespread use of CeO2 in the automobile industry, where it has been used to increase fuel efficiency and reduce particulate emissions.4–6 It appears that CeO2 nanoparticles may also be capable of acting as antioxidants, which has led some to postulate that these particles may be useful for the treatment of cardiovascular disease,7 neurodegenerative disease,8 and radiation-induced tissue damage.9,10 Nonetheless, other in vitro work has shown that CeO2 nanoparticles can also cause oxidative stress.11
The Organization for Economic Co-operation and Development Working Party on Manufactured Nanomaterials has demarcated CeO2 nanoparticles along with 14 other nanoparticles as a high-priority for evaluation.12 Given current industrial applications, it is thought that the most common route of CeO2 exposure is likely to be through inhalation and/or ingestion. Although previous studies have shown that intratracheal instillation of CeO2 nanoparticles can cause a toxicological response in the lung, whether these particles also exhibit systemic toxicity is currently unclear.2,13 Therefore, the purpose of the current study was to determine if the intratracheal instillation of CeO2 nanoparticles is associated with alterations in the indices of systemic toxicity and pathological change. On the basis of previous work examining the translocation of carbon nanotubes from the lung,14 we hypothesized that intratracheal instillation of CeO2 nanoparticles could lead to nanoparticle deposition in other organs through the circulation. Consistent with this hypothesis, our data suggest that the intratracheal instillation of CeO2 nanoparticles is associated with increased liver ceria levels, reductions in liver weight, and evidence of liver damage.
Materials and methods
Particle characterization
CeO2 nanoparticles, 10 wt% in water (average diameter approximately 20 nm), were obtained from Sigma-Aldrich (St Louis, MO) as previously outlined.13 Normal saline was used as vehicle to suspend the nanoparticles prior to instillation. CeO2 samples diluted in saline were used for animal exposures. Since the CeO2 nanoparticles form agglomerates in suspension, the size distribution of the agglomerates of CeO2 was analyzed using field emission scanning electron microscopy and transmission electron microscopy (TEM).
The CeO2 suspension was analyzed using field emission scanning electron microscopy as follows: the CeO2 particle suspensions were diluted with distilled water (about 10-fold) and were dried on carbon planchet and sputter-coated. After sputter-coating, the specimens were examined with a Hitachi Model S-4800 field emission scanning electron microscope (Schaumburg, IL) between 5 kV and 20 kV. In addition, the particles were diluted in double distilled filtered water and a drop was placed on a formvar-coated copper grid to dry before viewing the samples with a JEOL 1220 TEM (Tokyo, Japan).
Animal handling and instillation of CeO2 nanoparticles
Specific pathogen-free male Sprague-Dawley (Hla: SD-CVF) rats (6 weeks old) were purchased from Hilltop Laboratories (Scottdale, PA). Rats were kept in cages individually and ventilated with HEPA filtered air in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. After acclimatization for one week, the rats were randomly divided into four groups (n = 7 per group) to receive vehicle control (saline, 0.9% NaCl), or instillation of 1.0, 3.5, or 7.0 mg/kg CeO2 nanoparticles. Rats were anesthetized with sodium methohexital (35 mg/kg, intraperitoneally) and placed on an inclined restraint board before instillation with 0.3 mL of saline suspension or CeO2 nanoparticles. The animals were euthanized 28 days post-exposure by drug overdose according to the Guide for the Care and Use of Laboratory Animals and as approved by the National Institute for Occupational Safety and Health Animal Care and Use Committee. All animals were humanely treated and were monitored for any potential suffering.
Determination of cerium content in the liver
Liver cerium content was estimated by induction coupled plasma-mass spectrometry (ICP-MS) at Elemental Analysis Inc (Lexington, KY) according to the standard protocol.14 Briefly, liver samples (n = 4 for each group) were prepared using Environmental Protection Agency method 3050B for the analysis of total cerium by ICP-MS. A 2.5 g sample was weighed to the nearest 0.0001 g and digested with concentrated nitric acid, 30% hydrogen peroxide, and concentrated hydrochloric acid. A method blank, laboratory control sample, a laboratory duplicate, and a predigestion matrix spike were prepared for each sample. After digestion, the extracts and the quality control samples were diluted to a final volume of 50 mL before analysis using an Agilent 7500cx ICP-MS. The instrument was calibrated for Ce-140 with 0, 0.1, 1.0, 10.0, and 100 μg/L standards prepared from a certified reference standard traceable to National Institute of Standards and Technology reference materials. A second source calibration verification standard traceable to National Institute of Standards and Technology reference materials was analyzed to verify the calibration standards. A continuing calibration verification standard and a continuing calibration blank were analyzed at the beginning of the run, after every ten samples, and at the conclusion of the run.
