Abstract
Primary cutaneous large B-cell lymphoma, leg type, is a rare and aggressive neoplasm as defined by the recently updated World Health Organization–European Organization for Research and Treatment of Cancer classification of cutaneous lymphomas. We present a case of a 74-year-old woman who presented with a cutaneous lesion on her forearm. Skin biopsy revealed pathology consistent with this entity. The patient was treated with systemic chemotherapy with rituximab combined with doxorubicin, cyclophosphamide, vincristine, and prednisone. Here, we review the available literature and summarize clinical features and management of this uncommon subtype of non-Hodgkin lymphoma.
CASE REPORT
A 74-year-old white woman was referred by her primary care physician for evaluation of a lesion on her right arm. She had a history of breast cancer, for which she underwent a unilateral mastectomy for breast cancer in 1992, and a second breast cancer on the opposite side in 1994 treated with mastectomy followed by chemotherapy with doxorubicin, cyclophosphamide, and 5-fluorouracil. On presentation, she was noted to have a hard, immobile, pea-sized nodule palpable just under the skin surface on the dorsum of her right forearm. She reported that the lesion had been present for approximately 1 month without significant change in size, color, or shape. She denied other symptoms including fever, chills, night sweats, or pruritus.
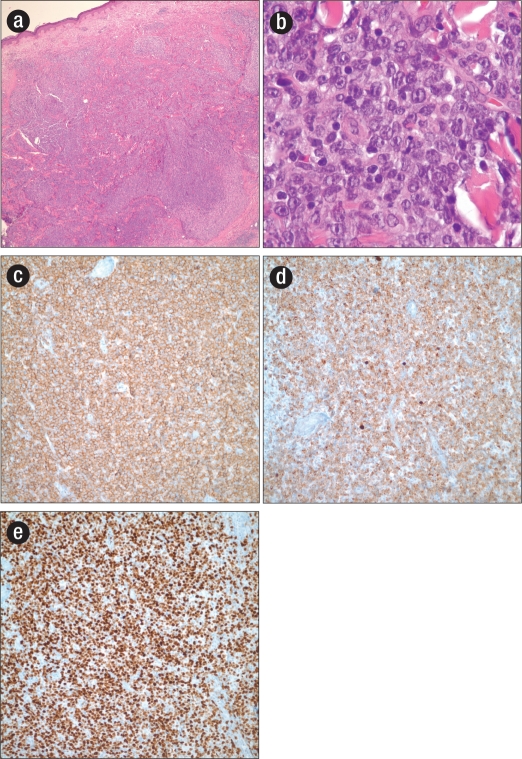
An excisional biopsy of the lesion revealed an infiltrate of monomorphic large cells in the dermis with vesicular chromatin, prominent nucleoli, and frequent mitoses (Figure). Immunohistochemical evaluation revealed the abnormal cells to be CD20, BCL-2, BCL-6, and MUM-1 positive and CD10 negative, suggestive of a germinal center (B-cell) origin and with a high proliferative index (MIB-1 >90%) (Figure). The morphology and staining pattern were consistent with a diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (PCLBLC, LT). Further staging evaluation, including bone marrow biopsy and computed tomographic imaging, was unremarkable.
Figure 1.
(a) Hematoxylin and eosin stain showing nodular infiltration of the superficial and deep dermis by primary cutaneous diffuse large B-cell lymphoma, leg type (low power). (b) Hematoxylin and eosin stain showing large monomorphic cells with a vesicular chromatin pattern, prominent nucleoli, and frequent mitoses (high power). (c) Immunohistochemical staining for CD20. (d) Immunohistochemical staining for Bcl-2. (e) Immunohistochemical staining for MIB-1.
The patient was subsequently started on systemic chemotherapy with rituximab combined with doxorubicin, cyclophosphamide, vincristine, and prednisone (R-CHOP) for three cycles, followed by replacement of doxorubicin with etoposide to avoid cardiac complications given her prior anthracycline exposure during breast cancer treatment in 1994. She tolerated therapy well and remains free of disease approximately 1½ years after her lymphoma diagnosis.
