Abstract
Objective
Lopinavir/ritonavir (Kaletra®) is first line therapy for pediatric HIV infection. In clinical practice, Kaletra® tablets are occasionally crushed for pediatric administration. This study compared lopinavir/ritonavir exposure between whole and crushed tablets in HIV-infected children.
Design
This was a randomized, open-label, cross-over study of pediatric patients taking lopinavir/ritonavir as part of their antiretroviral regimen. Each subject had two separate (within 30 days) steady-state 12-hour pharmacokinetic (PK) studies with crushed and whole 200/50 mg lopinavir/ritonavir tablets.
Methods
PK blood samples were drawn at 0 (pre-dose), 1, 2, 4, 6, 8, and 12 hours post-dose. Lopinavir and ritonavir plasma concentrations measured by high performance liquid chromatography were used to calculate non-compartmental area under the concentration versus time curve (AUC) and clearance (CL/F). Wilcoxon signed-rank tests compared PK values between crushed and whole tablets.
Results
Twelve children, median age of 13 years (10–16 years), took 550/138 mg/m2/day lopinavir/ritonavir divided every 12 hours. The median lopinavir AUC following crushed and whole tablets were 92 mg*hr/L and 144 mg*hr/L, respectively, with an AUC ratio of 0.55 (p=0.003). Median ritonavir AUC of crushed and whole tablets were 7 mg*hr/L and 13.3 mg*hr/L, respectively, with an AUC ratio of 0.53 (p=0.006).
Conclusions
Administration of crushed 200/50 mg lopinavir/ritonavir tablets to children significantly reduced lopinavir and ritonavir exposure with a decrease in AUC by 45% and 47%, respectively. The administration of crushed tablets would require higher doses and therapeutic drug monitoring to ensure adequate lopinavir exposure in patients requiring this practice. The use of crushed lopinavir/ritonavir tablets should be avoided, if possible.
Keywords: lopinavir, ritonavir, pharmacokinetics, pediatrics
INTRODUCTION
International, European and USA pediatric antiretroviral therapy guidelines recommend co-formulated protease inhibitor (PI) lopinavir/ritonavir (Kaletra®) as a first-line agent for the treatment of HIV infection in infants, children and adolescents.(1–3) For administration in infants and younger children, Kaletra® is available as an oral suspension (80/20mg/1mL lopinavir/ritonavir). This liquid formulation has an extremely unpleasant taste with a high content (42%) of alcohol, creating the potential for significant alcohol toxicity with overdose, especially in infants.(4) In addition to poor palatability, Kaletra® suspension must be taken with food and requires refrigeration, which limits its use in resource-limited settings.
The older gelatin capsule Kaletra® formulation (133.3/33.3mg LPV/RTV) represented a major tolerability barrier in pediatric HIV therapy. Two film-coated tablet formulations (200/50 mg and 100/50 mg lopinavir/ritonavir) have entered the practice in the US and successfully replaced the previous capsule version. These tablet formulations do not require refrigeration and may be taken without regards to meals.(5) The prescribing information states that these tablets may not be crushed, broken or chewed. In the development of this formulation, Abbott Pharmaceuticals, the producer of Kaletra®, found that the crushed tablets were poorly absorbed in ten beagle dogs; 33% lower lopinavir and 61% lower ritonavir exposure was seen with a single crushed tablet compared to a single intact tablet dose.(6) Single-dose pharmacokinetics do not predict steady-state lopinavir concentrations due to complex interactions with the ritonavir component. No information as to the extent of the decrease in absorption in humans has been reported.
Administration of capsules and tablets is a common barrier to pediatric antiretroviral therapy.(7–13) Swallowing an adult size solid oral dosage form can be challenging in school aged children and adolescents, and may represent a choking hazard in younger pediatric patients. Children under seven years of age are frequently unable to swallow even smaller tablets.(14) To overcome the poor palatability of the liquid Kaletra® formulation, pediatric patients and their caregivers are frequently requesting early initiation of the tablet Kaletra® formulations. When pill swallowing difficulties become the next barrier in taking the drug, the caregivers consider dosing children with broken or crushed tablets. If crushed tablet administration lowers systemic lopinavir exposure, then patients using this administration technique can be at higher risk for development of viral resistance and treatment failure. This lopinavir/ritonavir administration technique has not been studied in humans, and providers lack evidence to support or discourage this dosing strategy. We conducted the study among pediatric patients to compare lopinavir/ritonavir exposure between whole and crushed 200/50 mg lopinavir/ritonavir tablet doses in HIV-infected children. The smaller tablet (100/25 mg lopinavir/ritonavir) formulation of Kaletra® was just entering the market in the US at the time of this study and was not evaluated in our cohort of patients.