Serum biochemical and lipid profile analysis
Blood was collected by cardiac puncture into a serum collection tube (BD Vacutainer®) before centrifugation at 800× g for 15 minutes. Serum was collected and used for biochemical assays using an Abaxis VetScan® analyzer (Abaxis, Union City, CA). Serum biochemical parameters, ie, alanine aminotransferase, alkaline phosphatase, bilirubin, blood urea nitrogen, albumin, calcium (Ca2+), creatinine, amylase, globulin, potassium (K+), sodium (Na+), phosphorus, total bilirubin, and total protein were evaluated with a Comprehensive Diagnostic Profile Disk. The lipid profile, ie, total cholesterol, triglycerides, and high-density lipoprotein was measured using lipid profile-Glu cassettes (Cholestech LDX) and a Cholestech LDX® analyzer. The remaining serum was stored at −80°C.
Multiplexed serum protein immunoassays
Pooled serum samples from all seven animals in each experimental group were shipped on dry ice to Rules-Based Medicine (Austin, TX) for Rodent MAP® version 2.0 antigen analysis using a Luminex 100 instrument, as detailed elsewhere.14 The antigen panel consisted of 59 proteins, which included proteins involved in inflammation, cytokines, growth factors, and tissue factors. Each analyte was quantified using 4 and 5 parameter, weighted and nonweighted curve fitting algorithms using proprietary data analysis software developed at Rules-Based Medicine.
Tissue collection and histopathological examination
Liver, kidney, spleen, and heart were collected at the time of death. Each tissue was weighed and then fixed in FineFIX™ (Milestone medicals, Shelton, CT) preservative for later histopathological examination. Tissues from liver, spleen, kidney, and heart were embedded in paraffin wax, sectioned at 5 μm, mounted on glass slide and stained with hematoxylin-eosin using standard histopathological techniques. Sections were examined by light microscopy in a blinded fashion by a board certified pathologist.
Data analysis
Results are presented as the mean ± standard error of the mean. Data were analyzed using the SigmaPlot 11.0 statistical program. One-way analysis of variance was performed for overall comparisons, while the Student–Newman–Keuls post hoc test was used to determine differences between groups. Values of P < 0.05 were considered to be statistically significant.
Results
Nanoparticle characterization
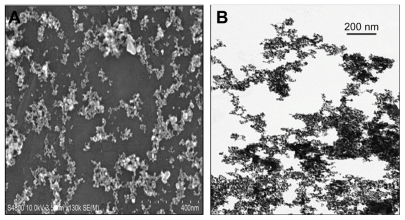
Similar to previous work using the same batch of CeO2 nanoparticles,13 analysis of nanoparticle size by TEM and scanning electron microscopy confirmed the presence of single and agglomerated CeO2 nanoparticles in the suspensions (Figure 1A and B). Field emission scanning electron microscopy showed that the CeO2 nano particles were generally dispersed into submicron groups with an average size of 9.26 ± 0.58 nm. The diameter of the primary CeO2 particles was determined to be 10.14 ± 0.76 nm by TEM.
Figure 1.
Characterization of the cerium oxide nanoparticles by (A) field emission scanning electron microscopy and (B) transmission electron microscopy (scale bar = 200 nm) of a dilute cerium oxide suspension.
CeO2 instillation decreases liver wet weight
CeO2 instillation at the 1, 3.5, or 7 mg/kg dosages had no significant effect on rat body, heart, kidney, or spleen weight (Table 1). Compared with control animals, only the highest CeO2 dosage (7 mg/kg) decreased liver weight (saline control 14.55 ± 0.27 versus CeO2 7.0 mg/kg 12.50 ± 0.54; P < 0.05, Table 1).
