CASE REPORT
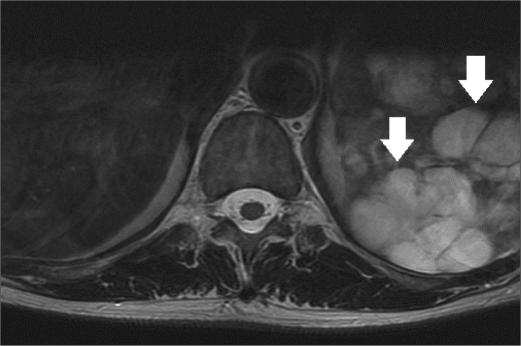
A 56-year-old woman saw her primary care provider for progressive left flank pain with bulging of the left side of the abdomen. A magnetic resonance imaging (MRI) study of the thoracic spine without contrast enhancement incidentally discovered the spleen to be enlarged and largely replaced with cystic lesions (Figure 1). Further discussions with the patient revealed that she had presented to an outside hospital 3 years earlier and had been diagnosed with biliary pancreatitis. A computed tomography (CT) study of the abdomen had been performed and was reported to show splenomegaly with multiple splenic cysts of various sizes. An emergent laparoscopic cholecystectomy was performed during the patient's admission to the outside hospital. The operation was complicated by postoperative bleeding, requiring exploration. No active bleeding was encountered; however, a large amount of hemoperitoneum was seen. Rupture of a splenic cyst was proposed as the source of the hemoperitoneum. The patient recovered well and in the interim between presentations also underwent an open Roux-en-Y gastric bypass for morbid obesity.
Figure 1.
Axial T2-weighted image of the lower thoracic spine shows diffuse replacement of the spleen with hyperintense lesions of various sizes (arrows).
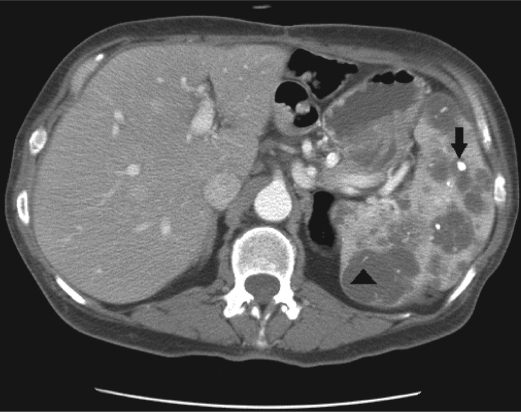
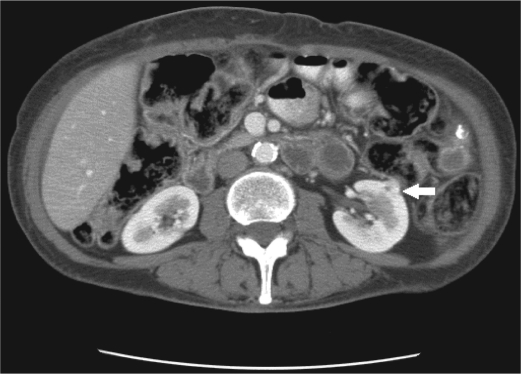
On examination at Baylor University Medical Center at Dallas, a reducible midline incisional hernia was found and the spleen was palpated four fingerbreadths below the left costal margin. A CT study of her abdomen with intravenous contrast enhancement showed splenomegaly with near complete replacement of the normal splenic parenchyma by cysts of various sizes, some of which had peripheral curvilinear calcification or central coarse calcification (Figure 2). An incidental 1-cm exophytic solid mass was also discovered arising from the interpolar left kidney, concerning for renal cell carcinoma (Figure 3).
Figure 2.
Axial CT image of the upper abdomen shows splenomegaly with multiple hypoattenuating lesions of various sizes. These lesions contain both coarse central calcifications (arrow) as well as curvilinear peripheral calcifications (arrowhead).
Figure 3.
Axial CT image at the level of the kidneys shows a 1-cm solid exophytic lesion arising from the anterior interpolar left kidney (arrow).
