Abstract
Background
Despite decades of research, anastomotic intimal hyperplasia remains a major cause of delayed prosthetic arterial graft failure. Previously, we reported profound upregulation of Thrombospondin-2 (TSP-2) mRNA in neointimal smooth muscle cells following prosthetic arterial bypass graft placement. TSP-2 is an anti-angiogenic matricellular protein with specific functions yet unknown. In this study, we hypothesized that inhibition of TSP-2 in human aortic smooth muscle cells (HAoSMCs) would reduce cell proliferation and migration in-vitro, providing a therapeutic target to mitigate intimal hyperplasia.
Study Design
HAoSMCs were transfected with TSP-2 siRNA using a commercial transfection reagent. Gene silencing was evaluated using qRT-PCR. ELISA was used to measure TSP-2 protein levels in cell culture supernatants. Cell migration and proliferation were assessed using scratch wound assays and alamar blue assays respectively. Attachment assays were performed to assess the effect of TSP-2 silencing on HAoSMC adhesion to fibronectin.
Results
TSP-2 siRNA achieved consistent target gene silencing at 48 hours post-transfection in HAoSMCs. This single transfection allowed suppression of TSP-2 protein expression for over 30 days. TSP-2 gene silencing did not affect HAoSMC migration or proliferation. MMP-2 levels were also unaffected by changes in TSP-2 protein levels. However, HAoSMC attachment to fibronectin improved significantly in cells treated with TSP-2 siRNA.
Conclusions
siRNA-mediated TSP-2 silencing of human aortic HAoSMCs improved cell attachment but had no effect on cell migration or proliferation. The effect on cell attachment was unrelated to changes in MMP activity.
Introduction
Peripheral arterial disease affects approximately five million Americans [1]. This number is expected to rise with increasing average national age. Patients suffering advanced disease often require arterial bypass surgery. While autologous veins are the preferred choice of graft for infra-inguinal bypass, prosthetic grafts are an acceptable alternative when an appropriate vein is unavailable [2]. Despite decades of research, patency rates of synthetic bypass grafts remain poor. Over time, prosthetic grafts develop anastomotic intimal hyperplasia leading to stenoses and occlusions at the distal anastomosis [3]. Various modifications to synthetic grafts have been employed without significant improvements [4].
In our previous study, prosthetic arterial grafts were implanted in canine carotid arteries. Neointimal tissue samples from distal anastomotic sites were analyzed and differentially regulated genes were identified [5]. Micro-array analysis of hyperplastic tissue sections revealed profound upregulation of Thrombospondin-2 (TSP-2) mRNA to 66, 144, and 311-fold higher than that of control samples at 7, 14, and 30 days respectively. Elevated levels of TSP-2 protein in the tissue sections were confirmed by immunohistochemistry at each time point. These findings led us to investigate the role of TSP-2 in aortic smooth muscle cell biology as it pertains to the development of intimal hyperplasia.
TSP-2 is a matricellular protein from the Thrombospondin family of proteins. Matricellular proteins are thought to interact with various receptors in the extracellular matrix (ECM), but do not fulfill a structural role [6]. TSP-2 is considered an anti-angiogenic protein but the exact functions remain elusive. It is secreted by various stromal cells and its expression is linked to tissue injury, notably during the proliferative and remodeling phases of wound healing [6].
Before TSP-2 can be considered a potential therapeutic target for the treatment of intimal hyperplasia, we sought to determine whether TSP-2 upregulation in arterial wound healing constitutes a physiologic response leading to arterial repair or a pathologic response leading to graft failure. Because TSP-2 was consistently upregulated across all time points in the in-vivo model, we hypothesized that inhibition of TSP-2 in human aortic smooth muscle cells (HAoSMCs) would reduce cell proliferation and migration in-vitro, and thereby limit the development of intimal hyperplasia.
Successful silencing of TSP-2 gene and protein using small interfering RNAs (siRNAs) did not yield significant differences in HAoSMC proliferation or migration in-vitro. Low endogenous levels of TSP-2 at baseline restricted our ability to create large differences in TSP-2 concentration between treatments. However, contrary to the reported effects of TSP-2 on dermal fibroblasts, TSP-2 gene silencing achieved improved attachment of HAoSMC to fibronectin without affecting matrix metalloproteinase-2 (MMP-2) levels, suggesting a new role for TSP-2 in the development of intimal hyperplasia.
