Abstract
Objective
To evaluate the cost-effectiveness of universal neonatal screening for T cell lymphocytopenia in enhancing quality of life and life expectancy for children with severe combined immunodeficiency (SCID).
Methods
Decision trees were created and analyzed to estimate the cost, life years, and quality adjusted life years (QALYs) across a population when universal screening for lack of T cells is used to detect SCID, as implemented in five states, compared to detection based on recognizing symptoms and signs of disease. Terminal values of each tree limb were derived through Markov models simulating the natural history of three cohorts: unaffected subjects; those-diagnosed with SCID as neonates (early diagnosis); and those diagnosed after becoming symptomatic and arousing clinical suspicion (late diagnosis). Models considered the costs of screening and of care including hematopoietic cell transplantation for affected individuals. Key decision variables were derived from the literature and from a survey of families with children affected by SCID, which was used to describe the clinical history and healthcare utilization for affected subjects. Sensitivity analyses were conducted to explore the influence of these decision variables.
Results
Over a 70 year time horizon, the average cost per infant was $8.89 without screening and $14.33 with universal screening. The model predicted that universal screening in the U.S. would cost approximately $22.4 million/year with a gain of 880 life years and 802 QALYs. Sensitivity analyses showed that screening test specificity and disease incidence were critical driving forces affecting the incremental cost-effectiveness ratio (ICER). Assuming a SCID incidence of 1/75,000 births and test specificity and sensitivity each at 0.99, screening remained cost-effective up to a maximum cost of $15 per infant screened.
Conclusion
At our current estimated screening cost of $4.22/infant, universal screening for SCID would be a cost effective means to improve quality and duration of life for children with SCID.
Keywords: severe combined immunodeficiency (SCID), newborn screening, cost-effectiveness, Markov models, T-cell receptor excision circle (TREC), primary immunodeficiency, hematopoietic cell transplant
INTRODUCTION
Severe combined immunodeficiency (SCID) is a life-threatening defect in both cellular and humoral immunity [1]. Most infants with SCID are not diagnosed until a series of increasingly severe infections raises suspicion. This typically occurs around 6 months of age, after maternally derived antibodies have waned. Unless there is a family history of SCID, correct diagnosis depends on the physician’s inclusion of immunodeficiency in the differential diagnosis of presentation with recurrent infections and other manifestations of SCID. However, family histories are typically absent, thereby delaying diagnosis. Untreated SCID is fatal early in life because of overwhelming infections [2, 3] but survival is prolonged by hematopoietic cell transplantation (HCT) to reconstitute the immune system[4, 5] . HCT within the first 3.5 months of life yields the best outcome [3, 6]. This survival advantage depends on early detection which is challenging without universal newborn screening.
In the setting of scarce resources, the decision to universally screen depends on the costs of screening programs compared to the benefits of early detection. Chan and Puck showed that subjects with SCID are differentiated from unaffected neonates by measuring T cell receptor excision circles (TRECs), a DNA product produced during normal T cell development, in DNA isolated from dried blood spots (DBS) already collected routinely from newborns [7]. Wisconsin and Massachusetts began TREC screening; and, following the 2010 recommendation of the U.S. Secretary of Health and Human Serivces to add SCID to the uniform panel of screened conditions, California, Louisiana, New York and Puerto Rico have followed. The newborn screening programs in these States have successfully identified infants with SCID and T cell lymphocytopenia [8-10]. Screening for the SCID hallmark of T lymphocytopenia is feasible by measuring TRECs, and statewide trials of TREC screening of newborn DBS began in Wisconsin and Massachusetts [10-12], California, New York and others. However, cost-effectiveness and improved outcomes with TREC screening remain unproven. A prior estimate of the cost-effectiveness of SCID newborn screening incorporated limited information about care costs or outcome differences between infants identified prior to vs. than after becoming symptomatic [13]. Here, we evaluated the cost-effectiveness of universal screening using a model incorporating the impact of early detection on the natural history of SCID. We simulated the natural history of SCID using Markov models of subjects’ progressions through multiple health states, allowing us to test the hypothesis that the benefits of earlier SCID detection exceed the costs of universal screening.