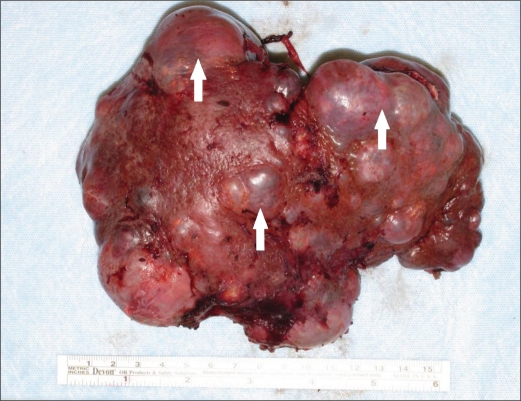
An open surgery was planned for wedge resection of the kidney lesion, splenectomy, and incisional hernia repair. Two weeks prior to the planned operation, the patient received pneumococcal, meningococcal, and haemophilus influenza type B vaccinations. Intraoperatively, an extensive adhesiolysis was performed. This was followed by splenectomy, wedge resection of the left kidney lesion, and ventral hernia repair with mesh. There was no evidence of echinococcal disease or splenosis in the abdomen. The spleen was enlarged and appeared to be almost completely replaced by a cystic process (Figure 4).
Figure 4.
Gross photograph shows extensive replacement of the spleen by a cystic process (arrows).
DIAGNOSIS: Splenic hemangiomatosis with incidental clear cell renal carcinoma.
DISCUSSION
The spleen is a highly vascular organ composed of red pulp, which makes up the majority of the spleen and is the site of phagocytosis, and white pulp, which is the lymphoid component. With these distinct components, the spleen performs a variety of functions, including filtration, erythrocyte culling, platelet reservoir, hematopoiesis, as well as immune surveillance and response (1).
Given its varying functions, the spleen is susceptible to a wide range of conditions: infectious and inflammatory processes, vascular and hematologic disorders, benign and malignant neoplasms (whether primary, secondary, or hematolymphoid), and storage diseases. Classically, these conditions have been divided into those affecting the red pulp and those affecting the white pulp. While not perfect, these categories serve as a useful method to provide a systematic approach to splenic pathology. Disorders predominantly affecting the white pulp include the varying causes of reactive hyperplasia (e.g., rheumatoid arthritis, immune thrombocytopenic purpura, and acquired immune deficiency syndrome) as well as malignant lymphomas and related lymphoproliferative disorders (e.g., chronic lymphocytic leukemia, large B-cell lymphoma, T-cell lymphoma, and Hodgkin lymphoma). Disorders predominantly affecting the red pulp include infections (e.g., mononucleosis), congestion (e.g., hemolytic anemia), storage disorders (e.g., Gaucher disease), leukemia (e.g., chronic myelogenous leukemia and hairy cell leukemia), nonhematopoietic tumors (e.g., cysts, hamartomas, hemangiomas, lymphangiomas, littoral cell angiomas, angiosarcomas, and metastases), and peliosis (2).