Materials and Methods
Cell culture
HAoSMCs (Lonza Walkersville Inc., Walkersville, MD) passage 3 to 9 were cultured in growth media; 1) Vasculife HAoSMC basal medium supplemented with Vasculife Life Factors kit (Lifeline Cell Technology, Walkersville, MD) and 2) Dulbecco's Modified Eagle's Medium (DMEM) with low glucose, L-glutamine, and sodium pyruvate, (Invitrogen, Carlsbad, CA) supplemented with 5% or 10% fetal bovine serum (Hyclone, Logan, UT). Cellular assays were performed in 24-well plates or 96-well microplates (Corning, Lowell, MA), with an initial seeding density of 10,000 cells per well or 1,700 cells per well respectively.
siRNA Transfection
HAoSMCs were plated on “Day 0” and then transfected the following day. HiPerFect transfection reagent (Qiagen Inc., Valencia, CA) and siRNAs targeting the Thrombospondin-2 (THBS2 Stealth RNAi™ siRNA HSS110726, Invitrogen, Carlsbad, CA) as well as siGENOME Non-Targeting siRNA Pool #1 control siRNA (Dharmacon Inc., Lafayette, CO) were used following the manufacturer's protocols. Three candidate sequences of siRNA against TSP-2 were compared and the most effective sequence was chosen. Control groups were as follows: Untreated HAoSMCs (NT), HAoSMCs treated with transfection reagent alone (HPF alone), and HAoSMCs transfected with siGENOME Non-Targeting siRNA Pool #1, a control sequence of siRNA (Dharmacon, Lafayette, CO) with no known homology to the human genome (CTRL siRNA). Cell media was changed on the day following transfection and then every two days thereafter.
RNA extraction and complementary DNA synthesis
At 48 hours following HAoSMC transfection, RNA was harvested using the RNEasy Mini kit (Qiagen Inc., Valencia, CA). The amount of RNA was quantified using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE) and complimentary DNA (cDNA) was synthesized from equal amounts of RNA using the iScript cDNA Synthesis kit (BioRad, Hercules, CA) according to manufacturer's protocols.
Semi-Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using the Stratagene MX3005P system together with Brilliant SYBR Green qRT-PCR Master Mix (Stratagene, LaJolla, CA) as specified by the manufacturer's protocols. β2-microglobulin and TSP-2 primers were obtained from Integrated DNA Technologies (Coralville, IA) [Forward: 5’-ACG AGT TTG GGT CTG TGG ACT TCA-3’; Reverse: 5’-TAG AAG CGG CTG CTT GAC TGG TAA-3’]. Target gene mRNA levels were normalized to β2-microglobulin levels. Relative TSP-2 mRNA expression levels were calculated with the 2-ΔΔCT method. All gene-amplification reactions were performed in duplicates. Experiments were repeated at least three times using each of the two different growth media.
Protein quantification with enzyme-linked immunosorbent assay (ELISA)
Cells were plated on day 0 and transfected on day 1. Cell culture supernatants were subsequently collected at days 3, 7, 14, 21, and 28 for protein quantification. Cells remained at its original passage for the duration of sample collection. TSP-2 and MMP-2 protein levels were quantified using the Quantikine Human TSP-2 and MMP-2 Immunoassay (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations. Target protein levels were normalized to total protein levels in the corresponding supernatant samples using the BCA Protein Assay Kit (Pierce, Rockford, IL). All individual samples were analyzed in duplicates. Each experiment was repeated at least three times.
Scratch Wound Migration Assay
Scratch assay for cell migration was performed as described by Liang et al. with minor modifications [7]. HAoSMCs were plated in 24-well plates, transfected as described, and cultured complete media with 10% FBS for approximately three days. When the HAoSMCs reached >90% well-confluence, the cells were serum-starved in 0.1% serum media overnight. After approximately 16 hours, a linear scratch wound was created across the middle of the well surface with a sterile 200μL pipette tip. Scratched wells were gently washed with serum-free media to remove cell debris. Complete media with 10% FBS was then added to the cells for stimulation. The initial cell positions were determined using the “bright-field” and “confluence” modes of an adherent cell cytometer (Celigo™, Cyntellect, San Diego, CA), which allows repeated measurements within the exact location of a microplate well. The initial cell number was obtained by counting stained nuclei (Hoechst 33342, Invitrogen, Carlsbad, CA) using the fluorescence expression mode of the adherent cytometer. Changes in cell position and number were evaluated over the next several days in the same manner. Sample photographs of cell migration over time are shown in Figure 1. Complete growth media was added to the HAoSMCs each day following measurements. Migration and proliferation data are presented as percentages of the initial well-confluence or cell count respectively.
Figure 1.
Photographs from scratch assay. (A) HAoSMCs begin to infiltrate the horizontal scratch defect created in the lower half one day after scratch. (B) By three days post-scratch, the cells occupy the lesion.
Cell Proliferation Assay
Following transfection, the HAoSMCs were allowed to rest in complete media for one day before they were serum starved using DMEM supplemented with 0.5% fetal bovine serum overnight. On the following day, complete media with 10% FBS was added to initiate the assay. Relative cell proliferation was quantified using Alamar Blue (Invitrogen, Carlsbad, CA) at this time, and every two days thereafter. Colorimetric readouts were quantified with μQuant microplate reader and KC4 software (BioTek, Winooski, VT). Individual samples were analyzed in duplicate and each experiment was repeated at least three times.
