Abstract
Seven commercial heparin active pharmaceutical ingredients and one commercial low molecular weight from different manufacturers were characterized with a view profiling their physico-chemical properties. All heparins had similar molecular weight properties as determined by polyacrylamide gel electrophoresis (MN 10–11 kDa, MW 13–14 kDa, polydispersity (PD) 1.3–1.4) and by size exclusion chromatography (MN 14–16 kDa, MW 21–25 kDa, PD 1.4–1.6). 1D 1H- and 13C-NMR evaluation of the heparin samples was performed and peaks were fully assigned using 2D NMR. The percentage of glucosamine residues with 3-O-sulfo groups and the percentage of N-sulfo groups and N-acetyl groups ranged from 5.8–7.9, 78–82 and 13–14 %, respectively. There was substantial variability observed in the disaccharide composition with, as determined by high performance liquid chromatography (HPLC)-mass spectral analysis of heparin lyase I–III digested heparins. Heparin oligosaccharide mapping was performed using HPLC following separate treatments with heparin lyase I, II and III. These maps were useful in qualitatively and quantitatively identifying structural differences between these heparins. The binding affinities of these heparins to antithrombin III and thrombin were evaluated by using a SPR competitive binding assay. This study provides the physico-chemical and activity characterization necessary for the appropriate design and synthesis of a generic bioengineered heparin.
Keywords: heparin, polyacrylamide gel electrophoresis, size exclusion chromatography analysis, molecular weight properties, disaccharide composition, high performance liquid chromatography-mass spectrometry, oligosaccharide mapping, nuclear magnetic resonance spectroscopy, surface plasmon resonance
INTRODUCTION
Heparin and heparin-derived low molecular weight heparins (LMWHs) are the most widely used clinical anticoagulants.1 Modern medical procedures including treatment of thrombotic disorders, such as deep vein thrombosis, extracorporeal therapy, such as renal dialysis and blood oxygenation, the use of indwelling catheters and shunts, and the post-surgical control of clots, have increased the need for heparin and LMWH.2, 3 The introduction of modern medical procedures in developing countries has further increased the demand for heparin. Heparin is a highly sulfated, linear glycosaminoglycan that is abundantly found in mucosal tissues such as the lungs and intestines.4 The structure of this ~10–20 kDa polysaccharide is predominantly made up of a major repeating disaccharide unit, α-L-IdoA2S (1→4)-α-D-GlcNS6S (where IdoA is idopyranosyluronic acid, S is sulfo and GlcN is 2-deoxy, 2-amino glucopyranose).1 The structure and sulfation pattern of the heparin molecule are integral to its therapeutic value.5 In particular, a unique pentasaccharide sequence present in heparin having the structure, →GlcNAc6S→GlcA→GlcNS3S6S→IdoA2S→GlcNS6S→ (where GlcA is D-glucopyranosyluronic acid and Ac is acetyl), is responsible for its specific binding to the serine protease inhibitor antithrombin III (AT) resulting in its conformational activation and leading to the inhibition of major coagulation cascade proteases, including thrombin (factor (F) IIa) and FXa.1
Heparin is currently produced from the tissues of food animals, primarily pig intestine, sheep intestine and beef lung. There are drug safety concerns related to heparin products. For example, the appearance of bovine spongiform encephalopathy in Europe in the 1990s and the concerns of prion contamination have decreased the production and use of bovine and ovine heparin worldwide.6 As a result, there has been an increased demand on porcine intestinal heparin with over half the current world supply coming from China. The appearance of blue-ear disease,7 a viral infection that killed up to 10% of the pigs in China, further stressed the tight supply of animal-sourced heparin. A lack of control on Chinese heparin production resulted in the introduction of OSCS contaminated product in 2007–2008, associated with numerous adverse reactions and multiple deaths.8,9 This comes at a time of critical need for a reliable and controlled source of heparin that is uniform in structure and activity and free of viral, prion and chemical contaminants.
An improved understanding of heparin biosynthesis,10,11 the availability of all the heparin biosynthetic enzymes as recombinant proteins expressed in E. coli,12,13 and a method for cofactor recycling14 has led to the preparation of multi-milligrams quantities of heparin that is chemically similar to animal-sourced USP heparins in our laboratory.15, 16 Our ultimate goal to prepare a bioengineered heparin that is chemically, biologically and pharmacologically identical to animal-sourced heparin. The introduction of a generic bioengineered heparin requires the use of sophisticated chemical analysis as well as in vitro and in vivo (animal and human) studies. Because heparin is a polydisperse mixture of polysaccharide chains with sequence heterogeneity, a multifaceted approach is required for their chemical and structural analysis. This paper examines seven heparins in the form of active pharmaceutical ingredient (API) collected from a variety of manufacturers and one LMWH for comparison. The physico-chemical properties including molecular weight, disaccharide composition, oligosaccharide maps, chemical fine structure and thrombin and AT binding affinity were characterized to provide the basis for the design and preparation of a generic bioengineered heparin.
EXPERIMENTAL
Materials
Seven heparin sodium API (Table 1, #1–3, 5–8) and one LMWH API (#4) produced by nitrous acid depolymeriztion were provided by manufacturers from USA, China, and Europe. Sodium heparin used in the protein binding studies (200 IU/mg) was from Celsus (Cincinnati, OH). Recombinant Flavobacterial heparin lyase I, II, and III were expressed in our laboratory using E. coli strains, provided by Professor Jian Liu, University of North Carolina, College of Pharmacy, Chapel Hill, NC).12 Reagents for polyacrylamide gel electrophoresis, alcian blue dye, 2-cyanoacetamide, tetra-n-butylamonium hydrogen sulfate, and other reagents used in this study were from Sigma (St. Louis, MO). Heparin oligosaccharides of different molecular weights for use as calibrants for molecular weight determination by size exclusion chromatography (SEC) were purchased from Iduron (Manchester, UK). Unsaturated heparin/HS disaccharides standards (Di-0S, ΔUA-GlcNAc; Di-NS, ΔUA-GlcNS; Di-6S, ΔUA-GlcNAc6S; Di-UA2S, ΔUA2S-GlcNAc; Di-UA2SNS, ΔUA2S-GlcNS; Di-NS6S, ΔUA-GlcNS6S; Di-UA2S6S, ΔUA2S-GlcNAc6S; and Di-triS, ΔUA2S-GlcNS6S, where ΔUA is deoxy-α-L-threo-hex-4-enopyranosyl uronic acid) were obtained from Seikagaku Corporation (Japan).
Table 1.
Anti-factor IIa and anti-factor Xa activities provided by the manufacturer.
| Heparin # | Anti-factor IIa activity (IU/mg)a |
Anti-factor Xa activity (U/mg) |
Anti-Xa/Anti-IIa ratiob |
|---|---|---|---|
| 1 | 197 | 197 | 1.0 |
| 2 | 203 | -- | -- |
| 3 | 201 | 197 | 0.98 |
| 5 | 203 | -- | -- |
| 6 | 204 | -- | -- |
| 7 | 210 | -- | -- |
| 8 | 200 | -- | -- |
USP heparin is required to have an anti-factor IIa activity of >180 IU/mg.
USP heparin is required to have an anti-factor Xa/anti-factor IIa activity ratio of 0.9 to 1.1.