METHODS
This was a prospective, open-label, cross-over PK study in pediatric patients infected with HIV who were taking Kaletra® tablets twice daily as part of their antiretroviral regimen. Subjects were recruited from the Special Immunology Program at Children’s National Medical Center (CNMC) in Washington, DC. The study was approved by the University of California, San Diego and CNMC Institutional Review Boards, was conducted in accordance with ethical standards of the institutional review committees and the Helsinki Declaration of 1975, as revised in 2000, and required informed consent of the legal guardian and assent in children ages 7–18 years of age. To be eligible for study participation patients were required to be 6–17 years of age, to have documented HIV infection and to take Kaletra® 200/50 mg lopinavir/ritonavir tablets at standard pediatric doses for more than two weeks. Concomitant medications and/or natural products, including potentially interacting products, had to be stable for more than two weeks and not expected to change over the course of the study. Exclusion criteria included acute serious medical illness or infection requiring treatment and/or hospitalization within 14 days prior to study entry and pregnancy.
PK sampling
Following study enrollment, patients were randomly assigned equally (1:1) in two study arms: Arm A and Arm B. The subjects in study Arm A were administered whole Kaletra® tablets at Study Visit 1, and crushed tablets at Study Visit 2, while Arm B subjects received the drug in the reverse order. The patients received standard prescribed dose of Kaletra® dispensed to them through their local pharmacies during routine refills.
Study Visit 1
The patients fasted overnight and arrived at the research center prior to eating or taking their morning lopinavir/ritonavir dose or any other medications. Drinking water prior to the study visit was allowed. The patients brought their own prescription 200/50 mg Kaletra® tablets, and other antiretroviral and concomitant medications that they needed to take throughout the day. Following drawing of the pre-dose blood sample (3 mL), children took a witnessed dose of lopinavir/ritonavir tablets. The whole Kaletra® tablet(s) was administered with 6 ounces of water. For the crushed dose, a standard commercially available pill crusher (CVS/pharmacy store brand) was used to crush the tablet(s) and the crushed tablet(s) was/were mixed in 4 ounces of Jell-O brand pudding. Any crushed medication that remained in the pill-crusher device was scraped out with a metal spatula and stirred into the pudding. Following the drug intake, children ate a standardized breakfast consisting of 7 calories/kg, 20% protein, 50% carbohydrates and 30% fat. The meal was finished within 30 minutes of study drug administration. Subjects took the rest of their antiretroviral regimen one hour after the study drug and ate at will throughout the day. Following the Kaletra® intake blood samples (3 mL) were drawn at 1, 2, 4, 6, 8, and 12 hours post-dose.
Study Visit 2
After the completion of Study Visit 1, the subjects returned for Study Visit 2 (within 30 days of Visit 1). The procedures were identical to Study Visit 1, with the exception of taking the alternate dosage administration method, crushed vs. whole tablets, according to the Study Arm assignment.
Drug assays
The blood was collected in a heparin tube and separated by centrifugation at 3000 rpm for 15 minutes at 4°C. Plasma was placed in cryovials and frozen at −20°C, shipped on ice and stored at −20°C pending analysis. All plasma samples were assayed for lopinavir and ritonavir concentrations at the University of California, San Diego (UCSD) Pediatric Clinical Pharmacology Laboratory using a validated, reversed-phase multiplex high performance liquid chromatography (HPLC) method. The lower limit of detection was 0.091 mcg/mL for lopinavir and 0.094 mcg/mL for ritonavir. The inter-assay coefficient of variation (CV) was 10.9% at the limit of detection for both drugs and was < 10% CV for low, middle and high controls. Overall recovery from plasma was 98% for lopinavir and 117.3% for ritonavir. The UCSD laboratory has been enrolled in the ACTG QA/QC proficiency testing program since 2001 with twice a year testing.(15)
PK analyses
Pre-dose concentrations, maximum concentrations, 12-hour concentrations and time of maximum concentrations were observed from the data. Area under the concentration vs. time curve (AUC0–12) for the dose interval was estimated by the linear trapezoidal rule. For subjects with undetectable pre-dose concentrations, AUC0-∞ was calculated; AUClast-∞ was estimated as C12 divided by the elimination rate constant, the latter determined from a log-linear regression of the terminal slope. For concentrations below the assay limit of detection, a value of one-half of the detection limit (0.045 mcg/mL for lopinavir, 0.047 mcg/mL for ritonavir) was used in other summary calculations. Apparent clearance (CL/F) from plasma was calculated as dose divided by AUC. Ritonavir concentrations were summarized in the same manner, and ritonavir AUC0–12 was also calculated. All pharmacokinetic parameters were calculated using WinNonlin version 5.0.1 (Pharsight®, a Certara™ company, St. Louis, MO, USA).
Statistical analyses
Our hypothesis was that exposure to lopinavir would be lower when the dose is administered as crushed tablets instead of whole tablets. We considered a reduction in AUC of 35% or more to be clinically important. The intra-patient lopinavir AUC0–12 variability was estimated at 15%. Using a two-sided paired t-test and an alpha value of 0.05, a sample size of 12 patients had >90% power to detect a within-patient difference in AUC0–12 of 35%.