Cell Attachment Assay
Cell attachment assays were based on a method described by Murphy-Ullrich and Hook [8]. Flat-bottom, black-walled 96-well microplates (Corning, Lowell, MA) were coated with 0.5μg of fibronectin in 100μL of PBS and incubated at 4°C overnight. Unspecific protein binding sites were blocked with DMEM containing 1% BSA for one hour at 37°C. Meanwhile, transfected HAoSMCs that were grown to near-confluence over a three-day period were serum starved overnight and trypsinized and re-suspended in serum-free media the next day. A 24-hour sample of cell culture supernatant was obtained prior to the assay for TSP-2 and MMP-2 protein quantification. Collected cells were counted and 10,000 cells per well were plated in the pretreated wells and allowed to attach to the substratum at 37°C. After one hour of incubation, the wells were washed gently with serum-free DMEM three times to remove loosely adherent cells. The attached cell nuclei were visualized with Hoechst stain for counting using the Celigo cytometer as described above. Each experiment was analyzed in triplicate.
Gelatin Zymography
Cell culture supernatants of siRNA treated HAoSMCs were collected three to five days following transfection. Media changes were made on the day prior to collection to ensure a 24-hour supernatant sample. MMP-2 activity was detected by gelatin zymography using Ready Gel Zymogram Gels with 10% gelatin (Biorad, Hercules, CA) and a Mini-Protean II Electrophoresis Cell (Biorad) were used following the manufacturer's instructions.
Data and Statistical Analysis
Statistical analysis was performed using Prism 5 software (GraphPad Software, San Diego, CA). Data are presented as means ± standard deviations. Significance of association was assessed using paired, two-tailed Student's t-test or randomized-block one or two-way ANOVA with Bonferroni's multiple comparisons test as appropriate. A p-value of <0.05 was considered statistically significant.
Results
TSP-2 siRNA Mediated Gene Silencing
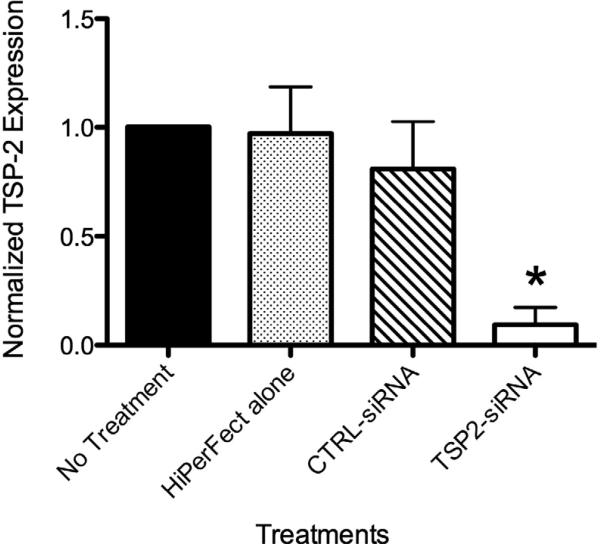
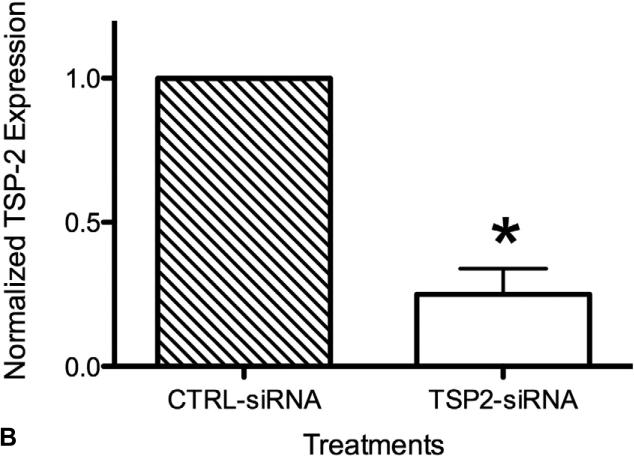
The effect of siRNA transfection on gene silencing was confirmed using qRT-PCR. Figure 2 shows TSP-2 mRNA expression of HAoSMCs that were a) untreated [NT], b) treated with transfection reagent alone [HPF alone], c) transfected with non-targeting control siRNA [CTRL-siRNA], and d) transfected with TSP-2 siRNA [TSP2-siRNA], normalized to corresponding ‘NT’ values for each experiment. Two days following transfection of HAoSMCs with TSP-2 siRNA, TSP-2 mRNA expression was significantly reduced (p<0.001) compared to: a) ‘NT’ group (90.6%), b) ‘HPF alone’ group (87.8%±10.7%), and c) ‘CTRL-siRNA’ group (71.6%±10.9%). No significant differences in TSP-2 mRNA levels were found between the ‘NT’ group and the ‘HPF alone’ group (p=0.76), or between the ‘NT’ group and ‘CTRL-siRNA’ group (p=0.08) suggesting minimal effect of the transfection reagent or non-targeting control siRNA on TSP-2 gene expression.
Figure 2.