Polyacrylamide gel electrophoresis (PAGE) analysis
Heparin samples were separated by PAGE on a 15% total acrylamide (15% T) resolving gel containing 14.8% (w/v) acrylamide, 0.92% (w/v) N,N′-methylene-bis-acrylamide, and 5% (w/v) sucrose. All monomer solutions were prepared in resolving buffer (0.1 M boric acid, 0.1 Tris, 0.01 M disodium EDTA, pH 8.3). Stacking gel monomer solution was prepared in resolving buffer with the pH adjusted to 6 using HCl, and it contained 4.75% (w/v) acrylamide and 0.25% (w/v) N,N’-methylene-bisacrylamide. Gels were subjected to electrophoresis at a constant power of 200 V for 25 min, stained by Alcian blue for 30 min, and destained in water. PAGE images were acquired on an HP Scanjet 7400 at 600 dpi and processed for densitometry data using Un-Scan-it software (Silk Science Inc., Orem, Utah). These data were used to calculate weight averaged molecular weight (MW), number averaged molecular weight (MN), and polydispersity (PD) of pharmaceutical heparin.17
Size exclusion chromatography (SEC) of heparin
SEC was performed using a TSK-GEL G3000PWxl size exclusion column with a sample injection volume of 20 µL and a flow rate of 0.6 mL/min on an apparatus composed of a Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector.18 The mobile phase consisted of 0.1 M NaNO3. The column was maintained at 40°C with an Eppendorf column heater during the chromatography. The SEC chromatograms were recorded with the LCsolution Version 1.25 software and analyzed with its “GPC Postrun” function. Heparin oligosaccharides of different molecular weights (dp6, dp10, dp16 and dp20) were used as calibrants for the standard curve.
Disaccharide analysis using LC/MS
Heparin (20 mg/mL) was treated with heparin lyase I, II, III (Hep I, II and III at 3 m-unit each in 10 µL of sodium phosphate (5 mM, pH 7.1) buffer) and incubated at 37°C overnight.19 The products were filtered using 10,000 molecular weight cut-off (MWCO) centrifugal filters and the disaccharides were recovered in the filtrate. The disaccharides were freeze-dried and exactly 100 µL of water was added prior to their analysis. Disaccharide analysis was performed on a high performance liquid chromatography-mass spectrometry (HPLC-MS) system (Agilent LC/MSD trap MS).19 Solutions A and B for the HPLC separation contained 37.5 mM NH4HCO3 and 11.25 mM tributylamine in 15% and 70% acetonitrile, respectively. The pH values of these solutions were adjusted to 6.5 with acetic acid. Separation was performed on a C-18 column (Agilent) at a flow rate was 10 µL/min using solution A for 20 min, followed by a linear gradient from 20 to 45 min of 0% to 50% solution B. The column effluent entered the source of the ESI-MS for continuous detection by MS. The electrospray interface was set in the negative ionization mode with the skimmer potential of −40.0 V, capillary exit at −40.0 V, and a source of temperature at 325 °C to obtain the maximum abundance of the ions in full scan spectra (150–1500 Da, 10 full scans/s). Nitrogen was used as a drying (5 L/min) and nebulizing gas (20 psi).
Heparin oligosaccharide mapping using analytical strong anion exchange (SAX)- HPLC
Each individual heparin sample (4.0 mg) was dissolved in 4.0 mL of 50 mM, pH 7.5, sodium phosphate buffer and was incubated in a 30 °C water bath with heparin lyase 1 (Hep I, 1.5 unit, activity against heparin), heparin lyase 2 (Hep II, 0.35 unit, activity against heparin), or heparin lyase 3 (Hep III, 5.0 unit activity against heparan sulfate).20 The reaction completion was monitored by taking out small amount of aliquots of the reaction mixture for PAGE analysis. The same amount of each heparin lyase was added to the reaction at each 12 h interval for another two times for an exhaustive digestion on the substrates. Reactions were quenched by heating in a 100 °C water bath for 10 min.
SAX-HPLC analysis of heparin lyase digestion products were performed on a Shimadzu LC-10Ai LC system equipped with an SPD-20A ultraviolet-visible (UV) detector using a 4.6 × 250 mm Waters Spherisorb S5 SAX column. A two-segment gradient elution was achieved using mobile phase A (water, pH 3.5, adjusted with HCl) and mobile phase B (2.0 M NaCl, pH 3.5, adjusted with HCl) at a flow rate of 1.0 mL/min. 10µL of each disaccharide standard was loaded onto the column in a concentration of 0.5 µg/µl and washed with 0% to 60% B over 60 min. The elution was monitored at 232 nm.
Nuclear Magnetic Resonance (NMR) analysis
Approximately 20 mg of each porcine mucosal heparin samples from different companies were analyzed by one dimensional 1H, 13C and two-dimensional correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), nuclear Overhauser effect spectroscopy (NOESY), and total correlation spectroscopy (TOCSY) experiments.21 All samples were dissolved in 0.5 mL of 99.996 % deuterium oxide (2H2O, Sigma, St. Louis, MO, USA) and freeze dried to remove exchangable protons. NMR experiments were obtained at a Bruker Avance Ultrashield 600 MHz (14.1-Tesla) NMR instrument equipped with an ultrasensitive HCN cryoprobe with a z-axis gradient. The spectra were acquired at a probe temperature of 298 K. Proton and carbon resonances were assigned with the use of conventional HMQC, COSY and TOCSY spectra. For one-dimensional 1H-NMR spectra, sweep width of 20.5 ppm and acquisition time of 2.65 s were used. Sweep width was 236 ppm and number of scans was 20,480 for one-dimensional 13C spectra. For the COSY, TOCSY and NOESY spectra, 512 experiments resulting in 4096 data points for a spectral width of 12.4 ppm were measured. Proton-detected HMQC experiments used 12.4 and 78.8 ppm spectral widths in the 1H dimension and 13C dimension, respectively. A mixing time of 250 ms with 1.5 s relaxation delay was used in NOESY experiment. The 2D NMR data sets were processed by Topspin version 2.1.4.
Surface Plasma Resonance (SPR) analysis
Biotinylated heparin prepared from Celsus heparin was immobilized to streptavidin (SA) chip based on the manufacturer’s protocol. Solution competition study between surface heparin and soluble different heparins to measure IC50 were performed using SPR.22 AT (250 nM) mixed with different of concentrations of heparin in HBS-EP buffer (10 mM 4-(2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 150 mM sodium chloride, 3 mM ethylenediaminetetraacetic acid (EDTA), 0.005% polysorbate surfactant P20, pH 7.4) were injected over heparin chip at a flow rate of 30 µL/min, respectively. After each run, the dissociation and the regeneration were performed as described above. For each set of competition experiments on SPR, a control experiment (only protein without heparin) was performed to make sure the surface was completely regenerated and that the results obtained between runs were comparable. The same analysis was performed with 63 nM thrombin in place of the AT.