DISCUSSION
Primary cutaneous B-cell lymphoma (PCBCL) belongs to a distinct group of B-cell lymphoproliferative disorders defined by its presentation in the skin, without evidence of extracutaneous spread at the time of diagnosis (1). Extranodal involvement occurs in approximately 25% of non-Hodgkin lymphomas, with the gastrointestinal tract being the most common site of extranodal involvement, followed by the skin (2). The annual incidence of cutaneous lymphomas is approximately 0.5 to 1 per 100,000 (1). While B-cell lymphomas account for the majority of nodal lymphomas, PCBCLs represent only 20% to 25% of all primary cutaneous lymphomas (3).
The new 2008 World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas identifies three main subtypes of PCBCLs: primary cutaneous marginal zone lymphoma (PCMZL), primary cutaneous follicular center lymphoma (PCFCL), and PCLBCL, LT. The consensus guidelines for the management of CBCL have been published only in the last 3 to 4 years (3).
The pathogenesis of PCBCL is unclear. There is some speculation that PCBCL may represent a lymphoproliferative response to antigenic stimuli in the cutis, a skin-associated lymphoid tissue–related B-cell lymphoma (a process similar to mucosa-associated lymphoid tissue lymphomas in the gastrointestinal tract) (4). In Europe, there is evidence linking Borrelia burgdorferi infection to the development of PCBCL (2). Jelic and colleagues reported borrelial serology in 12 of 22 (55%) PCBCL cases (5). Another study evaluating cases of PCBCL in an area with endemic borrelia infections in the Scottish Highlands found B. burgdorferi–specific DNA in 35% of cases using polymerase chain reaction, with a statistically significant positive association between B. burgdorferi and PCBCL (6). However, this association has not been readily demonstrated in US data. The relatively smaller frequency of PCBCL in the US (4.5% compared with 20% in Europe) may reflect geographic differences in the strains of B. burgdorferi (7). Molecular analysis of PCBCL supports the hypothesis of an antigen-driven germinal center origin based on findings of a characteristic pattern of somatic hypermutation and the presence of intraclonal diversity in B-cell immunoglobulin genes (8).
The subtype identified in our patient, PCLBCL, LT, represents approximately 20% of all PCBCLs and 4% of all cutaneous lymphomas (9, 10). It is more common in the elderly, with a median age in the mid 70s (11, 12). The male to female ratio ranges from 1:3 to 1:4 (10, 12, 13). Patients with PCLBCL, LT present with red to bluish nodules or tumors on one or both lower legs. Only about 10% to 15% of these patients are noted to develop lesions outside of the lower extremities, as was the case with our patient, who presented with a right arm nodule (10). Compared with other subtypes of PCBCLs (PCMZL and PCFCL), these tumors are more aggressive with worse outcomes, as they frequently disseminate to lymph nodes and visceral organs (3).
Staging of PCBCL includes a complete history and physical examination, and laboratory studies include a complete blood count, comprehensive serum chemistries, serum protein electrophoresis, and serum lactate dehydrogenase, along with radiographic imaging with computed tomographic or positron emission tomography scans to evaluate for extracutaneous disease. Bone marrow aspiration and biopsy should be performed routinely for initial staging of PCLBCL, LT, though it is optional with indolent cutaneous B-cell lymphomas (14).