HAoSMC TSP-2 mRNA expression at 48 hours. TSP-2 siRNA mediated gene silencing. TSP-2 mRNA expression was significantly reduced at 48 hours following TSP-2 siRNA transfection compared to controls as measured using qRT-PCR. * p<0.05.
One-time Transfection with TSP-2 siRNA Leads to 30 Days of Protein Silencing
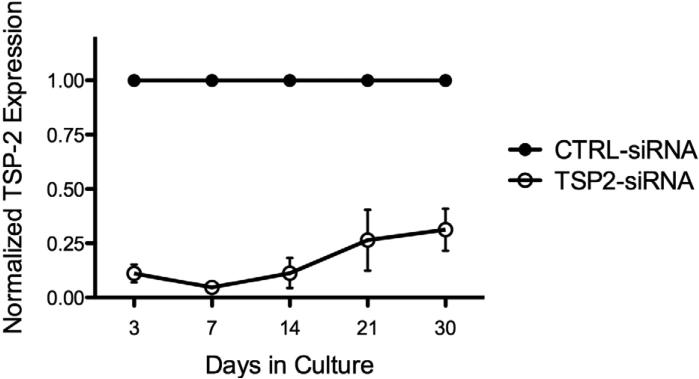
Gene silencing was further confirmed by measuring TSP-2 protein concentrations in supernatants of transfected HAoSMCs using ELISA. TSP-2 siRNA transfection significantly reduced protein concentration of TSP-2 compared to the control group at all time points (p < 0.05) as shown in Figure 3 (89 ± 7% at day 3, 95 ± 4% at day 7, 89 ± 12% at day 14, 74 ± 24% at day 21, and 69 ± 17% at day 30). This data suggests that a single TSP-2 siRNA transfection results in 30 days of significant TSP-2 protein silencing.
Figure 3.
HAoSMC TSP-2 protein expression (n=3). TSP-2 protein concentrations in supernatants of transfected HAoSMCs as measured using ELISA. One-time transfection with TSP-2 siRNA leads to 30 days of TSP-2 protein silencing. p<0.05 at all time points.
No detectable differences were seen in TSP-2 protein levels amongst the three control groups; ‘NT’, ‘HPF alone’, and ‘CTRL-siRNA’ groups (data not shown).
TSP-2 siRNA Mediated Gene Silencing Does Not Affect HAoSMC Migration
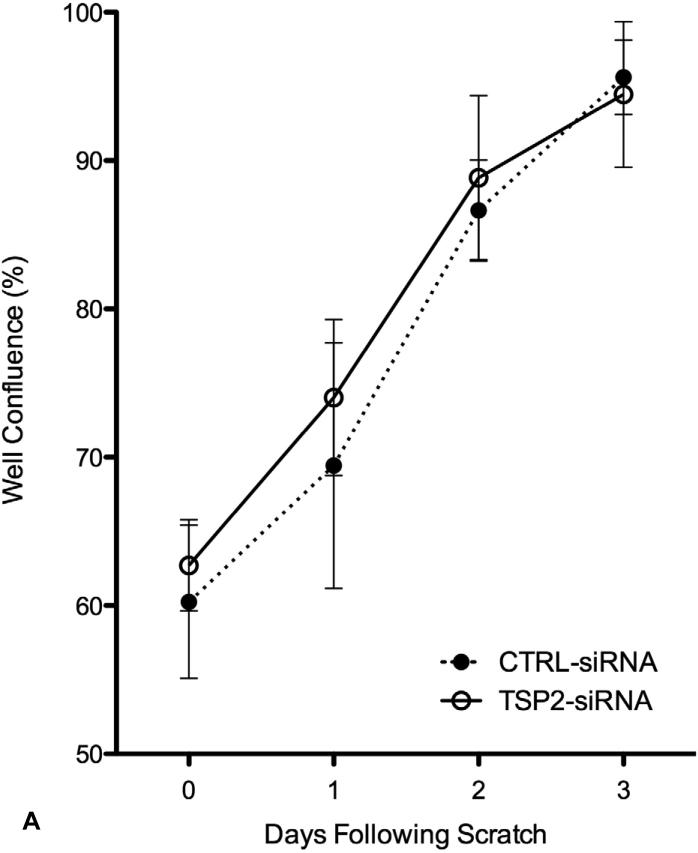
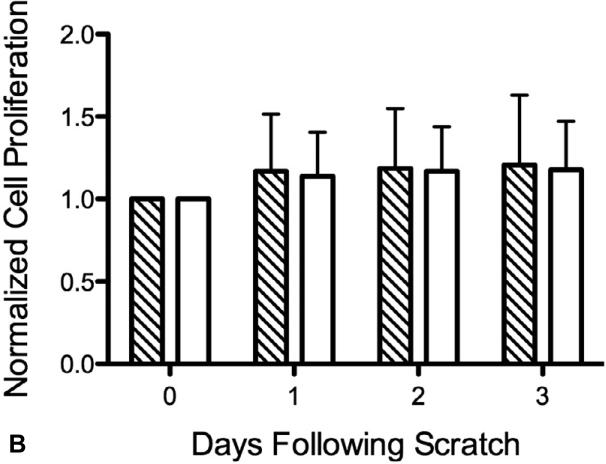
The effect of TSP-2 gene silencing on cell migration was assessed with scratch wound assays. When HAoSMCs reached >90% confluence, scratch wounds were created in the culture wells. Measurements of well confluence were made on subsequent days using an adherent cytometer. Scratch wound size was consistent between individual wells; approximately 40% of the area under investigation. Cells migrated into the scratch wound area and covered the scratch area within three days. TSP-2 silencing did not affect the rate of cell migration compared to the ‘CTRL-siRNA’ group (Figure 4-A). HAoSMC proliferation was assessed at each corresponding time point. As shown in Figure 4-B, cell growth was minimal during the migration period, verifying that cell migration, and not cell proliferation, was the primary mode of scratch wound closure.