The primary endpoint was a within-subject comparison of lopinavir AUC0–12 estimated from each of the two different administration strategies. This endpoint, along with the secondary endpoints of maximum, 12-hour and pre-dose concentrations and oral clearance, were assessed using the Wilcoxon signed-rank test in STATA/SE 10 (StataCorp LP, College Station, TX). Descriptive statistics were used to summarize PK parameters for each of the two different administration strategies. Medians and range were determined for AUC, maximum and pre-dose concentration and oral clearance for the entire cohort for each different administration method. In addition, PK parameters were log-transformed, and the geometric mean ratio and 90% confidence intervals were calculated.
RESULTS
Subject characteristics and outcomes
Thirteen pediatric patients (6 males, 7 females) aged from 10 to 16 years (median 13 years) were enrolled between August 2008 and August 2009. One subject (12 years old male) refused to take the crushed Kaletra® tablet and was replaced. Median [range] weight (44 kg [31 – 94 kg]) and height (157 cm [131 – 177 cm]) of the enrolled patients were representative of children of this age group. All enrolled had been taking Kaletra® as part of their antiretroviral regimen twice daily. Nine subjects were receiving two 200/50 mg lopinavir/ritonavir tablets (400/100mg lopinavir/ritonavir per dose) twice daily, and three were prescribed one and half 200/50 mg lopinavir/ritonavir tablets (300/75mg lopinavir/ritonavir per dose) twice daily using a broken tablet for the half tablet. During the study all subjects received two 200/50 mg lopinavir/ritonavir tablets (400/100mg lopinavir/ritonavir per dose) with the median lopinavir/ritonavir dose at 275/69 mg/m2 (193/48 – 372/93 mg/m2). All patients were treated with lopinavir/ritonavir plus two nucleoside reverse transcriptase inhibitors. No other classes of antiretroviral drugs were used. None of the concomitant medications had known interactions with lopinavir or ritonavir. No natural product use was reported. No subject vomited within one hour of study drug administration.
