Abstract
Id3−/− mice represent a model for T cell mediated primary Sjogren's syndrome (PSS). An intriguing feature of this disease model is the early appearance of impaired salivary function or exocrinopathy prior to lymphocytic infiltration of the salivary glands. This phenomenon prompted us to examine the role of cytokines produced by T cells in the systemic regulation of gland function. A comprehensive examination of serum cytokine profiles revealed elevated levels of IL-13 in Id3−/− mice. We found that the increase in serum IL-13 levels in Id3−/− mice was largely dependent on αβ T cells. Removal of αβ T cells in Id3−/− mice also eliminates disease symptoms, including lymphocytic infiltration in the gland tissues, and impaired saliva production. We further show that the number of mast cells in the salivary glands of Id3−/− mice is significantly increased, in a trend inversely related to the saliva production. This increase in the number of mast cells is also dependent on the presence of αβ T cells. Treatment of young Id3−/− mice with anti-IL-13 antibodies over a two-month period resulted in a reduction of both serum IL-13 levels and the number of mast cells in the salivary gland tissues, as well as correspondingly improved saliva production. These findings indicate a potentially important role for IL-13 in gland regulation and disease pathology.
Keywords: Id3 deficient mice, IL-13, Exocrinopathy, Mast cells
1. Introduction
Sjogren's syndrome (SS) is a chronic autoimmune disease in which host-derived immune cells chronically attack the lachrymal and salivary glands, resulting in reduced tear and saliva production and subsequent keratoconjuntivitis sicca and xerostomia[1]. SS is one of the most prevalent rheumatic autoimmune disorders, affecting millions of individuals world wide, and has a strong female bias comparable to that of rheumatoid arthritis[2]. SS can be classified as either primary, if the disease occurs alone, or secondary if the disease is found in conjunction with other autoimmune conditions such as Systemic Lupus Erythematosus (SLE) or Rheumatoid Arthritis (RA)[3]. Numerous studies have demonstrated the involvement of both T cell mediated and humoral autoimmunity in SS, though much work remains to be done in order to further understand the mechanisms underlying the initiation and early disease manifestations of this complex disorder[4, 5]. This is due in part to the fact that there are few animal models that can fully recapitulate primary SS[6].
Id3−/− mice develop hallmark symptoms of PSS and, similar to human patients, disease severity progresses with age[7]. The first sign of disease is impaired saliva production, which occurs at two months of age even in the absence of lymphocytic infiltration. Lymphocytes appear in the salivary and lachrymal glands by three months, and by 12 months of age production of autoantibodies is detected. Altered glandular homeostasis has been reported in Id3−/− mice prior to the presence of lymphocytic infiltration, suggesting that additional factors likely contribute to the early exocrinopathy observed in this SS mouse model[7]. T cells play an integral role in the initiation and progression of the pathogenesis observed in Id3−/− mice, as shown through T cell transfer experiments and T cell specific deletion of Id3−/− [7, 8]. It still remains unclear how T cells influence gland function prior to significant tissue infiltration. Elevated cytokine levels have been reported in a number of autoimmune disorders, including SS, and this raises the possibility that cytokine dysregulation could be contributing to the observed exocrinopathy in Id3−/− mice[9].
IL-13 was initially recognized as a TH2 cytokine due to its effects on monocytes and B cells, promoting the upregulation of MHC II expression, IgE class switching, and inhibiting inflammatory cytokine production[10–13]. IL-13 has important roles in the development of TH2 cells and regulation of cell-mediated immunity including resistance to pathogens such as Leishmania major [14, 15]. IL-13 exhibits stimulatory activity on a number of cell types including B cells, mast cells, and fibroblasts[10]. Mouse models have shown that IL-13 plays an essential role in allergic asthma by regulating airway hyperresponsiveness, eosinophilic inflammation, and mucus secretion[16]. In addition, IL-13 has been shown to be a potent mediator of tissue fibrosis in schistosomiasis infection in mice, indicating that it is an important regulator of the extracellular matrix[17]. Elevated levels of IL-13 have been reported to correlate to increased autoantibody levels in various rheumatic autoimmune disorders, and IL-13 mRNA has been detected in the gland tissues of SS patients, indicating this cytokine might play an important role in disease pathology[18–20].