Figure 4.
(A) HAoSMC migration (n=4). Results of scratch wound migration assay. TSP-2 siRNA mediated gene silencing does not affect HAoSMC migration. (B) HAoSMC proliferation (n=4). Migrating HAoSMCs from the scratch wound assay was measured for cell proliferation simultaneously. Cells show limited growth in the three days of cell migration (see Figure 3A). Diagonal striped bar, CTRL-siRNA; white bar, TSP-2-siRNA.
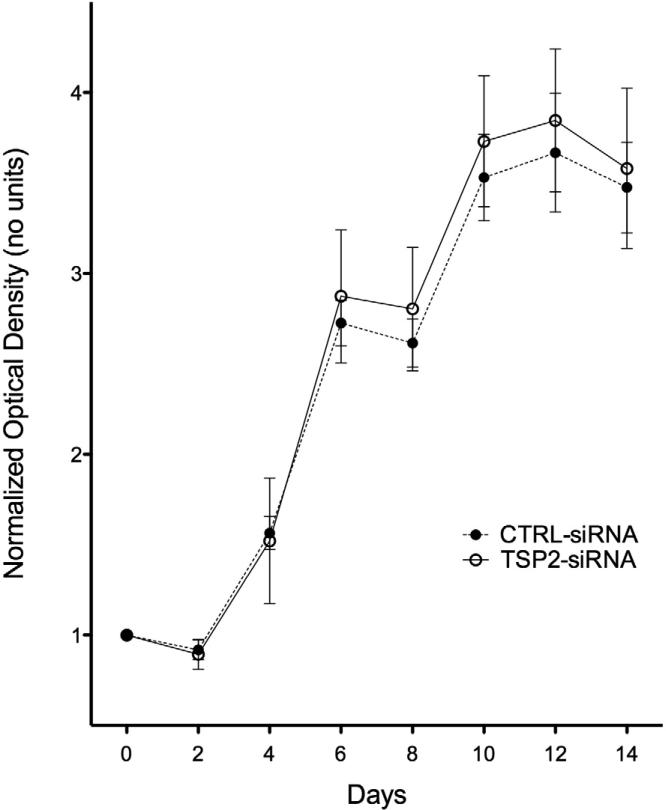
TSP-2 siRNA Mediated Gene Silencing Does Not Affect HAoSMC Proliferation
The effect of TSP-2 gene silencing on HAoSMC proliferation was assessed using Alamar Blue. ‘Day 0’ measurements for each well served as baseline readings for subsequent time points (Figure 5). TSP-2 siRNA treated HAoSMCs displayed greater proliferation at later time points, but statistical differences were not achieved (p >0.05 at all time points). HAoSMCs treated with TSP-2 siRNA appeared more spindle-like and tightly organized than control siRNA treated cells (observational data).
Figure 5.
HAoSMC proliferation (n=4). Results of Alamar Blue assay to measure cell proliferation. Transfection of TSP-2 siRNA does not affect HAoSMC growth over time compared to control siRNA treated cells.
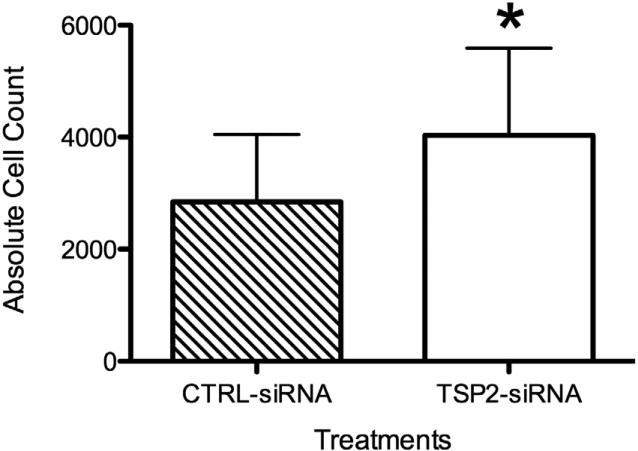
TSP-2 siRNA Mediated Gene Silencing Improves HAoSMC Attachment
Compared to controls, transfection with TSP-2 siRNA significantly improved cell attachment to fibronectin coated cell culture plates (Figure 6). Cells from the ‘TSP-2 siRNA’ group resulted in significantly higher attachment (3728 ± 791 cells per well) compared to cells from the ‘CTRL siRNA’ group (2719 ± 564 cells per well, p<0.05) or ‘NT’ group (2667 ± 545 cells/well, p<0.05, data not shown).