RESULTS AND DISCUSSION
Molecular weight properties of heparin samples
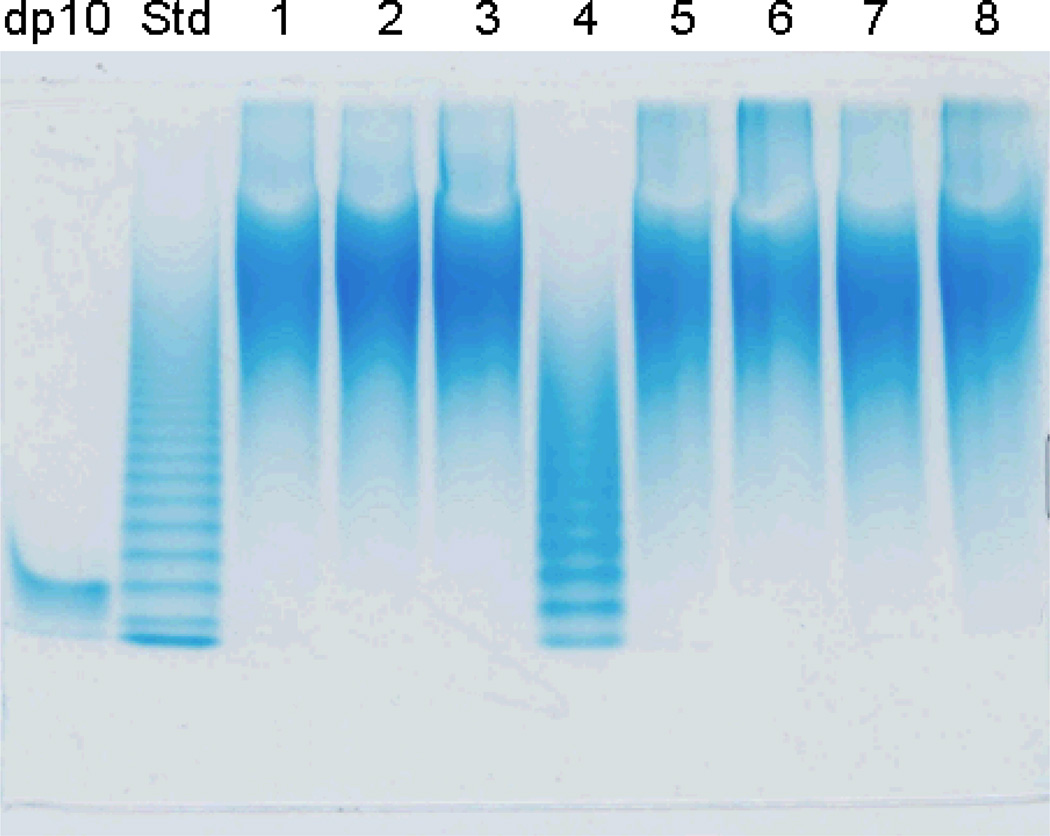
PAGE was used to determine the number averaged molecular weight (MN) and weight averaged molecular weight (MW), as well as the polydispersity (PD), of the heparin samples.17 Furthermore, PAGE offers a qualitative map of the components within heparin and can be used to establish the presence of polyanionic impurities including OSCS.23 The analysis of these heparins all show a broad, continuous and virtually identical smears of unresolved polysaccharide bands indicating the high and uniform level of microheterogeneity of these preparations (Figure 1). Qualitative evaluation of Figure 1 confirms that 1–3 and 5–8 are heparin and that 4 is a LMWH and that none of the samples show the presence of obvious impurities or contaminants. Molecular weight is calculated based on a banding ladder of heparin oligosaccharides (Std in Figure 1) prepared through the partial heparin lyase I depolymerization of heparin.17 The size of the oligosaccharides present in this banding ladder begins by identifying one of the bands by using a pure heparin decasaccharide (labeled degree of polymerization (dp) 10 in Figure 1). Alignment of the dp standard affords a counting frame from which the bands in the Std lane of Figure 1 can be identified, assigned a molecular mass required for the preparation of a standard curve. The LMWH, shown in lane 6, is clearly distinguished from heparin by both its lower molecular weight and the characteristic banding pattern associated with the presence of chains having only an even number of saccharide units (heparin shows no clear banding due to the presence of even and odd saccharide chains).17 PAGE analysis indicated that all 7 heparin samples had MN 10–11 kDa, MW 13–14 kDa and PD of 1.3–1.4 (Table 2).
Figure 1.
Polyacrylamide gel electrophoresis (PAGE) analysis on heparin samples. Lanes are: dp10, corresponding to the decasaccharide ΔUA2S(GlcNS6S-IdoA2S)4GlcNS6S; Std, an oligosaccharide ladder prepared by partial (30%) Hep I depolymerization of bovine lung heparin; lanes 1 to 8, correspond to heparin samples #1 to 8 (sample #4 is LMWH).
Table 2.
PAGE analysis of heparin samples for weight averaged molecular weight (MW), number averaged molecular weight (MN), and polydispersity (PD) (standard deviation based on triplicate measurements).
| Heparin Sample # | MW | MN | PD |
|---|---|---|---|
| 1 | 14,000 ± 1,000 | 10,200 ± 2,300 | 1.41 |
| 2 | 13,700 ± 800 | 10,400 ± 2,000 | 1.35 |
| 3 | 13,700 ± 900 | 10,200 ± 2,300 | 1.37 |
| 4 | 6,300 ± 400 | 4,600 ± 200 | 1.38 |
| 5 | 13,700 ± 800 | 10,100 ± 1,200 | 1.36 |
| 6 | 15,700 ± 1,400 | 11,200 ± 2,100 | 1.42 |
| 7 | 13,400 ± 1,000 | 10,100 ± 1,600 | 1.34 |
| 8 | 14,600 ± 1,100 | 10,800 ± 1,500 | 1.37 |
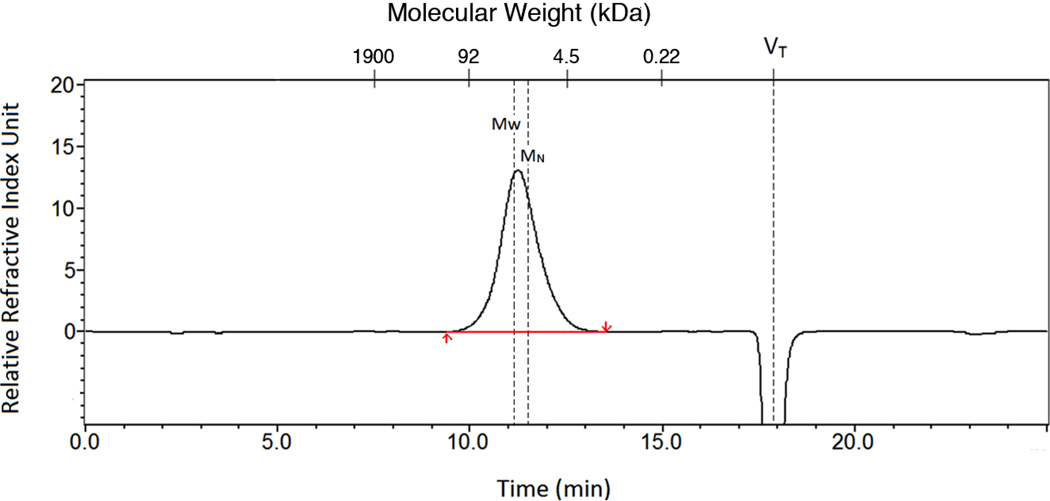
Next, the molecular weight properties of the heparin samples were assessed using SEC this time using individual heparin oligosaccharide standards. A typical SEC chromatogram for heparin #1 is shown in Figure 2. SEC analysis showed that all heparins had MN 14–16 kDa, MW 21–25 kDa and PD of 1.4–1.6 (Table 3). The molecular weight properties determined by PAGE and SEC (Tables 2 and 3), while similar are not identical, with SEC showing slightly higher molecular weights and polydispersities. The differences are method associated as PAGE assumes equal staining intensity of different chain sizes17 and SEC can be complicated by interactions between the heparin polyelectrolyte and the supporting column matrix.24 Despite these complicating issues, both sets of values are consistent with those reported in the literature (Table 1).17, 25
Figure 2.
