Abstract
Optical coherence tomography (OCT) has become a well established imaging tool in ophthalmology. The unprecedented depth resolution that is provided by this technique yields valuable information on different ocular tissues ranging from the anterior to the posterior eye segment. Polarization sensitive OCT (PS-OCT) extends the concept of OCT and utilizes the information that is carried by polarized light to obtain additional information on the tissue. Several structures in the eye (e.g. cornea, retinal nerve fiber layer, retinal pigment epithelium) alter the polarization state of the light and show therefore a tissue specific contrast in PS-OCT images. First this review outlines the basic concepts of polarization changing light–tissue interactions and gives a short introduction in PS-OCT instruments for ophthalmic imaging. In a second part a variety of different applications of this technique are presented in ocular imaging that are ranging from the anterior to the posterior eye segment. Finally the benefits of the method for imaging different diseases as, e.g., age related macula degeneration (AMD) or glaucoma is demonstrated.
Keywords: Optical coherence tomography, Polarization sensitive imaging, Retina, Age related macula degeneration, Glaucoma, Cornea
1. Introduction
Optical coherence tomography (OCT) (Drexler and Fujimoto, 2008a; Fercher et al., 2003; Huang et al., 1991) is now a well established tool for high-resolution cross sectional and three-dimensional imaging of transparent and translucent tissues. Based on low-coherence interferometry (Fercher et al., 1991, 1988; Hitzenberger, 1991), it measures the echo time delay and magnitude of backscattered or –reflected light to build up two- and three-dimensional images with a resolution of ∼20 μm (transversal) × 5 μm (axial) for commercial systems, and of 2–3 μm (isotropic) for high-end research systems (Cense et al., 2009b; Fernandez et al., 2008; Zawadzki et al., 2008). Although in the meantime several imaging applications of OCT have been reported – from medical applications like dermatology, cardiology, neurology, gastro-intestinal imaging, etc., to developmental biology to materials sciences – the first, and still dominating, application field was ophthalmology (Fercher et al., 1993; Hee et al., 1995; Huang et al., 1991; Swanson et al., 1993). Ocular imaging is an ideal application field for OCT because the transparency of major ocular media provides a nearly unrestricted access of optical radiation to the most important structures like cornea and retina. It is, therefore, not surprising that roughly 50% of all scientific publications on OCT were published in ophthalmology journals (Drexler and Fujimoto, 2008b), and a still growing number of companies is marketing ophthalmic OCT systems.
The enormous progress and success of OCT in recent years can largely be attributed to a paradigm shift in OCT technology. While the first generation of OCT systems was based on the time domain principle (TD-OCT) – for each A-scan (depth profile), a reference mirror was shifted over the probing depth, which limited the speed to ∼100–400 A-scans/s – the newer generation is based on the spectral domain (or Fourier domain) approach (SD-OCT) (Fercher et al., 1995; Häusler and Lindner, 1998; Wojtkowski et al., 2002): instead of shifting the reference mirror, a stationary mirror is used. The light exiting the interferometer is spectrally dispersed by a grating onto a line scan camera, and a fast Fourier transform of the spectral interference signal provides the depth profile, i.e. the entire depth profile is recorded in a single shot. The A-scan speed is only limited by the data acquisition rate and transfer speed of the camera. Moreover, this massively parallel approach provides a huge sensitivity improvement of about 2 orders of magnitude (Choma et al., 2003; de Boer et al., 2003; Leitgeb et al., 2003a). These advantages enabled 3D retinal imaging at speeds of ∼30,000 A-lines/s for commercial instruments and up to 300,000 A-lines/s in experimental systems (Potsaid et al., 2008; Srinivasan et al., 2008). More details on the principles of the OCT technique, its historic development, and state of the art, can be found in several review papers and textbooks (Bouma and Tearney, 2002; Drexler and Fujimoto, 2008a, b; Fercher, 1996; Fercher et al., 2003; Fercher and Hitzenberger, 2002; Geitzenauer et al., 2011; Schuman et al., 2004; van Velthoven et al., 2007; Wojtkowski, 2010).
Despite its great success that revolutionized ocular imaging and diagnostics, conventional, intensity based OCT still has a considerable drawback: it cannot directly differentiate between different tissues. Especially in ocular diseases where retinal layers are damaged, distorted, displaced, disrupted or vanished, it is often difficult to identify specific layers based on backscattered intensity. This, however, is mandatory for correct diagnosis, monitoring, and follow up. To overcome this and other limitations, intense research is going on to gain additional information beyond signal intensity. The most promising of these are Doppler OCT which provides information on blood flow (Chen et al., 1997; Izatt et al., 1997; Leitgeb et al., 2003b; Wang et al., 2007; Werkmeister et al., 2008) and can be used for generating vessel contrast (An et al., 2010; Makita et al., 2006; Szkulmowska et al., 2009; Zotter et al., 2011), and polarization sensitive (PS)-OCT (de Boer et al., 1997; Hee et al., 1992), the topic of this review.
PS-OCT measures the polarization state of light and is based on the fact that some tissues can change the polarization state of the probing light beam. Different mechanisms of light–tissue interaction can cause such changes of the polarization state: birefringence, diattenuation, and depolarization. While diattenuation is regarded to be negligible in biologic tissue (Kemp et al., 2005b; Park et al., 2004; Todorovic et al., 2004), the other two interaction mechanisms can be found in various ocular tissues: examples of birefringent tissue are the corneal stroma (Bour, 1991; Götzinger et al., 2004), the sclera (Baumann et al., 2007; Yamanari et al., 2008a), ocular muscles and tendons, trabecular meshwork (Baumann et al., 2007; Yamanari et al., 2008a), the retinal nerve fiber layer (Cense et al., 2004b; Weinreb et al., 1990), Henle’s fiber layer (klein Brink and van Blokland, 1988; Pircher et al., 2004b), and scar tissue (Michels et al., 2008). Depolarization can be found in melanin containing tissue like the retinal pigment epithelium (RPE) (Götzinger et al., 2008c; Pircher et al., 2006; Pircher et al., 2004b), the iris pigment epithelium (Pircher et al., 2004a), choroidal naevi and melanoma (Götzinger et al., 2009b), but also in accumulations of pigment loaded macrophage and – to a smaller amount – in the choroid (Baumann et al., 2010; Götzinger et al., 2008c).
In this review, we will provide an overview of the fundamentals of polarization changing light–tissue interactions, of the technical principles of PS-OCT, and of various applications of PS-OCT to generate improved tissue contrast, perform quantitative measurements, and to segment retinal layers and lesions based on their intrinsic, tissue specific contrast.
2. Fundamentals of polarization changing light–tissue interactions
As known from microscopy many objects yield poor contrast if imaged only on the basis of light intensity. To increase image contrast and/or to retrieve additional information on the sample, polarization properties of light can be utilized. Light can be described as an electromagnetic wave, where both field vectors (of the magnetic and electric field, respectively) are orthogonal to the propagation direction of the light and orthogonal to each other (Born and Wolf, 1999). Because of the wave properties of the light the field vectors are oscillating, resulting in a change of the vector with time and space. To assess the wave properties the electromagnetic wave can be described by amplitude (related to the light intensity) and phase. The phase determines the state of the electric field within the cycle of one oscillation of the field vector.
For simplicity we shall neglect in the following the magnetic field vector and focus only on the electric field vector. Light is known as linear polarized when the electric field vector remains within a single plane (that is spanned by the vector of propagation direction and the electric field vector) as the wave propagates. In general, a polarized light beam can be in an arbitrary, so called elliptical polarization state. Any arbitrary polarization state can be described as a combination of two orthogonal linear polarization states (Huard, 1997).
A special case of elliptically polarized light is circularly polarized light. In this case there is a phase difference of 90° between two orthogonal polarization states of the same amplitude which results in a rotation of the total electric field vector (sum of both electric field vectors) during its propagation that can be clockwise or counter clockwise around the axis of light propagation. Depending on the direction of the rotation, the polarization state is known as right hand or left hand circularly polarized light. The propagation of polarized light can mathematically be described using either Jones (Jones, 1941) or Mueller formalism (Mueller, 1943). While the Jones matrix formalism is sufficient for all cases utilizing fully polarized light, the Mueller calculus also can describe partially or depolarized light.
Several light–tissue interactions may alter the polarization state of light: birefringence, diattenuation and depolarization. Here and in the following context we want to consider only linear materials (i.e. the polarization vector is linearly proportional to the electric field) (Haskell et al., 1989). Birefringence is introduced by anisotropy of material. In that case the refractive index of the material (determined by the speed of light within the material) depends on the direction of light propagation and on the polarization state of the light. In biological tissue this anisotropy is often generated by a compounding of at least two homogeneous dielectric materials. However, to observe measurable birefringence, these materials have to be arranged in an organized manner as in, e.g., fibrils that are surrounded my material of different refractive index. This type of birefringence is referred to as form birefringence (Haskell et al., 1989; Wiener, 1912) that can be found in several tissue types (e.g. tendons, muscle, nerve, cartilage).
When light traverses a birefringent material, a relative delay between two orthogonal polarization states is introduced that accumulates with thickness of the material. This delay is known as phase retardation and is ambiguous with respect to the period of light oscillation (delays that exceed multiples of the wavelength of the light cannot be distinguished from delays that are smaller than the wavelength of the light and lead to a banding structure in the images, see chapter 4 and Fig. 7 for an example).
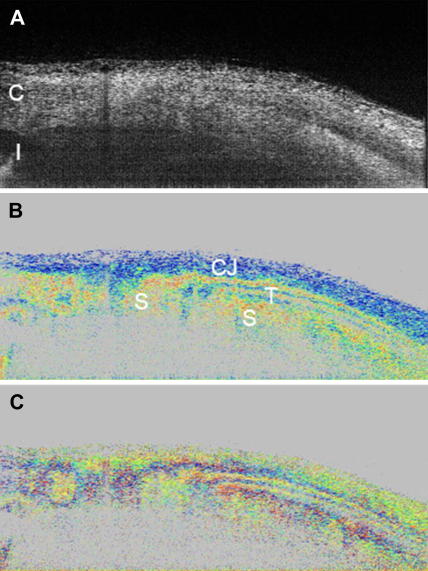
Fig. 7.
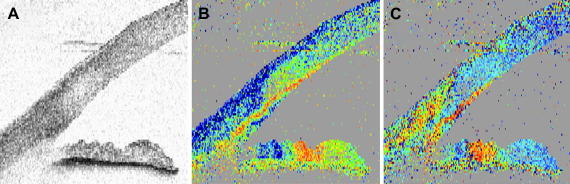
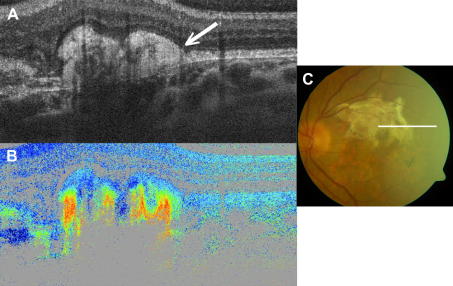
PS-OCT of external ocular tissue. The intensity image is displayed on a log. scale (A). Retardation image shows strong birefringence in this tissue region (B). Color scale: blue δ = 0°, red δ = 90°. (C) Fast axis orientation. Color scale: blue θ = −90°, red θ = +90°. C: cornea, I: iris, CJ: conjunctiva, S: sclera, T: ocular tendon (reprinted from Baumann et al. (2007)).
Scattering, on the other hand, may also alter the polarization state of the light. Even in the case of a single scattering event the polarization state of the scattered light depends on the scattering particle (size and shape), the direction of the scattered light and the incident polarization state (de Boer and Milner, 2002; Schmitt and Xiang, 1998). In multiply scattered light even small changes introduced to the polarization state by a single scattering event are accumulated which finally leads to a completely random polarization state or depolarization (Mishchenko and Hovenier, 1995).
3. Technical principles of PS-OCT
OCT is based on measuring the backscattered light intensity from nearly transparent and translucent tissues. In addition, the polarization state of the backscattered light contains valuable information on characteristic tissue parameters as, e.g., birefringence or depolarization that can be exploited using polarization sensitive OCT. Therefore, only one year after the introduction of OCT in 1991, a first PS low-coherence interferometer had been realized (Hee et al., 1992). Up to now several different PS-OCT techniques have been developed and demonstrated to access the information inherently present in the polarization state. These techniques differ in the number of measurements required per sample location, the number of polarization states with which the sample has to be illuminated, and in the number of accessible polarization parameters (from a simple phase retardation measurement to fully determined Mueller and Jones matrices), as well as the used OCT technique. A full description of all these techniques is beyond the scope of this paper. Therefore we restrict this review to techniques that have been widely used in ophthalmology.
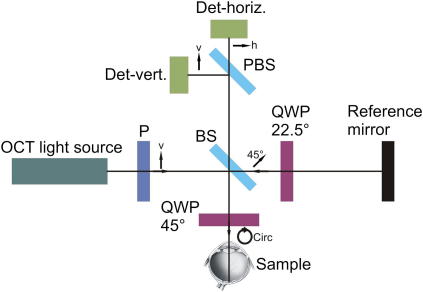
Although the first presented PS-OCT system was based on the meanwhile outdated time domain OCT, some of the modern systems are based on this initial setup and therefore we decided to use this setup to explain several key features of PS-OCT in more detail. Fig. 1 shows a scheme of the setup. The light from the OCT light source (broadband light source or swept laser source, i.e., a fast tunable light source) is vertically linear polarized by a polarizer before entering a Michelson interferometer. A beam splitter splits the incoming light into a sample beam and a reference beam. The light in the reference arm traverses a quarter wave plate (QWP) oriented at 22.5° which ensures that the light is linearly polarized with a polarization plane of 45° with respect to the incident light after traversing the QWP twice. This provides equal reference light intensity in both detection channels. In the sample arm the light traverses a QWP oriented at 45° which results in circularly polarized light that is incident on the sample. This ensures that the measured polarization parameters are independent from the birefringent axis orientation of the sample. The light backscattered from the sample is, in general, in an elliptical polarization state; it is recombined with the light from the reference arm at the beam splitter.
Fig. 1.
Schematic diagram of a polarization sensitive optical coherence tomography system. P – polarizer, BS – beam splitter, QWP – quarter wave plate, PBS – polarizing beam splitter, Det – detector, v – vertical, h – horizontal.