In this report, we show that young Id3−/− mice display elevated IL-13 levels in the serum and significant populations of IL-13+ T cells exist in the thymus and lymph nodes. Genetic ablation of αβ T cells results in reduced serum IL-13 levels, improved gland function, an absence of lymphocytic infiltration, and a reduction in mast cell presence in gland tissue. In addition to the contribution of T cells to disease development and progression, we have identified mast cells as an important regulator of gland function. Id3−/− mice have significantly elevated numbers of mast cells in the mandibular gland tissue and a proportion of these mast cells stain positive for IL-13. These results should help to further understand potential mechanistic links between T cells, mast cells and IL-13 during disease initiation and manifestation in Id3−/− mice.
2. Methods and Materials
2.1. Mice
The Id3−/− strain has been backcrossed to C57Bl/6 for 11 generations before use in the present studies. This strain is now deposited in Jackson Labs (Bar Harbor). TCRβ deficient mice (Jackson Laboratory) were crossed with the Id3 knockout strain for a minimum of 5 generations. All animal procedures were approved by the Duke University Animal Use and Care Committee.
2.2. ELISA and Luminex Assays
Cytokine levels were determined using mouse serum from 12–24 week old wild type, Id3−/−, and Id3−/−/β−/− mice using a Luminex Multiplex platform. Type I Interferon levels were quantified using the Verikine Mouse Interferon Alpha ELISA kit (PBL Interferon Source #42100-1) according to the manufacturer's instructions.
2.3. Saliva Secretion Test
Mice were anesthetized by intraperitoneal injection of Avertin (Sigma Cat#. T4, 840-2) (15uL/g body weight) and then injected with pilocarpine hydrochloride (Sigma, Cat#. P-6503) (0.5ug/g body weight) dissolved in ddH2O to stimulate saliva production. Saliva was collected with a 100uL microcapillary pipette (VWR, Cat#. 53432-921) immediately after pilocarpine injection for a duration of 9 minutes. Saliva secretion volumes are normalized to body weight.
2.4. Histology
Histology sections were prepared from paraffin-embedded tissues and stained with Haematoxylin and Eosin (H+E). Each lymphocytic focus was defined by the presence of 50 or more nucleated cells in a cluster. The foci score for each mouse is from one mandibular gland section. Mast cells were counted using Toluidine blue staining. Tissue remodeling and fibrosis was assessed by Masson's Trichrome staining.
2.5. FACS Analysis
Thymocytes, splenocytes, and lymph node-derived lymphocytes were isolated from wild type and Id3−/− mice and were resuspended in 1× PBS containing 5% FBS (Gemini #900-108) and surface staining was done on ice for 20 minutes in the dark. Intracellular staining was performed following surface staining by fixing cells in 2% paraformaldehyde for 20 minutes on ice in the dark. Cells were then permeabilized in 0.5% Saponin (Sigma #S4521) in 1× PBS for 30 minutes on ice in the dark. Intracellular staining was then performed using anti-IL-13 (eBioscience #51-7133-80) in 1× PBS containing 5% FBS for 20 minutes on ice in the dark.
2.6. RT-PCR
Total RNA was isolated from total thymocytes, total splenocytes, and sorted CD4+ lymph node derived T cells with Trizol (Sigma). Following cDNA synthesis, RT-PCR was performed using the following primers: IL-13 Forward, 5'-GGGTGACTGCAGTCCTGGCT-3', IL-13 Reverse, 5'-GGTGCTCAGCTCCTCAATAAGC-3', GAPDH Forward 5'-CCTGGAGAAACCTGCCAAGTATG-3', GAPDH Reverse 5'- AGAGTGGGAGTTGCTGTTGAAGTC-3'.
2.7. Immunofluorescent Microscopy
Immunofluorescent microscopy involved flash freezing tissues in a dry ice/ethanol bath for sectioning. Five-micron sections were fixed in methanol/acetone at -20 C for 20 minutes then blocked in 3% BSA at room temperature for 20 minutes. Tissues were then stained with various combinations of the following antibodies: CD4 (Biolegend #100408), CD8 (Biolegend # 126608), TCRβ (Biolegend 109208), TCRδ (eBioscience 12-5711-82), IL-13 (eBioscience # 13-7135-81), and c-Kit (eBioscience #12-1171-81), and were mounted using Fluoromount and stored overnight at room temperature.