A sample chromatogram using SEC for heparin molecular weight determination of heparin #1. The y-axis corresponds to the mass detected using a refractive index detector and the x-axes correspond to elution time (min) and molecular mass (calculated from a standard curve). VT marks the total volume of the column where a change in salt concentration results in a negative peak.
Table 3.
SEC of commercial heparins for molecular weight determination (average error based on duplicate measurements)
| Heparin Sample # | MW | MN | PD |
|---|---|---|---|
| 1 | 22,900 ± 100 | 15,400 ± 190 | 1.49 |
| 2 | 20,900 ± 600 | 15,000 ± 500 | 1.39 |
| 3 | 21,900 ± 300 | 15,900 ± 100 | 1.38 |
| 4 | 6,400 ± 200 | 4,700 ± 100 | 1.38 |
| 5 | 21,300 ± 300 | 14,300 ± 80 | 1.49 |
| 6 | 24,400 ± 1,100 | 15,500 ± 200 | 1.57 |
| 7 | 21,000 ± 20 | 14,600 ± 100 | 1.44 |
| 8 | 24,000 ± 400 | 14,600 ± 300 | 1.64 |
Disaccharides analysis
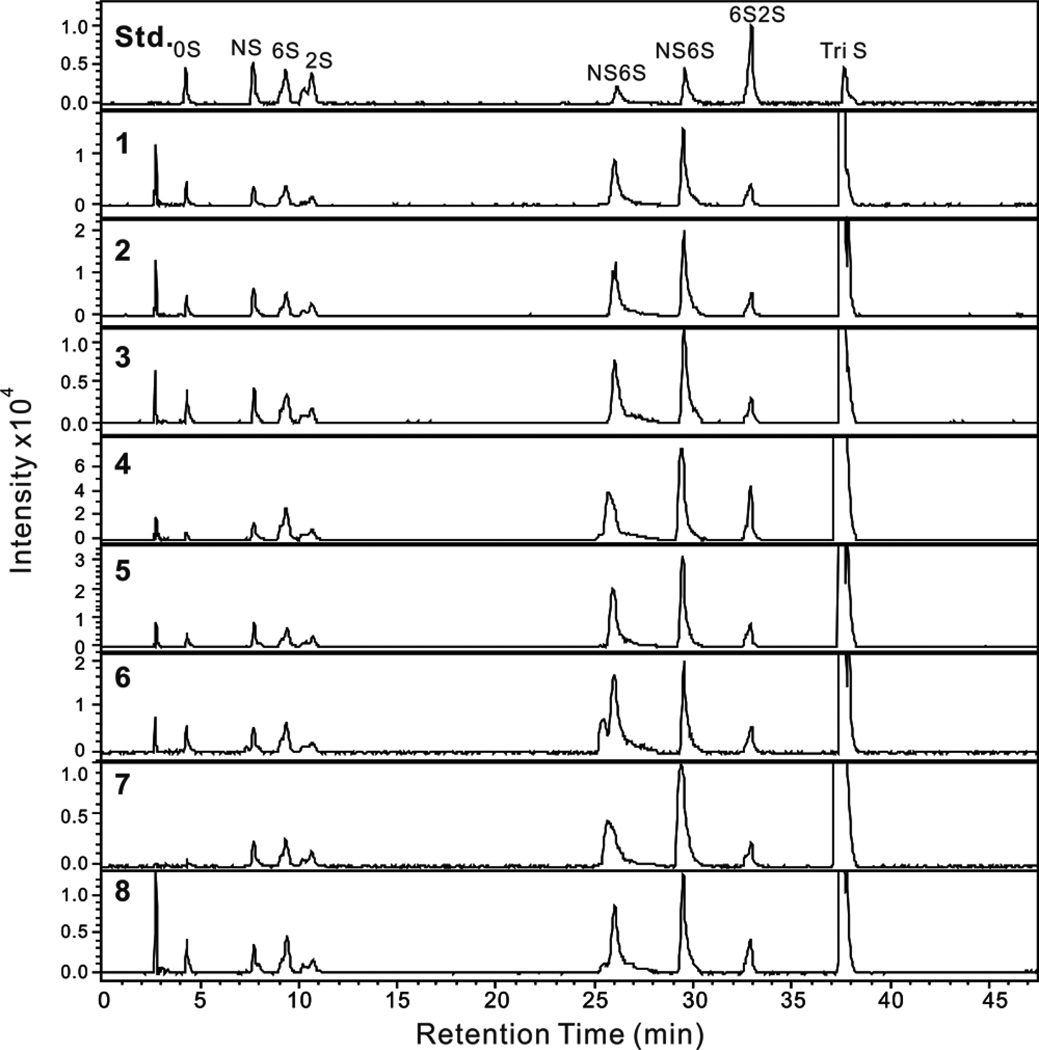
Heparin is composed of different disaccharide units.1 The most prominent of these is the trisulfated disaccharide α-L-IdoA2S (1→4)-α-D-GlcNS6S. On treatment with a mixture of heparin lyase I, II and III (Hep I, II and III), heparin is broken into unsaturated disaccharides26 (a small quantity of resistant tetrasaccharide associated with the AT binding site is also formed).27 The major trisulfated disaccharide affords the ΔUA2S (1→4)-α-D-GlcNS6S disaccharide (where ΔUA corresponds to 4-deoxy-α-L-threo-hex-4-eno-pyranosyluronic acid). After treatment with heparin lyases, the heparin and LMWH samples were analyzed reversed phase ion pairing (RPIP)-ultraperformance liquid chromatography (UPLC)-mass spectrometry (MS). The extracted ion chromatograms (EIC) of disaccharides analyses are presented in Figure 3. Eight common heparin disaccharides were detected in all samples, their quantity was calculated from a linear equation derived from a calibration curve of these 8 disaccharide standards28 and their disaccharide compositions are given in Table 4. Analyses show that the major disaccharide in heparin is the trisulfated disaccharide as expected but it is present in a range from 63–85 mole %, suggesting that manufacturing processes and/or source material have an impact disaccharide composition.
Figure 3.
EIC of disaccharide analysis of 7 heparin samples and one LMWH sample by RPIP-UPLC-MS. Std shows the analysis of an equimolar mixture of 8 disaccharide standards used for peak identification and injection of different amounts afford a standard curve for quantification. Chromatograms 1–8 show the disaccharide analysis of the eight commercial heparin samples #1–#8, respectively. The peak eluting prior to 0S in each chromatogram is salt.
Table 4.