Table 1.
Alterations in absolute organ wet weight 28 days after intratracheal instillation of cerium oxide nanoparticles
| Organ weight (g) | Saline control (n = 7) | CeO2 1.0 mg/kg (n = 7) | CeO2 3.5 mg/kg (n = 7) | CeO2 7.0 mg/kg (n = 7) |
|---|---|---|---|---|
| Heart (g) | 1.52 ± 0.15 | 1.35 ± 0.05 | 1.27 ± 0.07 | 1.23 ± 0.05 |
| Liver (g) | 14.55 ± 0.27 | 14.30 ± 1.04 | 14.78 ± 0.57 | 12.50 ± 0.54* |
| Kidney (g) | 2.67 ± 0.31 | 2.55 ± 0.21 | 2.54 ± 0.33 | 2.43 ± 0.31 |
| Spleen (g) | 0.58 ± 0.06 | 0.65 ± 0.10 | 0.56 ± 0.08 | 0.64 ± 0.04 |
Note: Significantly different from vehicle control (P < 0.05).
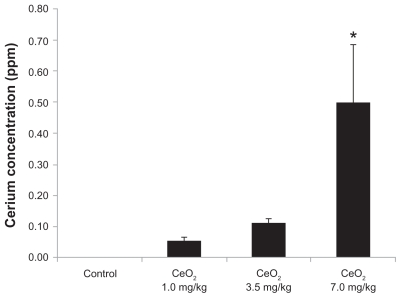
CeO2 instillation increases liver ceria content
The ceria content of animals instilled with 7.0 mg/kg CeO2 nanoparticles was higher than that observed in the other groups (saline control nondetectable versus 1.0 mg/kg CeO2: 0.05 ± 0.01 ppm versus 3.5 mg/kg CeO2: 0.11 ± 0.02 ppm versus CeO2 7.0 mg/kg: 0.50 ± 0.18 ppm; P < 0.05; Figure 2).
Figure 2.
Concentration of cerium in liver after intratracheal instillation of cerium oxide nanoparticles.
Note: *Significantly different from the vehicle control (P < 0.05).
Effect of CeO2 instillation on serum biochemical profile
Table 2 shows the alterations of the serum biochemical parameters following CeO2 nanoparticle exposure. Compared with control animals, CeO2 instillation at 1, 3.5, or 7 mg/kg diminished the sodium to potassium ratio (P < 0.05), while the CeO2 dosage of 7 mg/kg increased serum alanine aminotransferase levels and reduced albumin levels (P < 0.05). The serum lipid profile analysis (Table 2B) indicated a reduction in the triglyceride levels with 7 mg/kg CeO2 nanoparticle exposure.
Table 2.
Changes in serum biochemical parameters (A) and lipid profile (B) 28 days after the intratracheal instillation of cerium oxide nanoparticles
| Analyte | Saline control (n = 7) | CeO2 1.0 mg/kg (n = 7) | CeO2 3.5 mg/kg (n = 7) | CeO2 7.0 mg/kg (n = 7) |
|---|---|---|---|---|
| A | ||||
| Glucose | 186.4 ± 25.7 | 208 ± 43.0 | 197.6 ± 40.2 | 231 ± 93.5 |
| ALP | 276.1 ± 53.7 | 263 ± 55.4 | 242 ± 35.3 | 222.23 ± 81.9 |
| ALT | 58.3 ± 10.7 | 83.4 ± 28.5 | 88.3 ± 31.4 | 130.5 ± 94.5* |
| Amylase | 974.7 ± 97.4 | 1055.1 ± 124.2 | 991.4 ± 116 | 908.4 ± 277.0 |
| Total protein | 6.0 ± 0.1 | 5.9 ± 0.6 | 6.2 ± 0.5 | 5.4 ± 1.3 |
| Albumin | 4.2 ± 0.2 | 4.1 ± 0.5 | 4.5 ± 0.4 | 3.5 ± 1.1* |
| Globulin | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 |
| ALB-GLOB ratio | 2.3 ± 0.3 | 2.3 ± 0.3 | 2.2 ± 0.3 | 1.9 ± 0.6 |