2.8. Anti-IL-13 Treatment
Eight week old Id3−/− mice were injected IV with 25 ug anti-IL-13 (R+D Systems MAB413) or an IgG2b isotype control antibody (PharMingen #11031D) every two weeks until sacrifice at 16 weeks of age. Serum was collected prior to initial treatment and following sacrifice to determine serum IL-13 levels.
2.9. Statistical Analysis
Statistical significance was performed using an unpaired two tailed student's t-test using Prism software.
3. Results
3.1. Increased serum IL-13 levels in Id3−/− mice
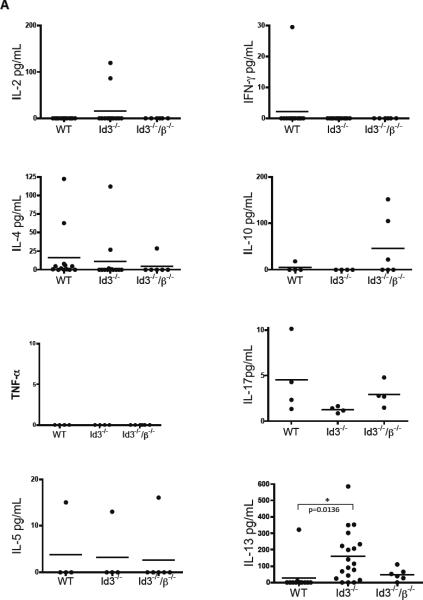
Elevated cytokine levels have been shown to be associated with various rheumatic autoimmune disorders including SS[9], so we sought to determine whether or not there were elevated cytokine levels in the serum of 12–24 week old Id3−/− mice. There was no significant difference in the levels of IL-2 and IFN-γ and other TH1 associated cytokines. In addition, IL-10 and IL-17, cytokines frequently associated with autoimmunity, did not show any differences as well (Figure 1A). The TH2 locus, which contains the IL-4, IL-5 and IL-13 genes, was also analyzed for variance in serum levels and neither IL-4 or IL-5 showed significant increases, though serum IL-13 levels in 12–24 week old mice was dramatically increased (Figure 1A). T cells represent an important source of IL-13[11], so we tested the serum from 12–24 week old Id3−/−/β−/− double deficient mice. By removing αβ T cells from Id3−/− mice, serum IL-13 levels were greatly reduced, though not to the baseline levels seen in wild type mice (Figure 1A). This result indicates that αβ T cells, and possibly other cells or tissues, work in concert to increase IL-13 levels.
Figure 1.
Serum cytokine levels found in 12–24 week old wild type, Id3−/− and Id3−/−/β−/− double deficient mice were determined by Luminex assays.
3.2. αβ T cells regulate gland infiltration and secretory function
Previous publications have confirmed that T cells play a critical role in disease initiation in Id3−/− mice[7, 8], so we tested two key disease parameters in 12–24 week old Id3−/− mice that lack αβ T cells, namely, lymphocytic infiltration and saliva production. Histological analysis of salivary gland tissues revealed that Id3−/− mice lacking αβ T cells fail to develop the hallmark lymphocytic infiltration found in Id3−/− mice (Figure 2A). Id3−/− mice lacking αβ T cells also showed significant improvement in saliva production, reaching levels comparable to that of wild type control mice as compared to Id3−/− mice containing αβ T cells (Figure 2B). These finding further exemplify the importance of αβ T cells to disease pathology.
Figure 2.
Lymphocytic foci were quantified in the mandibular glands from wild type (n=19), Id3−/− (n=17) and Id3−/−/β−/− (n=9) double deficient mice (Figure 2A). Saliva production was assessed in wild type (n=9), Id3−/− (n=22) and Id3−/−/β−/− (n=7) double deficient mice (Figure 2B). All mice were age matched and ranged from 12–24 weeks of age.