METHODS
Study design: decision tree and strategies
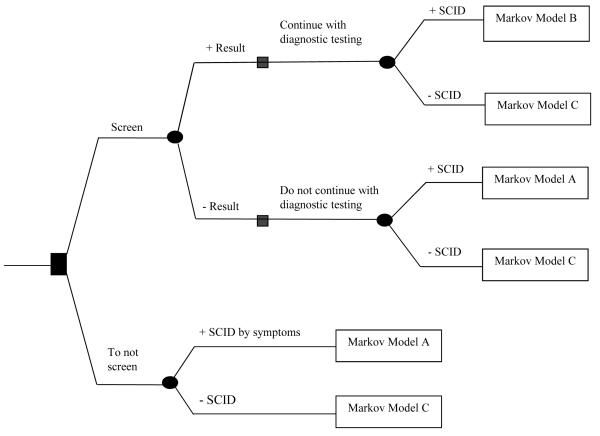
Decision analysis was used to evaluate cost, life years, and quality adjusted life years (QALYs) of universal TREC assay newborn screening for T lymphocytopenia to identify SCID compared to no screening in Fig. 1. Decision trees integrate information about three populations characterized using separate Markov models (Fig. 2): “Model A” describes subjects affected with SCID not detected through screening; “Model B” describes subjects with SCID detected by screening; and “Model C” describes unaffected subjects. We adopted a societal perspective for our model following recommendations of the Panel on Cost Effectiveness in Health and Medicine [14].
Figure 1.
Markov model analytic decision tree comparing two strategies: 1) newborn screening, and 2) no screening. The following models at the terminal end of each limb depict the possible stochastic processes of affected SCID identified early by screening (Markov model B), affected SCID identified after manifestation of symptoms (Markov model A), or unaffected non-SCID infants (Markov model C).
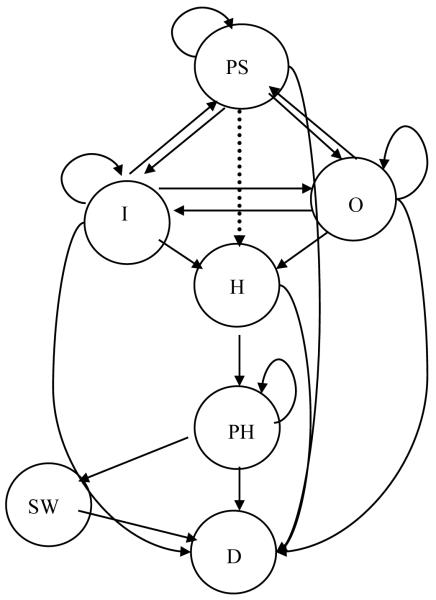
Figure 2.
Transition state Markov model A and B: Both models have all the same health states,; In Model B, one additional transition is allowed as shown by the dotted arrow to demonstrate moving from presymptomatic SCID directly to HCT following screening. Abbreviations as in Table 1.
The first strategy [no screening] describes the default status in which SCID is diagnosed after an infant develops infections. We assume that the diagnosis is confirmed by test with 100% sensitivity and specificity. After diagnosis, SCID infants would continue through any of the possible transition states in Markov Model A, representing the clinical history of SCID identified late [Fig. 2 without dotted line transition]. Health states, Presymptomatic SCID, Outpatient, Inpatient, HCT, Post-HCT, SCID Well, and Deceased, are described in Table 1 and depicted in Figure 2.
Table 1.
Descriptions of the States Used in Models
| Abbreviation | State | Definition |
|---|---|---|
| PS | Presymptomatic SCID | No overt signs of illness; the only medical encounters are for routine baby care. |
| O | Outpatient | Two or more trips/month to either doctor’s office or emergency department because of illness related to SCID—infections, graft versus host diseases (GVHD), diarrhea, fail to thrive (FTT). |
| I | Inpatient | Hospitalization for one or more nights per month for a medical condition related to SCID infections. |
| H | HCT | A one-month period including preparation and administration of bone marrow. |
| PH | Post-HCT | Inpatient SCID patient remains in the hospital. Hospitalization might be required for T cell lymphopenia, isolation, infection, GVHD, etc. |
| Outpatient SCID patient is out of the hospital, but still needs one or more medical visits/month for tests and interventions. |
||
| SW | SCID Well | A post-BMT state in which a SCID patient is thriving but still receiving immunoglobulin to provide antibody protection. |
| D | Decreased | Death |
| UW | Unaffected Well | Child unaffected with SCID |
| UD | Unaffected Death | Death of a child unaffected with SCID |
The natural history of patients with SCID detected by newborn screening was described using Markov Model B (Fig. 2), which assumes that subjects identified through screening could transition directly from Presymptomatic SCID to HCT. Otherwise, health states are similar to those in Model A although transition probabilities differ.