| BUN | 15.4 ± 1.1 | 15 ± 3.1 | 15.7 ± 1.9 | 14.4 ± 4.2 |
| Creatinine | 0.3 ± 0.1 | 0.27 ± 0.1 | 0.23 ± 0.1 | 0.28 ± 0.1 |
| Ca2+ | 11.4 ± 0.7 | 10.7 ± 1.3 | 11.5 ± 1.1 | 10.4 ± 2.4 |
| Phosphorus | 8.6 ± 0.9 | 7.9 ± 1.2 | 8.7 ± 1.0 | 8.2 ± 1.9 |
| Na+ | 142.3 ± 0.9 | 138 ± 10.7 | 138.1 ± 10.7 | 132.1 ± 16.3 |
| K+ | 5.5 ± 0.4 | 6.0 ± 0.5 | 6.5 ± 0.6 | 5.8 ± 0.9 |
| Na+-K+ ratio | 25.8 ± 2.0 | 22.9 ± 1.7* | 21.2 ± 1.4* | 22.8 ± 2.5* |
| B | ||||
| Total cholesterol | 100.7 ± 1.9 | 100 ± 0 | 100 ± 0 | 103.1 ± 8.3 |
| Triglycerides | 143 ± 53 | 109.6 ± 50.9 | 190.3 ± 83.7 | 93.1 ± 22.3* |
| HDL | 21 ± 6.0 | 19.4 ± 5.4 | 20 ± 6.4 | 19 ± 5.1 |
Note: Significantly different from the vehicle control (P < 0.05).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ALB-GLOB ratio, albumin to globulin ratio; BUN, blood urea nitrogen; Ca, calcium; Na, sodium; K, potassium; Na-K ratio, sodium to potassium ratio; HDL, high density lipoproteins.
CeO2 nanoparticle exposure is associated with evidence of liver pathology
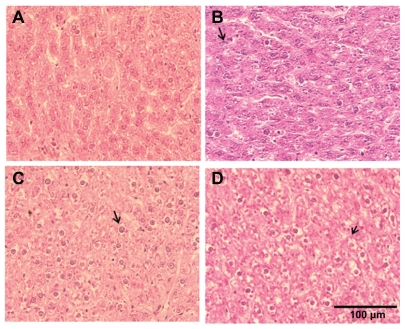
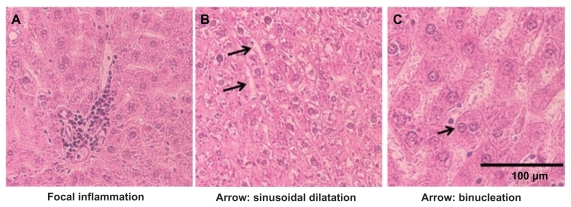
The primary alterations considered for liver tissue damage were hydropic degeneration of the hepatocytes, dilation of the sinusoids, portal inflammation, and fibrosis of the liver compared to tissues obtained from control animals. CeO2 nanoparticle exposure showed widespread hydropic degeneration of hepatocytes around the central vein region with sparing of the immediate periportal region (Figure 3). These changes were panlobular in nature. Along with hydropic degeneration, we also observed enlargement of the hepatocytes, enlargement of the nucleus in the hepatocyte, binucleation of some hepatocytes, dilatation of the sinusoids, and occasional focal inflammation areas in a few of the exposed animals (Figure 4). As the dose of the nanoparticles was increased, the number of hepatocytes that show hydropic degeneration was also elevated suggesting that changes in hepatocyte structure were dose-dependent.
Figure 3.
Cerium oxide nanoparticle exposure alters histopathological architecture of the liver. (A) Saline control (400×), (B) CeO2 at 1.0 mg/kg (400×), (C) CeO2 3.5 mg/kg (400×), and (D) CeO2 7.0 mg/kg (400×). Note evidence of hydropic degeneration (arrow) with CeO2 instillation.
Figure 4.
Histopathological alterations with the CeO2 nanoparticle exposure (7.0 mg/kg) include (A) focal inflammation, (B) sinusoidal dilatation, and (C) binucleation of the hepatocyte (400×).