3.3. Increased IL-13 transcription and IL-13+ CD4+ T cells
Based on the reduced levels of IL-13 present in the Id3−/−β−/− double deficient mice, we investigated whether or not there was an increase in IL-13 expressing T cells in Id3−/− mice. Using FACS analysis, we find that a small fraction of Id3−/− mice (2 out of 15) have an increased percentage of intracellular IL-13 in TCRβ+ CD4+ thymocytes and in lymph node-residing TCRβ+ CD4+, but not in resident CD8+ T cells (data not shown). To determine if there was an increase in IL-13 transcription in T cells, RT-PCR was performed using a variety of tissues and cell types. A fraction of Id3−/− mice showed an increase in IL-13 mRNA levels in the thymus and from purified lymph node CD4+ T cells, while splenic derived T cells from Id3−/− or wild type samples did not (Figure 3A). Immunofluorescent microscopy of cervical lymph nodes revealed that there are significant populations of IL-13+ TCRβ+ T cells present in Id3−/− mice (Figures 3B–C). The presence of these cells in the lymph nodes proximal to the salivary gland tissues raises the possibility that these cells might be contributing to the exocrinopathy observed in young Id3−/− mice.
Figure 3.
RT-PCR analysis of IL-13 mRNA in Id3−/− thymocytes and lymph node derived CD4+ T cells (Figure 3A). Immunofluorescent microscopy of cervical lymph nodes gives visualization of TCRβ+ IL-13+ T cells in 16 week old Id3−/− mice, populations that are largely absent in wild type mice (Figures 3B–C)
3.4. Gland tissue and mast cells positive for IL-13
Further analysis of salivary gland tissue revealed a number of interesting phenomena. First, the gland tissues of both wild type and Id3−/− mice stained positive for IL-13, especially around the gland ductal tissue, and the specificity of this staining was confirmed by using IL-13−/− gland tissue (Figures 4A–C). Second, lymphocytic infiltration is one of the defining characteristics of disease pathology, so we sought to determine if there were IL-13+ T cells present in the salivary gland tissues of Id3−/− mice. TCRβ+ T cells were detected within the salivary gland tissues of both wild type and Id3−/− mice, though immunofluorescent microscopy failed to reveal any significant populations of IL-13+ T cells in the gland tissues (Figure 4B). TCRβ+ T cells in the lymphocytic foci found in the glands of Id3−/− mice also stained negative for IL-13 (Figure 4C). Third, given that a number of other cell types can produce IL-13, we stained for a number of other markers including c-Kit, used as a surface marker for mast cells[21]. A significant proportion of Id3−/− mice (4 out of 11) had c-Kit+ mast cells that also stained positive for IL-13, though this was not observed in any of the wild type mice analyzed (0 out of 5) (Figures 4D–F).
Figure 4.
Immunofluorescent microscopy of mandibular gland tissue sections from 12–24 week old IL-13−/−, Id3−/− and wild type mice show specific binding anti-IL-13 antibodies to wild type and Id3−/− mice (Figures 4A–F). IL-13−TCRβ+ T cells are present in 16 week old wild type and Id3−/− mice (Figure 4A–C). Immunofluorescent microscopy of mandibular gland tissue sections staining for IL-13+ and c-Kit+ mast cells in wild type and Id3−/− mice (Figure 4D–F).
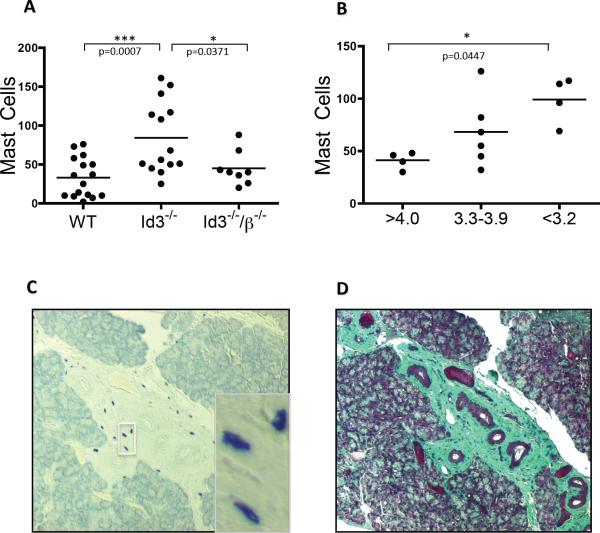
3.5. Increased presence of mast cells in salivary gland tissues in Id3−/− mice
When comparing various aspects of disease pathology in the salivary gland tissue of wild type and Id3−/− mice, we found that Id3−/− mice had a significant increase in the number of mast cells present in the mandibular gland tissue and that the removal of αβ T cells was sufficient to reduce this number significantly (Figure 5A). To determine whether this increase in mast cell presence in the gland tissues of Id3−/− mice correlates to gland function, we compared saliva production to mast cell numbers and found a correlation to reduced saliva in those mice with increased mast cell numbers, while foci and saliva production had no obvious connection (Figures 5B and data not shown). Histological analysis of gland tissues revealed that a majority of mast cells were largely confined to regions of fibrous and remodeled tissue, suggesting that these cells are likely involved in the extensive gland tissue remodeling seen in Id3−/− mice (Figures 5C–D).