Disaccharide compositional analysis of 7 heparin samples and one LMWH sample.
| Heparin sample # |
Disaccharide composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| ΔDi-0S | ΔDi-NS | ΔDi-6S | ΔDi-2S | ΔDi-NS6S | ΔDi-NS2S | ΔDi-6S2S | ΔDi-TriS | |
| 1 | 1.9 | 2.0 | 3.4 | 1.8 | 11.8 | 12.1 | 3.7 | 63.3 |
| 2 | 1.1 | 1.8 | 2.1 | 1.6 | 8.5 | 9.2 | 2.1 | 73.6 |
| 3 | 1.8 | 2.2 | 3.2 | 1.6 | 10.1 | 11.6 | 2.6 | 66.9 |
| 4 | 0.3 | 0.4 | 1.6 | 0.7 | 4.9 | 5.1 | 2.1 | 84.9 |
| 5 | 0.6 | 1.1 | 1.6 | 1.1 | 6.7 | 7.4 | 1.8 | 79.7 |
| 6 | 1.1 | 1.6 | 2.3 | 1.0 | 10.8 | 8.9 | 1.9 | 72.4 |
| 7 | 0.3 | 0.8 | 1.2 | 0.8 | 5.4 | 6.6 | 1.1 | 83.8 |
| 8 | 1.4 | 1.4 | 2.6 | 1.1 | 8.8 | 7.8 | 2.5 | 74.4 |
Heparin oligosaccharide mapping
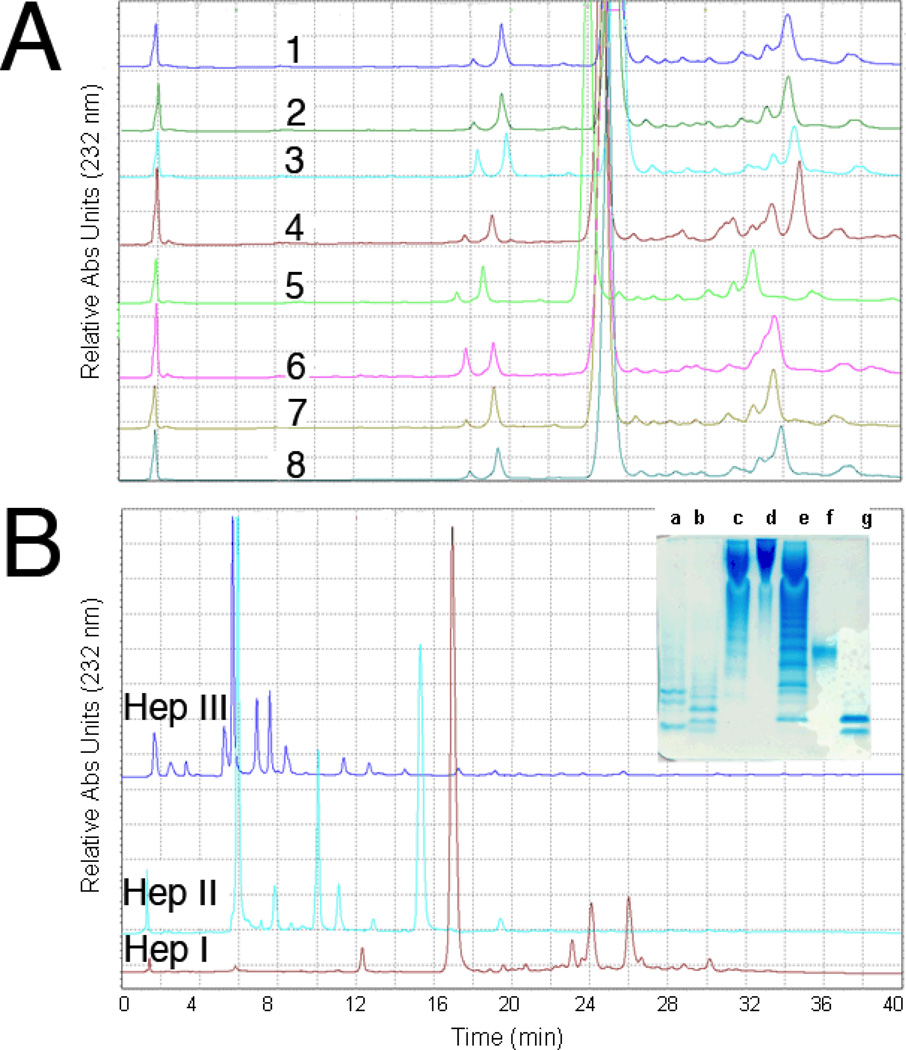
Heparin samples were next individually digested by each of the three heparin lyases (Hep I, II and III) and were analyzed in triplicate by SAX-HPLC to obtain quantitative oligosaccharide maps (Figure 4). This mapping technique is similar to that used to prepare peptide maps of proteins and is useful for comparing structural differences among heparins.29 The assigned disaccharide peaks, where pure standards were available, were integrated with their mole percentages with respect to the total disaccharides present in each mixture were determined (Table 4). Each heparin lyase affords a unique map containing different disaccharides (of known structures) and oligosaccharides (some of known structure),30 providing more structural information for each individual heparin sample than would a simple disaccharide analysis.20 The relative amount of certain disaccharides varied significantly between the different commercial heparin samples providing a qualitative evaluation of heparin variability based on the manufacturing process and animal/tissue source of each heparin (for example, some porcine heparins comes from whole intestine while others are derived only from porcine intestinal mucosa31). The manufacturing process may also modify the structure of the core protein linkage region in the reducing end of intact heparin, GlcAβ1-3Galβ1- 3Galβ1-4Xylβ1-O-Ser (where GlcA is D-glucuronic acid, Gal is D-galactose, Xyl is D-xylose and Ser is serine) affording modified tetrasaccharide fragments of the linkage region such as ΔUA-Gal-Gal-Xyl-O-CH2CONHCH2COOH. The amount of linkage region fragments in the 7 heparin samples ranged from ~1 to ~1.3 %.
Figure 4.
Quantitative disaccharide mapping of heparins using SAX-HPLC. A. SAX-HPLC analysis of heparins #1–3 and 5–8 and LMWH #4 digested by Hep I; B. HPLC analysis of heparin #1 digested by HepI, HepII and HepIII, respectively Insert: PAGE analysis of digested heparin. Lanes a, b and c, heparin #1 digested by Hep I, II and III, respectively, d, heparin #1, e, oligosaccharide ladder (see Figure 1 legend), f, oligosaccharide standard dp10 (see Figure 1 legend), g, oligosaccharide standard dp4 (most intense band corresponds to ΔUA2SGlcNS6S-IdoA2SGlcNS6S.