CeO2 nanoparticle exposure does not appear to affect spleen, kidney, and heart
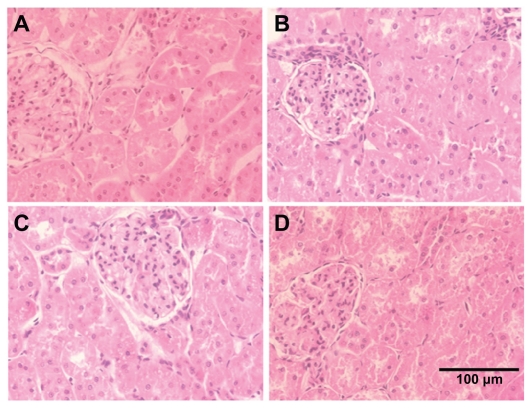
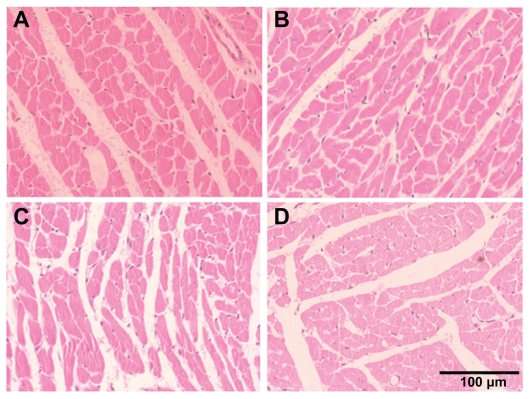
Alterations considered for the kidney pathologies were necrosis of the proximal tubular epithelium, tubular accumulation of proteinaceous material, and inflammatory reaction in the interstitial areas of the cortex and medulla. Spleen and heart tissues were examined for any histological alterations in structure along with the infiltration of inflammatory cells. We did not observe any alterations in the histological appearance or the infiltration of inflammatory cells in the kidney, spleen, and heart with CeO2 nanoparticle exposure (Figures 5–7).
Figure 5.
Cerium oxide nanoparticle exposure has no effect on the histological appearance of the kidney. (A) Saline control (400×), (B) CeO2 at 1.0 mg/kg (400×), (C) CeO2 3.5 mg/kg (400×), and (D) CeO2 7.0 mg/kg (400×).
Figure 7.
Cerium oxide nanoparticle exposure has no effect on histological appearance of heart. (A) saline control (400×), (B) CeO2 at 1.0 mg/kg (400×), (C) CeO2 3.5 mg/kg (400×), and (D) CeO2 7.0 mg/kg (400×).
Effect of CeO2 instillation on serum protein expression
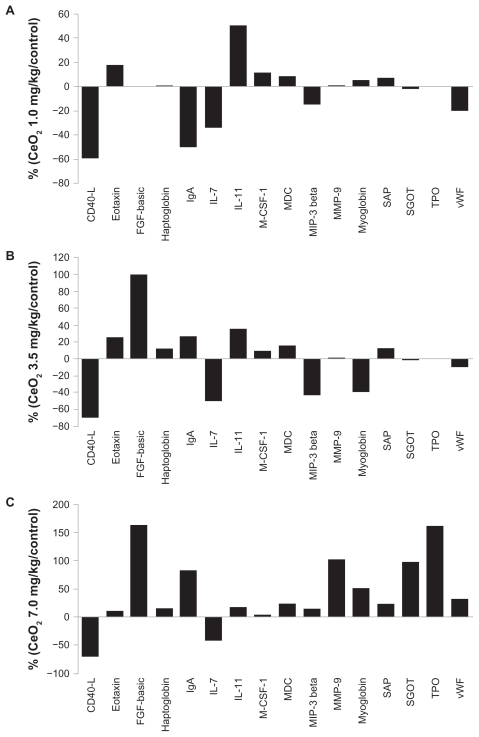
A panel of 59 protein biomarkers comprising cytokines, inflammatory markers, growth factors, and tissue factors were quantified in the serum samples collected in this study using the RBM RodentMAP® V2.0 multiplex immune assay service. Compared with levels observed in the control animals, the levels of 16 different analytes appeared to exhibit a trend towards being increased or decreased by at least 15% or more following the instillation procedure with the CeO2 nanoparticles (Figure 8A, B, and C). At the 7.0 mg/kg CeO2 dosage, ten of the analytes (fibroblast growth factor-basic, haptoglobin, immunoglobulin A, interleukin-11, matrix metalloproteinase- 9, myoglobin, serum amyloid protein, serum glutamic oxaloacetic, transaminase thrombopoietin, and von Willebrand factor) exhibited a trend towards increased expression whereas two (tumor necrosis factor-related activation protein (CD-40 L) and interleukin-7) appeared to exhibit a trend towards decreased expression (Figure 8C).