Before detection, the two linear polarization states (horizontal and vertical) are separated by a polarizing beam splitter. The detectors record the amplitude as well as the phase of the interferometric signals in order to extract the polarization parameters. The polarization state of the light traversing the OCT system can be described using Jones matrix formalism (de Boer et al., 1998; Everett et al., 1998; Hitzenberger et al., 2001) or Stokes vector and Mueller matrix formalism (de Boer et al., 1999; Jiao et al., 2000). Several parameters can be calculated from the recorded data. The total backscattered light reflectivity R(z), which is encoded in the sum of the squared amplitudes (A1 and A2) of the two channels (Hee et al., 1992):
| (1) |
The phase retardation δ(z), which is encoded in the quotient of the amplitudes (Hee et al., 1992):
| (2) |
The birefringent axis orientation θ(z), which is encoded in the phase difference between the channels (Hitzenberger et al., 2001):
| (3) |
And finally the Stokes vector elements (I, Q, U, V) that can be calculated via (Götzinger et al., 2008c).
| (4a) |
| (4b) |
| (4c) |
| (4d) |
In this context it should be noted that OCT is a coherent imaging technique. Therefore the degree of polarization (DOP) at a single measurement point will always be one (Jiao et al., 2000), preventing a direct measurement of the DOP. Nevertheless, as will be shown later on, information on depolarization of the backscattered light can be assessed and quantified with PS-OCT, providing tissue specific contrast.
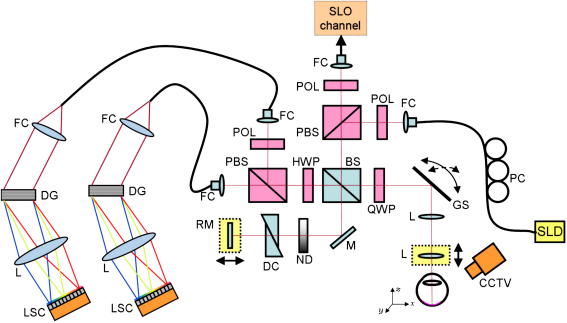
A typical PS-OCT instrument that has been used on a large number and variety of patients is shown in Fig. 2. To enable a fast alignment of the patient the instrument incorporates a scanning laser ophthalmoscope (SLO) channel (Baumann et al., 2010). The system is based on Fourier domain OCT and uses two identical spectrometers. The typical imaging speed of the instrument is 20,000 A-scans per second. One problem of spectrometer based SD PS-OCT is that the polarization sensitive detection as shown in Fig. 2 requires two identical spectrometers and therefore increases the experimental efforts. To reduce these efforts different groups introduced single camera FD-OCT systems (Baumann et al., 2007; Cense et al., 2007) for ophthalmic imaging, which, however, have the drawback of limited bandwidth accommodated by the single camera which limits the axial resolution.
Fig. 2.
PS-OCT instrument used for clinical studies with incorporated SLO channel for patient alignment. Components: SLD, superluminescent diode. PC, polarization controller. FC, fiber coupler. POL, polarizer. PBS, polarizing beam splitter. BS, non-polarizing beam splitter. QWP, quarter wave plate. GS, galvanometer scanner. L, lens. M, mirror. ND, variable neutral density filter. DC, dispersion compensation. RM, reference mirror. HWP, half wave plate. DG, diffraction grating. LSC, line scan camera. CCTV, camera for pupil observation. (reprinted from Baumann et al. (2010)).
It should be noted that different PS-OCT configurations have been used for ophthalmic imaging. Besides bulk optics systems, similar to the ones described above (Ducros et al., 1999; Götzinger et al., 2004), fiber based systems have been utilized. However, because of the fibers which may alter the polarization state of the light these systems need two measurements per sample location (with different incident polarization states that are orthogonal on the Poincaré sphere (Cense et al., 2002)) to retrieve the polarization sensitive data. This system has the advantage that the influence of the cornea (which is birefringent) on retinal polarization sensitive measurements is automatically eliminated. Instruments that use only a single input state need an additional post-processing step to eliminate this influence (Pircher et al., 2007). Recently, an instrument has been introduced that is based on polarization maintaining fibers which overcomes the need of two measurements per sample location (Götzinger et al., 2009a).
Finally, we want to mention that polarization sensitivity can be incorporated into all known OCT techniques such as spectrometer based Fourier domain systems (Götzinger et al., 2005; Yamanari et al., 2008b) or swept source OCT systems (Yamanari et al., 2008a). Therefore, identical resolutions (transverse and in depth) can be obtained with PS-OCT as in state of the art intensity based OCT. Moreover we want to emphasize that PS-OCT provides both, polarization sensitive images and reflectivity images (as standard OCT), simultaneously. To improve the depth resolution in OCT, broadband light sources have to be utilized (Drexler, 2004) which is no limitation for PS-OCT (Götzinger et al., 2009a). The transverse resolution in OCT is limited by the optics of the eye, which may introduce aberrations to the imaging beam, finally degrading image quality. To compensate these aberrations, adaptive optics (AO) can be used (Bigelow et al., 2007; Jonnal et al., 2003; Pircher and Zawadzki, 2007; Pircher et al., 2008; Torti et al., 2009; Zawadzki et al., 2005; Zhang et al., 2006) to achieve a diffraction limited transverse resolution of 2–3 μm. Recently a first combination of AO with PS-OCT has been demonstrated (Cense et al., 2009a).
4. Anterior eye segment imaging with PS-OCT
It is well known that the cornea is optically birefringent (Bour, 1991) and several papers investigating its birefringent properties with conventional (i.e., non-OCT) methods have been published so far (Bille et al., 1997; Francois and Feher, 1972; Stanworth and Naylor, 1950; Van Blokland and Verhelst, 1987). However, none of these techniques provided depth resolved information. The origin of corneal birefringence lies in the anatomical structure of the corneal stroma which consists of several lamellae, each lamella containing parallel fibrils that introduce form birefringence. Although this basic mechanism of corneal birefringence is beyond controversy, the studies mentioned above suggested different models of total corneal birefringence which are partly contradictory. E.g., the cornea has been modeled as a uniaxial (Stanworth and Naylor, 1950) and a biaxial crystal (Van Blokland and Verhelst, 1987).
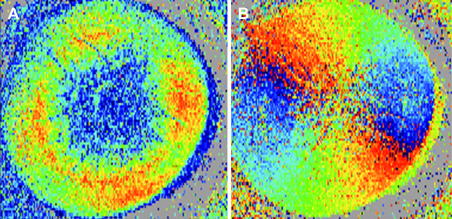
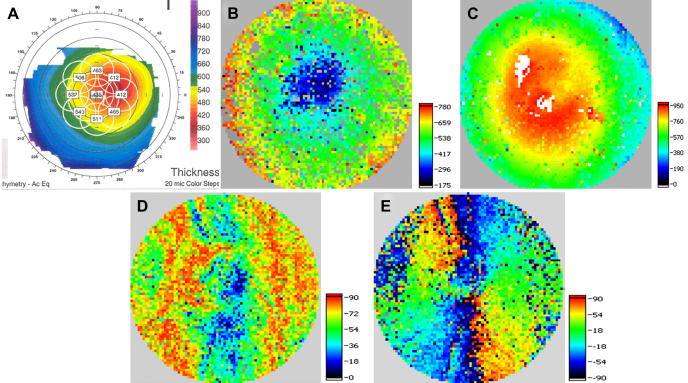
First PS-OCT instruments that investigated the cornea were limited to in vitro experiments because the imaging speed at that time was very low (Ducros et al., 1999; Götzinger et al., 2004). Fig. 3 shows a representative PS-OCT B-scan located at the center of the cornea (Pircher et al., 2004a). The intensity image (Fig. 3A) shows the shape of the cornea but otherwise the contrast within the cornea is very weak. On the other hand the additional contrast provided by the PS-OCT images is clearly visible (c.f. Fig. 3B and C). In all images only values above a certain intensity threshold are displayed, values below this threshold are displayed in gray. Because of different fibrils orientation with respect to the imaging beam (caused by the shape of the cornea and the fibril orientation within the cornea) an increase of retardation can be observed at the periphery of the cornea, whereas in the center of the cornea the retardation is rather low. A better impression of the retardation pattern introduced by the cornea is obtained by 3 dimensional imaging of the cornea. Fig. 4 shows en face images retrieved from the posterior surface of the cornea out of a 3D data set consisting of 490 × 100 × 100 (x, y and z direction) pixels and covering an area of 8 × 8 × 2.5 mm3. Clearly visible is a radially symmetric retardation pattern and a change of axis orientation which is an approximately linear function of the azimuth angle. Note that Fig. 4B shows a cumulative axis orientation which is related to the fibril orientation within the cornea. Recently, different theoretical models were introduced in order to explain the observed polarization sensitive image patterns (Fanjul-Vellez et al., 2010). These normal polarization patterns can be severely distorted in the presence of corneal diseases. Fig. 5 shows images of the cornea retrieved from a patient (male, 52 years) with keratoconus (Götzinger et al., 2007). The Orbscan (Fig. 5A) and the OCT thickness map (Fig. 5B) clearly indicate the thinning of the cornea that is caused by the disease. Although the local surface inclination is similar to the normal corneas investigated (as can be seen in the surface elevation map in Fig. 5C) the retardation (Fig. 5D) as well as the axis orientation patterns (Fig. 5E) are heavily distorted. Therefore, it is speculated that the origin of this distortion lies in a change of the lamellar structure of the cornea that has also been observed in this disease by x-ray scattering techniques (Daxer and Fratzl, 1997; Meek et al., 2005).
Fig. 3.
Representative B-scan of human cornea in vitro. (A) Intensity (standard OCT image), (B) retardation δ (Blue δ = 0, red δ = 90°), and (C) fast axis orientation θ (blue θ = –90°, red θ = +90°) (reprinted from Pircher et al. (2004a)).
Fig. 4.
En face images retrieved from the posterior surface of a human cornea in vitro. (A) retardation, (B) fast axis orientation (same color scales as in Fig. 3, reprinted from Pircher et al. (2004a)).
Fig. 5.
Images of keratoconus cornea. Orbscan thickness map in vivo (A) (color bar: μm), corneal thickness map derived from 3D PS-OCT data set in vitro (color bar: μm) (B), and anterior surface elevation map (color bar: μm) (C). The polarization sensitive images show a severely distorted pattern in the retardation at posterior corneal surface (D) as well as in the cumulative slow axis (deg) ∼100 μm anterior to posterior corneal surface (E). (reprinted from Götzinger et al. (2007)).
Such a change of the lamellar structure will eventually influence the corneal shape and its mechanical stability. PS-OCT might be useful to detect early changes of fibril orientation, prior to any macroscopically visible structural changes, and therefore might be a valuable tool for early diagnostics of keratoconus, possibly enabling the investigation and follow up of the development of the disease.
With the development of advanced OCT techniques, in vivo measurements became possible. Fig. 6 shows one of the first PS-OCT images of a human chamber angle in vivo. Beside the structural information provided by the intensity image (Fig. 6A) similar polarization sensitive information (increasing birefringence at the periphery of the cornea) as in the in vitro case can be obtained (Fig. 6B and C). Noticeable is the polarization preserving character of the stroma of the iris. The polarization state measured from this tissue resembles the polarization state obtained at the posterior surface of the cornea (the same retardation and axis orientation patterns are observed at the posterior surface of the cornea and at the stroma of the iris). Other interesting polarization features are observed at the pigment epithelium of the iris which scrambles the backscattered polarization state (characterized by the randomly varying retardation and axis orientation values measured at this layer) and a change within axis orientation at the corneal limbus which can be attributed to the different fibril orientation within the cornea in the fusion region between cornea and sclera.
Fig. 6.
Images of human anterior chamber angle in vivo. (A) Intensity, (B) retardation δ (blue δ = 0, red δ = 90°), and (C) fast axis orientation θ (blue θ = −90°, red θ = +90°) (each image covers an area of 5 × 5 mm2, reprinted from Pircher et al. (2004a)).
The sclera itself is birefringent as shown in Fig. 7. Within the polarization sensitive images (c.f. Fig. 7B and C) different tissue types as tendon, conjunctiva and sclera can be distinguished. Especially tendon, composed of parallel fibrils, shows very strong birefringence, giving rise to a banding structure that does not correspond to an anatomical banding. Instead it is caused by the 2π ambiguity of phase retardation measurements (delays of δ, δ + 2π, δ + n(2π) cannot be distinguished). For the special algorithm used here (Eq. (2)), this ambiguity causes that, whenever the phase retardation exceeds 90° (red color) the true phase retardation value will be mapped into a region between 0° and 90° leading to the observed color change (blue–red–blue–red). Additionally, the measured axis orientation is changed apparently because of this ambiguity and oscillates between two values even though the axis orientation of the highly birefringent tissue stays constant.
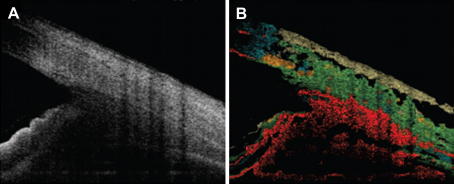
An even better differentiation between different tissues types can be achieved if more parameters (backscattered intensity, extinction coefficient and birefringence) are used for discrimination. Fig. 8 shows an example of tissue discrimination based on PS-OCT data (Miyazawa et al., 2009). Different tissue types can be distinguished: conjunctiva (light brown), sclera (green), trabecular meshwork (dark yellow), cornea (blue), and uvea (red).
Fig. 8.
Example of tissue discrimination based on PS-OCT. (A) intensity image, (B) pseudo color coded structural images. The light brown corresponds to conjunctiva, green indicates sclera, dark yellow indicates trabecular meshwork, blue indicates cornea, and red indicates uvea. (reprinted from Miyazawa et al. (2009)).
The capability of PS-OCT to discriminate between tissue types may improve the measurement of clinically relevant parameters of the anterior segment in glaucoma diagnostics (Yasuno et al., 2010).
5. PS-OCT in retinal imaging
5.1. PS-OCT in the macular region
Many diseases (e.g. age related macula degeneration (AMD), diabetic retinopathy) affect the macular region of the retina. The center of this region, the fovea, is most precious because (in the healthy eye) it corresponds to the location on the retina with the best vision. The retinal pigment epithelium (RPE) plays a fundamental role in the metabolism of the photoreceptors and is therefore a most relevant structure in all forms of AMD and other diseases.