Figure 5.
Number of mandibular gland mast cells counted in 12–24 week old wild type, Id3−/−, and Id3−/− mice (Figure 5A). Saliva production (x-axis, measured in uL/gram body weight) compared to number of mast cells present in mandibular glands in 12–24 week old Id3−/− mice (Figure 5B). Toluidine blue staining of mandibular gland tissue sections collected from 16 week old Id3−/− mice showing an accumulation of mast cells in remodeled gland tissue (Figure 5C). Trichrome staining of mandibular gland tissue showing fibrous tissue co-localized with mast cell accumulation in 16 week old Id3−/− mice (Figure 5D).
3.6. Anti-IL-13 Treatment
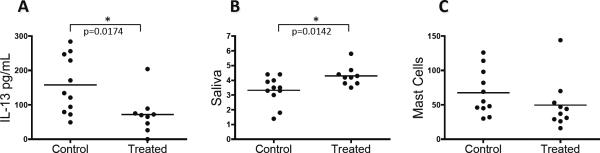
To further understand the potential role of IL-13 in early exocrinopathy, we sought to neutralize IL-13 in young Id3−/− mice prior to the detection of gland infiltration. Id3−/− mice treated with an anti-IL-13 monoclonal antibody for two months showed a significant reduction in serum IL-13 levels as compared to the Id3−/− mice that received control antibody, indicating that the treatment was effective in reducing serum IL-13 (Figure 6A). Saliva secretion tests showed that there was a significant increase in saliva production in Id3−/− mice treated with anti-IL-13 as compared to control Id3−/− mice (Figure 6B). Mast cell numbers were reduced in the mandibular glands in all but one individual of the treated group compared to the control mice (Figure 6C). Thus, anti-IL-13 treatment resulted in an improvement in salivary secretory function.
Figure 6.
Eight week old Id3−/− mice were treated with either an isotype control antibody or anti-IL-13 antibodies every two weeks for eight weeks and were analyzed for various aspects of disease pathology. IL-13 levels from anti-IL-13 treated Id3−/− mice compared to isotype control treated Id3−/− mice after two months of treatment (Figure 6A). Saliva production after anti-IL-13 as compared to control antibody treated mice (Figure 6B). Mast cell numbers from control and anti-IL-13 treated (Figure 6C).
4. Discussion
Disease initiation remains one of the most challenging issues facing autoimmunity with regards to diagnosis and treatment, with the application of preventative measures based on early clinical signs representing a major goal. Here we report the finding of increased serum IL-13 levels in 12–24 week old Id3−/− mice, an age where salivary dysfunction begins to appear. We have identified an increase in IL-13 transcription in the thymus and lymph nodes of Id3−/− mice in addition to visual confirmation IL-13+ TCRβ+ CD4+ T cells present in the periglandular lymph nodes. Expression of IL-13 transcripts in the thymus is unexpected, as IL-13 production from T cells is predominately from TH2 polarized cells, not thymocytes[22]. We do not know whether these IL-13 producing cells are first developed in the thymus before migrating to peripheral lymph nodes or vice versa. It is possible that there is only differentiation of these IL-13+ T cells when exposed to glandular antigens by periglandular APCs, and that some of these cells migrate back to the thymus, yielding detectable IL-13 transcription. These cells could be partially responsible for the elevated serum IL-13 levels, since genetic ablation of αβ T cells in Id3−/− mice resulted in a significant reduction of serum IL-13, though they could also function to induce IL-13 production from other cells and/or tissues. Because these IL-13+ CD4 T cells are frequently detected in periglandular lymph nodes it raises the possibility that these cells may play an important role in the salivary gland dysfunction observed in Id3−/− mice.