Definitive diagnosis is based on the combination of morphologic, histologic, immunohistochemical, and genotypic analyses of preferably an excisional biopsy specimen (3). Pathology reveals diffuse nonepidermotropic infiltrates made up of a monotonous population of large noncleaved B cells usually extending into the subcutaneous tissue. There is generally a paucity of reactive or inflammatory cells (2, 12). The neoplastic cells express B-cell markers (CD12, CD20, CD22, CD79a) and also often express surface immunoglobulin (3, 15). They are strongly positive for bcl-2; however, unlike PCFCL, the t(14;18) which often leads to bcl-2 overexpression is not seen in PCLBCL, LT (2, 16). Bcl-6, MUM-1, and FOXP1 are usually positively expressed, while CD10 and CD138 are negative (3, 12, 17). Similar to its systemic counterpart, diffuse large B-cell lymphoma, but unlike PCFCL, fluorescent in situ hybridization studies reveal that 80% of PCLBCL, LT cases have breakpoints in at least one loci involving IGH, MYC, BCL6, or MALT1 genes (2). Inactivation of the CDKN2A gene by deletion of chromosome 9p21.3 has been reported in 67% of PCLBCL, LT cases and is associated with an inferior prognosis (18). A recent paper investigating the expression of polycomb-group genes in primary nodal and cutaneous large B-cell lymphomas found that unlike primary nodal large B-cell lymphoma, PCLBCL, LT lacked BMI-1 oncogene expression (which plays a role in regulation of p16 cell cycle inhibitor). The HPH1 gene was also noted to be rare in PCLBCL of the head and trunk, while it was abundant in the leg primaries, supporting “leg-type” as a distinct entity (2, 19).
As previously mentioned, PCLBCL, LT has an inferior prognosis compared with PCFCL and PCMZL. Disease-related 5-year survivals for PCLBCL, LT range from 36% to 55% compared with over 95% for the other subtypes of primary cutaneous lymphomas (3, 11, 12). In an analysis of 60 patients with PCLBCL, LT, Grange and colleagues showed that patients with skin lesions on the leg had a statistically significantly worse prognosis compared with patients having nonleg lesions, with 3-year disease-specific survival rates of 43% vs. 77%, respectively (P = 0.02). Age over 75 and multiple lesions were also noted to be poor prognostic factors (11). Other factors contributing to the poor prognosis of PCLBCL, LT include a higher incidence of multiple relapses, shorter time to progression, and more frequent extracutaneous spread (20).
Given the rarity of PCLBCL, LT, prospective randomized therapeutic trials are lacking. Current patterns of care are based on retrospective and anecdotal evaluations. While watch-and-wait strategies are often employed with the other subtypes, aggressive systemic therapy with combination chemotherapy with or without involved-field radiation therapy remains the recommended approach for PCLBCL, LT. Radiation therapy alone is much less effective in PCLBCL, LT than in other CBCLs, with higher rates of relapse and frequent distant metastases (21, 22). A recent review of literature revealed that of 32 patients with PCLBCL, LT who received multiagent chemotherapy (without rituximab), the complete response rate was 81% and the relapse rate, 58% (3). A more recent study of 12 patients treated with R-CHOP demonstrated a complete response in 92% of patients (11/12) with only one relapse (11). Current treatment guidelines recommend that in the absence of an available clinical trial, these patients should be treated similar to patients with limited-stage diffuse large B-cell lymphoma given their similar clinical behaviors. Alternative therapy with systemic single-agent rituximab may be appropriate in elderly patients unable to tolerate multiagent chemotherapy; however, long-term data are lacking (23, 24).
Trials of experimental therapies utilizing autologous transplantation combined with rituximab, gene therapy, immunotherapy with interleukin 2, and radioimmunotherapy are ongoing for patients with relapsed or refractory disease. However, most data are currently extrapolated from positive responses seen in patients with nodal B-cell non-Hodgkin lymphomas (25).
In summary, PCLBCL, LT has only recently been recognized as a distinct clinical subtype of PCBCL based on its unique clinical features, morphology, immunophenotype, and molecular characteristics. Given its aggressive nature and the lack of large trials, PCLBCL, LT is treated similar to diffuse large B-cell lymphoma. However, relapse rates remain >50% with frequent extracutaneous dissemination. On presentation, patients should be referred for enrollment in a clinical trial, when one is available. Otherwise, treatment with multiagent chemotherapy with or without involved-field radiation therapy is most appropriate.
Acknowledgments
The authors thank Marvin Stone, MD, for his helpful comments on this manuscript.