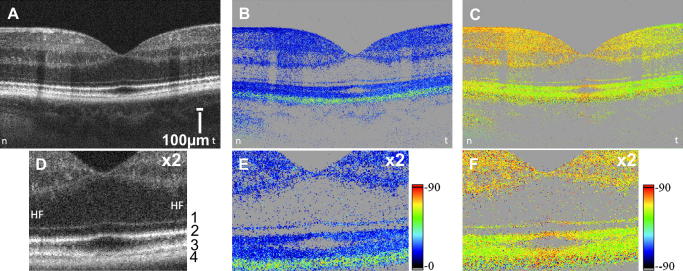
To demonstrate the capabilities of PS-OCT, Fig. 9 shows typical PS-OCT images of the macula of a healthy volunteer. Within the reflectivity image (standard OCT, c.f. Fig. 9A) different retinal layers can be visualized, however, the RPE (c.f. Fig. 9 D, layer 4) shows very similar contrast as other highly backscattering structures, e.g., the junction between inner and outer segments of photoreceptors (layer 2 in Fig. 9D) and end tips of photoreceptors (layer 3 in Fig. 9D). PS-OCT retrieves additional information that is available because of differing physical properties of the individual layers. As can be seen in the retardation image (Fig. 9B and E) as well as in the axis orientation image (Fig. 9C and F) the RPE clearly is distinct from other layers (c.f. greenish band in the retardation image). A detailed analysis of the polarization sensitive data reveals that the polarization state of the light backscattered from the RPE randomly varies from speckle to speckle, i.e. the RPE scrambles the polarization state of the backscattered light (Pircher et al., 2006, 2004b), while the polarization state of the light backscattered from other layers is either preserved (anterior retinal layers, photoreceptor layer) or smoothly altered over large distances because of birefringence (RNFL, Henle’s fiber layer, sclera). This is indicated in the PS-OCT images by the rather constant retardation and axis orientation values. It should be noted that the PS-OCT images presented in Fig. 9 were not corrected for the influence of anterior segment birefringence. However, for depolarization effects (as caused by the RPE) this influence can be neglected.
Fig. 9.
PS-OCT images of the fovea region of a healthy subject (left eye, reprinted from Pircher et al. (2006)). (A) Intensity image, (B) retardation image, (C) axis orientation image (horizontal axis orientation corresponds to 0°); magnified (×2) views are depicted in (D), (E) and (F). The posterior retinal layers are labeled with numbers from 1 to 4. HF: Henle’s fiber layer. Values below a certain intensity threshold are displayed in gray in (B), (C), (E), (F). (Images A, B, C consist of 3400 × 500 pixels).
As mentioned in chapter 3, the degree of polarization at a single point is not accessible with OCT, nevertheless depolarization can be observed by a polarization state that randomly varies from speckle to speckle (c.f. Fig. 9E). To quantify this observation we introduced the degree of polarization uniformity (DOPU), a value that is related to the degree of polarization (a kind of averaged DOP). DOPU is calculated via the averaged Stokes vector elements (Q, U, V) within a rectangular evaluation window (Götzinger et al., 2008c):
| (5) |
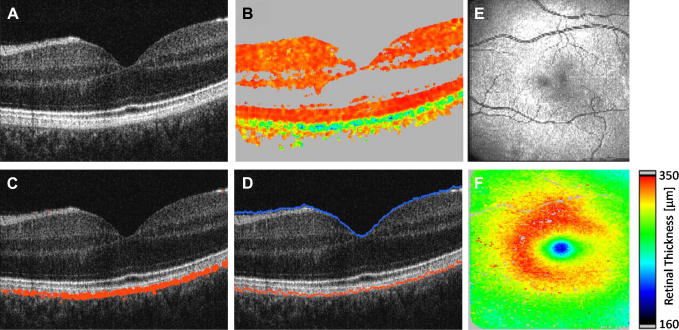
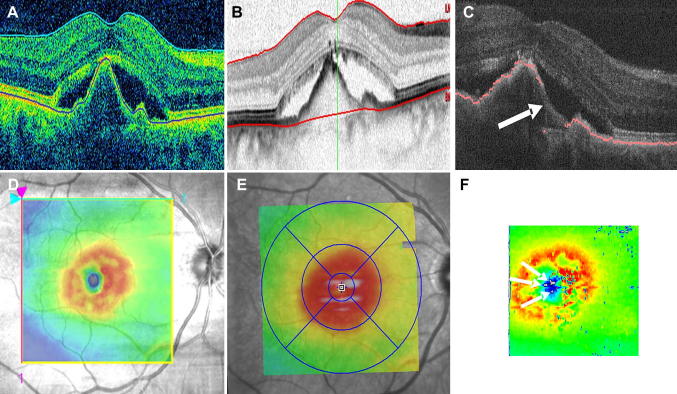
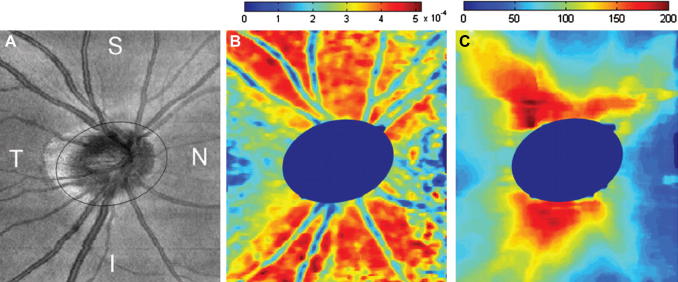
where the indices m indicate the mean normalized Stokes vector elements. In case of polarization preserving or birefringent tissue the DOPU value is approximately 1, while in depolarizing tissue this value is well below 1. By regarding structures with DOPU values below 0.65 (empirical value) as depolarizing, these structures (e.g. RPE) can be easily segmented within PS-OCT images. Note that in contrast to other segmentation algorithms that are based on intensity, this algorithm uses a different physical quantity which is changed by tissue specific properties. Fig. 10 shows different steps in the segmentation procedure. Within the DOPU image (c.f. Fig. 10B) the RPE can be clearly identified as greenish band (indicating low DOPU values). The fully automated segmentation algorithm applies a threshold to the DOPU image and provides an excellent estimation of the location of the RPE (c.f. Fig. 10C). Together with an intensity based algorithm that detects the inner limiting membrane (ILM) (c.f. Fig. 10D) retinal thickness maps can be generated (c.f. Fig. 10F).
Fig. 10.
PS-OCT images of the fovea region of a healthy volunteer (reprinted from Baumann et al. (2010)). (A) Intensity B-scan image. (B) DOPU B-scan image [color scale: DOPU = 0 (dark blue) to DOPU = 1 (red)]. Pixels with intensities below a certain threshold are displayed in gray. (C) Automatically segmented RPE (red) overlaid on the intensity image. (D) Location of ILM and RPE overlaid on reflectivity image in blue and red, respectively. (E) En face fundus image reconstructed from 3D OCT data set. (F) Retinal thickness map computed as geometric axial distance between ILM position and RPE position.
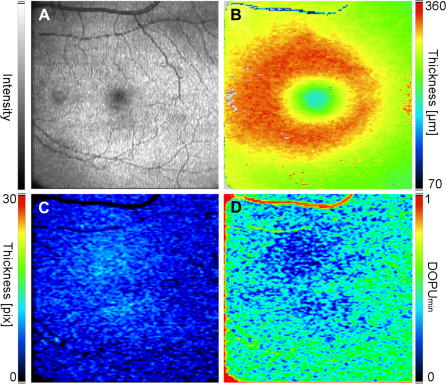
In addition the polarization sensitive data can be used to obtain quantitative data on the polarization scrambling characteristics of the RPE (Baumann et al., 2009). As shown in Fig. 11 the apparent thickness of the depolarizing layer changes with eccentricity from the fovea (it should be noted, however, that the measured thickness is a convolution of the actual thickness of the layer with the evaluation window of the DOPU calculation). The thickness shows a maximum in the fovea whereas it is decreased in the periphery. On the other hand stronger depolarization can be observed in the fovea which is reduced with larger eccentricities. The observed depolarization is most likely caused by multiple scattering at large non spherical particles (e.g. pigments) which show the highest density in the fovea. This scattering explains the above mentioned observations. Because of multiple scattering the path length of the light is increased which manifests itself within OCT images in a signal that apparently originates from a somewhat deeper position (and therefore, because not all of the photons undergo the same number of scattering events, to an apparent increase of the thickness of a layer). Additionally, each scattering event alters the polarization state which leads in the case of multiple scattering to a random polarization state or very low DOPU values.
Fig. 11.
En face images of the fovea region in a healthy volunteer (reprinted from Baumann et al. (2009)). (A) Fundus image reconstructed from PS-OCT data set. (B) Retinal thickness map. (C) Thickness Map of segmented RPE tissue. The polarization scrambling layer (PSL) appears slightly thickened in the center of the fovea, while its thickness decreases in the periphery. (D) DOPUmin map. Values of DOPUmin increase in the periphery indicating higher depolarization of light in the center and lower depolarization in the periphery.
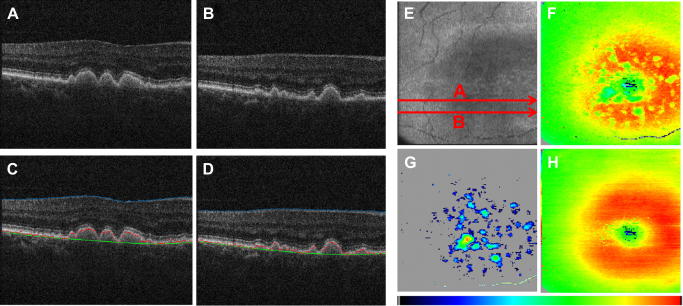
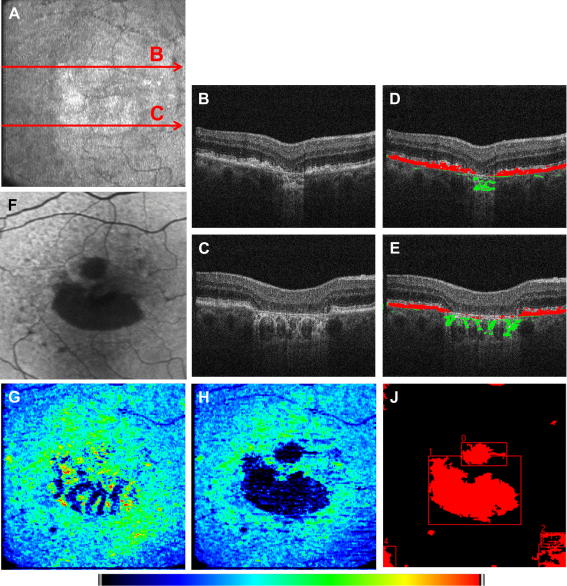
The automated RPE segmentation based on PS-OCT data can be used in a variety of different diseases. Typically, in a first step the RPE is segmented by its depolarizing effect. In a second step, the RPE is used as a “backbone” for segmentation of adjacent structures or associated lesions. Fig. 12 shows the performance of this method for drusen segmentation. As can be seen in the B-scan images with the segmentation lines (c.f. Fig. 12C and D) the RPE is correctly found by the algorithm. Additionally, the normal RPE position is estimated (c.f. green line in Fig. 12C and D) which allows the automated measurement of drusen area and volume. Both quantities can be measured reliably using PS-OCT with a coefficient of variation of ∼7% (Baumann et al., 2010) and are considered as a clinically significant risk factor for further disease progression (Anand et al., 2000).
Fig. 12.
Drusen segmentation using PS-OCT (reprinted from Baumann et al. (2010)). (A, B) Intensity B-scan images. (C, D) Overlaid positions of ILM (blue), segmented RPE (red), and assumed normal RPE (green). (E) En face fundus image reconstructed from 3-D OCT data set. (F) Retinal thickness map showing the axial distance between ILM and RPE position (color map: 70–350 μm). (G) Drusen thickness map computed as axial distance between segmented RPE position and approximated normal RPE position. The color map was scaled to the maximum elevation value of 55 pixels corresponding to ∼128 μm. (H) Total retinal thickness map showing the axial distance between ILM and approximated normal RPE position (color map: 70–350 μm).
In standard, intensity based OCT, segmentation of geographic atrophies remains difficult because Bruch’s membrane or the photoreceptor layer is mistakenly regarded as RPE in many intensity based algorithms. In contrast, PS-OCT can reliably segment the RPE even in the presence of atrophic areas. Fig. 13 shows an example of segmentation of geographic atrophies using PS-OCT. In principle an RPE thickness map (similar to the one in Fig. 11C) would clearly show the atrophic zones. However, depolarizing material within the choroid that is mainly visible at the location of the atrophic zones (c.f. green marked structures in Fig. 13D and E) obscure the atrophic zone (c.f. Fig. 13G). In order to exclude these structures from the RPE segmentation the estimated normal position of the RPE is used as a boundary to differentiate between RPE and choroid. All structures beneath the estimated RPE are regarded as choroid and are marked in green in Fig. 13D and E. The resulting en face map of the thickness of the depolarizing layer (Fig. 13H) clearly shows the atrophic areas and corresponds well with the auto-fluorescence (AF) image (c.f. Fig. 13F). Moreover, an increased thickness can be observed at the margins of the lesion which already indicates an altered RPE at these locations which partly corresponds to a lower AF signal visible in Fig. 13F. Based on the RPE thickness map, area and number of atrophic zones can be determined automatically. In a preliminary study on 5 patients, the repeatability of the geographic atrophy area measurement has been determined with ∼9% (Baumann et al., 2010).
Fig. 13.
Example of RPE segmentation in a patient with geographic atrophy (reprinted from Baumann et al. (2010)): (A) En face fundus image reconstructed from 3D-PS-OCT data set, (B, C) intensity B-scan images, (D, E) Overlay of depolarizing structures within and outside the evaluation band (associated with the RPE and the choroid) shown in red and green color, respectively, and (F) auto-fluorescence image. (G) Map of over all number of depolarizing pixels per A-line. Zones of RPE atrophy are masked by choroidal depolarization. (H) Map displaying the thickness of the depolarizing layer associated with the RPE. (J) Binary map of atrophic zones. The color map scales from 0 to 39 pixels for (G) and (H).
In more advanced forms of AMD a development of fibrosis is very common. In intensity based OCT an unspecific thickening of the RPE-photoreceptor band is observed (c.f. Fig. 14A). PS-OCT reveals that the highly backscattering area is birefringent as can be seen in Fig. 14B by increasing retardation (color change from blue to red). It is very likely that form birefringence caused by a microscopic regular arrangement of collagen fibers within the fibrosis is the origin of the observed birefringence. Interestingly, the amount of birefringence varies within the corresponding highly backscattering area which might be caused by a macroscopic inhomogeneous arrangement of the fibers.