Figure 8.
Cerium oxide nanoparticles exposure results in alterations in the expression of serum protein biomarkers.
Discussion
Investigation of the effects that nanomaterials may have on cellular function is essential for ensuring that the utilization of these materials in industrial or medical applications is safe. Although CeO2 nanoparticles have demonstrated excellent potential for biomedical use,7,8,10 limited knowledge exists concerning their potential systemic toxicity. The primary finding of this investigation was that intratracheal instillation of CeO2 nanoparticles (Figure 1) results in increased liver ceria levels (Figure 2), and that these changes in liver ceria are associated with evidence of liver pathology (Figures 3 and 4), decreases in liver weight (Table 1), and alterations in blood chemistry (Table 2). Consistent with other reports examining CeO2,15 titanium dioxide,16 silica,17 and copper18 nanoparticles, our data suggest it is possible that CeO2 nanoparticles are capable of translocating from the lung to the liver via the circulation.
The histopathological appearance of the liver following CeO2 nanoparticle instillation is consistent with the possibility that ceria can induce several different pathological alterations, including hydropic degeneration of hepatocytes, enlargement of hepatocytes, dilatation of the sinusoids, and nuclear enlargement (Figures 3 and 4). There was no evidence of granuloma, portal inflammation, fibrosis, or bile duct abnormalities, except for the presence of some local inflammation of the lobules in some animals.
Because the liver is the major organ for biotransformation of toxins, it may be the first organ to be exposed to nanoparticles that are able to enter into the circulation. It is thought that hydropic degeneration can be caused by hypoxia,19 ischemia,20 or the treatment of hepatocytes with endotoxins21 or chemicals.22 Consistent with our findings, this response has also been observed following exposure to other toxic materials, including copper nanoparticles23 and carbon tetrachloride,24 or following the inhalation of anesthetics such as sevofulrane and desflurane.25 How exposure to CeO2 nanoparticles may induce hydropic degeneration or if these changes are reversible is currently unclear. Sinusoidal dilatation is the increased gap between the hepatic cords in the hepatic lobule that has also been observed in aluminum-induced hepatic toxicity,26 carbon tetrachloride-induced hepatic toxicity,27 and ischemia,28 as well as with the organophosphate insecticide, methidathion. 29 In addition, we also noted the accumulation of granular material inside the hepatocytes which appeared to be dose-dependent and perhaps related to reduction of liver weight (Table 1).
Our serum biochemical profile data suggest that CeO2 nanoparticle instillation in the rat may be associated with an elevation of alanine aminotransferase and reduction in albumin (Table 2). It is well established that hepatocyte damage is associated with the release of liver enzymes into the circulation and reduced albumin levels.26 In addition to changes in the level of circulating liver enzymes, CeO2 nanoparticle instillation also appears to decrease the sodium-potassium ratio and the amount of triglycerides (Table 2).
Similar to other work examining other types of nanoparticles, 30,31 we observed a trend towards an increasing serum concentration of haptoglobin (16%), serum amyloid P protein (24%), and von Willebrand factor (33%) following exposure to CeO2 nanoparticles. Consistent with our histopathological findings, and the possibility of hepatic injury, we also found evidence that CeO2 nanoparticle instillation was associated with a trend toward increases in the amount of serum thrombopoietin, fibroblast growth factor, serum glutamic oxaloacetic transaminase, and transaminase thrombopoietin (Figure 4). Elevation in these serum biomarkers is thought to be highly correlated with acute hepatic injury.22,32 Taken together, these data suggest that ceria deposition may be associated with liver damage. Given our findings that CeO2 nanoparticle instillation, at least at the levels used in the current study, does not induce appreciable damage to the heart, kidney, or spleen. It is possible that the liver, by acting to clear CeO2 nanoparticles from the circulation, is functioning to prevent additional secondary or tertiary pathological changes elsewhere.