Fine structural analysis
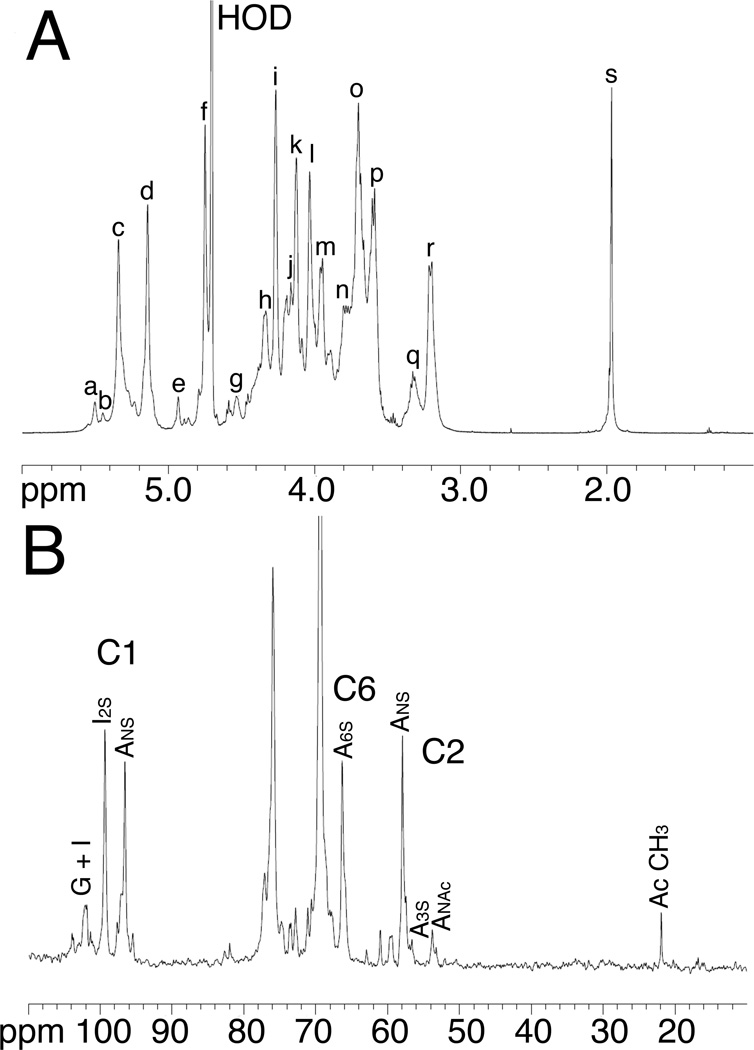
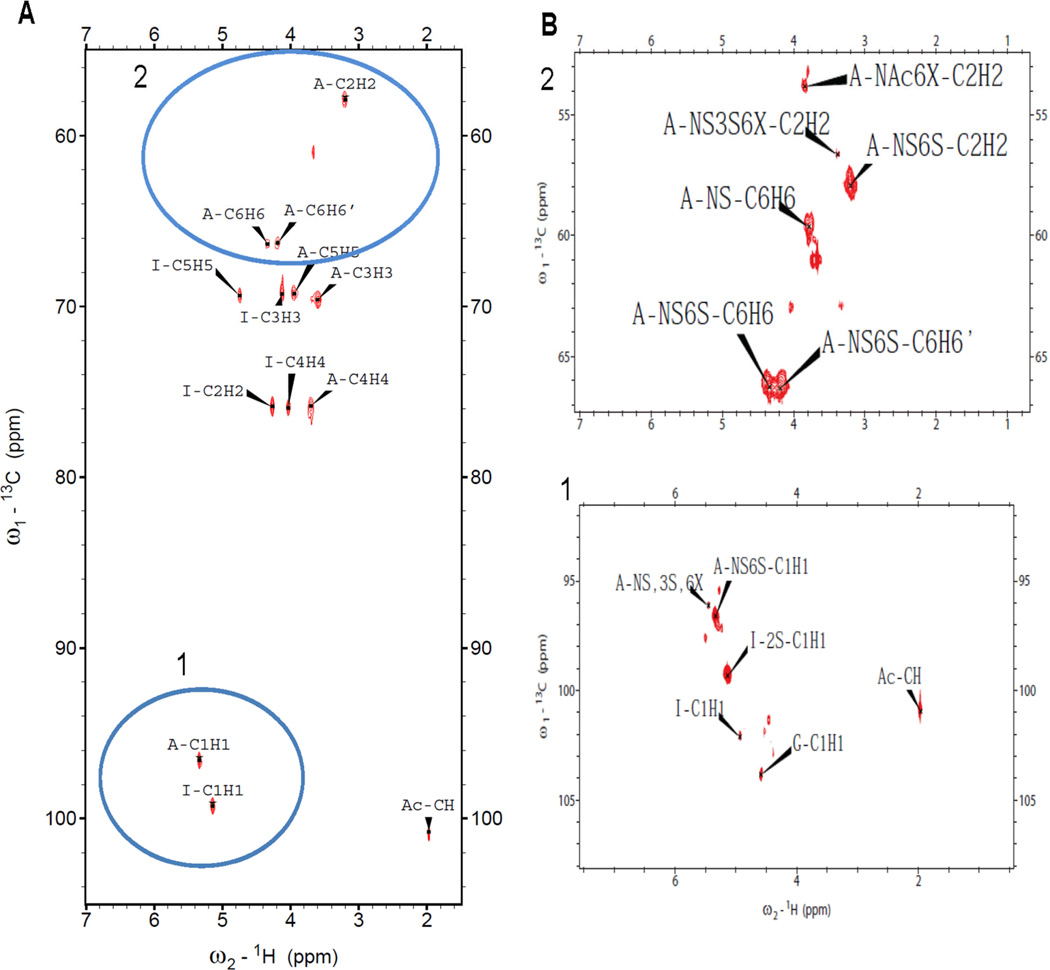
1D 1H and 13C NMR evaluation of the heparin samples was performed with and without prior lyophilization to allow the detection of volatile solvents such as ethanol (Figure 5 A and B). Process impurities were present in all 7 heparin samples. Half of these contained ethanol while the other half contained sodium acetate. All peaks were fully assigned (Table 6) by 2D NMR including HMQC (Figure 6), HHCOSY, TOCSY, and NOESY studies. Critical features in the GlcN residues, including N-sulfo, N-acetyl, and 3-O-sulfo, 6-O-sulfo content vary amongst heparins. Critical features in the uronic acid residues include the content of IdoA, IdoS2S and GlcA also show a range of values (Table 6).
Figure 5.
A sample NMR analysis of a pharmaceutical heparin 1H-NMR and 13C-NMR spectra of heparin #1. The spectra were acquired at 600 MHz on a Bruker Avance Ultrashield 600 MHz (14.1-Tesla) NMR instrument. A. Peak assignments for proton spectrum, a, H1 ANS-(G); b, H1 A3S; c, H1 ANS6x; d, H1 I2S; e, H1 I; f, H5 I2S; g, H1 G; h, H6 ANS6S; i, H2 I2S; j, H6’ ANS6S; k, H3 I2S; l, H4 I2S; m, H5 ANS6S; n, H6 ANS; o, H4 ANS6S; p, H3 ANS6x; q, H2 G, H2 A3S; r, H2 ANS6x; s, acetyl CH3 (A, Glucosamine; I, Iduronic acid; G, Glucuronic acid). B. 13C-spectrum of heparin #1 (20 mg) in 0.5 mL of deuterium oxide.
Table 6.
Percent substitution of glucosamine (A) and uronic acids (iduronic acid I or glucuronic acid G) in heparin samples #1–8.
| Heparin Sample # | ANS % |
ANAc % |
A3S % |
A6Sa % |
I2S % |
I % |
G % |
Impurities observed |
|---|---|---|---|---|---|---|---|---|
| 1 | 81 | 13 | 6.2 | 83.4 | 61.3 | 11.5 | 27.2 | ethanol |
| 2 | 80 | 13 | 7.9 | 83.4 | 60.8 | 10.0 | 29.1 | acetate |
| 3 | 78 | 14 | 7.8 | 82.0 | 61.8 | 9.5 | 28.6 | ethanol |
| 4b | 80 | 14 | 6.6 | 87.2 | 57.0 | 13.5 | 29.5 | ethanol |
| 5 | 80 | 14 | 6.0 | 83.7 | 62.6 | 10.3 | 27.0 | acetate |
| 6 | 81 | 13 | 6.3 | 84.1 | 54.2 | 11.9 | 33.9 | acetate |
| 7 | 82 | 13 | 5.8 | 82.9 | 65.3 | 9.9 | 24.8 | acetate |
| 8 | 80 | 13 | 6.9 | 84.1 | 60.9 | 10.7 | 28.4 | ethanol |
| Avg. | 80 | 13 | 6.7 | 83.4 | 61 | 10.5 | 28.4 | ethanol |
| Std. dev. | 0.9 | 0.5 | 0.7 | 0.6 | 2.6 | 0.7 | 2.0 | |
| Var. | 0.7 | 0.2 | 0.4 | 0.3 | 5.8 | 0.4 | 3.4 |
A6S values obtained by the integration of the 13C spectra.