Fig. 14.
Example of fibrosis imaged with PS-OCT (reprinted from Michels et al. (2008)). (A) Intensity (conventional) OCT image, (B) retardation and (C) Fundus image. The white line marks the approximate location of the OCT B-scan. The external limiting membrane overlying the fibrosis appears to be intact (marked with an arrow in A).
Fig. 15 shows an example of RPE detection with different spectral domain OCT instruments in a patient with neovascular AMD (Ahlers et al., 2010). The elevated retinal layers can clearly be visualized in the OCT B-scans. However, a differentiation between different highly backscattering layers is difficult because of the heavily distorted retinal structure. Therefore, the automated RPE segmentation provided by commercial instruments often yields erroneous results (c.f. bottom red line in Fig. 15B). Moreover, the commercially available RPE detection algorithms fail to detect RPE atrophies. Since RPE segmentation using PS-OCT data is based on an intrinsic tissue specific contrast, RPE atrophies can be detected even in such a case of RPE distortion (c.f. Fig. 15C). The corresponding retinal thickness maps (c.f. Fig. 15D and F) show the lateral extension of the lesion. Note the presence of multiple locations of RPE atrophy that can only be detected with PS-OCT (c.f. arrows in Fig. 15F). These atrophies might give an explanation why in these patients after restoration of retinal anatomy that is visible in OCT B-scans (e.g. after antiangiogenic treatment) the visual acuity is not improved (Ahlers et al., 2010).
Fig. 15.
Different performances of RPE detection in a patient with neovascular AMD. (A) Cirrus OCT (Zeiss Meditec), (B) Spectralis OCT (Heidelberg Engineering), (C) PS-OCT. (D) Retinal thickness map (inner limiting membrane to RPE) retrieved from Cirrus OCT, (E) retinal thickness map retrieved from Spectralis OCT, (F) retinal thickness map obtained with PS-OCT (areas with RPE atrophy are marked in gray). The arrows in (C) and (F) point to locations with RPE atrophy (reprinted from Ahlers et al. (2010)).
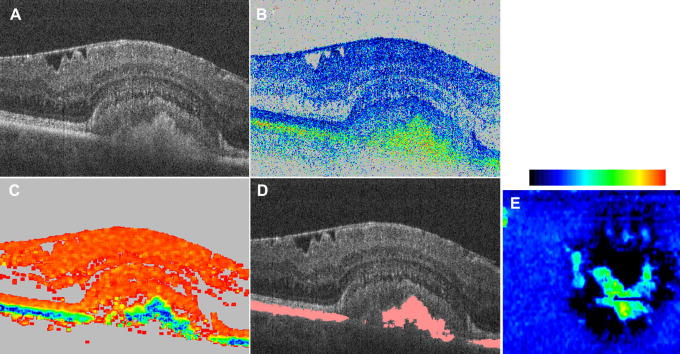
Another example of improved image contrast with PS-OCT is shown in Fig. 16. The image was taken from a patient with pseudo-vitelliform pattern dystrophy (adult-onset foveomacular vitelliform dystrophy). The standard OCT (intensity) image (c.f. Fig. 16A) shows a dome like lesion beneath the junction of the inner and outer segments of photoreceptors that is partly filled with material of unknown origin. The RPE cannot be identified within the lesion of this image. The retardation and the DOPU images reveal that part of the lesion is filled with depolarizing material which might therefore consist of RPE cells or pigment loaded macrophages (inflammatory cells). Apart from the lesion the RPE remains intact. The en face view of the thickness of the depolarizing layer (c.f. Fig. 16E) clearly shows the accumulation of depolarizing material in the central part of the lesion as well as complete absence of this layer (RPE atrophy) in the surrounding region.
Fig. 16.
Example of PS-OCT in a pseudo-vitelliform pattern dystrophy. (reprinted from Götzinger et al. (2008c)). (A) Intensity image; (B) retardation (color bar: 0°–90°); (C) DOPU (color bar: 0–1); (D) reflectivity overlaid with segmented RPE. Image size: 15° (horizontal) × 0.75 mm (vertical). (E) En face map of the thickness of segmented layer. Color bar: 0–200 μm.
Finally, it should be mentioned that other depolarizing structures within the diseased retina can be detected using PS-OCT. Initial studies in patients with diabetic macular edema showed that hard exudates are depolarizing structures. Therefore PS-OCT can be used to automatically detect and quantify (size, number) hard exudates in this disease (Lammer et al., 2010).
These initial studies demonstrate the potential of PS-OCT in various diseases affecting the macula region of the retina. One clear advantage of this technique is the possibility to segment the RPE reliably and fully automated. For example in drusen, which represent the first clinical findings in early stage AMD, automated segmentation is essential in order to establish entry criteria and end points for disease progression. Although algorithms relying on standard intensity based OCT are capable to segment the RPE (e.g. (Ahlers et al., 2008; Bagci et al., 2008; Fabritius et al., 2009; Fernandez et al., 2005)), the reliability of these methods in patients is rather limited. Commercially available algorithms incorporated in clinical SD-OCT instruments detect only ∼30% of drusen with negligible error (Schlanitz et al., 2010). This value is increased using PS-OCT to more than 95% (Schlanitz et al., IOVS in press) which clearly emphasizes the benefit of PS-OCT technology.
Quantification of lesion size in geographic atrophy is essential in order to monitor disease progression and therapeutic outcome. In fundus photography the contrast of these lesions is moderate and therefore the reproducibility of lesion size measurement is rather poor. Auto-fluorescence imaging (AF) (Holz et al., 2001, 2007; vonRuckmann et al., 1997) and depth integrated SD-OCT imaging (Yehoshua et al., 2011) provide improved contrast and enable a more accurate measurement of lesion growth. However, the information provided by both imaging modalities is sometimes complimentary, therefore only the combination of both methods provides optimum reliability (Wolf-Schnurrbusch et al., 2008). Especially at the margins of the lesion AF provides additional information that is based on a change in the AF signal which appears probably prior to a complete RPE loss at this location (Holz et al., 2007). Due to the polarization scrambling characteristics of the pigments of the RPE, PS-OCT may combine benefits of both, AF and SD-OCT imaging modality in a single instrument. Changes in depolarization at the rim of the atrophy might provide information on RPE cell viability, while depth resolution might provide details on structural changes occurring at the lesion rim prior to atrophy growth.
Initial studies confirmed the good correspondence of PS-OCT with AF imaging in lesion size detection (Schuetze et al., 2011).
Diabetes mellitus may lead to diabetic macular edema (DME) which severely degrades vision and is therefore regarded as one of the leading causes of visual impairment in the western world (Kempen et al., 2004). In order to better understand the therapeutic effect of various treatments (e.g. photocoagulation, anti-vascular endothelial growth factor agents) a detailed analysis of the integrity of the RPE in the presence of DME is of importance. Furthermore a quantification of focal deposits within the inner retina that are visible as highly backscattering structures in OCT images (Bolz et al., 2009) may further improve prognostic value and guide therapeutic decisions. Both structures depolarize the backscattered light and can be visualized with enhanced contrast using PS-OCT. PS-OCT might therefore be a valuable tool in order to quantify these structures reliably.
5.2. PS-OCT in the nerve head and peripheral retina
The main tissue of interest for PS-OCT imaging in the region of the optic nerve head (ONH) is the birefringent retinal nerve fiber layer (RNFL). The RNFL is damaged by glaucoma, one of the leading causes of blindness in the world (Quigley, 1996; Thylefors et al., 1995). It has been shown that thinning of the RNFL can be observed before visual field loss can be detected (Quigley et al., 1982). Untreated, this thinning leads to irreversible vision loss and finally to blindness. Therefore, it is important to detect RNFL thinning as early as possible. Based on these considerations, scanning laser polarimetry (SLP) (Dreher et al., 1992; Weinreb et al., 1990) has been introduced as a tool to measure the RNFL thickness via its birefringence: if a constant RNFL birefringence is assumed, the retardation observed between the two orthogonally polarized components of a sampling laser beam is directly proportional to the thickness of the RNFL. SLP is based on a confocal scanning laser ophthalmoscope with an integrated ellipsometer; it measures the retardation in the area of the ONH and calculates RNFL thickness maps that can be used for glaucoma diagnosis. Several studies have shown the usefulness of SLP for diagnosing glaucoma (Bagga et al., 2003; Brusini et al., 2005; Weinreb et al., 1995).
While SLP is quite successful, it also has some drawbacks: (i) It is known to produce images obscured by artifacts, called atypical GDx scans, in a considerable subset of healthy and glaucomatous eyes (Bowd et al., 2007; Mai et al., 2007; Sehi et al., 2007; Zhou et al., 2003). Patients whose SLP images show these atypical patterns cannot be reliably diagnosed by SLP. (ii) SLP does not provide any depth information. The total birefringence of the ocular fundus is integrated in depth, producing 2D-maps. The origin of the birefringence is not exactly known – RNFL and other layers cannot be differentiated. (iii) Since no depth information is available, birefringence – i.e. retardation per unit depth – cannot be measured. Instead, a constant birefringence value is used for the retardation to thickness conversion (Zhou et al., 2003). Therefore, the maps provided by SLP are not true thickness maps but retardation maps.
On the other hand, OCT has been used to directly measure the RNFL thickness (Bowd et al., 2006; Greenfield et al., 2010; Hoh et al., 2000; Medeiros et al., 2005, 2004; Schuman et al., 1995, 2007, 1996; Townsend et al., 2009; Weinreb and Khaw, 2004). This method, however, requires an accurate delineation of the borders of the RNFL. While the anterior border, the inner limiting membrane, can be found reliably in most of the cases, the posterior border is hard to detect, especially in glaucoma patients because the contrast of the RNFL is degraded. This effect causes significant differences in RNFL thickness measurements between commercially available instruments (Lee et al., 2011).
Since PS-OCT combines the depth information of OCT with the polarization sensitivity of SLP, it can solve several of the problems mentioned above.
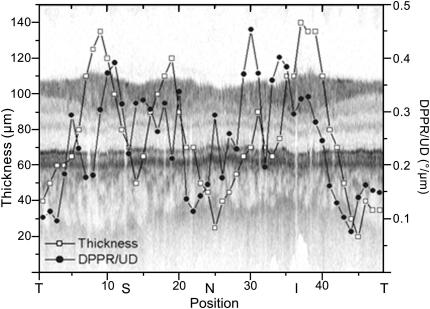
Because polarimetry of the RNFL for glaucoma diagnostics was already well known from SLP, these applications were also the first ones pursued by PS-OCT. Similar to intensity based OCT, the early studies were done by time domain instruments. First measurements were carried out in a primate retina in vitro (Ducros et al., 2001) and provided a birefringence of 3.4 × 10−4 at 859 nm in the arcuate nerve fiber bundle ∼350 μm superior to the ONH. First in vivo measurements in the healthy human retina were reported by B. Cense et al. (Cense et al., 2002). A double pass phase retardation of 39°/100 μm, equivalent to a birefringence of 4.5 × 10−4, was found in the RNFL near the ONH. Later, measurements by the same group demonstrated that the RNFL birefringence is not constant but varies with location (Cense et al., 2004a,b), ranging from 1.2 × 10−4 to 4.1 × 10−4 (at a wavelength of 840 nm), with higher values observed inferior and superior to the ONH and lower values obtained on the nasal and temporal side. At a given azimuthal angle, birefringence was constant with radial distance from the ONH. Fig. 17 shows a typical example of a circumpapillary OCT scan, overlaid with plots of RNFL thickness and birefringence.
Fig. 17.
Example of combined RNFL thickness and birefringence measurements along a circular scan around the ONH. The intensity image is plotted in the background. The RNFL is relatively thicker superiorly (S) and inferiorly (I). A similar development can be seen in the birefringence plot. The birefringence is relatively higher in the thicker areas, whereas it is lower in the thinner temporal (T) and nasal (N) areas. (Reprinted from Cense et al. (2004b)).
One of the main limitations of quantitative PS-OCT is speckle noise (Pircher et al., 2003; Schmitt et al., 1999), which reduces the precision available for birefringence measurements. This problem can be overcome by using an enhanced algorithm that exploits data from several polarization states and averaging of data obtained from several uncorrelated speckle fields (Kemp et al., 2005a). Because the multiple measurements slow down the total acquisition procedure, this method could not be used in humans but only in anesthetized primate retina. Table 1 shows the results of these high-precision measurements of RNFL birefringence in a rhesus monkey at locations inferior and nasal to the ONH. The data clearly show that birefringence is higher in the inferior location.
Table 1.
Thickness and birefringence of in vivo primate RNFL (Reprinted from Kemp et al. (2005a)).
| Location (session) | ∆zRNFL (μm) | δRNFL(°) | ∆nRNFL(°/100 μm) |
|---|---|---|---|
| Inferior (day 1) | 170 | 29.5 | 17.3 |
| Inferior (day 2) | 167 | 27.8 | 16.6 |
| Nasal (day 1) | 50 | 3.4 | 6.8 |
| Nasal (day 2) | 51 | 3.9 | 7.6 |
Another parameter of birefringent tissue, in addition to retardation, is the orientation of the birefringent axis. This orientation corresponds to the orientation of the nerve fibers in the RNFL. Therefore, PS-OCT allows measuring the orientation of the nerve fibers. A first demonstration of axis orientation measurement of the human RNFL was carried out by a transverse scanning TD PS-OCT system (Pircher et al., 2004b). The radial orientation of the nerve fibers around the ONH was clearly shown.