LPV and RTV exposure
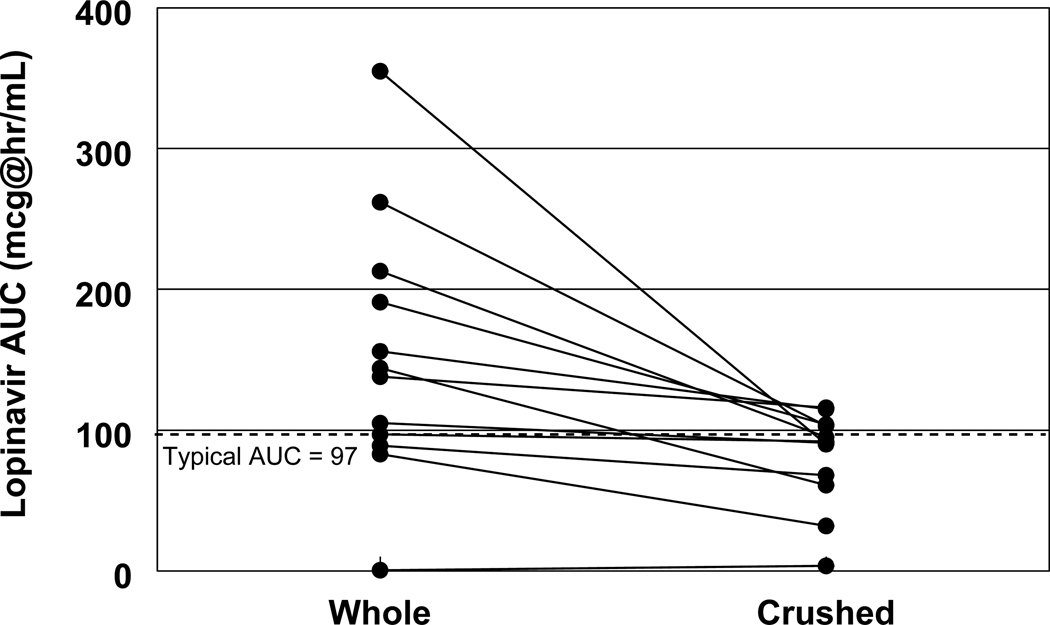
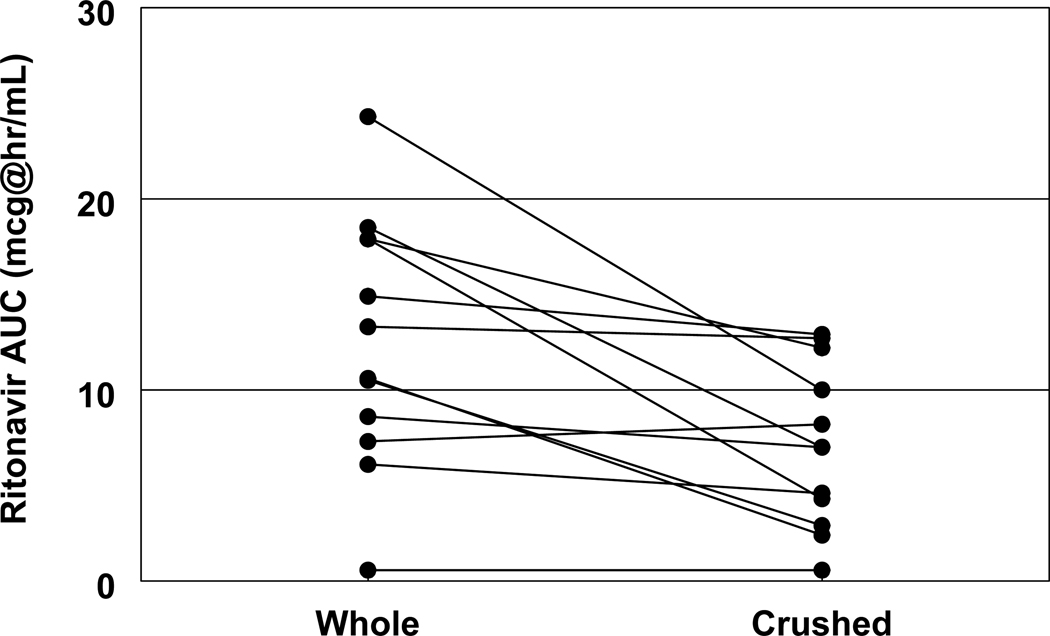
The median concentrations of lopinavir and ritonavir in study subjects are presented in Figures 1 and 2. One subject had undetectable lopinavir and ritonavir concentrations in all blood samples after administration of whole tablets, and had very low or undetectable concentrations after the crushed tablet administration also. This subject was excluded from the summary statistics and Figures 1 and 2. The exposure to both lopinavir and ritonavir was decreased in crushed tablets compared to that of whole tablets with lopinavir whole and crushed AUC being 141 mg*hr/L and 92 mg.hr/L, respectively, and ritonavir whole and crushed AUC being 11.9 mg*hr/L and 7.0 mg.hr/L, respectively. This translates into a 45% decrease in AUC for lopinavir (p=0.003) and a 47% decrease in AUC for ritonavir (p=0.005; Figures 3 and 4). Almost all subjects had a decrease in AUC for both lopinavir and ritonavir. The sharpest drop in lopinavir AUC was from 355 mg*hr/L to 90 mg*hr/L, representing almost a 75% decrease in AUC. Only three subjects had < 20% change in lopinavir AUC. These three subjects did not have any obvious similarities (one male, two female, ages 10, 14 and 16 years, weights 32 to 61 kg, and AUCs within the interquartile ranges of the whole population). The change in lopinavir and ritonavir AUCs with study drug administration method were strongly correlated (r=0.76). The majority (8/12; 66.7%) of patients had lopinavir AUC higher than 80 mcg*hr/mL, and lopinavir AUC lower than 50 mcg*hr/mL was only observed in 2 subjects. The statistical comparisons did not differ whether the one subject with mostly undetectable concentrations was included or excluded from the analyses.
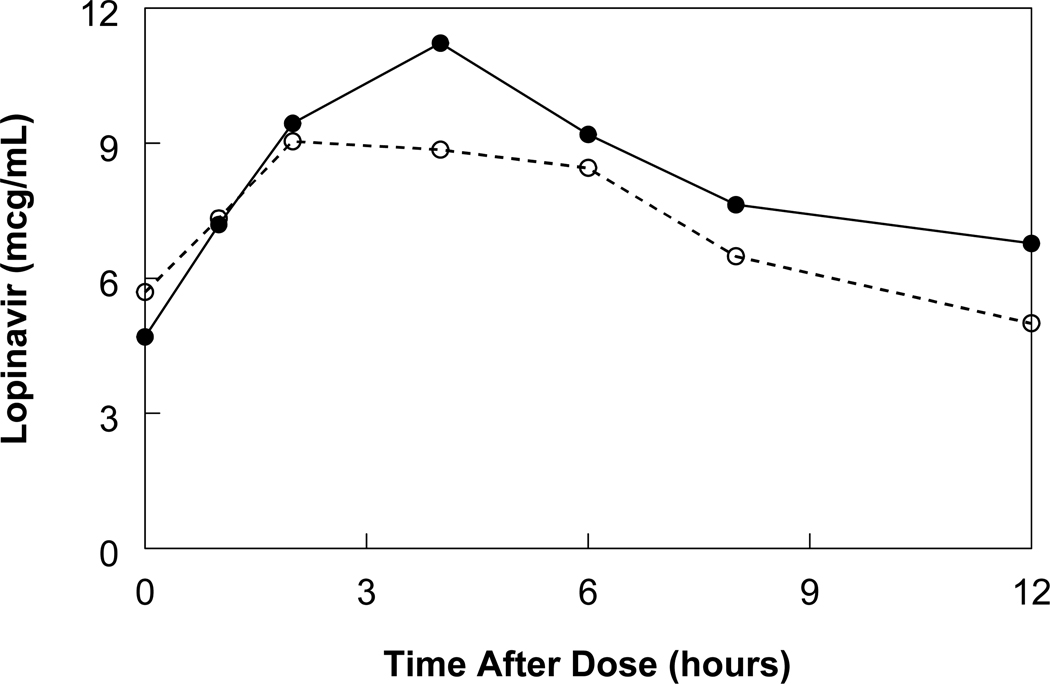
Figure 1. Median lopinavir concentrations with whole and crushed tablets.