Sample # 4 is LMWH.
Figure 6.
Two-dimensional 1H-13C correlation spectrum (HMQC) of heparin #1. A. Entire HMQC spectrum. B. The selected regions of the spectrum. Blue circles 1 and 2 indicate anomeric region and H2/C2-H6/C6 glucosamine resonances, respectively.
Surface Plasma Resonance (SPR) analysis
Two proteins (AT and thrombin), having important roles in coagulation system, were used for the heparin binding SPR experiments. Solution/surface competition experiments were performed by SPR to examine the relative binding affinity of different heparins to AT and thrombin interaction. AT (250 nM) mixed with different of concentrations of heparin in HBS-EP buffer were injected over heparin chip. Once the active binding sites on AT molecules were occupied by heparin in the solution, the binding of AT to the surface-immobilized heparin should decrease resulting in a reduction in SPR. The IC50 values (concentration of competing analyte resulting in a 50% decrease in response units (RU)) can be calculated from the solution/surface competition SPR. The injections were in performed in duplicate (with less than 5% variation). Similar solution/surface competition experiments were conducted with human thrombin. The calculated IC50 values for different heparins are shown in Table 7.
Table 7.
IC50 of different commercial heparins measured by solution/surface competition SPR (based on duplicate experiments with less than 5% of errors)
| Heparin Sample # | IC50 (U/ml) to AT | IC50 (U/ml) to human thrombin |
|---|---|---|
| 1 | 1.8 | 1.5 |
| 2 | 1.3 | 1.8 |
| 3 | 1.2 | 1.3 |
| 5 | 1.2 | 1.5 |
| 6 | 1.5 | 2.1 |
| 7 | 1.1 | 1.8 |
| 8 | 0.9 | 1.7 |
CONCLUSIONS
The contemporaneous (prepared after the end of the heparin crisis of 2008) heparin APIs examined were prepared from animals raised on three continents and on heparins processed in different manufacturing facilities using different processes. Despite these differences in source material and process, there was a surprising similarity in the physico-chemical characteristics of these products. There were, however, unique features associated with each heparin API that begins to define the acceptable range of structural features that define pharmaceutical heparins that have qualified as EP and USP heparin. This information should help inform our evaluation of the chemical equivalence of generic heparin preparations, particularly the bioengineered heparin currently being developed in our laboratory.32
Table 5.
SAX-HPLC analysis of 7 commercial heparin samples digested with Hep I, II or III.
| Heparin Sample # | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Heparin lyase 1 |
1 | 2 | 3 | 5 | 6 | 7 | 8 | Average | Std. dev.b |
| %a | % | % | % | % | % | % | % | ||
| 2ac | 0.87 | 0.86 | 0.75 | 0.78 | 0.77 | 0.81 | 0.79 | 0.80 | 0.05 |
| 2b | 1.22 | 1.01 | 1.18 | 0.99 | 1.05 | 1.08 | 1.15 | 1.10 | 0.09 |
| 2c | 4.44 | 4.65 | 4.51 | 4.55 | 3.99 | 4.11 | 3.98 | 4.32 | 030 |
| 2d | 80.40 | 79.7 | 79.08 | 80.07 | 80.89 | 80.58 | 80.85 | 80.22 | 0.70 |
| Heparin Sample # | |||||||||
|
Heparin lyase 2 |
1 | 2 | 3 | 5 | 6 | 7 | 8 | Average | Std. dev. |
| % | % | % | % | % | % | % | % | ||
| 2a | 0.93 | 0.89 | 0.97 | 0.98 | 0.95 | 1.10 | 1.02 | 0.98 | 0.07 |
| 2b | 15.5 | 14.50 | 15.30 | 14.90 | 15.20 | 15.10 | 13.50 | 14.86 | 0.70 |
| 2c | 9.10 | 9.00 | 10.10 | 8.90 | 8.10 | 7.90 | 7.40 | 8.64 | 0.90 |
| 2d | 61.60 | 62.02 | 60.54 | 63.24 | 62.29 | 64.13 | 65.97 | 62.83 | 1.8 |
| 2e | 3.51 | 3.31 | 3.49 | 3.41 | 3.11 | 2.99 | 2.89 | 3.24 | 0.30 |
| 2f | 3.11 | 2.99 | 3.08 | 3.02 | 3.01 | 2.70 | 3.09 | 3.00 | 0.10 |
| 2g | 1.44 | 1.69 | 1.72 | 1.71 | 1.70 | 2.10 | 1.79 | 1.74 | 0.20 |
| 2h | 1.41 | 1.40 | 1.45 | 1.39 | 1.42 | 1.51 | 1.40 | 1.43 | 0.04 |
| Heparin Sample # | |||||||||
|
Heparin lyase 3 |
1 | 2 | 3 | 5 | 6 | 7 | 8 | Average | Std. dev. |
| % | % | % | % | % | % | % | % | ||
| 2a | 43.19 | 42.69 | 43.46 | 49.78 | 43.50 | 52.05 | 39.76 | 44.92 | 4.3 |
| 2b | 4.66 | 4.28 | 3.85 | 4.06 | 5.70 | 4.30 | 4.75 | 4.51 | 0.60 |
| 2e | 12.76 | 12.73 | 11.99 | 11.60 | 12.25 | 11.77 | 14.25 | 12.48 | 0.90 |
| 2f | 11.61 | 11.2 | 12.1 | 13.92 | 12.75 | 13.91 | 15.20 | 12.96 | 1.5 |
Tetrasaccharide and higher oligosaccharide products were not quantified so that the mole % of disaccharides did not sum to 100%. Quantitative analysis of the disaccharide products (2a–2h) was performed in triplicate.
Analysis was performed on each chromatogram and the average value of each disaccharide (2a–2h) in the 7 heparins treated with Hep I, II or III was calculated and the standard deviations across the 7 heparin samples were calculated.
The structures determined for each peak are: 2a ΔUA-GlcNAc, 2b ΔUA-GlcNS6S, 2c ΔUA2S-GlcNS, 2d ΔUA2S-GlcNS6S, 2e ΔUA-GlcNS, 2f ΔUA-GlcNAc6S, 2g ΔUA2S-GlcNAc, 2h ΔUA2S-GlcNAc6S
ACKNOWLEDGEMENTS
This work was supported by grants funded by the National Institutes of Health HL101721 and HL096972 (RJL) and the Bioengineered Heparin Consortium.