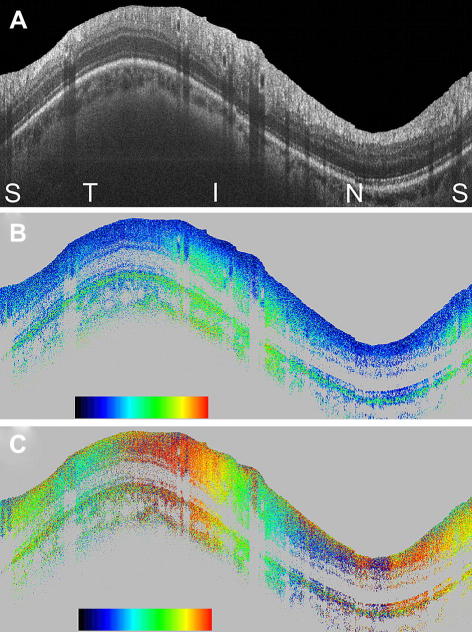
Similar to the case of intensity based OCT, also PS-OCT shifted to spectral domain methods in the last decade. After an initial demonstration in human skin in vitro (Yasuno et al., 2002), the first high speed SD PS-OCT for imaging human retina in vivo was demonstrated in 2005 (Götzinger et al., 2005). Operating at 20,000 A-lines/s, both, the retardation and optic axis orientation distribution around the ONH could be imaged in two and three dimensions. In addition to RNFL birefringence, strong birefringence was observed at the rim of the scleral canal, and birefringence of varying strength and orientation was demonstrated in the lamina cribrosa. While the first version of this instrument was a bulk optic setup, an improved setup based on polarization maintaining fibers and incorporating a broadband light source for enhanced depth resolution was reported later (Götzinger et al., 2009a). Fig. 18 shows a circumpapillary scan in a healthy volunteer recorded with that instrument. Fig. 18A shows the intensity image. The increased thickness of the superior and inferior RNFL bundles is clearly visible. Fig. 18B shows the retardation image, where the increased retardation caused by these nerve fiber bundles is clearly observed (color change from blue to green). Fig. 18C shows the axis orientation image, where the two full color oscillations from left to right indicate the radial orientation of the nerve fibers (360° rotation of axis orientation).
Fig. 18.
Circumpapillary PS-OCT scan from healthy human retina in vivo. Scan diameter: ∼10 deg (corresponds to a circumference of ∼9.4 mm, equal to horizontal image width; optical image depth: 1.8 mm). (A) Intensity (log scale); (B) retardation (color bar: 0°–90°); (C) optic axis orientation (color bar: 0°–180°). Orientation of scan from left to right: (S)uperior, (T)emporal, (I)nferior, (N)asal, (S)uperior. (Reprinted from Götzinger et al. (2009a)).
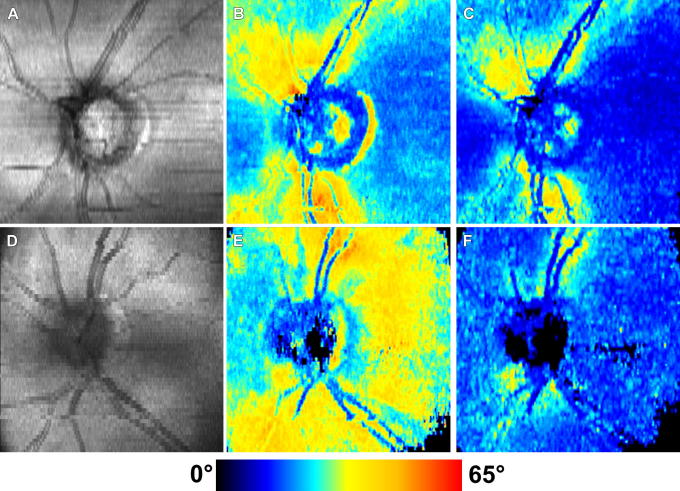
Quantitative birefringence measurements along a circle around the ONH of a healthy eye were demonstrated by a single camera SD PS-OCT instrument (Cense et al., 2007) and provided values of birefringence ranging from 2.2 × 10−4 to 4.8 × 10−4, approximately similar to earlier results obtained by TD PS-OCT. Similar results were reported in a healthy eye by a two-camera based setup, while measurements in a glaucomatous eye showed a pronounced localized reduction of RNFL birefringence (Yamanari et al., 2008b). In the same paper, 2D-maps of retardation measured by PS-OCT are compared with results obtained by SLP. Fig. 19 shows results obtained in a healthy volunteer, demonstrating that PS-OCT (Fig. 19D) provides results comparable to those of SLP (Fig. 19B).
Fig. 19.
Fundus image (A), nerve fiber thickness map measured by GDx-VCC (B), en-face projection image of the OCT intensity (C), and en-face phase retardation image measured by the PS-SD-OCT (D) in a healthy eye. (Reprinted from Yamanari et al. (2008b)).
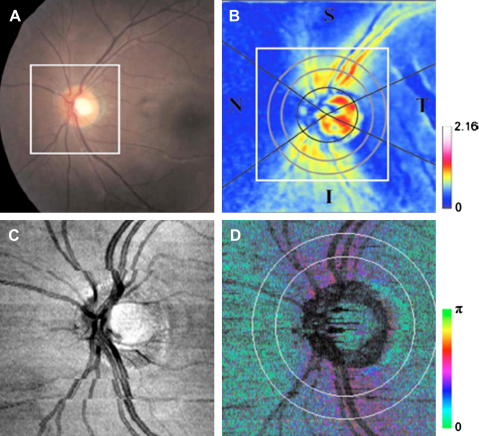
The first paper presenting a 2D birefringence map around the ONH was published by Mujat et al. (Mujat et al., 2007). Fig. 20 shows the result. As in the circumpapillary scans, increased birefringence is observed superior and inferior to the ONH. Götzinger et al. published a comparison of SLP retardation, PS-OCT retardation, and PS-OCT birefringence maps in a healthy and a glaucomatous eye (Götzinger et al., 2008b), demonstrating not only reduced retardation but also reduced birefringence in the glaucomatous case. Later, a comparison between 8 healthy and 12 glaucoma suspect eyes demonstrated decreased birefringence in the RNFL of glaucoma suspects (Götzinger et al., 2009c). The birefringence decrease was of higher statistical significance as the decrease of RNFL thickness in the same eyes. Birefringence could be a sensitive measurement of density of ganglion cell axons or their microtubules. Based on observations of early structural changes of retinal ganglion cells in glaucomatous neuropathy (Shou et al., 2003; Weber et al., 1998), it has been speculated that ganglion cell axon density changes before a reduction of thickness of the RNFL can be measured (Huang et al., 2004). If this can be confirmed in a larger study, birefringence measurements could provide the earliest information on structural changes within the RNFL, possibly enabling diagnosis of glaucoma earlier than other instruments. A prerequisite would, however, be to establish a normative database of RNFL birefringence distribution in the normal population. Significant deviations of birefringence patterns from this normal distribution, which can be measured only by PS-OCT, could then be regarded as early signs of glaucoma and be treated accordingly.
Fig. 20.
OCT scan (4.24 × 5.29 mm2) of the retina of a normal volunteer, centered on the ONH. (A) Integrated reflectance map; (B) birefringence map; (C) RNFL thickness map (color bar scaled in microns). (S = superior, N = nasal, I = inferior, T = temporal). (Reprinted from (Mujat et al., 2007)).
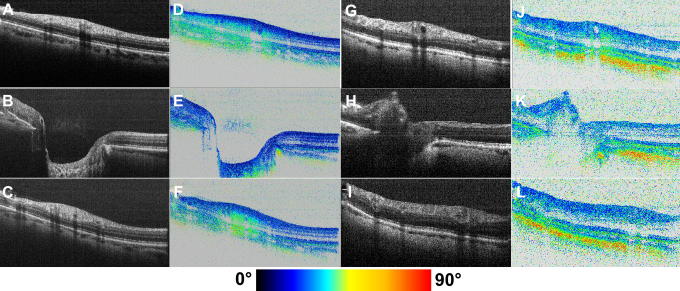
As mentioned before, a shortcoming of SLP is that artifacts are observed in a considerable fraction of measured eyes, preventing the use of SLP for glaucoma diagnostics in that case. In these so called “atypical GDx scans” patches of abnormally increased retardation are observed in areas where they are not expected, e.g. temporally and nasally to the ONH (Bagga et al., 2005; Bowd et al., 2007; Mai et al., 2007; Sehi et al., 2007). PS-OCT was used to investigate the reason for these atypical scans (Götzinger et al., 2008a). It was found that in most of the eyes where these atypical patterns are observed, the sampling light beam penetrates deeper into the eye than usual, down to the birefringent sclera. Because SLP has no depth discrimination, the birefringence is integrated over all depths. Therefore, the scleral birefringence distorts the normal retardation patterns. Fig. 21 illustrates this finding, comparing a normal eye (Fig. 21A–F) to an eye with atypical patches (Fig. 21G–L) where the scleral birefringence is clearly observed. The same paper demonstrates how PS-OCT can generate retardation patterns without atypical patches, even in eyes with deeper light penetration: because OCT provides depth information, signals from the sclera can be excluded. Fig. 22 demonstrates the elimination of atypical artifacts. Fig. 22A–C were recorded in a normal eye, Fig. 22D–F in an atypical eye. Fig. 22B,E include scleral signals, Fig. 22C,F exclude them. The generation of artifact free retardation patterns might be another way to improve glaucoma diagnostics, whereby advantage might be drawn from the normative databases already existing for SLP.
Fig. 21.
PS-OCT B-scan images of healthy eyes. Analysis of the origin of atypical scanning laser polarimetry retardation patterns. (A–C) Intensity images and (D–F) retardation images from eye with normal retardation pattern. (G–I) Intensity and (J–L) retardation images from eye with atypical retardation pattern. (Reprinted from Götzinger et al. (2008a)).
Fig. 22.
PS-OCT en-face images corresponding to Fig. 21. (A–C) Images from eyes with normal retardation patterns. (D–F) Images from eyes with atypical retardation patterns. (A, D) Intensity image (pseudo-SLO). (B, E) Retardation image including scleral signals. (C, F) Retardation image excluding scleral signals. (Reprinted from Götzinger et al. (2008a)).
While imaging of deeper layers in the ocular fundus is limited to the above mentioned atypical cases at the usual OCT wavelength around 800 nm, deeper penetration, down to the sclera, is improved at longer wavelengths around 1050 nm because they are less absorbed and scattered by the RPE (Lee et al., 2006; Unterhuber et al., 2005; Yasuno et al., 2007). First applications of PS-OCT at 1050 nm were recently reported (Yamanari et al., 2009, 2010), demonstrating that birefringence induced retardation can clearly be observed in the sclera and in the lamina cribrosa. More detailed studies are required to evaluate the usefulness of this information.
Since PS-OCT combines the advantages of SLP and OCT in a single instrument this technology might become a valuable tool in glaucoma diagnostics. Especially the capability of PS-OCT to measure birefringence, a more sensitive parameter of RNFL damage (as initial studies showed), will most likely enable a more accurate monitoring of disease progression and therapeutic outcome. However, in order to use this technology for glaucoma diagnostics in clinical routine more studies with higher patient numbers have to be performed. This will enable to establish a normative database (similar to SLP) which is essential to detect deviations from normal RNFL birefringence.
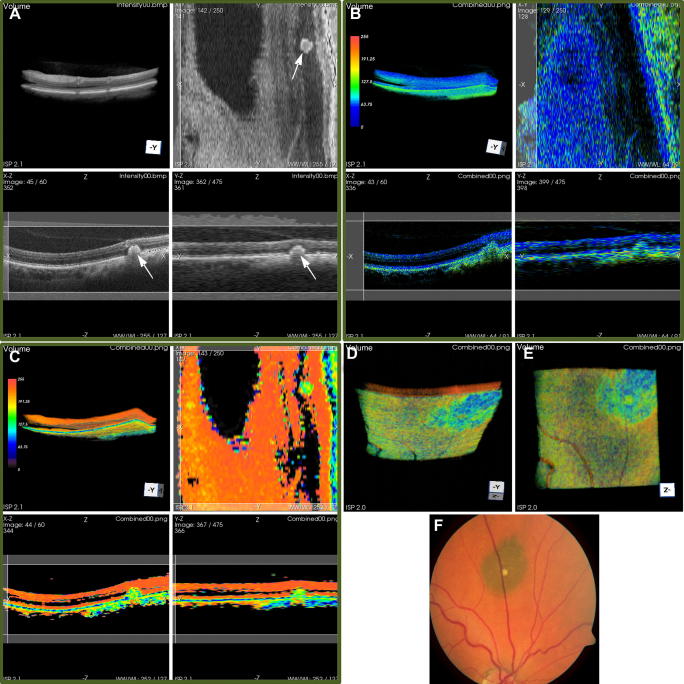
A completely different application of PS-OCT in the peripheral retina should finally be mentioned: PS-OCT can be used to image choroidal naevi and melanoma based on the depolarization caused by the melanin they contain. The contrast mechanism is similar to that reported for the RPE contrast in chapter 5.1. Fig. 23 shows an example of a 3D data set obtained from an eye with a choroidal naevus (Götzinger et al., 2009b). Fig. 23A–C show intensity, retardation, and DOPU images, respectively (each image contains sub-images of volume rendering, transverse scan, horizontal and vertical B-scan). Fig. 23D and E show different aspects of a volume rendered DOPU data set, clearly visualizing the extension of the naevus (blue-green color). Fig. 23F shows a corresponding color fundus photo. A limitation of these PS-OCT images is that the strong scattering and absorption of the melanin prevents the measurement of total thickness of the pigmented tumors; PS-OCT at 1050 nm might overcome that limitation.
Fig. 23.
3D PS-OCT data set of the retina of a patient with a choroidal nevus. (A) Intensity; (B) retardation (color bar: 0 = 0°, 255 = 90°); (C) DOPU (color bar: 0 = 0, 255 = 1). Figure arrangement: top left: volume rendering; top right: en face section; bottom left: horizontal B-scan; bottom right: vertical B-scan. (D) and (E) additional views of the volume rendered DOPU data set: (D) inclined upwards and (E) upwards. For better comparison with the fundus photo (F), these reverse-direction images are mirrored. Image size of OCT images: ∼14.25°(x) × 15°(y) × 1.5 mm(z, in air). (Reprinted from Götzinger et al. (2009b)).
6. Conclusion and future directions
The eye consists of several structures that alter the polarization state of light. This valuable information can be exploited using PS-OCT. As outlined in this review many different applications of this technique have been introduced for ocular imaging ranging from the anterior to the posterior eye segment. To improve clinical diagnostics and therapy, long term studies are essential in order to monitor disease progression and/or effectiveness of therapies. These studies require accurate, reliable and comprehensive information on each individual disease. PS-OCT complements the information that is provided by other modern imaging technologies as standard OCT or auto-fluorescence imaging.
Currently, probably the most promising application of PS-OCT in the eye is the automated segmentation of the RPE and other pigmented structures (e.g. hard exudates) based on the polarization scrambling effect. Since a different physical property is used for this detection it overcomes many limitations that are present in standard, intensity based OCT images or data from other imaging modalities (e.g. auto-fluorescence imaging). This will allow for more accurate follow up studies and therapy monitoring. Additionally, a quantitative evaluation of the depolarizing effect can provide information on the status of the underlying structure (e.g. the RPE), a valuable quantity that cannot be provided by standard OCT.