Some subjects will have false negative screening tests. We assumed that SCID infants with negative screening results would eventually be identified by becoming symptomatic and will either have a course similar to unscreened subjects with SCID or die without diagnosis, as modeled by Markov Model A (Fig. 2). Unaffected subjects with true negative tests are treated as normal healthy infants (Markov Model C) (Fig. 3). Infants with false-positive tests will incur additional costs because of the misdiagnosis and increased parental anxiety. However, we assume this cost is transient and can be minimized by educating providers and parents.
Figure 3.
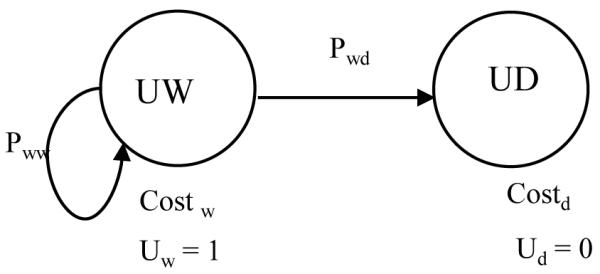
Transition state Markov model of two states of an unaffected infant (Markov model C).
Survey to collect data for transition probabilities
To gather information about the early natural history of SCID, we administered a structured interview to 39 consenting parents of patients diagnosed with SCID since 2000, performing a medical record review for 5 of these cases. To preserve confidentiality, patient identifiers were not recorded on the diagrams used to determine Markov transition probabilities. Transition probabilities relating to HCT treatment were derived from the medical literature, the national marrow donor registry, and parent responses and medical charts. To calculate transitions from states following HCT, we extrapolated data from the Kaplan-Meier survival curves of SCID transplanted after 3 months of life from the national marrow registry. Published survival curves were used to model the survival of SCID patients transplanted within the first 3 months of life [15, 16]. Published Kaplan-Meier curves were smoothed by fitting to exponential curves, and percent survival per month was used to predict transitions through the tunnel states post-HCT.
Costs
We used a gross-costing approach to estimate medical and non-medical costs for each health state [17], expressing costs in 2005 dollars discounted over time at 3% yearly. We estimated average costs for the following variables: 1) screening and confirmatory testing, 2) late HCT, 3) early HCT, 4) hospital inpatient and outpatient visits, and 5) intravenous immunoglobulin (IVIG) therapy.
We estimated the cost of screening at $4.22 per test based on machine usage, labor, and reagents [6]. Costs for confirmatory testing were estimated at $250 per patient including complete and differential blood counts and lymphocyte phenotyping [6]. Late HCT charges were obtained from SCID patients diagnosed between 6 and 9 months of age who received treatment at the Dana-Farber Cancer Institute and Children’s Hospital Boston from 2003-2005 (SYP and FAB, unpublished). These infants had infections at the time of diagnosis. Because charges specific to early HCT for SCID are not available and may vary between individual institutions, we took the conservative approach of using mean HCT data not stratified by patient age, found in the Healthcare Cost and Utilization Project (HCUP) Kids’ Inpatient (KID) database for the ICD-9-CM principal diagnosis code for HCT. These SCID transplants were presumed to be a mixture of early and late, so that using this source for our early HCT estimate would tend to minimize differences between the two HCT types in our model. We converted charges to costs using a cost to charge ratio of 0.39, which was similar to both the publicly available reports for the Dana-Farber Cancer Institute and Children’s Hospital of Boston (Massachusetts Uncompensated Care Pool PFY05 Annual Report, Division of Health Care Finance and Policy 2005-2006) and the HCUP database. Thus the mean cost of a late HCT from charges provided by Dana-Farber Cancer Institute and Children’s Hospital Boston was $360,000, while the cost of an early HCT using HCUP data was $120,000.
We derived average numbers of outpatient visits and inpatient hospital days from our patient survey. The cost of an outpatient visit, $37.89, was estimated by multiplying the relative value units (RVUs) for an intermediate (level 3) office visit, from the current procedure terminology, by the Center for Medicare services payment conversion factor. Costs of inpatient days were estimated from average charges for patients with an immunity disorder during 2003 in the HCUP-KIDS database and applying the cost-to-charge ratio of 0.39. Monthly IVIG was estimated at $1070 per month based on average cost per dose of the most common immunoglobulin brands (www.primaryimmune.org).