Median lopinavir concentration-time curves in patients after administration of whole tablets (solid line, n=11) and crushed tablets (dotted line, n = 11).
Figure 2. Median ritonavir concentrations with whole and crushed tablets.
Median ritonavir concentration-time curves in patients after administration of whole tablets (solid line, n=11) and crushed tablets (dotted line, n = 11).
Figure 3. Lopinavir AUC with whole tablets vs. crushed tablets.
Changes in lopinavir AUC from whole tablets (left column) to crushed tablets (right column) in the same patients (n=12). The dotted line represents the typical lopinavir AUC of approximately 97 mcg*hr/mL in adult patients following administration of two 200mg/500mg lopinavir/ritonavir tablets.
Figure 4. Ritonavir AUC with whole tablets vs. crushed tablets.
Changes in ritonavir AUC from whole tablets (left column) to crushed tablets (right column) in the same patients (n=12).
Lopinavir and ritonavir pharmacokinetic parameters for whole and crushed tablets are presented in Table 1. Lopinavir and ritonavir were below detection at 0 time point (pre-dose) at 7 study visits (5 whole, 2 crushed) out of 24. The maximum post-dose plasma (Cmax) concentrations of both lopinavir and ritonavir decreased, significantly for lopinavir and trended for ritonavir, with crushed tablet administration (p=0.021 and 0.075; Table 1). Time to reach maximal concentration (Tmax) was also sooner for both lopinavir and ritonavir in crushed tablets. Oral CL/F of both lopinavir and ritonavir significantly increased with crushed tablet administration, by 1.4 and 1.6 times, respectively. After 12 hours, trough plasma concentrations (C12) of both lopinavir and ritonavir in crushed tablets were below that of whole tablets, significantly for lopinavir (p=0.016). With whole tablet administration, all subjects had C12 above 4 mcg/mL. With crushed tablet administration, four of the subjects had concentrations below 4 mcg/mL, and one subject had a C12 of 0.72 mcg/mL, less than the minimum trough target for treatment-naïve patients of 1 mcg/mL.
Table 1.
Pharmacokinetic parameters of crushed vs. whole lopinavir/ritonavir tablets
| Parameter | Whole1 | Crushed1 | Crushed/Whole2 | p-value | |
|---|---|---|---|---|---|
| Lopinavir | AUC (mg*hr/L) | 144 (101 – 202) | 92 (79 – 103) | 0.55 (0.45 – 0.69) | 0.003* |
| CL/F (L/hr/m2) | 2.3 (1.3 – 2.6) | 3.2 (2.5 – 3.5) | 1.8 (1.5 – 2.2) | 0.003* | |
| Tmax (hr) | 4 (2 – 4) | 2 (1.5 – 4) | n/a | n/a | |
| Cmax (mg/L) | 11.3 (9.3 – 13.8) | 9.4 (7.2 – 11.4) | 0.75 (0.61 – 0.92) | 0.021* | |
| C0 (mg/L) | 4.7 (<0.1 – 8.8) | 5.7 (4.2 – 8) | 1.3 (0.64 – 2.6) | 0.505 | |
| C12 (mg/L) | 6.8 (5.2 – 10.1) | 5 (2.8 – 5.9) | 0.56 (0.4 – 0.78) | 0.016* | |
| Ritonavir | AUC (mg*hr/L) | 13.3 (9.6 – 17.9) | 7 (4.5 – 11.1) | 0.53 (0.4 – 0.71) | 0.006* |
| CL/F (L/hr/m2) | 5 (4.5 – 7.1) | 8.2 (6.7 – 16.1) | 1.89 (1.41 – 2.53) | 0.008* | |
| Tmax (hr) | 4 (3 – 5) | 2 (2 – 4) | n/a | n/a | |
| Cmax (mg/L) | 0.9 (0.8 – 1.7) | 0.8 (0.6 – 1.2) | 0.7 (0.51 – 0.97) | 0.075 | |
| C0 (mg/L) | 0.2 (<0.1 – 0.8) | 0.5 (0.2 – 0.8) | 1 (0.56 – 1.85) | 0.69 | |
| C12 (mg/L) | 0.5 (0.3 – 0.6) | 0.4 (0.2 – 0.6) | 0.87 (0.67 – 1.12) | 0.45 |
Median (Interquartile range);
Geometric mean (90% Confidence Interval);
Statistically significant difference between whole and crushed at p < 0.05;
AUC0–12 = area under the plasma concentration-time curve;
Cmax = maximum concentration;
Tmax = time post-dose of maximum concentration;
C0 = pre-dose concentration;
C12 = 12-hour post-dose concentration;
CL/F = oral clearance;
DISCUSSION
Lopinavir/ritonavir remains the first line drug for the initial antiretroviral regimen in pediatric HIV-infected children in the US and Europe starting with children as young as 2 weeks old.(2,3) In resource-limited settings, lopinavir/ritonavir has become the first choice antiretroviral drug for the inclusion in first line regimens in children younger than 2 years of age with history of perinatal non-nucleoside reverse transcriptase inhibitor exposure as part of prevention of mother to child transmission of HIV.(1) Treatment in infants under 24 months of age is recommended regardless of the CD4 cell count or WHO clinical stage. While the availability of pediatric smaller lopinavir/ritonavir tablet (100mg/25 mg) has improved the acceptance of this protease inhibitor in children, a significant proportion of children and adolescents continue to experience difficulties with swallowing lopinavir/ritonavir tablets or oral liquid formulations. In our large pediatric HIV practice in the US, we face many clinical scenarios where caregivers consider dividing the antiretroviral drugs into smaller sizes through splitting or breaking of tablets. Due to the limitations in the availability of certain liquid antiretroviral formulations and child-sized pills in many regions, adult pills continue to constitute the main delivery of antiretroviral therapy to children in diverse settings worldwide.(16–18) Moreover, even in the area of established access to pediatric antiretroviral formulations, many providers have been reported to hesitate using them due to their less reliable supply, as compared to more plentiful adult drug access.(19)