PS-OCT is an extension of the standard OCT technique. Therefore new developments e.g. in terms of imaging speed can directly be transferred to PS-OCT. Using high speed cameras or high speed swept sources, A-scan rates beyond 100 kHz up to the MHz range, can be achieved (Huber et al., 2007; Klein et al., 2011; Potsaid et al., 2008). Recently, we demonstrated PS-OCT imaging beyond a 100 kHz A-scan rate (Götzinger et al. Optics Express, in press). Beside the reduction of eye motion artifacts, high speed imaging enables averaging over several B-scans without the use of a hardware implemented eye tracker (e.g. Spectralis, Heidelberg Engineering). This procedure greatly improves the image quality in PS-OCT. Moreover, depolarizing effects can be assessed using multiple B-scans recorded successively instead of using a spatial evaluation window, which greatly improves the spatial resolution of DOPU images. This will enable the detection of very small depolarizing structures as e.g. micro-exudates in diabetic retinopathy. The higher image quality will also improve quantitative evaluation of PS-OCT data and therefore improve the accuracy of birefringence measurements of the retinal nerve fiber layer.
For some applications a different imaging wavelength is beneficial. At 1050 nm scattering is less pronounced which yields an enhanced penetration depth into the choroid and to an improved image quality in patients with cataract. PS-OCT at 1050 nm has been demonstrated (Yamanari et al., 2009). The enhanced penetration into the choroid allows the visualization of the sclera and therefore a better estimation of the choroidal thickness. These and other improvements that are currently under development are likely to further extend the application range of PS-OCT in the future.
Acknowledgments
The authors would like to acknowledge contributions of B. Baumann, E. Götzinger, H. Sattmann from the Center for Medical Physics and Biomedical Engineering of the Medical University of Vienna, and M. Bolz, C. Schütze, C. Ahlers, J. Lammer, F.G. Schlanitz, W. Geitzenauer from the Department of Ophthalmology and Optometry, Medical University of Vienna.
Financial support from the Austrian science fund (FWF grants P16776-N02 and P19624-B02), and from the European Union (FP7 HEALTH program grant 201880, FUN-OCT) are acknowledged.
References
- Ahlers C., Gotzinger E., Pircher M., Golbaz I., Prager F., Schutze C., Baumann B., Hitzenberger C.K., Schmidt-Erfurth U. Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2010;51:2149–2157. doi: 10.1167/iovs.09-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers C., Simader C., Geitzenauer W., Stock G., Stetson P., Dastmalchi S., Schmidt-Erfurth U. Automatic segmentation in three-dimensional analysis of fibrovascular pigmentepithelial detachment using high-definition optical coherence tomography. British Journal of Ophthalmology. 2008;92:197–203. doi: 10.1136/bjo.2007.120956. [DOI] [PubMed] [Google Scholar]
- An L., Subhush H.M., Wilson D.J., Wang R.K. High-resolution wide-field imaging of retinal and choroidal blood perfusion with optical microangiography. Journal of Biomedical Optics. 2010;15:026011. doi: 10.1117/1.3369811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Bressler S.B., Davis M.D., Ferris F.L., Klein R., Lindblad A.S., Milton R.C., Sperduto R.D., Grp, A.R.E.D.S.R Risk factors associated with age-related macular degeneration – A case-control study in the age-related eye disease study: age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci A.M., Shahidi M., Ansari R., Blair M., Blair N.P., Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. American Journal of Ophthalmology. 2008;146:679–687. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga H., Greenfield D.S., Feuer W., Knighton R.W. Scanning laser polarimetry with variable corneal compensation and optical coherence tomography in normal and glaucomatous eyes. American Journal of Ophthalmology. 2003;135:521–529. doi: 10.1016/s0002-9394(02)02077-9. [DOI] [PubMed] [Google Scholar]
- Bagga H., Greenfield D.S., Feuer W.J. Quantitative assessment of atypical birefringence images using scanning laser polarimetry with variable corneal compensation. American Journal of Ophthalmology. 2005;139:437–446. doi: 10.1016/j.ajo.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Baumann B., Götzinger E., Pircher M., Hitzenberger C.K. Single camera based spectral domain polarization sensitive optical coherence tomography. Optics Express. 2007;15:1054–1063. doi: 10.1364/oe.15.001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Götzinger E., Pircher M., Hitzenberger C.K. Measurements of depolarization distribution in the healthy human macula by polarization sensitive OCT. Journal of Biophotonics. 2009;2:426–434. doi: 10.1002/jbio.200910031. [DOI] [PubMed] [Google Scholar]
- Baumann B., Götzinger E., Pircher M., Sattman H., Schutze C., Schlanitz F., Ahlers C., Schmidt-Erfurth U., Hitzenberger C.K. Segmentation and quantification of retinal lesions in age-related macular degeneration using polarization-sensitive optical coherence tomography. Journal of Biomedical Optics. 2010;15:061704. doi: 10.1117/1.3499420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow C.E., Iftimia N.V., Ferguson R.D., Ustun T.E., Bloom B., Hammer D.X. Compact multimodal adaptive-optics spectral-domain optical coherence tomography instrument for retinal imaging. Journal of the Optical Society of America A-Optics Image Science and Vision. 2007;24:1327–1336. doi: 10.1364/josaa.24.001327. [DOI] [PubMed] [Google Scholar]
- Bille J.F., Pelz B., Weschenmoser C., Goelz S., Fischer J.P. Examination of the corneal birefringence in vivo with an electrooptical laser scanning ellipsometer. Physica Medica. 1997;13:308–312. [Google Scholar]
- Bolz M., Schmidt-Erfurth U., Deak G., Mylonas G., Kriechbaum K., Scholda C., Vienn D.R.R.G. Optical coherence Tomographic Hyperreflective Foci A Morphologic sign of Lipid Extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–920. doi: 10.1016/j.ophtha.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Born M., Wolf E. seventh ed. Cambridge University Press; Camridge: 1999. Principles of Optics. [Google Scholar]
- Bouma B., Tearney G. Marcel Dekker; New York: 2002. Handbook of Optical Coherence Tomography. [Google Scholar]
- Bour L.J. Polarized light and the eye. In: Charman W.N., editor. Visual Optics and Instrumentation. CRC Press; Boca Raton, FL: 1991. pp. 310–325. [Google Scholar]
- Bowd C., Medeiros F.A., Weinreb R.N., Zangwill L.M. The effect of atypical birefringence patterns on glaucoma detection using scanning laser polarimetry with variable corneal compensation. Investigative Ophthalmology & Visual Science. 2007;48:223–227. doi: 10.1167/iovs.06-0787. [DOI] [PubMed] [Google Scholar]
- Bowd C., Zangwill L.M., Medeiros F.A., Tavares I.M., Hoffmann E.M., Bourne R.R., Sample P.A., Weinreb R.N. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Investigative Ophthalmology & Visual Science. 2006;47:2889–2895. doi: 10.1167/iovs.05-1489. [DOI] [PubMed] [Google Scholar]
- Brusini P., Salvetat M.L., Parisi L., Zeppieri M., Tosoni C. Discrimination between normal and early glaucomatous eyes with scanning laser polarimeter with fixed and variable corneal compensator settings. European Journal of Ophthalmology. 2005;15:468–476. doi: 10.1177/112067210501500409. [DOI] [PubMed] [Google Scholar]
- Cense B., Chen H.C., Park B.H., Pierce M.C., de Boer J.F. In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. Journal of Biomedical Optics. 2004;9:121–125. doi: 10.1117/1.1627774. [DOI] [PubMed] [Google Scholar]
- Cense B., Chen T.C., Park B.H., Pierce M.C., de Boer J.F. In vivo depth-resolved birefringence measurements of the human retinal nerve fiber layer by polarization-sensitive optical coherence tomography. Optics Letters. 2002;27:1610–1612. doi: 10.1364/ol.27.001610. [DOI] [PubMed] [Google Scholar]
- Cense B., Chen T.C., Park B.H., Pierce M.C., de Boer J.F. Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2004;45:2606–2612. doi: 10.1167/iovs.03-1160. [DOI] [PubMed] [Google Scholar]
- Cense B., Gao W.H., Brown J.M., Jones S.M., Jonnal R.S., Mujat M., Park B.H., de Boer J.F., Miller D.T. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Optics Express. 2009;17:21634–21651. doi: 10.1364/OE.17.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cense B., Koperda E., Brown J.M., Kocaoglu O.P., Gao W.H., Jonnal R.S., Miller D.T. Volumetric retinal imaging with ultrahigh-resolution spectral-domain optical coherence tomography and adaptive optics using two broadband light sources. Optics Express. 2009;17:4095–4111. doi: 10.1364/oe.17.004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cense B., Mujat M., Chen T.C., Park B.H., de Boer J.F. Polarization-sensitive spectral-domain optical coherence tomography using a single line scan camera. Optics Express. 2007;15:2421–2431. doi: 10.1364/oe.15.002421. [DOI] [PubMed] [Google Scholar]
- Chen Z.P., Milner T.E., Srinivas S., Wang X.J., Malekafzali A., vanGemert M.J.C., vanGemert M.J.C., Nelson J.S. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Optics Letters. 1997;22:1119–1121. doi: 10.1364/ol.22.001119. [DOI] [PubMed] [Google Scholar]
- Choma M.A., Sarunic M.V., Yang C.H., Izatt J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Optics Express. 2003;11:2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- Daxer A., Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Investigative Ophthalmology & Visual Science. 1997;38:121–129. [PubMed] [Google Scholar]
- de Boer J.F., Cense B., Park B.H., Pierce M.C., Tearney G.J., Bouma B.E. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Optics Letters. 2003;28:2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- de Boer J.F., Milner T.E. Review of polarization sensitive optical coherence tomography and Stokes vector determination. Journal of Biomedical Optics. 2002;7:359–371. doi: 10.1117/1.1483879. [DOI] [PubMed] [Google Scholar]
- de Boer J.F., Milner T.E., Nelson J.S. Determination of the depth-resolved Stokes parameters of light backscattered from turbid media by use of polarization-sensitive optical coherence tomography. Optics Letters. 1999;24:300–302. doi: 10.1364/ol.24.000300. [DOI] [PubMed] [Google Scholar]
- de Boer J.F., Milner T.E., van Gemert M.J.C., Nelson J.S. Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography. Optics Letters. 1997;22:934–936. doi: 10.1364/ol.22.000934. [DOI] [PubMed] [Google Scholar]
- de Boer J.F., Srinivas S.M., Malekafzali A., Chen Z.P., Nelson J.S. Imaging thermally damaged tissue by polarization sensitive optical coherence tomography. Optics Express. 1998;3:212–218. doi: 10.1364/oe.3.000212. [DOI] [PubMed] [Google Scholar]
- Dreher A.W., Reiter K., Weinreb R.N. Spatially resolved birefringence of the retinal nerve-fiber layer assessed with a retinal laser ellipsometer. Applied Optics. 1992;31:3730–3735. doi: 10.1364/AO.31.003730. [DOI] [PubMed] [Google Scholar]
- Drexler W. Ultrahigh-resolution optical coherence tomography. Journal of Biomedical Optics. 2004;9:47–74. doi: 10.1117/1.1629679. [DOI] [PubMed] [Google Scholar]
- Drexler W., Fujimoto J.G. Springer; Berlin: 2008. Optical Coherence Tomography. Technology and Applications. [Google Scholar]
- Drexler W., Fujimoto J.G. State-of-the-art retinal optical coherence tomography. Progress in Retinal and Eye Research. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ducros M.G., de Boer J.F., Huang H.E., Chao L.C., Chen Z.P., Nelson J.S., Milner T.E., Rylander H.G. Polarization sensitive optical coherence tomography of the rabbit eye. IEEE Journal of Selected Topics in Quantum Electronics. 1999;5:1159–1167. doi: 10.1109/2944.796347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducros M.G., Marsack J.D., Rylander H.G., Thomsen S.L., Milner T.E. Primate retina imaging with polarization-sensitive optical coherence tomography. Journal of the Optical Society of America A-Optics Image Science and Vision. 2001;18:2945–2956. doi: 10.1364/josaa.18.002945. [DOI] [PubMed] [Google Scholar]
- Everett M.J., Schoenenberger K., Colston B.W., Da Silva L.B. Birefringence characterization of biological tissue by use of optical coherence tomography. Optics Letters. 1998;23:228–230. doi: 10.1364/ol.23.000228. [DOI] [PubMed] [Google Scholar]
- Fabritius T., Makita S., Miura M., Myllyla R., Yasuno Y. Automated segmentation of the macula by optical coherence tomography. Optics Express. 2009;17:15659–15669. doi: 10.1364/OE.17.015659. [DOI] [PubMed] [Google Scholar]
- Fanjul-Vellez F., Pircher M., Baumann B., Götzinger E., Hitzenberger C.K., Arce-Diego J.L. Polarimetric analysis of the human cornea measured by polarization-sensitive optical coherence tomography. Journal of Biomedical Optics. 2010;15:056004. doi: 10.1117/1.3486540. [DOI] [PubMed] [Google Scholar]
- Fercher A.F. Optical coherence tomography. Journal of Biomedical Optics. 1996;1:157–173. doi: 10.1117/12.231361. [DOI] [PubMed] [Google Scholar]
- Fercher A.F., Drexler W., Hitzenberger C.K., Lasser T. Optical coherence tomography – principles and applications. Reports on Progress in Physics. 2003;66:239–303. [Google Scholar]
- Fercher A.F., Hitzenberger C., Juchem M. Measurement of intraocular optical distances using partially coherent laser-light. Journal of Modern Optics. 1991;38:1327–1333. [Google Scholar]
- Fercher A.F., Hitzenberger C.K. Optical coherence tomography. Progress in Optics. 2002;44:215–302. [Google Scholar]
- Fercher A.F., Hitzenberger C.K., Drexler W., Kamp G., Sattmann H. In-vivo optical coherence tomography. American Journal of Ophthalmology. 1993;116:113–115. doi: 10.1016/s0002-9394(14)71762-3. [DOI] [PubMed] [Google Scholar]