REFERENCES
- 1.Linhardt RJ. Heparin: Structure and activity. J Med Chem. 2003;46:2551–2554. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub AY, Sheiner E. Anticoagulant therapy and thromboprophylaxis in patients with thrombophilia. Arch Gynecol Obstet. 2007;276:567–571. doi: 10.1007/s00404-007-0409-2. [DOI] [PubMed] [Google Scholar]
- 3.Bick RL, Frenkel EP, Walenga J, Fareed J, Hoppensteadt DA. Unfractionated heparin, low molecular weight heparins, and pentasaccharide: basic mechanism of actions, pharmacology, and clinical use. Hematol Oncol Clin North Am. 2005;19:1–51. doi: 10.1016/j.hoc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Nader HB, Dietrich CP. In: Natural occurrence and possible biological role of heparin. In Heparin Chemical and Biological Properties, Clinical Applications. Lane DA, Lindahl U, editors. Boca Raton, FL: CRC Press; 1989. pp. 115–133. [Google Scholar]
- 5.Lindahl U, Backstrom G, Thunberg L, Leder IG. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci (USA) 1980;77:6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonberger LB. New variant Creuzfeldt-Jakob disease and bovine spongiform encephalopathy. Infect Disease Clinics North Am. 1998;12:111–121. doi: 10.1016/s0891-5520(05)70412-8. [DOI] [PubMed] [Google Scholar]
- 7.Cho JG, Dee SA. Porcine reproductive and respiratory syndrome virus. Theriogenology. 2006;66:655–662. doi: 10.1016/j.theriogenology.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Guerrini M, Beccati D, Shriver Z, Naggi AM, Bisio A, Capila I, Lansing J, Guglieri S, Fraser B, Al-Hakim A, Gunay S, Viswanathan K, Zhang Z, Robinson L, Venkataraman G, Buhse L, Nasr M, Woodcock J, Langer R, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a major contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–775. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindahl U, Feingold DS, Roden L. Biosynthesis of heparin. Trends Biochem Sci. 1986;11:221–225. [Google Scholar]
- 11.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Jones CL, Liu J. Using an enzymatic combinatorial approach to identify anticoagulant heparan sulfate structures. Chem & Biol. 2007;14:986–993. doi: 10.1016/j.chembiol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edavettal SC, Lee KA, Negishi M, Linhardt RJ, Liu J, Pedersen LC. Crystal structure and mutational analysis of heparan sulfate 3-O-sulfotransferase isoform 1. J Biol Chem. 2004;279:25789–25797. doi: 10.1074/jbc.M401089200. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Moon A, Song D, Pedersen LC, Liu J. Engineering sulfotransferases to modify heparan sulfate. Nat Chem Biol. 2008;4:200–202. doi: 10.1038/nchembio.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, McCallum SA, Xie J, Nieto L, Corzana F, Jiménez-Barbero J, Chen M, Liu J, Linhardt RJ. Solution structures of chemoenzymatically synthesized heparin and its precursors. J Am Chem Soc. 2008;130:12998–13007. doi: 10.1021/ja8026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Xu Y, Chen M, Weïwer M, Bridges A, DeAngelis PL, Zhang Q, Linhardt RJ, Liu J. Chemoenzymatic design of heparan sulfate oligosaccharides. J Biol Chem. 2010;285:34240–34249. doi: 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of the molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Yang B, Zhang Z, Ly M, Takieddin M, Mousa S, Liu J, Dordick JS, Linhardt RJ. Control of heparosan N – deacetylation leads to an improved bioengineering heparin. Appl Microbiol Biotechnol. 2011;91:91–99. doi: 10.1007/s00253-011-3231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Xie J, Liu H, Liu J, Linhardt RJ. Quantitification of heparan sulfate and heparin disaccharides using ion pairing, reverse-phase, micro-flow, high performance liquid chromatography coupled with electrospray ionization trap mass spectrometry. Anal Chem. 2009;81:4349–4355. doi: 10.1021/ac9001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Z, Tappen BR, Ly M, Zhao W, Canova LP, Guan H, Linhardt RJ. Heparin mapping using heparin lyases and the generation of a novel low molecular weight heparin. Med Chem. 2011;54:603–610. doi: 10.1021/jm101381k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrini M, Zhang Z, Shriver Z, Naggi A, Masuko S, Langer R, Casu B, Linhardt RJ, Torri G, Sasisekharan R. Orthogonal analytical approaches to detect potential contaminants in heparin. Proc Nat Acad Sci (USA) 2009;106:16956–16961. doi: 10.1073/pnas.0906861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaudet JM, Weyers A, Solakyildirim K, Yang B, Takieddin M, Mousa S, Zhang F, Linhardt RJ. Affect of autoclave sterilization on the activity and structure of formulated heparin. J Pharm Sci. 2011;100:3396–3404. doi: 10.1002/jps.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Xiao Z, Masuko S, Zhao W, Sterner E, Bansal V, Fareed J, Dordick JS, Zhang F, Linhardt RJ. Mass balance analysis of contaminated heparin product. Anal Biochem. 2011;408:147–156. doi: 10.1016/j.ab.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Condra M, Kimura K, Berth G, Dautzenberg H, Dubin PL. Determination of molecular weight of heparin by size exclusion chromatography with universal calibration. Anal Biochem. 2003;312:33–39. doi: 10.1016/s0003-2697(02)00428-1. 2003. [DOI] [PubMed] [Google Scholar]
- 25.Bertini S, Bisio A, Torri G, Bensi D, Terbojevich M. Molecular weight determination of heparin and dermatan sulfate by size exclusion chromatography with a triple detector array. Biomacromolec. 2005;6:168–173. doi: 10.1021/bm049693s. [DOI] [PubMed] [Google Scholar]
- 26.Linhardt RJ. In: In current protocols in molecular biology, Analysis of Glycoconjugates. Varki A, editor. Wiley-Interscience; 1994. pp. 17.13.17–17.13.32. [Google Scholar]
- 27.Merchant ZM, Kim YS, Rice KG, Linhardt RJ. Structure of heparin-derived tetrasaccharides. Biochem J. 1985;229:369–377. doi: 10.1042/bj2290369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Weyers A, Baik JY, Sterner E, Sharfstein S, Mousa SA, Zhang F, Dordick JS, Linhardt RJ. Ultraperformance ion-pairing liquid chromatography with on-line electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal Biochem. 2011;415:59–66. doi: 10.1016/j.ab.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linhardt RJ, Loganathan D, Al-Hakim A, Wang HM, Walenga JM, Hoppensteadt D, Fareed J. Oligosaccharide mapping of low molecular weight heparins: structure and activity differences. J Med Chem. 1990;33:1639–1645. doi: 10.1021/jm00168a017. [DOI] [PubMed] [Google Scholar]
- 30.Linhardt RJ, Kerns RJ, Vlahov IR. In: Heparin and heparin oligosaccharides: Preparation, analysis, applications and biological activities, biochemical functions and biotechnology of natural and artificial polymers. Yalpani M, editor. Mt. Prospect, IL: ATL Press, Science Publishers; 1996. pp. 46–62. [Google Scholar]
- 31.Linhardt RJ. Heparin: an important drug enters its seventh decade. Chem Ind. 1991;2:45–50. [Google Scholar]
- 32.Wang Z, Ly M, Zhang F, Zhong W, Suen A, Dordick JS, Linhardt RJ. E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol Bioengin. 2010;107:968–977. doi: 10.1002/bit.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]