References
- 1.Pandolfino TL, Siegel RS, Kuzel TM, Rosen ST, Guitart J. Primary cutaneous B-cell lymphoma: review and current concepts. J Clin Oncol. 2000;18(10):10–2152. doi: 10.1200/JCO.2000.18.10.2152. [DOI] [PubMed] [Google Scholar]
- 2.Santucci M, Pimpinelli N. Primary cutaneous B-cell lymphomas. Current concepts. I. Haematologica. 2004;89(11):11–1360. [PubMed] [Google Scholar]
- 3.Senff NJ, Noordijk EM, Kim YH, Bagot M, Berti E, Cerroni L, Dummer R, Duvic M, Hoppe RT, Pimpinelli N, Rosen ST, Vermeer MH, Whittaker S, Willemze R. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112(5):5–1600. doi: 10.1182/blood-2008-04-152850. [DOI] [PubMed] [Google Scholar]
- 4.Slater DN. MALT and SALT: the clue to cutaneous B-cell lymphoproliferative disease. Br J Dermatol. 1994;131(4):4–557. doi: 10.1111/j.1365-2133.1994.tb08560.x. [DOI] [PubMed] [Google Scholar]
- 5.Jelić S, Filipović-Ljesković I. Positive serology for Lyme disease borrelias in primary cutaneous B-cell lymphoma: a study in 22 patients; is it a fortuitous finding? Hematol Oncol. 1999;17(3):3–107. doi: 10.1002/(sici)1099-1069(199909)17:3<107::aid-hon644>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Goodlad JR, Davidson MM, Hollowood K, Ling C, MacKenzie C, Christie I, Batstone PJ, Ho-Yen DO. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotland. Am J Surg Pathol. 2000;24(9):9–1279. doi: 10.1097/00000478-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Wood GS, Kamath NV, Guitart J, Heald P, Kohler S, Smoller BR, Cerroni L. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28(10):10–502. doi: 10.1034/j.1600-0560.2001.281002.x. [DOI] [PubMed] [Google Scholar]
- 8.Gellrich S, Rutz S, Golembowski S, Jacobs C, von Zimmermann M, Lorenz P, Audring H, Muche M, Sterry W, Jahn S. Primary cutaneous follicle center cell lymphomas and large B cell lymphomas of the leg descend from germinal center cells. A single cell polymerase chain reaction analysis. J Invest Dermatol. 2001;117(6):6–1512. doi: 10.1046/j.0022-202x.2001.01543.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang CT, Yang WC, Liu YC, Lin SF. Primary cutaneous diffuse large B-cell lymphoma, leg type, with unusual clinical presentation of bluish-reddish multicolored rainbow pattern. J Clin Oncol. 2011;29(17):e497–e498. doi: 10.1200/JCO.2010.34.4796. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 11.Grange F, Beylot-Barry M, Courville P, Maubec E, Bagot M, Vergier B, Souteyrand P, Machet L, Dalac S, Esteve E, Templier I, Delaporte E, Avril MF, Robert C, Dalle S, Laroche L, Delaunay M, Joly P, Wechsler J, Petrella T. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143(9):9–1144. doi: 10.1001/archderm.143.9.1144. [DOI] [PubMed] [Google Scholar]
- 12.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):10–3768. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 13.Senff NJ, Hoefnagel JJ, Jansen PM, Vermeer MH, van Baarlen J, Blokx WA, Canninga-van Dijk MR, Geerts ML, Hebeda KM, Kluin PM, Lam KH, Meijer CJ, Willemze R. Reclassification of 300 primary cutaneous B-cell lymphomas according to the new WHO-EORTC classification for cutaneous lymphomas: comparison with previous classifications and identification of prognostic markers. J Clin Oncol. 2007;25(12):12–1581. doi: 10.1200/JCO.2006.09.6396. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, Dummer R, Hoppe RT, ISCL and EORTC TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110(2):2–479. doi: 10.1182/blood-2006-10-054601. [DOI] [PubMed] [Google Scholar]