Non-medical costs associated with travel, waiting and care time were calculated assuming that one parent would lose 4 hours of work at the average federal wage rate of $16.50 per hour to assist a child ill with SCID. Roundtrip transportation costs were estimated at $5.00 per medical visit [18, 19].
Utility values
The value or utility of time spent in each state is expressed in quality adjusted life years (QALYs) where 1 QALY is defined as a year spent in perfect health. QALYs for SCID subjects after HCT were estimated by analogy to estimates for pediatric conditions that create similar limitations in activity. Specifically, we averaged the utilities published for children with cystic fibrosis, sickle cell anemia, pediatric HIV-AIDS, medium chain acyl CoA dehydrogenase (MCAD) deficiency and leukemia [20-23].
Analyses
Decision trees and Markov models were solved using TreeAge (Williamstown, MA) assigning costs and QALYs associated with each cycle over a time horizon of 70 years [24, 25]. For each individual in our hypothetical cohort, the total cost and health outcomes were determined by accumulating the costs and utilities associated with each specific health state before and after transplantation, respectively, of each occupied state over time for all cycles [24]. The difference in cost between screening and no screening was divided by the difference in health outcome in QALYs to measure the cost per life year saved, expressed as the incremental cost-effectiveness ratio (ICER). We tested the impact of varying incidence, test sensitivity, test specificity, cost of TREC test, cost of diagnostic testing, and ratio of cost of early versus late HCT (Table 2) using sensitivity analyses. We also determined confidence intervals around ICER estimates and the acceptability curve at different ranges of societal willingness to pay (WTP), using second-order Monte Carlo simulation with triangular distributions of four variables: cost of screening, incidence, test sensitivity and test specificity [26]. We assumed a test specificity and sensitivity of 99% for our base case, examining ranges of values (0.80-1.00) in our sensitivity analyses to determine the robustness of test parameters.
Table 2.
Variables and ranges used in sensitivity analyses
| Variables | Base -Case Value | Analysis Range |
|---|---|---|
| Incidence | 1/75,000 | 1/25,000 -500,000 |
| Screening test performance | ||
| Sensitivity rate | 0.99 | 0.85 – 1.00 |
| Specificity rate | 0.99 | 0.85 – 1.00 |
| Cost | ||
| Screening Test | $ 4.22 | $ 0.50 - 30.00 |
| Diagnostic Test | $ 250 | $ 50 - 1000 |
| HCTlate/HCTearly | 3 | 0.50 - 10 |
| Discount rate | 0.03 |
RESULTS
Survey results to generate probabilities for modeling
Timing of HCT and/or death in 39 SCID infants with and without a known family history is shown in Table 3. Thirty-two interviews captured sporadic SCID cases, with no family history. The infections experienced by sporadic SCID infants at or before diagnosis often compromised their outcome. The average age of diagnosis was 9.0 ± 7.6 months (between 1.4 to 16.6 months of age); 8 did not survive to be considered for HCT or were too ill to receive this treatment (Table 3). One critically ill infant diagnosed at 7 months with ADA deficient SCID died a month later despite receiving PEG-ADA enzyme treatment. Of the 9 of 23 transplanted SCID infants who died, the average age of diagnosis was 6.9 ± S.D. 3.6 months.
Table 3.
Age of clinical events in SCID patients (n=39)*
| Months (mean ± SD) | |||
|---|---|---|---|
| diagnosis | treatment | death | |
| SCID infants identified early+ (n=7) |
1.0 ± 0 | 3.7 ± 4.3 | all alive |
|
| |||
| SCID infants identified late− (n=32) |
9.0 ± 7.6 | 9.6 ± 5.4 | 17.6 ± 10.4% |
| SCID infants with HCT (n=23) |
6.9 ± 5.0 | 9.8 ± 5.5 | 17.3 ± 7.5** |
| SCID infants with no HCT (n=8) |
15.4 ± 10.3 | 19.4 ± 14.0 | |
| SCID infant with PEG- ADA therapy^ (n=1) |
7 | 7 | 8 |
Based on survey responses from physicians and families of SCID children.
Identified early is based on known family history of SCID, prior to manifestation of infections.
Identified late is defined as confirmed with SCID after manifestation of infections.
Twenty out of 32 SCID patients identified late died.
Ten out of the 23 SCID patients transplanted died.
All eight SCID patients without HCT died.