The marked increase in mast cell counts in the salivary gland tissues of Id3−/− mice correlates with reduced saliva production, indicating that mast cells play an important role in regulating salivary gland function. The mechanism of gland regulation remains unclear, though histological analysis shows that mast cells localize in areas of significant tissue remodeling, providing an important clue as to how mast cells may regulate glandular homeostasis. Mast cells can act as effector cells that amplify inflammation, as well as regulatory cells that suppress immune responses. Despite substantial evidence that mast cells are critical players in the pathogenesis of some autoimmune conditions, their precise mode of action is still unclear. Mast cells may regulate T cell priming, localization, and migration[21]. Mast cells may also regulate autoimmune disease severity by inflicting direct damage on local tissue structures[21]. SS patients have been reported to have increased mast cells in the salivary glands, and the fact that Id3−/− mice have increased populations of IL-13+ mast cells located within the salivary gland tissue adds a complex yet intriguing layer to the possible regulation of gland function and disease progression by mast cells[21, 23].
The relationship between T cells, mast cells, and IL-13 with respect to glandular function and regulation is still correlative and likely complex. The proximal localization of IL-13+ T cells to gland tissues that contain large numbers of mast cells indicates a potentially important aspect of disease progression. Human studies have shown that extended exposure of mast cells to IL-13 resulted in the upregulation of FcεRI expression, increased histamine release following IgE/anti-IgE activation, and increased proliferation[24]. These findings indicate the possibility of IL-13+ T cells influencing mast cell proliferation, activation and subsequent production of IL-13, and increased histamine release in the gland tissues of Id3−/− mice.
The importance of T cells in the initiation and progression of SS and, more specifically, exocrinopathy has been established, though the precise mechanisms remain unclear. Numerous studies have demonstrated various ways in which T cells and mast cells can regulate the function and homing of one another[25, 26], but how these effector cells contribute to the function of salivary glands is poorly understood. It is likely that T cells are important in the recruitment of mast cells to the gland tissues through the secretion of various chemoattractants and cytokines, with IL-13 being a likely candidate. Future studies involving T cell specific ablation of IL-13 or other relevant genes may help to resolve the issue.
One of the major goals of autoimmunity research is to find potential therapeutic approaches to relieve and potentially prevent the development of many of the often devastating disease symptoms. By using an experimental approach aimed at neutralizing serum IL-13, we found that treatment of young Id3−/− mice over a two month period was sufficient to reduce serum IL-13 levels to a significant degree. Additionally, we found that saliva production returned to wild type levels following antibody treatment, though lymphocytic infiltration in the gland tissue was unaffected (data not shown). Additionally, mast cell populations were reduced in the salivary gland tissue in anti-IL-13 treated Id3−/− mice, with the exception of one individual, indicating that IL-13 plays an important role in the recruitment of mast cells to the gland tissue. Because removal of αβ T cells also results in a reduced number of mast cells in the gland tissue, it is possible that T cell derived IL-13 could be an important mediator in this recruitment.
5. Conclusions
Examination of a panel of cytokines revealed that young Id3−/− mice have significantly higher levels of IL-13 than wild type controls and that these levels are reduced in the absence of αβ T cells. Immunofluorescent microscopy revealed large populations of IL-13+ TCRβ+ T cells exist in the cervical lymph nodes proximal to the salivary gland tissue, while these populations were absent in wild type tissue. The fact that these cells are present proximal to areas of specific autoimmune destruction further supports a role for these cells in the progression of disease pathology. Quantification of mast cells demonstrated that Id3−/− mice have a significant increase in the number of mast cells present in the mandibular gland tissue compared to wild type controls. Additionally, there is a correlation between the number of mast cells present in the gland tissue and reduced saliva production, indicating that these cells play an important role in regulating saliva production. Treatment of young Id3−/− mice with neutralizing anti-IL-13 monoclonal antibody over a two month period resulted in a significant reduction in serum IL-13 levels, increased saliva production and the reduction of mast cells present in the mandibular gland tissue. These findings provide evidence that gland tissue function can be regulated by IL-13, and that T cells and mast cells play important roles in this regulation.