- 15.Koens L, Vermeer MH, Willemze R, Jansen PM. IgM expression on paraffin sections distinguishes primary cutaneous large B-cell lymphoma, leg type from primary cutaneous follicle center lymphoma. Am J Surg Pathol. 2010;34(7):7–1043. doi: 10.1097/PAS.0b013e3181e5060a. [DOI] [PubMed] [Google Scholar]
- 16.Geelen FA, Vermeer MH, Meijer CJ, Van der Putte SC, Kerkhof E, Kluin PM, Willemze R. bcl-2 protein expression in primary cutaneous large B-cell lymphoma is site-related. J Clin Oncol. 1998;16(6):6–2080. doi: 10.1200/JCO.1998.16.6.2080. [DOI] [PubMed] [Google Scholar]
- 17.Kodama K, Massone C, Chott A, Metze D, Kerl H, Cerroni L. Primary cutaneous large B-cell lymphomas: clinicopathologic features, classification, and prognostic factors in a large series of patients. Blood. 2005;106(7):7–2491. doi: 10.1182/blood-2005-03-1175. [DOI] [PubMed] [Google Scholar]
- 18.Senff NJ, Zoutman WH, Vermeer MH, Assaf C, Berti E, Cerroni L, Espinet B, de Misa Cabrera RF, Geerts ML, Kempf W, Mitchell TJ, Paulli M, Petrella T, Pimpinelli N, Santucci M, Whittaker SJ, Willemze R, Tensen CP. Fine-mapping chromosomal loss at 9p21: correlation with prognosis in primary cutaneous diffuse large B-cell lymphoma, leg type. J Invest Dermatol. 2009;129(5):5–1149. doi: 10.1038/jid.2008.357. [DOI] [PubMed] [Google Scholar]
- 19.Raaphorst FM, Vermeer M, Fieret E, Blokzijl T, Dukers D, Sewalt RG, Otte AP, Willemze R, Meijer CJ. Site-specific expression of polycomb-group genes encoding the HPC-HPH/PRC1 complex in clinically defined primary nodal and cutaneous large B-cell lymphomas. Am J Pathol. 2004;164(2):2–533. doi: 10.1016/S0002-9440(10)63143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinzani PL, Quaglino P, Pimpinelli N, Berti E, Baliva G, Rupoli S, Martelli M, Alaibac M, Borroni G, Chimenti S, Alterini R, Alinari L, Fierro MT, Cappello N, Pileri A, Soligo D, Paulli M, Pileri S, Santucci M, Bernengo MG, Italian Study Group for Cutaneous Lymphomas Prognostic factors in primary cutaneous B-cell lymphoma: the Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24(9):9–1376. doi: 10.1200/JCO.2005.03.6285. [DOI] [PubMed] [Google Scholar]
- 21.Senff NJ, Hoefnagel JJ, Neelis KJ, Vermeer MH, Noordijk EM, Willemze R, Dutch Cutaneous Lymphoma Group Results of radiotherapy in 153 primary cutaneous B-cell lymphomas classified according to the WHO-EORTC classification. Arch Dermatol. 2007;143(12):12–1520. doi: 10.1001/archderm.143.12.1520. [DOI] [PubMed] [Google Scholar]
- 22.Smith BD, Glusac EJ, McNiff JM, Smith GL, Heald PW, Cooper DL, Wilson LD. Primary cutaneous B-cell lymphoma treated with radiotherapy: a comparison of the European Organization for Research and Treatment of Cancer and the WHO classification systems. J Clin Oncol. 2004;22(4):4–634. doi: 10.1200/JCO.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Bonnekoh B, Schulz M, Franke I, Gollnick H. Complete remission of a primary cutaneous B-cell lymphoma of the lower leg by first-line monotherapy with the CD20-antibody rituximab. J Cancer Res Clin Oncol. 2002;128(3):3–161. doi: 10.1007/s00432-001-0313-2. [DOI] [PubMed] [Google Scholar]
- 24.Fink-Puches R, Wolf IH, Zalaudek I, Kerl H, Cerroni L. Treatment of primary cutaneous B-cell lymphoma with rituximab. J Am Acad Dermatol. 2005;52(5):5–847. doi: 10.1016/j.jaad.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 25.Dreno B. Standard and new treatments in cutaneous B-cell lymphomas. J Cutan Pathol. 2006;33(Suppl 1):47–51. doi: 10.1111/j.0303-6987.2006.00544.x. [DOI] [PubMed] [Google Scholar]