Figure 6.
HAoSMC attachment (n=10). Attachment of HAoSMCs to fibronectin is significantly improved after TSP-2 siRNA transfection. *p<0.05.
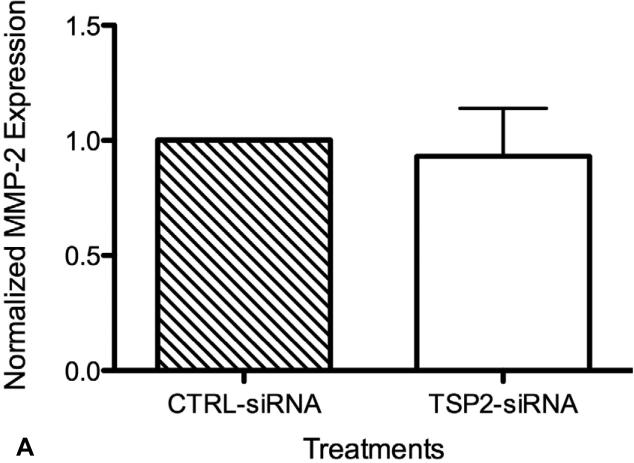
TSP-2 Protein Silencing Does Not Affect MMP-2 Levels
Conditioned media from HAoSMC cultures subsequently used in attachment assays were measured for MMP-2 content. As shown in Figure 7-A, no significant differences in MMP-2 levels were observed between supernatants of TSP-2 siRNA and control siRNA treated HAoSMCs (56.1 ± 12.2 ng/mL and 61.1 ± 22.6 ng/mL, respectively). Zymography from matching samples did not show significant differences in MMP-2 enzyme activities between the treatment groups (data not shown). TSP-2 silencing was confirmed in the same samples that were examined for MMP-2 content and enzyme activity (Figure 7-A). As expected, the average TSP-2 content in TSP-2 siRNA treated HAoSMCs (3.0 ± 1.7ng/mL) was significantly lower than control siRNA treated HAoSMCs (10.9 ± 3.9 ng/mL) (p <0.05).
Figure 7.
(A) HAoSMC MMP-2 protein expression (n=6). Transfection of HAoSMCs with TSP-2 siRNA does not affect MMP-2 levels. Conditioned media, collected from cultures inhabited by HAoSMCs before conducting the cell attachment assay, were measured for MMP-2 concentration using ELISA. (B) HAoSMC TSP-2 protein expression (n=6). Conditioned media, collected from cultures inhabited by HAoSMCs before conducting the cell attachment assay, were measured for TSP-2 concentration using ELISA. Taken together with results from Figure VI-A, TSP-2 gene silencing does not affect MMP-2 concentration.
Discussion
TSP-2 is a matricellular protein, highly expressed in the proliferative and remodeling phases of wound healing [6]. It is recognized as an antiangiogenic protein, but its exact functions remain uncharacterized. Published in-vitro work on TSP-2 as related to vascular biology has mainly focused on its effects on endothelial cells and fibroblasts [8-18]. Despite evidence of pronounced TSP-2 expression in hyperplastic smooth muscle cells, very little is known about the role of TSP-2 in intimal hyperplasia. We therefore hypothesized that TSP-2 was pathologically over-expressed in intimal hyperplasia and by silencing this gene, HAoSMC migration and proliferation could be attenuated. We developed a TSP-2 knockdown model in HAoSMCs invitro, and evaluated cellular functions to test this hypothesis.
TSP-2 siRNA transfection with HiPerFect transfection reagent provided consistent and reproducible silencing of the target gene to approximately 10% of controls at 48 hours post transfection (Figure 2). This transfection procedure provided the basis for all experiments described.
Results from ELISA showed target protein knockdown out to thirty days following transfection (Figure 3). In a recently published report, similar methods were used to transfect TSP-2 siRNA in MC3T3-E1 pre-osteoblasts, but the effect lasted less than 14 days [19]. Differences in transfection efficiency may be attributed to cell type, transfection reagent effect, cell density at the time of transfection, and cell growth rate, with varying degrees of contribution from each [20]. Weakening of the protein silencing effect over the last three weeks in our cultures may be attributed to a combination of siRNA dilution with successive cell division and deactivation of RNA-induced silencing complexes (RISCs) as a result of reduction in target mRNA [21]. Based on initial cell seeding density and cell count at confluence, two to three cell divisions occur in the first two weeks of culture and remain confluent with little change in the appearance of the cell population during the last two weeks of culture (observed data). Our demonstration that TSP-2 silencing persists at thirty days following a single transfection will be an asset in future in-vivo applications to modify intimal hyperplasia and improve outcomes following prosthetic arterial bypass graft surgeries.