Conclusion
In summary, our data suggest that intratracheal instillation of CeO2 nanoparticles may be associated with hepatotoxicity. The toxicity induced by CeO2 nanoparticles appears to be dose-dependent, because the rats instilled with 7.0 mg/kg body weight of CeO2 nanoparticles exhibited the greatest evidence of toxicological response. The toxicological response appears to be limited to the liver and may occur through extrapulmonary translocation of the CeO2 nanoparticles into the systemic circulation. Given these findings, additional research to evaluate the health effects of CeO2 nanoparticles is likely warranted.
Figure 6.
Cerium oxide nanoparticle exposure has no effect on the histological appearance of the spleen. (A) Saline control (400×), (B) CeO2 at 1.0 mg/kg (400×), (C) CeO2 3.5 mg/kg (400×), and (D) CeO2 7.0 mg/kg (400×).
Acknowledgments
Grant support for this project came from Department of Energy funding (No DE-SC0005162) to ERB. The authors would like to thank Eli Shleser for assisting in sample collection and Stephanie Woods for preparation of the paraffin-embedded sections for hematoxylin-eosin staining.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The findings and conclusions in this report have not been formally disseminated by the NIOSH and should not be construed to represent any agency determination or policy.
References
- 1.Bumajdad A, Eastoe J, Mathew A. Cerium oxide nanoparticles prepared in self-assembled systems. Adv Colloid Interface Sci. 2009;147–148:56–66. doi: 10.1016/j.cis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Cassee FR, van Balen EC, Singh C, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41(3):213–229. doi: 10.3109/10408444.2010.529105. [DOI] [PubMed] [Google Scholar]
- 3.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 2007;(10):1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 4.Park B, Martin P, Harris C, et al. Initial in vitro screening approach to investigate the potential health and environmental hazards of Envirox-trade mark – a nanoparticulate cerium oxide diesel fuel additive. Part Fibre Toxicol. 2007;4:12. doi: 10.1186/1743-8977-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park B, Donaldson K, Duffin R, et al. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive – a case study. Inhal Toxicol. 2008;20(6):547–566. doi: 10.1080/08958370801915309. [DOI] [PubMed] [Google Scholar]
- 6.Nikolaou K. Emissions reduction of high and low polluting new technology vehicles equipped with a CeO2 catalytic system. Sci Total Environ. 1999;235(1–3):71–76. doi: 10.1016/s0048-9697(99)00191-6. [DOI] [PubMed] [Google Scholar]
- 7.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73(3):549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das M, Patil S, Bhargava N, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salyer DC, Eggleston JC. Oat cell carcinoma of the bronchus and the carcinoid syndrome. Arch Pathol. 1975;99(10):513–515. [PubMed] [Google Scholar]
- 10.Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5(2):225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Eom HJ, Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett. 2009;187(2):77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Simonelli F, Marmorato P, Abbas K, et al. Cyclotron production of radioactive nanoparticles and their application for uptake studies. IEEE Trans Nanobioscience. 2011;10(1):44–50. doi: 10.1109/TNB.2011.2119491. [DOI] [PubMed] [Google Scholar]
- 13.Ma JY, Zhao H, Mercer RR, et al. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology. 2011;5:312–325. doi: 10.3109/17435390.2010.519835. [DOI] [PubMed] [Google Scholar]
- 14.Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca(2+)-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37(10):2134–2146. doi: 10.1016/j.biocel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.He X, Zhang H, Ma Y, et al. Lung deposition and extrapulmonary translocation of nano-ceria after intratracheal instillation. Nanotechnology. 2010;21(28):285103. doi: 10.1088/0957-4484/21/28/285103. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li J, Yin J, et al. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J Nanosci Nanotechnol. 2010;10(12):8544–8549. doi: 10.1166/jnn.2010.2690. [DOI] [PubMed] [Google Scholar]