The crushing of the coated Kaletra® pill has the potential to alter drug exposure. The new tablet formulation helps ensure solubility in the gastrointestinal tract and thus facilitates absorption. Disruption of the extrude matrix environment may adversely impact this formulation affect. The crushing of the pill leaves part of the drug(s) on the walls of the container or crushing device, and the transfer of the crushed substance to the food or liquid for mixing may also generate loss of the active drug. In our study we used a commercial pill crushing device sold over the counter in the US, and transferred the crushed Kaletra® tablet directly into the prepackaged commercially prepared pudding to help absorption following crushed tablet administration.
Kaletra® tablets can be administered on an empty stomach or with food. The administration of a moderate fat meal (500–682 Kcal; 23–25% fat calories) has been reported to increase the lopinavir AUV by 26.9%, while a high fat meal (872 Kcal; 56% fat calories) has more modest effect on lopinavir AUC, increasing it by 18.9% only.(5) In our study we have used a standardized moderate fat meal (7 calories/kg; 30% fat calories) for both forms of tablet administration in order to optimize the lopinavir/ritonavir pharmacokinetics and to approximate the intake to the real life pediatric scenario where the crushed or intact preparations are usually taken with meal. The ingredients in pudding (salt, disodium phosphate, tetra sodium phosphate, diglycerides and sugar) are not expected to affect the absorption of lopinavir/ritonavir, as they are similar to the ingredients in the oral solution, which contains salts, sugars, and glycerin.
Following the intake of the Kaletra® tablets, lopinavir and ritonavir plasma concentrations rose faster with crushed tablets immediately after administration, possibly because of easier absorption due to larger surface area of drug particles, but fell off as time progressed, leading to a lower total exposure in crushed compared to whole tablets. As a result, the maximum plasma concentrations of both lopinavir and ritonavir were lower with crushed tablets. Due to the limited sampling duration during the elimination phase, the half-lives of lopinavir and ritonavir could not be estimated with good precision. However, no large differences were seen in the terminal portions of the plasma curves, suggesting that the increase in oral clearance is due to a decrease in bioavailability (F) and not due to faster elimination.
The absolute bioavailability of lopinavir co-formulated with ritonavir in humans is not established.(5) The equal degree (45% and 47%) of decrease in AUC for lopinavir and ritonavir, respectively, is most likely due to the unknown changes in bioavailability of both drugs. Both drugs are substrates of the MDR1 drug transporter and lopinavir is also transported by the organic anion transporter polypeptide (OATP).(20,21) Recently, we reported the significant association of the SLCO1B1 T521C polymorphism with increased LPV AUC in pediatric HIV-infected patients.(22) The potential changes in the CYP450 based metabolism in the gut and intracellular drug transport may also be responsible for the observed changes in AUC for lopinavir and ritonavir. Equally, the significant increases in lopinavir and ritonavir CL/F could have contributed to the decreased AUCs for both drugs.(23)
Consistent with pediatric lopinavir/ritonavir study by La Porte et al.,(24) we have observed a high level of inter-individual variability in lopinavir pharmacokinetics. This finding is consistent with higher variability of lopinavir/ritonavir in children and is possibly due to age, physical and sexual maturation and weight differences of the subjects.(25–27) In our study, the decrease in AUC ranged from 5% to as high as 75%. Similarly, decreases in Cmax and C12 varied greatly, albeit to a lesser extent than AUC. Younger children tend to have lower C12, and children in general have a much greater variability in trough concentrations.(28) This poses an additional challenge in estimating the expected reduction in drug exposure in each individual patient and raises particular concern with using crushed lopinavir/ritonavir in younger children.