Despite our original hypothesis based on the fact that TSP-2 is highly upregulated in SMCs in intimal hyperplasia, TSP-2-null mice are known to produce irregular collagen fibrils and a less organized collagen matrix [9, 10, 12], which may lead to a reduction in ECM integrity and actually improve cell migration. A cell migration assay was performed to aid in the understanding of these conflicting observations. The scratch wound cell migration assay was chosen for its suitability to study the regulation of cell migration by cell-ECM and cell-cell interactions [7]. Our results revealed that siRNA mediated silencing of TSP-2 did not change the rate of migration of HAoSMCs in culture compared to controls (Figure 4-A). These findings were in contrast to published reports that have shown recombinant TSP-2 to inhibit growth factor-induced endothelial cell migration [22, 23]. However, concentrations exceeding 1.0μg/mL of exogenous TSP-2 was used to achieve those results. The average TSP-2 concentrations in our culture supernatant samples were considerably lower, ranging between 3-11ng/mL. Despite achieving significant relative target protein silencing, the low baseline concentrations of TSP-2 in control siRNA treated HAoSMC cultures were perhaps insufficient to allow for differences in TSP-2 concentrations that would affect cell migration. Given the fact that matricellular proteins change behavior and function according to temporo-spatial patterns, and that TSP-2 expression increases during times of injury and inflammation [6], its roles may also change as a function of concentration. Therefore, at low concentrations, TSP-2 does not affect SMC migration. High concentrations of TSP-2 may affect SMC migration, however, there is no proof that concentrations exceeding 1.0μg/mL are achievable in-vivo.
The use of an adherent cytometer allowed monitoring of cell migration over time as well as corresponding cell proliferation. The traditional method uses inverted microscopy for data collection and imaging software to analyze cell migration [24]. Determining the leading edge of cell migration can become difficult and arbitrary when migration is not uniform. The adherent cytometer allowed repeated measurements of cell confluence over the designated surface area. Simultaneous measurements for cell proliferation allowed determination of the level of contribution from cell proliferation on cell migration. Figures 4-A and 4-B confirm that the scratch wounds closed as a result of cell migration, independent of cell proliferation over four days in culture.
To confirm these preliminary results on cell proliferation, differences in cell growth with or without TSP-2 gene silencing were measured over a two-week period. TSP-2 appears to limit cell proliferation in endothelial cells [25, 26], but not in dermal fibroblasts [10]. In the current study, we showed that silencing TSP-2 gene and protein levels with siRNA do not alter HAoSMC proliferation compared to those treated with control siRNA treated HAoSMCs (Figure 5).
Thrombospondins have been implicated to modify cell attachment to the surrounding extracellular matrix [10, 17, 18, 27]. In dermal fibroblasts taken from TSP-2 gene knockout mice, a pro-adhesive function for TSP-2 has been suggested [10]. Cell attachment defects in TSP-2-null cells were attributed to increased levels of MMP-2 found associated with the loss of TSP-2 expression [17], leading to increased proteolysis of cell surface tissue transglutaminase [27]. Given the ubiquitous nature of tissue transglutaminases, we expected TSP-2 silenced HAoSMCs to demonstrate attachment defects in a similar fashion.
Instead, we provide evidence that TSP-2 siRNA treatment of HAoSMCs significantly improved cell attachment compared to controls (Figure 6) without affecting MMP-2 levels (Figure 7-A). Conditioned supernatant samples, taken from HAoSMC cultures used in attachment assays, were analyzed but no significant differences in MMP-2 content or activity (data not shown) between TSP-2 siRNA treated HAoSMCs and controls were detected. Appropriate TSP-2 silencing was confirmed in samples tested for MMP-2 content (Figure 7-B). These results suggest that TSP-2 can limit vascular smooth muscle-ECM attachment in a manner independent of MMP-2 activity.
To understand why siRNA-mediated TSP-2 silencing did not affect MMP-2 levels, protein concentrations in our supernatant samples were compared to the reported data [17]. The concentration of MMP-2 found in supernatant samples taken from fibroblast cultures (average 84.3-170μg/L, or 84.3-170ng/mL) were similar to that found in our culture samples (average 59.5ng/mL). The concentration of recombinant TSP-2 required to rescue TSP-2 null mouse dermal fibroblast attachment (40nM of TSP-2 in 100μL, approximately equal to 5.2μg/mL) was much greater than the amount of TSP-2 protein produced by our HAoSMCs in culture (3-13ng/mL). Therefore it is possible that the physiologic concentration of endogenous TSP-2 produced by our HAoSMCs in culture was insufficient to affect MMP-2 levels.