- 17.Jin C, Jin Y, Wang J, Zhao C. Comparative study of the effect on oxidative damage in rats inhaled by nano-sized and micro-sized silicon dioxide. Wei Sheng Yan Jiu. 2008;37(1):16–18. 36. Chinese. [PubMed] [Google Scholar]
- 18.Yang B, Wang Q, Lei R, et al. Systems toxicology used in nanotoxicology: mechanistic insights into the hepatotoxicity of nano-copper particles from toxicogenomics. J Nanosci Nanotechnol. 2010;10(12):8527–8537. doi: 10.1166/jnn.2010.2481. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda J, Syuto B, Too K, Ohfuji S. Lactate dehydrogenase isoenzyme patterns in bovine liver tissue. Nihon Juigaku Zasshi. 1989;51(4):733–739. doi: 10.1292/jvms1939.51.733. [DOI] [PubMed] [Google Scholar]
- 20.Chen JW, Chen DZ, Lu GZ. Asymptomatic process of hepatic artery thrombosis in a patient after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int. 2004;3(1):149–151. [PubMed] [Google Scholar]
- 21.Memis D, Hekimoglu S, Sezer A, Altaner S, Sut N, Usta U. Curcumin attenuates the organ dysfunction caused by endotoxemia in the rat. Nutrition. 2008;24(11–12):1133–1138. doi: 10.1016/j.nut.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Chaung SS, Lin CC, Lin J, Yu KH, Hsu YF, Yen MH. The hepato-protective effects of Limonium sinense against carbon tetrachloride and beta-D-galactosamine intoxication in rats. Phytother Res. 2003;17(7):784–791. doi: 10.1002/ptr.1236. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Gao Y, Zhang L, et al. Potential health impact on mice after nasal instillation of nano-sized copper particles and their translocation in mice. J Nanosci Nanotechnol. 2009;9(11):6335–6343. doi: 10.1166/jnn.2009.1320. [DOI] [PubMed] [Google Scholar]
- 24.Bogers M, Appelman LM, Feron VJ, Beems RB, Notten WR. Effects of the exposure profile on the inhalation toxicity of carbon tetrachloride in male rats. J Appl Toxicol. 1987;7(3):185–191. doi: 10.1002/jat.2550070307. [DOI] [PubMed] [Google Scholar]
- 25.Arslan M, Ozkose Z, Akyol G, Barit G. The age- and gender-dependent effects of desflurane and sevoflurane on rat liver. Exp Toxicol Pathol. 2010;62(1):35–43. doi: 10.1016/j.etp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Turkez H, Yousef MI, Geyikoglu F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem Toxicol. 2010;48(10):2741–2746. doi: 10.1016/j.fct.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Kamalakkannan N, Rukkumani R, Varma PS, Viswanathan P, Rajasekharan KN, Menon VP. Comparative effects of curcumin and an analogue of curcumin in carbon tetrachloride-induced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol. 2005;97(1):15–21. doi: 10.1111/j.1742-7843.2005.pto_97103.x. [DOI] [PubMed] [Google Scholar]
- 28.Baykara B, Tekmen I, Pekcetin C, et al. The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochem. 2009;111(1):42–51. doi: 10.1016/j.acthis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Sutcu R, Altuntas I, Yildirim B, Karahan N, Demirin H, Delibas N. The effects of subchronic methidathion toxicity on rat liver: role of antioxidant vitamins C and E. Cell Biol Toxicol. 2006;22(3):221–227. doi: 10.1007/s10565-006-0039-7. [DOI] [PubMed] [Google Scholar]
- 30.Higashisaka K, Yoshioka Y, Yamashita K, et al. Acute phase proteins as biomarkers for predicting the exposure and toxicity of nanomaterials. Biomaterials. 2011;32(1):3–9. doi: 10.1016/j.biomaterials.2010.08.110. [DOI] [PubMed] [Google Scholar]
- 31.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 32.Schiodt FV, Balko J, Schilsky M, Harrison ME, Thornton A, Lee WM. Thrombopoietin in acute liver failure. Hepatology. 2003;37(3):558–561. doi: 10.1053/jhep.2003.50113. [DOI] [PubMed] [Google Scholar]