The high inter-individual variability may also be responsible for the non-detectable lopinavir and ritonavir C0 after unobserved doses which can be due to the longer period of time since the previous dose. Nevertheless, the most plausible explanation of undetectable C0 remains the lack of steady adherence, which correlates with the clinical histories in study patients, the majority of whom (8/12) had a history of stable undetectable HIV viremia, while 4 subjects (2 using mixed whole and halves of the 200/50 mg tablets and 2 using whole 200/50 mg tablets) had a history of suboptimal virologic suppression and had identified adherence difficulties in the past and present medical history. Three subjects in the study had used mixture of whole and halves of the 200/50 mg lopinavir/ritonavir tablets as their standard of care (in order to meet the dose) on regular bases, which could have also affected their baseline plasma trough concentrations.
While a 45% reduction in lopinavir and ritonavir AUC represents a clinical concern (particularly for the treatment-experienced patients), we recognize that the majority of patients in the study had lopinavir AUC >80 mcg*hr/mL. This is most likely due to a higher dosing of the children in this study (275/69 mg/m2/dose versus currently recommended 230/57.5 mg/m2/dose) and much-higher-than-average lopinavir concentrations observed in patients taking whole tablets. With standard-of-care lower lopinavir/ritonavir dosing, the chances of reaching sub-therapeutic lopinavir exposure would be significantly higher. In addition to a decrease in AUC and Cmax, plasma trough lopinavir concentration (C12) was also reduced by 33%. Only one of the patients had C12 below the suggested minimum efficacy cut-off of 1 mg/L after crushed tablet administration. Nevertheless, lower plasma troughs in combination with AUC and Cmax declines, may lead to earlier development of lopinavir resistance. This is particularly relevant to the treatment-experienced children and treatment-naïve adolescents who are prescribed lopinavir/ritonavir once a day. Lopinavir/ritonavir therapy is not currently recommended for once daily use in pediatric patients and its use has been associated with lower troughs and worse virologic control in ARV-experienced children.(29)
As discussed above, our study had certain limitations. The start time during the day of the PK study visit (7 am) might have resulted in pre-dose samples greater than 12 hours after an unobserved dose (since many children take their drugs with the meal after school). Adherence was not evaluated in the study and the patients were assumed to be at steady state, while some of them might have been missing medication doses at home and in reality presented as a single dose pharmacokinetic evaluation. To attempt to adjust for this, AUC0-∞ were estimated for those specific visits with pre-dose concentrations only below detection. Despite these limitations, our data clearly suggest that the lopinavir/ritonavir pharmacokinetics are significantly impacted by crushing the lopinavir/ritonavir tablets with AUC, Cmax, and C12 all being significantly reduced, posing potential therapy failure and antiretroviral medication resistance risks in pediatric HIV patients. The reduced and variable exposure with crushed tablet dosing reinforces the need to discourage this dosing practice in children or consider therapeutic drug monitoring in the settings where this cannot be avoided.
CONCLUSIONS
Crushing of antiretroviral drugs is considered to deliver or approximate pediatric doses, especially in children who are unable to swallow pills. Since the published data on the bioequivalence of crushed antiretroviral drugs are very limited, most antiretroviral drug recommendations advise against this practice. In our study of the steady state single dose of the whole versus crushed Kaletra® tablet in pediatric patients, administration of crushed 200/50mg lopinavir/ritonavir tablet decreased AUC of lopinavir and ritonavir by approximately 40%. The magnitude was highly variable and unpredictable between subjects, ranging from 5 to 75% reduction in AUC. The extent and variability of reduced exposure after multiple crushed doses at steady-state in HIV-infected children remains unknown. Increased doses and therapeutic drug monitoring are needed to ensure adequate lopinavir/ritonavir exposure in patients requiring crushed Kaletra® tablets. The reduced exposure with crushed Kaletra® tablet dosing reinforces the need to discourage this dosing practice.
ACKNOWLEDGEMENTS
We would like to thank the children and their families for their participation in this study.
Sources of Funding: Funding support for this study was provided by the American Foundation for Pharmaceutical Education-American Association of Colleges of Pharmacy New Investigator Program Grant, 2007 (BB); NICHD Pediatric Pharmacology Research Unit Network: U10 HD031318 (EC, BB), U10 HD45993 (NR, EW, JV); NICHD K23 HD060452-01A1 (NR); and NCRR M01 RR020359 (NR, JV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have no personal or financial relationships that might bias this work.
Presented: Presented in part at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, February, 2010; Abstract number 629.
REFERENCES
- 1.World Health Organization. Geneva: WHO; 2010. Antiretroviral therapy of HIV Infection in infants and children: towards universal access: recommendations for a public health approach-2010 revision. [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed January 01, 2011];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Updated August 16, 2010. Available at http://aidsinfo.nih.gov/contentfiles/PediatricGuidelines.pdf.