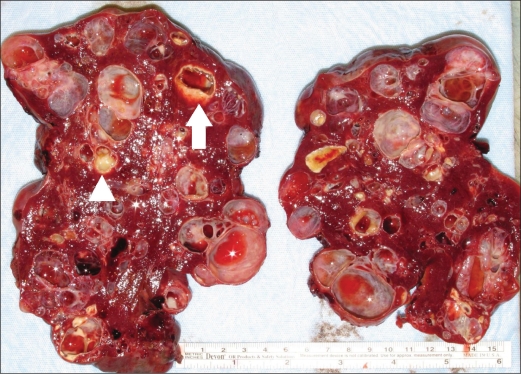
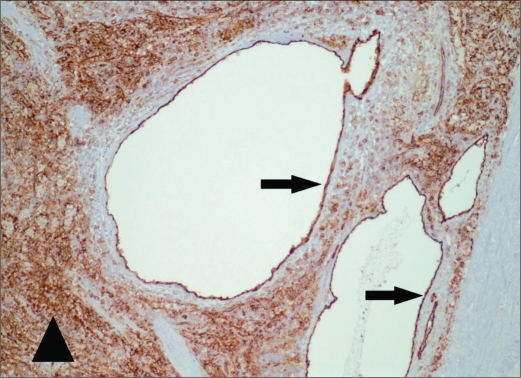
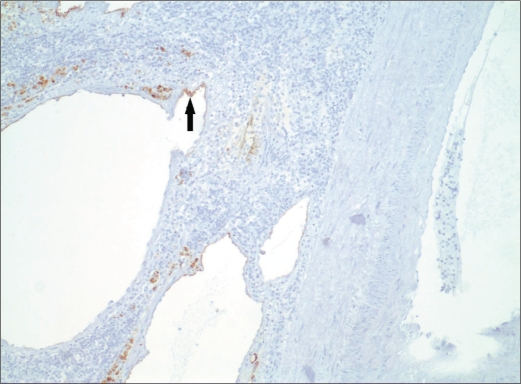
In our patient, the gross pathologic specimen showed a diffusely lobulated spleen weighing 422 g (average spleen weight in adults, 150 g) (1). Numerous cysts ranging in size from 0.1 to 6.0 cm replaced approximately 60% of the splenic parenchyma. On sectioning, these cysts contained either bloody or clear serous fluid, and many had areas of calcific deposits (Figure 5). A single layer of flattened cells lined the cystic spaces. This lining was strongly positive with CD31 stain, a vascular marker, and largely negative with D2-40 stain, a lymphatic marker (Figures 6 and 7). This finding confirmed the diagnosis of splenic hemangiomatosis as opposed to lymphangiomatosis or peliosis.
Figure 5.
Gross photograph of the spleen bisected along its long axis shows multiple cystic lesions ranging in size from 0.1 to 6 cm in diameter. Central calcifications (arrowhead) and peripheral calcifications (arrow) are present in many of these lesions.
Figure 6.
Photomicrograph of the spleen (original magnification ×100) shows a representative cystic lesion with CD31 immunohistochemical stain, a vascular marker. The thin cellular lining (arrows) is strongly positive, consistent with vascular endothelium. The sinusoidal endothelial cells of the red pulp (arrowhead) also show strong positivity, as expected.
Figure 7.
Photomicrograph of the spleen (original magnification ×100) shows the cystic lesions present in Figure 5 with D2-40 immunohistochemical stain, a lymphatic marker. This marker is largely negative, with sparse focal positivity in the cellular lining (arrow).
While hemangiomas are rare, they are the most common benign primary tumor of the spleen. They can range in size from capillary to cavernous, with most being cavernous type. Cavernous hemangiomas of the spleen can undergo thrombosis and infarction, resulting in the formation of central coarse calcifications. Rim calcification may also be present in the cystic spaces that compose cavernous hemangiomas (3). Splenic hemangiomatosis, with diffuse replacement of the normal splenic parenchyma, is even more rare and is most frequently seen in cases of diffuse angiomatosis, such as Klippel-Trenaunay syndrome. Hemangiomas of the spleen are usually asymptomatic and incidentally discovered. Patients with large hemangiomas or hemangiomatosis may experience symptoms referable to splenomegaly, as in our case. Kasabach-Merritt syndrome (anemia, thrombocytopenia, and coagulopathy) and portal hypertension have been reported in patients with hemangiomatosis of the spleen (4, 5). In prior decades, 25% of patients diagnosed with splenic hemangioma presented with acute abdominal symptoms related to rupture (6). Hemangioma rupture may well have been the source of hemoperitoneum following cholecystectomy in our patient.
The imaging characteristics of splenic hemangiomas vary depending on its size and internal composition. With sonography, splenic hemangiomas usually appear as solid echogenic masses or complex masses with well-defined cystic components that may contain anechoic fluid or echogenic debris. Acoustic shadowing may be seen in lesions that contain calcium (7). With unenhanced CT scans, splenic hemangiomas may appear as hypoattenuating or isoattenuating well-defined masses or as complex cystic and solid lesions. With contrast administration, the solid-type lesions show homogenous avid enhancement. Cavernous splenic hemangiomas have been described to have mottled areas of heterogeneous attenuation on delayed imaging, as opposed to the classic early peripheral nodular enhancement with delayed centripetal enhancement seen in cavernous hemangiomas of the liver. This difference is believed to be the result of scattered, but mostly central, cystic spaces that do not contain vascular channels (3, 8). With MRI, most splenic hemangiomas are hypointense on T1-weighted images, although some may be isointense. They become hyperintense on T2-weighted images. MRI characteristics can be more complex if there has been hemorrhage, infarction, or thrombosis in the lesion (9).