- Fercher A.F., Hitzenberger C.K., Kamp G., Elzaiat S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Optics Communications. 1995;117:43–48. [Google Scholar]
- Fercher A.F., Mengedoht K., Werner W. Eye-length measurement by interferometry with partially coherent-light. Optics Letters. 1988;13:186–188. doi: 10.1364/ol.13.000186. [DOI] [PubMed] [Google Scholar]
- Fernandez D.C., Salinas H.M., Puliafito C.A. Automated detection of retinal layer structures on optical coherence tomography images. Optics Express. 2005;13:10200–10216. doi: 10.1364/opex.13.010200. [DOI] [PubMed] [Google Scholar]
- Fernandez E.J., Hermann B., Povazay B., Unterhuber A., Sattmann H., Hofer B., Ahnelt P.K., Drexler W. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Optics Express. 2008;16:11083–11094. doi: 10.1364/oe.16.011083. [DOI] [PubMed] [Google Scholar]
- Francois J., Feher J. Light microscopical and polarization optical study of lattice dystrophy of cornea. Ophthalmologica. 1972;164:1–18. doi: 10.1159/000306698. [DOI] [PubMed] [Google Scholar]
- Geitzenauer W., Hitzenberger C., Schmidt-Erfurth U. Retinal optical coherence tomography: past, present and future perspectives. British Journal of Ophthalmology. 2011;95:171–177. doi: 10.1136/bjo.2010.182170. [DOI] [PubMed] [Google Scholar]
- Götzinger E., Baumann B., Pircher M., Hitzenberger C.K. Polarization maintaining fiber based ultra-high resolution spectral domain polarization sensitive optical coherence tomography. Optics Express. 2009;17:22704–22717. doi: 10.1364/OE.17.022704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Baumann B., Ahlers C., Geitzenauer W., Schmidt-Erfurth U., Hitzenberger C.K. Three-dimensional polarization sensitive OCT imaging and interactive display of the human retina. Optics Express. 2009;17:4151–4165. doi: 10.1364/oe.17.004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Baumann B., Hirn C., Vass C., Hitzenberger C.K. Analysis of the origin of atypical scanning laser polarimetry patterns by polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2008;49:5366–5372. doi: 10.1167/iovs.08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Baumann B., Hirn C., Vass C., Hitzenberger C.K. Retinal nerve fiber layer birefringence evaluated with polarization sensitive spectral domain OCT and scanning laser polarimetry: a comparison. Journal of Biophotonics. 2008;1:129–139. doi: 10.1002/jbio.200710009. [DOI] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Baumann B., Resch H., Vass C., Hitzenberger C.K. 2009. Comparison of Retinal Nerve Fiber Layer Birefringence and Thickness of Healthy and Glaucoma Suspect Eyes Measured with Polarization Sensitive Spectral Domain OCT. ARVO Annual Meeting Abstract, 5823. [Google Scholar]
- Götzinger E., Pircher M., Dejaco-Ruhswurm I., Kaminski S., Skorpik C., Hitzenberger C.K. Imaging of birefringent properties of keratoconus corneas by polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2007;48:3551–3558. doi: 10.1167/iovs.06-0727. [DOI] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Geitzenauer W., Ahlers C., Baumann B., Michels S., Schmidt-Erfurth U., Hitzenberger C.K. Retinal pigment epithelium segmentation by polarization sensitive optical coherence tomography. Optics Express. 2008;16:16410–16422. doi: 10.1364/oe.16.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Hitzenberger C.K. High speed spectral domain polarization sensitive optical coherence tomography of the human retina. Optics Express. 2005;13:10217–10229. doi: 10.1364/opex.13.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger E., Pircher M., Sticker M., Fercher A.F., Hitzenberger C.K. Measurement and imaging of birefringent properties of the human cornea with phase-resolved, polarization-sensitive optical coherence tomography. Journal of Biomedical Optics. 2004;9:94–102. doi: 10.1117/1.1629308. [DOI] [PubMed] [Google Scholar]
- Greenfield D.S., Goodkin M.L., Grewal D.S. Three-dimensional high-speed optical coherence tomography for diagnosis of hypotony maculopathy after glaucoma filtration surgery. Journal of Glaucoma. 2010;19:349–355. doi: 10.1097/IJG.0b013e3181bd59c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell R.C., Carlson F.D., Blank P.S. Form birefringence of muscle. Biophysical Journal. 1989;56:401–413. doi: 10.1016/S0006-3495(89)82686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler G., Lindner M.W. “Coherence radar” and “spectral radar” – new tools for dermatological diagnosis. Journal of Biomedical Optics. 1998;3:21–31. doi: 10.1117/1.429899. [DOI] [PubMed] [Google Scholar]
- Hee M.R., Huang D., Swanson E.A., Fujimoto J.G. Polarization-sensitive low-coherence reflectometer for birefringence characterization and ranging. Journal of the Optical Society of America B-Optical Physics. 1992;9:903–908. [Google Scholar]
- Hee M.R., Izatt J.A., Swanson E.A., Huang D., Schuman J.S., Lin C.P., Puliafito C.A., Fujimoto J.G. Optical coherence tomography of the human retina. Archives of Ophthalmology. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- Hitzenberger C.K. Optical measurement of the axial eye length by laser Doppler interferometry. Investigative Ophthalmology & Visual Science. 1991;32:616–624. [PubMed] [Google Scholar]
- Hitzenberger C.K., Götzinger E., Sticker M., Pircher M., Fercher A.F. Measurement and imaging of birefringence and optic axis orientation by phase resolved polarization sensitive optical coherence tomography. Optics Express. 2001;9:780–790. doi: 10.1364/oe.9.000780. [DOI] [PubMed] [Google Scholar]
- Hoh S.T., Greenfield D.S., Mistlberger A., Liebmann J.M., Ishikawa H., Ritch R. Optical coherence tomography and scanning laser polarimetry in normal, ocular hypertensive, and glaucomatous eyes. American Journal of Ophthalmology. 2000;129:129–135. doi: 10.1016/s0002-9394(99)00294-9. [DOI] [PubMed] [Google Scholar]
- Holz F.G., Bellman C., Staudt S., Schutt F., Volcker H.E. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2001;42:1051–1056. [PubMed] [Google Scholar]
- Holz F.G., Bindewald-Wittich A., Fleckenstein M., Dreyhaupt J., Scholl H.P.N., Schmitz-Valckenberg S., Grp F.S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. American Journal of Ophthalmology. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W., Hee M.R., Flotte T., Gregory K., Puliafito C.A., Fujimoto J.G. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.R., Bagga H., Greenfield D.S., Knighton R.W. Variation of peripapillary retinal nerve fiber layer birefringence in normal human subjects. Investigative Ophthalmology & Visual Science. 2004;45:3073–3080. doi: 10.1167/iovs.04-0110. [DOI] [PubMed] [Google Scholar]
- Huard S. Masson; Paris: 1997. Polarization of Light. [Google Scholar]
- Huber R., Adler D.C., Srinivasan V.J., Fujimoto J.G. Fourier domain mode locking at 1050 nm for ultra-high-speed optical coherence tomography of the human retina at 236,000 axial scans per second. Optics Letters. 2007;32:2049–2051. doi: 10.1364/ol.32.002049. [DOI] [PubMed] [Google Scholar]
- Izatt J.A., Kulkami M.D., Yazdanfar S., Barton J.K., Welch A.J. In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomograghy. Optics Letters. 1997;22:1439–1441. doi: 10.1364/ol.22.001439. [DOI] [PubMed] [Google Scholar]
- Jiao S.L., Yao G., Wang L.H.V. Depth-resolved two-dimensional Stokes vectors of backscattered light and Mueller matrices of biological tissue measured with optical coherence tomography. Applied Optics. 2000;39:6318–6324. doi: 10.1364/ao.39.006318. [DOI] [PubMed] [Google Scholar]
- Jones R.C. A new calculus for the treatment of optical systems I. Description and discussion of the calculus. Journal of the Optical Society of America. 1941;31:488–493. [Google Scholar]
- Jonnal R.S., Qu J., Thorn K., Miller D.T. En-face coherence gating of the retina with adaptive optics. Investigative Ophthalmology & Visual Science. 2003;44:U275. [Google Scholar]
- Kemp N.J., Park J., Zaatari H.N., Rylander H.G., Milner T.E. High-sensitivity determination of birefringence in turbid media with enhanced polarization-sensitive optical coherence tomography. Journal of the Optical Society of America A-Optics Image Science and Vision. 2005;22:552–560. doi: 10.1364/josaa.22.000552. [DOI] [PubMed] [Google Scholar]
- Kemp N.J., Zaatari H.N., Park J., Rylander H.G., Milner T.E. Form-biattenuance in fibrous tissues measured with polarization-sensitive optical coherence tomography (PS–OCT) Optics Express. 2005;13:4611–4628. doi: 10.1364/opex.13.004611. [DOI] [PubMed] [Google Scholar]
- Kempen J.H., O’Colmam B.J., Leske C., Haffner S.M., Klein R., Moss S.E., Taylor H.R., Hamman R.F., West S.K., Wang J.J., Congdon N.G., Friedman D.S., Grp E.D.P.R. The prevalence of diabetic retinopathy among adults in the United States. Archives of Ophthalmology. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- Klein Brink H.B., van Blokland G.J. Birefringence of the human foveal area assessed in vivo with Mueller-matrix ellipsometry. Journal of the Optical Society of America A-Optics Image Science and Vision. 1988;5:49–57. doi: 10.1364/josaa.5.000049. [DOI] [PubMed] [Google Scholar]
- Klein T., Wieser W., Eigenwillig C.M., Biedermann B.R., Huber R. Megahertz OCT for ultrawide-field retinal imaging with a 1050 nm Fourier domain mode-locked laser. Optics Express. 2011;19:3044–3062. doi: 10.1364/OE.19.003044. [DOI] [PubMed] [Google Scholar]
- Lammer J., Bolz M., Baumann B., Götzinger E., Pircher M., Hitzenberger C., Schmidt-Erfurth U. 2010. Automated Detection and Quantification of Hard Exudates in Diabetic Macular Edema Using Polarization Sensitive Optical Coherence Tomography. ARVO abstract 4660/D935. [Google Scholar]
- Lee E.C.W., de Boer J.F., Mujat M., Lim H., Yun S.H. In vivo optical frequency domain imaging of human retina and choroid. Optics Express. 2006;14:4403–4411. doi: 10.1364/oe.14.004403. [DOI] [PubMed] [Google Scholar]
- Lee E.S., Kang S.Y., Choi E.H., Kim J.H., Kim N.R., Seong G.J., Kim C.Y. Comparisons of nerve fiber layer thickness measurements between stratus, Cirrus, and RTVue OCTs in healthy and glaucomatous eyes. Optometry and Vision Science. 2011;88:751–758. doi: 10.1097/OPX.0b013e318215cc40. [DOI] [PubMed] [Google Scholar]
- Leitgeb R., Hitzenberger C.K., Fercher A.F. Performance of fourier domain vs. time domain optical coherence tomography. Optics Express. 2003;11:889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- Leitgeb R.A., Schmetterer L., Drexler W., Fercher A.F., Zawadzki R.J., Bajraszewski T. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Optics Express. 2003;11:3116–3121. doi: 10.1364/oe.11.003116. [DOI] [PubMed] [Google Scholar]
- Mai T.A., Reus N.J., Lemij H.G. Structure-function relationship is stronger with enhanced corneal compensation than with variable corneal compensation in scanning laser polarimetry. Investigative Ophthalmology & Visual Science. 2007;48:1651–1658. doi: 10.1167/iovs.06-1003. [DOI] [PubMed] [Google Scholar]
- Makita S., Hong Y., Yamanari M., Yatagai T., Yasuno Y. Optical coherence angiography. Optics Express. 2006;14:7821–7840. doi: 10.1364/oe.14.007821. [DOI] [PubMed] [Google Scholar]
- Medeiros F.A., Zangwill L.M., Bowd C., Vessani R.M., Susanna R., Weinreb R.N. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. American Journal of Ophthalmology. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- Medeiros F.A., Zangwill L.M., Bowd C., Weinreb R.N. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Archives of Ophthalmology. 2004;122:827–837. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- Meek K.M., Tuft S.J., Huang Y.F., Gill P.S., Hayes S., Newton R.H., Bron A.J. Changes in collagen orientation and distribution in keratoconus corneas. Investigative Ophthalmology & Visual Science. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- Michels S., Pircher M., Geitzenauer W., Simader C., Götzinger E., Findl O., Schmidt-Erfurth U., Hitzenberger C.K. Value of polarisation-sensitive optical coherence tomography in diseases affecting the retinal pigment epithelium. British Journal of Ophthalmology. 2008;92:204–209. doi: 10.1136/bjo.2007.130047. [DOI] [PubMed] [Google Scholar]
- Mishchenko M.I., Hovenier J.W. Depolarization of light backscattered by randomly oriented Nonspherical particles. Optics Letters. 1995;20:1356–1358. doi: 10.1364/ol.20.001356. [DOI] [PubMed] [Google Scholar]
- Miyazawa A., Yamanari M., Makita S., Miura M., Kawana K., Iwaya K., Goto H., Yasuno Y. Tissue discrimination in anterior eye using three optical parameters obtained by polarization sensitive optical coherence tomography. Optics Express. 2009;17:17426–17440. doi: 10.1364/OE.17.017426. [DOI] [PubMed] [Google Scholar]
- Mueller H. 1943. Memorandum on the Polarization Optics of the Photoelastic Shutter. Report No. 2 of the OSRD project OEMsr-576. [Google Scholar]
- Mujat M., Park B.H., Cense B., Chen T.C., de Boer J.F. Autocalibration of spectral-domain optical coherence tomography spectrometers for in vivo quantitative retinal nerve fiber layer birefringence determination. Journal of Biomedical Optics. 2007;12:041205. doi: 10.1117/1.2764460. [DOI] [PubMed] [Google Scholar]
- Park B.H., Pierce M.C., Cense B., de Boer J.F. Jones matrix analysis for a polarization-sensitive optical coherence tomography system using fiber-optic components. Optics Letters. 2004;29:2512–2514. doi: 10.1364/ol.29.002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher M., Goetzinger E., Leitgeb R., Hitzenberger C.K. Transversal phase resolved polarization sensitive optical coherence tomography. Physics in Medicine and Biology. 2004;49:1257–1263. doi: 10.1088/0031-9155/49/7/013. [DOI] [PubMed] [Google Scholar]