- 3.Welch S, Sharland M, Lyall EG, et al. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Boxwell D, Cao K, Lewis L, et al. Neonatal toxicity of Kaletra oral solution – LPV, ethanol or propylene glycol? [Poster #708]. Presented at: CROI 2011: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. [Google Scholar]
- 5.Kaletra® product labeling. North Chicago, IL, USA: Abbott Laboratories; 2005. [Google Scholar]
- 6.Liu W, Klein CE, March K, et al. Predicted pharmacokinetics of lopinavir after multiple-dose administration of lopinavir/ritonavir tablet to pediatric patients [Poster #366]. Presented at: 8th International Congress on Drug Therapy in HIV Infection; Glasgow, U.K. 2006. [Google Scholar]
- 7.Reddington C, Cohen J, Baldillo A, et al. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis J. 2000;19:1148–1153. doi: 10.1097/00006454-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Byrne M, Honig J, Jurgrau A, et al. Achieving adherence with antiretroviral medications for pediatric HIV disease. AIDS Read. 2002;12:151–154. 61–64. [PubMed] [Google Scholar]
- 9.Gibb DM, Goodall RL, Giacomet V, et al. Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatr Infect Dis J. 2003;22:56–62. doi: 10.1097/00006454-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Gavin PJ, Yogev R. The role of protease inhibitor therapy in children with HIV infection. Paediatr Drugs. 2002;4:581–607. doi: 10.2165/00128072-200204090-00004. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyke RB, Lee S, Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 12.King JR, Kimberlin DW, Aldrovandi GM, et al. Antiretroviral pharmacokinetics in the paediatric population: a review. Clin Pharmacokinet. 2002;41:1115–1133. doi: 10.2165/00003088-200241140-00001. [DOI] [PubMed] [Google Scholar]
- 13.Rakhmanina NY, van den Anker JN, Soldin SJ, et al. Can therapeutic drug monitoring improve pharmacotherapy of HIV infection in adolescents? Ther Drug Monit. 2010;32:273–281. doi: 10.1097/FTD.0b013e3181dca14b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunupuradah T, Wannachai S, Chuamchaitrakool A, et al. Use of taste-masking product, FLAVORx, to assist Thai children to ingest generic antiretrovirals. AIDS Res Ther. 2006;3:30. doi: 10.1186/1742-6405-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland DT, DiFrancesco R, Stone J, et al. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004;48:824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barigye H, Luyirika E. The challenges of paediatric ARV formulations in resource-poor countries – the Ugandan experience [Abstract #1080]. Presented at: 2nd IAS Conference on HIV Pathogenesis and Treatment; Paris, France. 2003. [Google Scholar]
- 17.O'Brien DP, Sauvageot D, Zachariah R, et al. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20:1955–1960. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- 18.Corbett AH, Hosseinipour MC, Nyirenda J, et al. Pharmacokinetics of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian children. Antivir Ther. 2010;15:83–90. doi: 10.3851/IMP1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelps B, Rakhmaina N. Antiretroviral Drugs in Pediatric HIV-infected Patients: Pharmacokinetic and Practical Challenges. Pediatric Drugs. doi: 10.2165/11587300-000000000-00000. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 20.Chandler B, Almond L, Ford J, et al. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J Acquir Immune Defic Syndr. 2003;33:551–556. doi: 10.1097/00126334-200308150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakhmanina NY, Neely MN, Van Schaik RH, et al. CYP3A5, ABCB1 and SLCO1B1 polymorphisms and pharmacokinetics and virologic outcome of lopinavir/ritonavir in HIV-infected children. Ther Drug Monit. 2011;33:417–424. doi: 10.1097/FTD.0b013e318225384f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubomirov R, di Iulio J, Fayet A, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20:217–230. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 24.la Porte C, van Heeswijk R, Mitchell CD, et al. Pharmacokinetics and tolerability of once- versus twice-daily lopinavir/ritonavir treatment in HIV-1-infected children. Antivir Ther. 2009;14:603–606. [PubMed] [Google Scholar]
- 25.Rakhmanina N, van den Anker J, Baghdassarian A, et al. Population pharmacokinetics of lopinavir predict suboptimal therapeutic concentrations in treatment-experienced human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 2009;53:2532–2538. doi: 10.1128/AAC.01374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouillon-Pichault M, Jullien V, Piketty C, et al. A population analysis of weight-related differences in lopinavir pharmacokinetics and possible consequences for protease inhibitor-naive and -experienced patients. Antivir Ther. 2009;14:923–929. doi: 10.3851/IMP1414. [DOI] [PubMed] [Google Scholar]
- 27.Rakhmanina NY, Capparelli EV, van den Anker JN. Personalized therapeutics: HIV treatment in adolescents. Clin Pharmacol Ther. 2008;84:734–740. doi: 10.1038/clpt.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Lee M, Verweel G, de Groot R, et al. Pharmacokinetics of a once-daily regimen of lopinavir/ritonavir in HIV-1-infected children. Antivir Ther. 2006;11:439–445. [PubMed] [Google Scholar]
- 29.Foissac F, Urien S, Hirt D, et al. Pharmacokinetics and virologic efficacy after switch to once daily lopinavir/r in treatment-experienced HIV-1 infected children. Antimicrob Agents Chemother. 2011 Jul 11; doi: 10.1128/AAC.00166-11. [Epup] [DOI] [PMC free article] [PubMed] [Google Scholar]