Unfortunately, the imaging characteristics of splenic hemangiomas are not specific. The differential considerations for a solid mass of the spleen include metastases, lymphoma, hamartoma, and angiosarcoma. The differential for a cystic lesion of the spleen includes lymphoma, metastases, abscess, cyst, lymphangioma, and hematoma (7). Of splenic metastases, melanoma and adenocarcinoma are most common (10). The diffuse involvement of the spleen in our case with areas of calcification pointed to a nonmalignant process, with hemangiomatosis the leading consideration. Echinococcal infection, lymphangiomatosis, and peliosis of the spleen were the main differential considerations in our case. Peliosis is poorly understood and has been described in association with anabolic steroid use, tuberculosis, hematologic diseases (myeloma, aplastic anemia), and disseminated cancer. In this disease, there is diffuse formation of blood-filled spaces in the spleen with concomitant involvement of the liver in most cases. This differs from hemangiomatosis in that the blood-filled spaces do not classically have an endothelial lining (3). Given the poor specificity of imaging and the risks associated with percutaneous biopsy of the spleen, splenectomy is frequently performed when definitive characterization of splenic lesions is needed.
References
- 1.Chadburn A. The spleen: anatomy and anatomical function. Semin Hematol. 2000;37(1 Suppl 1):13–21. doi: 10.1016/s0037-1963(00)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.Burke JS. The spleen. In: Mills SE, editor. Sternberg's Diagnostic Surgical Pathology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 849–878. [Google Scholar]
- 3.Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. Primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24(4):4–1137. doi: 10.1148/rg.244045006. [DOI] [PubMed] [Google Scholar]
- 4.Shanberge JN, Tanaka K, Gruhl MC. Chronic consumption coagulopathy due to hemangiomatous transformation of the spleen. Am J Clin Pathol. 1971;56(6):6–723. doi: 10.1093/ajcp/56.6.723. [DOI] [PubMed] [Google Scholar]
- 5.Pitlik S, Cohen L, Hadar H, Srulijes C, Rosenfeld JB. Portal hypertension and esophageal varices in hemangiomatosis of the spleen. Gastroenterology. 1977;72(5 Pt 1):937–940. [PubMed] [Google Scholar]
- 6.Husni EA. The clinical course of splenic hemangioma with emphasis on spontaneous rupture. Arch Surg. 1961;83:681–688. doi: 10.1001/archsurg.1961.01300170037008. [DOI] [PubMed] [Google Scholar]
- 7.Ros PR, Moser RP, Jr, Dachman AH, Murari PJ, Olmsted WM. Hemangioma of the spleen: radiologic-pathologic correlation in ten cases. Radiology. 1987;162(1 Pt 1):73–77. doi: 10.1148/radiology.162.1.3538155. [DOI] [PubMed] [Google Scholar]
- 8.Ferrozzi F, Bova D, Draghi F, Garlaschi G. CT findings in primary vascular tumors of the spleen. AJR Am J Roentgenol. 1996;166(5):5–1097. doi: 10.2214/ajr.166.5.8615251. [DOI] [PubMed] [Google Scholar]
- 9.Elsayes KM, Narra VR, Mukundan G, Lewis JS, Jr, Menias CO, Heiken JP. MR imaging of the spleen: spectrum of abnormalities. Radiographics. 2005;25(4):4–967. doi: 10.1148/rg.254045154. [DOI] [PubMed] [Google Scholar]
- 10.Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996;16(1):1–107. doi: 10.1148/radiographics.16.1.107. [DOI] [PubMed] [Google Scholar]