- Pircher M., Götzinger E., Baumann B., Hitzenberger C.K. Corneal birefringence compensation for polarization sensitive optical coherence tomography of the human retina. Journal of Biomedical Optics. 2007;12:041210. doi: 10.1117/1.2771560. [DOI] [PubMed] [Google Scholar]
- Pircher M., Götzinger E., Findl O., Michels S., Geitzenauer W., Leydolt C., Schmidt-Erfurth U., Hitzenberger C.K. Human macula investigated in vivo with polarization-sensitive optical coherence tomography. Investigative Ophthalmology & Visual Science. 2006;47:5487–5494. doi: 10.1167/iovs.05-1589. [DOI] [PubMed] [Google Scholar]
- Pircher M., Götzinger E., Leitgeb R., Fercher A.F., Hitzenberger C.K. Speckle reduction in optical coherence tomography by frequency compounding. Journal of Biomedical Optics. 2003;8:565–569. doi: 10.1117/1.1578087. [DOI] [PubMed] [Google Scholar]
- Pircher M., Gotzinger E., Leitgeb R., Sattmann H., Findl O., Hitzenberger C.K. Imaging of polarization properties of human retina in vivo with phase resolved transversal PS–OCT. Optics Express. 2004;12:5940–5951. doi: 10.1364/opex.12.005940. [DOI] [PubMed] [Google Scholar]
- Pircher M., Zawadzki R. Combining adaptive optics with optical coherence tomography: unveiling the cellular structure of the human retina in vivo. Expert Review of Ophthalmology. 2007;2:16. [Google Scholar]
- Pircher M., Zawadzki R.J., Evans J.W., Werner J.S., Hitzenberger C.K. Simultaneous imaging of human cone mosaic with adaptive optics enhanced scanning laser ophthalmoscopy and high-speed transversal scanning optical coherence tomography. Optics Letters. 2008;33:22–24. doi: 10.1364/ol.33.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potsaid B., Gorczynska I., Srinivasan V.J., Chen Y.L., Jiang J., Cable A., Fujimoto J.G. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. Optics Express. 2008;16:15149–15169. doi: 10.1364/oe.16.015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H.A. Number of people with glaucoma worldwide. British Journal of Ophthalmology. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H.A., Addicks E.M., Green W.R. Optic-Nerve damage in human glaucoma .3. Quantitative Correlation of nerve-fiber loss and visual-field Defect in glaucoma, Ischemic neuropathy, Papilledema, and Toxic neuropathy. Archives of Ophthalmology. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- Schlanitz F.G., Ahlers C., Sacu S., Schutze C., Rodriguez M., Schriefl S., Golbaz I., Spalek T., Stock G., Schmidt-Erfurth U. Performance of drusen detection by spectral-domain optical coherence tomography. Investigative Ophthalmology & Visual Science. 2010;51:6715–6721. doi: 10.1167/iovs.10-5288. [DOI] [PubMed] [Google Scholar]
- Schmitt J.M., Xiang S.H. Cross-polarized backscatter in optical coherence tomography of biological tissue. Optics Letters. 1998;23:1060–1062. doi: 10.1364/ol.23.001060. [DOI] [PubMed] [Google Scholar]
- Schmitt J.M., Xiang S.H., Yung K.M. Speckle in optical coherence tomography. Journal of Biomedical Optics. 1999;4:95–105. doi: 10.1117/1.429925. [DOI] [PubMed] [Google Scholar]
- Schuetze C., Bolz M., Sayegh R., Baumann B., Pircher M., Gotzinger E., Hitzenberger C., Schmidt-Erfurth U. 2011. Proof of Principle of Lesion Size Detection in Geographic Atrophy Using Polarization-sensitive Spectral Domain Optical Coherence Tomography and Correlation to Conventional Imaging Techniques ARVO Abstract. 52:1244. [DOI] [PubMed] [Google Scholar]
- Schuman J.S., Hee M.R., Puliafito C.A., Wong C., Pedutkloizman T., Lin C.P., Hertzmark E., Izatt J.A., Swanson E.A., Fujimoto J.G. Quantification of nerve-fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography – a Pilot-study. Archives of Ophthalmology. 1995;113:586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- Schuman J.S., Pedut-Kloizman T., Pakter H., Wang N., Guedes V., Huang L., Pieroth L., Scott W., Hee M.R., Fujimoto J.G., Ishikawa H., Bilonick R.A., Kagemann L., Wollstein G. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Investigative Ophthalmology & Visual Science. 2007;48:3645–3654. doi: 10.1167/iovs.06-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman J.S., PedutKloizman T., Hertzmark E., Hee M.R., Wilkins J.R., Coker J.G., Puliafito C.A., Fujimoto J.G., Swanson E.A. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–1898. doi: 10.1016/s0161-6420(96)30410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman J.S., Puliafito C.A., Fujimoto J.G. Slack Inc.; Thorofare, NJ: 2004. Optical Coherence Tomography of Ocular Diseases. [Google Scholar]
- Sehi M., Ume S., Greenfield D.S. Scanning laser polarimetry with enhanced corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Investigative Ophthalmology & Visual Science. 2007;48:2099–2104. doi: 10.1167/iovs.06-1087. [DOI] [PubMed] [Google Scholar]
- Shou T.D., Liu J., Wang W., Zhou Y.F., Zhao K.X. Differential dendritic shrinkage of alpha and beta retinal ganglion cells in cats with chronic glaucoma. Investigative Ophthalmology & Visual Science. 2003;44:3005–3010. doi: 10.1167/iovs.02-0620. [DOI] [PubMed] [Google Scholar]
- Srinivasan V.J., Adler D.C., Chen Y., Gorczynska I., Huber R., Duker J.S., Schuman J.S., Fujimoto J.G. Ultrahigh-Speed optical coherence tomography for three-dimensional and en face imaging of the retina and optic nerve head. Investigative Ophthalmology & Visual Science. 2008;49:5103–5110. doi: 10.1167/iovs.08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanworth A., Naylor E.J. The polarization optics of the Isolated cornea. British Journal of Ophthalmology. 1950;34:210–211. doi: 10.1136/bjo.34.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson E.A., Izatt J.A., Hee M.R., Huang D., Lin C.P., Schuman J.S., Puliafito C.A., Fujimoto J.G. In-vivo retinal imaging by optical coherence tomography. Optics Letters. 1993;18:1864–1866. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- Szkulmowska A., Szkulmowski M., Szlag D., Kowalczyk A., Wojtkowski M. Three-dimensional quantitative imaging of retinal and choroidal blood flow velocity using joint spectral and time domain optical coherence tomography. Optics Express. 2009;17:10584–10598. doi: 10.1364/oe.17.010584. [DOI] [PubMed] [Google Scholar]
- Thylefors B., Negrel A.D., Pararajasegaram R., Dadzie K.Y. Global data on blindness. Bulletin of the World Health Organization. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- Todorovic M., Jiao S.L., Wang L.V. Determination of local polarization properties of biological samples in the presence of diattenuation by use of Mueller optical coherence tomography. Optics Letters. 2004;29:2402–2404. doi: 10.1364/ol.29.002402. [DOI] [PubMed] [Google Scholar]
- Torti C., Povazay B., Hofer B., Unterhuber A., Carroll J., Ahnelt P.K., Drexler W. Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Optics Express. 2009;17:19382–19400. doi: 10.1364/OE.17.019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K.A., Wollstein G., Schuman J.S. Imaging of the retinal nerve fibre layer for glaucoma. British Journal of Ophthalmology. 2009;93:139–143. doi: 10.1136/bjo.2008.145540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterhuber A., Povazay B., Hermann B., Sattmann H., Chavez-Pirson A., Drexler W. In vivo retinal optical coherence tomography at 1040 nm-enhanced penetration into the choroid. Optics Express. 2005;13:3252–3258. doi: 10.1364/opex.13.003252. [DOI] [PubMed] [Google Scholar]
- Van Blokland G.J., Verhelst S.C. Corneal polarization in the living human-eye explained with a biaxial model. Journal of the Optical Society of America A-Optics Image Science and Vision. 1987;4:82–90. doi: 10.1364/josaa.4.000082. [DOI] [PubMed] [Google Scholar]
- van Velthoven M.E.J., Faber D.J., Verbraak F.D., van Leeuwen T.G., de Smet M.D. Recent developments in optical coherence tomography for imaging the retina. Progress in Retinal and Eye Research. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- vonRuckmann A., Fitzke F.W., Bird A.C. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Investigative Ophthalmology & Visual Science. 1997;38:478–486. [PubMed] [Google Scholar]
- Wang Y.M., Bower B.A., Izatt J.A., Tan O., Huang D. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. Journal of Biomedical Optics. 2007;12:041215. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- Weber A.J., Kaufman P.L., Hubbard W.C. Morphology of single ganglion cells in the glaucomatous primate retina. Investigative Ophthalmology & Visual Science. 1998;39:2304–2320. [PubMed] [Google Scholar]
- Weinreb R.N., Dreher A.W., Coleman A., Quigley H., Shaw B., Reiter K. Histopathologic validation of fourier-ellipsometry measurements of retinal nerve-fiber layer thickness. Archives of Ophthalmology. 1990;108:557–560. doi: 10.1001/archopht.1990.01070060105058. [DOI] [PubMed] [Google Scholar]
- Weinreb R.N., Khaw P.T. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Weinreb R.N., Shakiba S., Zangwill L. Scanning laser polarimetry to measure the nerve-fiber layer of normal and glaucomatous eyes. American Journal of Ophthalmology. 1995;119:627–636. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- Werkmeister R.M., Dragostinoff N., Pircher M., Götzinger E., Hitzenberger C.K., Leitgeb R.A., Schmetterer L. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Optics Letters. 2008;33:2967–2969. doi: 10.1364/ol.33.002967. [DOI] [PubMed] [Google Scholar]
- Wiener O. Die Theorie des Mischkorpers fur das Feld der stationaren Stromung. Abhandlung der Sachsischen Gesellschaft der Wissenschaft. 1912;32:509–604. [Google Scholar]
- Wojtkowski M. High-speed optical coherence tomography: basics and applications. Applied Optics. 2010;49:D30–D61. doi: 10.1364/AO.49.000D30. [DOI] [PubMed] [Google Scholar]
- Wojtkowski M., Leitgeb R., Kowalczyk A., Bajraszewski T., Fercher A.F. In vivo human retinal imaging by Fourier domain optical coherence tomography. Journal of Biomedical Optics. 2002;7:457–463. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- Wolf-Schnurrbusch U.E.K., Enzmann V., Brinkmann C.K., Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT–SLO combination. Investigative Ophthalmology & Visual Science. 2008;49:3095–3099. doi: 10.1167/iovs.07-1460. [DOI] [PubMed] [Google Scholar]
- Yamanari M., Lim Y., Makita S., Yasuno Y. Visualization of phase retardation of deep posterior eye by polarization-sensitive swept-source optical coherence tomography with 1-mu m probe. Optics Express. 2009;17:12385–12396. doi: 10.1364/oe.17.012385. [DOI] [PubMed] [Google Scholar]
- Yamanari M., Makita S., Lim Y., Yasuno Y. Full-range polarization-sensitive swept-source optical coherence tomography by simultaneous transversal and spectral modulation. Optics Express. 2010;18:13964–13980. doi: 10.1364/OE.18.013964. [DOI] [PubMed] [Google Scholar]
- Yamanari M., Makita S., Yasuno Y. Polarization-sensitive swept-source optical coherence tomography with continuous source polarization modulation. Optics Express. 2008;16:5892–5906. doi: 10.1364/oe.16.005892. [DOI] [PubMed] [Google Scholar]
- Yamanari M., Miura M., Makita S., Yatagai T., Yasuno Y. Phase retardation measurement of retinal nerve fiber layer by polarization-sensitive spectral-domain optical coherence tomography and scanning laser polarimetry. Journal of Biomedical Optics. 2008;13:014013. doi: 10.1117/1.2841024. [DOI] [PubMed] [Google Scholar]
- Yasuno Y., Hong Y.J., Makita S., Yamanari M., Akiba M., Miura M., Yatagai T. In vivo high-contrast imaging of deep posterior eye by 1-mu m swept source optical coherence tomography and scattering optical coherence angiography. Optics Express. 2007;15:6121–6139. doi: 10.1364/oe.15.006121. [DOI] [PubMed] [Google Scholar]
- Yasuno Y., Makita S., Sutoh Y., Itoh M., Yatagai T. Birefringence imaging of human skin by polarization-sensitive spectral interferometric optical coherence tomography. Optics Letters. 2002;27:1803–1805. doi: 10.1364/ol.27.001803. [DOI] [PubMed] [Google Scholar]
- Yasuno Y., Yamanari M., Kawana K., Miura M., Fukuda S., Makita S., Sakai S., Oshika T. Visibility of trabecular meshwork by standard and polarization-sensitive optical coherence tomography. Journal of Biomedical Optics. 2010;15 doi: 10.1117/1.3499421. [DOI] [PubMed] [Google Scholar]
- Yehoshua Z., Rosenfeld P.J., Gregori G., Feuer W.J., Falcao M., Lujan B.J., Puliafito C. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118:679–686. doi: 10.1016/j.ophtha.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki R.J., Cense B., Zhang Y., Choi S.S., Miller D.T., Werner J.S. Ultrahigh-resolution optical coherence tomography with monochromatic and chromatic aberration correction. Optics Express. 2008;16:8126–8143. doi: 10.1364/oe.16.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki R.J., Jones S.M., Olivier S.S., Zhao M.T., Bower B.A., Izatt J.A., Choi S., Laut S., Werner J.S. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging. Optics Express. 2005;13:8532–8546. doi: 10.1364/opex.13.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cense B., Rha J., Jonnal R.S., Gao W., Zawadzki R.J., Werner J.S., Jones S., Olivier S., Miller D.T. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Optics Express. 2006;14:4380–4394. doi: 10.1364/OE.14.004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Reed J., Betts R., Trost P., Lo P., Wallace C., Bienias R., Li G., Winnick R., Papworth W., Sinai M. Detection of glaucomatous retinal nerve fiber layer damage by scanning laser polarimetry with variable corneal compensation. Proceedings of SPIE. 2003;4951:32–41. [Google Scholar]
- Zotter S., Pircher M., Torzicky T., Bonesi M., Gotzinger E., Leitgeb R.A., Hitzenberger C.K. Visualization of microvasculature by dual-beam phase-resolved Doppler optical coherence tomography. Optics Express. 2011;19:1217–1227. doi: 10.1364/OE.19.001217. [DOI] [PMC free article] [PubMed] [Google Scholar]