Research Highlights
-
>
Id3 disruption results in elevated levels of serum IL-13, increased numbers of mast cells in salivary glands, and Sjogren's syndrome like disease.
-
>
Mast cell count in gland tissues is inversely correlated to secretory function.
-
>
Id3−/− mice treated with anti-IL-13 show improved saliva production.
Acknowledgments
We thank Dr. Andrew McKenzie and Coral Key for providing IL-13−/− gland tissues; Dr. Baojun Zhang for assistance with the anti-IL-13 antibody IV injections; Meifang Dai for assistance with saliva tests and animal care; Duke Human Vaccine Institute for assistance with the Luminex assay; Dr. Mary Elizabeth Jones-Mason, Ian Belle, and Ian McLeod for advice and assistance. This study was supported by the Arthritis Foundation and the National Institute of Health (R21 RR024927; R01 GM059638).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren's syndrome. Nat Rev Rheumatol. 2010;6:529–37. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- [3].Fox RI, Stern M, Michelson P. Update in Sjogren syndrome. Curr Opin Rheumatol. 2000;12:391–8. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- [4].Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren's syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252–64. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- [5].Hansen A, Lipsky PE, Dorner T. B cells in Sjogren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res Ther. 2007;9:218. doi: 10.1186/ar2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jonsson MV, Delaleu N, Jonsson R. Animal models of Sjogren's syndrome. Clin Rev Allergy Immunol. 2007;32:215–24. doi: 10.1007/s12016-007-8012-7. [DOI] [PubMed] [Google Scholar]
- [7].Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity. 2004;21:551–60. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [8].Guo Z, Li H, Han M, Xu T, Wu X, Zhuang Y. Modeling Sjogren's syndrome with Id3 conditional knockout mice. Immunol Lett. 2011;135:34–42. doi: 10.1016/j.imlet.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. The American journal of pathology. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- [11].McKenzie AN, Culpepper JA, de Waal Malefyt R, Briere F, Punnonen J, Aversa G, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993;90:3735–9. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–8. [PubMed] [Google Scholar]
- [15].McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- [16].Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, et al. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol. 2001;25:522–30. doi: 10.1165/ajrcmb.25.4.4620. [DOI] [PubMed] [Google Scholar]
- [17].Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–91. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- [18].Spadaro A, Rinaldi T, Riccieri V, Taccari E, Valesini G. Interleukin-13 in autoimmune rheumatic diseases: relationship with the autoantibody profile. Clin Exp Rheumatol. 2002;20:213–6. [PubMed] [Google Scholar]
- [19].Villarreal GM, Alcocer-Varela J, Llorente L. Differential interleukin (IL)-10 and IL-13 gene expression in vivo in salivary glands and peripheral blood mononuclear cells from patients with primary Sjogren's syndrome. Immunol Lett. 1996;49:105–9. doi: 10.1016/0165-2478(95)02490-5. [DOI] [PubMed] [Google Scholar]
- [20].Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–8. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–39. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- [22].Wills-Karp M. Interleukin-13 in asthma pathogenesis. Current allergy and asthma reports. 2004;4:123–31. doi: 10.1007/s11882-004-0057-6. [DOI] [PubMed] [Google Scholar]
- [23].Konttinen YT, Hietanen J, Virtanen I, Ma J, Sorsa T, Xu JW, et al. Mast cell derangement in salivary glands in patients with Sjogren's syndrome. Rheumatol Int. 2000;19:141–7. doi: 10.1007/s002960050118. [DOI] [PubMed] [Google Scholar]
- [24].Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, et al. Mast cells express IL-13R alpha 1: IL-13 promotes human lung mast cell proliferation and Fc epsilon RI expression. Allergy. 2006;61:1047–53. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- [25].Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ, Jr., Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. European journal of immunology. 2005;35:3478–86. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- [26].Gombert M, Dieu-Nosjean MC, Winterberg F, Bunemann E, Kubitza RC, Da Cunha L, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. Journal of immunology. 2005;174:5082–91. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]