Although the relatively small difference in absolute TSP-2 levels do not affect MMP-2 concentration, we observed significant differences in cell attachment to fibronectin. This suggests that at lower concentrations, TSP-2 may act to inhibit cell attachment to its surrounding matrix. The in-vivo concentration of TSP-2 protein at sites of arterial injury are difficult to determine. The development of an in-vitro inflammatory model for HAoSMCs that will enhance TSP-2 expression and simulate vascular injury will be considered, where a larger difference in TSP-2 protein levels can be achieved. A more direct, gain-of-function study to observe the effects of TSP-2 on HAoSMCs may further expand our knowledge regarding its effects on vascular smooth muscle cells and help correlate our findings with published data from other cell types.
In summary, siRNA-mediated TSP-2 silencing of human aortic HAoSMCs improved cell attachment but had no effect on cell migration or proliferation. The effect on cell attachment was unrelated to changes in MMP activity. These observations reject our original hypothesis. Upregulation of TSP-2 in-vivo may contribute to decreased cell attachment and lead to altered cell-cell or cell-ECM interactions in the neointimal layer of intimal hyperplasia. Given the temporo-spatial change in function that matricellular proteins are thought to display the clear role of TSP-2 in bypass graft failure remains elusive. Therefore, we cannot conclude that TSP-2 does not affect cell proliferation or migration in-vivo based on simplified in-vitro experimental results. Continued work is needed to uncover the potential link between increased TSP-2 expression and the development of intimal hyperplasia.
Acknowledgments
This study was supported in part by National Institutes of Health Grant T32 HL07734 and R01 HL21796.
Abbreviations
- TSP-2
Thrombospondin-2
- HAoSMC
Human Aortic Smooth Muscle Cell
- siRNA
Small Interfering Ribonucleic Acid
- ELISA
Enzyme Linked Immunosorbent Assay
- MMP-2
Matrix Metalloproteinase-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Abstract presented at the 6th Annual Academic Surgical Congress, Huntington Beach, CA, February 2011.
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104–114. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 3.Cantelmo NL, Quist WC, LoGerfo FW. Quantitative analysis of anastomotic intimal hyperplasia in paired Dacron and PTFE grafts. J Cardiovasc Surg (Torino) 1989;30:910–915. [PubMed] [Google Scholar]
- 4.Kapadia MR, Popowich DA, Kibbe MR. Modified prosthetic vascular conduits. Circulation. 2008;117:1873–1882. doi: 10.1161/CIRCULATIONAHA.107.714170. [DOI] [PubMed] [Google Scholar]
- 5.Malek JY, Monahan TS, Andersen ND, et al. New England Society for Vascular Surgery Annual Meeting. Ledyard, CT: 2007. Selective Isolation of Anastomotic Intimal Hyperplasia for Microarray Analysis Using Laser Capture Microdissection. [Google Scholar]
- 6.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 8.Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krady MM, Zeng J, Yu J, et al. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyriakides TR, Zhu YH, Smith LT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakides TR, Zhu YH, Yang Z, et al. The distribution of the matricellular protein thrombospondin 2 in tissues of embryonic and adult mice. J Histochem Cytochem. 1998;46:1007–1015. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakides TR, Zhu YH, Yang Z, et al. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159:1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, et al. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- 15.Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987;105:1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr AW, Pedraza CE, Pallero MA, et al. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 19.Alford AI, Terkhorn SP, Reddy AB, et al. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone. 2010;46:464–471. doi: 10.1016/j.bone.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabzdyk CS, Chun MC, Pradhan LP, et al. 6th Annual Academic Surgical Congress. Huntington Beach, CA: 2011. Differential Susceptibility of Human Primary Vascular Cells Towards siRNA Transfection. [Google Scholar]
- 21.Song E, Lee SK, Dykxhoorn DM, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh YH, Matsuda K, Hong SK, et al. An N-terminal 80 kDa recombinant fragment of human thrombospondin-2 inhibits vascular endothelial growth factor induced endothelial cell migration in vitro and tumor growth and angiogenesis in vivo. J Invest Dermatol. 2003;121:1536–1543. doi: 10.1046/j.1523-1747.2003.12643.x. [DOI] [PubMed] [Google Scholar]
- 23.Volpert OV, Tolsma SS, Pellerin S, et al. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- 24.Lyon CA, Koutsouki E, Aguilera CM, et al. Inhibition of N-cadherin retards smooth muscle cell migration and intimal thickening via induction of apoptosis. J Vasc Surg. 2010;52:1301–1309. doi: 10.1016/j.jvs.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong LC, Bjorkblom B, Hankenson KD, et al. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell. 2002;13:1893–1905. doi: 10.1091/mbc.E01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes N, Gregg D, Vasudevan S, et al. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol Cell Biol. 2003;23:5401–5408. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agah A, Kyriakides TR, Bornstein P. Proteolysis of cell-surface tissue transglutaminase by matrix metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am J Pathol. 2005;167:81–88. doi: 10.1016/S0002-9440(10)62955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]