PEG-ADA, polyethylene glycol-modified adenosine deaminase enzyme replacement, specifically for SCID with adenosine deaminase deficiency
In contrast to the sporadic cases, the 7 with a family history of SCID were confirmed affected by 1 month of age, received HCT by 3.7 ± 4.3 months, and are all alive and healthy (Table 3).
Survey responses documented longer average hospitalizations before HCT and during the post-HCT phase for SCID infants identified late (mean of 30 hospital days) compared to infants identified early (14 days). Longer hospital stays increased the cost of hospitalization and other medical and non-medical costs. Data from sporadic SCID subjects were used to develop Markov Model A, while data from familial cases, whose early diagnosis would also be achieved by newborn screening, were used to develop Model B.
Projected cost for both strategies and life years saved by screening
The total cost, life years saved, and QALYs saved in screening vs. no screening scenarios were discounted and calculated for the model as described at a 70-year time horizon (Table 4). The discounted incremental cost-effectiveness ratio (ICER) was $25,429/life year and $27,907/QALY. Implementation of the TREC assay to screen for SCID would cost $22.4 million with a gain of 880 life-years and 802 QALYs. The average infant screened would experience 28.684737 discounted life years or 28.684708 discounted QALYs. Without screening, the average infant would lose 0.000214 life year or 0.000195 QALYS. The incremental cost of screening was predicted to be $5.44 per infant.
Table 4.
Base case cost -effectiveness and projected health outcomes measures: cost, life year saved, and quality-adjusted life year (QALYs) saved at time horizon of 70 years
| Variables | Both cost and effectiveness | US screening program for SCID * |
|---|---|---|
| Cost ($) | ||
| no screen | $8.892548 | |
| screen | $14.334449 | |
| incremental | $5.441901 | |
| Cost | $ 22,377,379 | |
| Effectiveness (life years) | ||
| no screen | 28.684523 | |
| screen | 28.684737 | |
| incremental | 0.000214 | |
| life year | 880 | |
| Effectiveness (QALYs) | ||
| no screen | 28.684513 | |
| screen | 28.684708 | |
| incremental | 0.000195 | |
| QALYs | 802 | |
| ICER ($ / life year saved) | $25,429 | |
| ICER ($ / QALY saved) | $27,907 | |
US cohort included 4,112,052 births in 2003;
2003 US dollar, life year and QALY were discounted at 3%
Sensitivity analyses
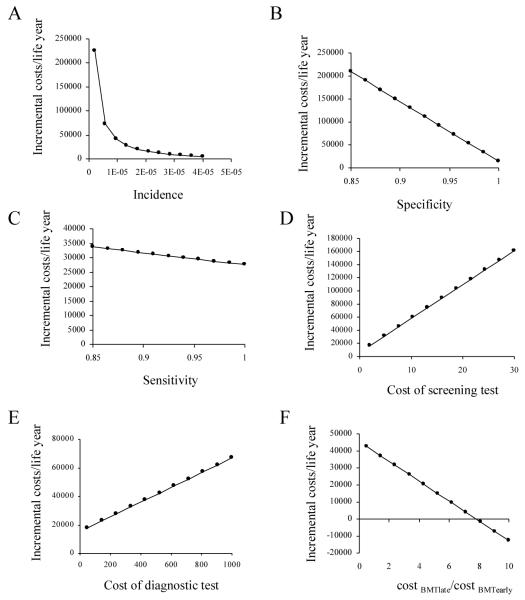
One-way and two-way sensitivity analyses were performed using ranges for the numeric values in Table 4 of key parameters: SCID incidence, screening test cost, specificity and sensitivity, cost of diagnostic testing, and ratio of cost of early vs. late HCT. One-way examples are shown in Fig. 4. Sensitivity analyses also determined the parameter values at which the cost per life year gained equaled a preferred willingness to pay (WTP). Threshold values for the key parameters are shown in Table 5. For example, assuming WTP of $50,000/QALY, screening was preferred if the SCID incidence is at least 1/250,000 (Fig. 4A). When cost of late HCT is 7.5 times as much as early HCT, screening is the preferred strategy to minimize costs and also improve life expectancy, all other factors being equal (Fig. 4F). Thus, screening appears to be dominant and cost-saving in all scenarios where WTP is less than $50,000/QALY.
Figure 4.
One-way sensitivity analysis of incremental cost per quality-adjust life year (QALYs) as function of A) prevalence, B) test sensitivity, C) test specificity, D) cost of screening test and E) cost of confirmation and diagnostic testing; and F) the ratio of cost of BMTlate vs. BMTearly (cost BMTlate/cost BMTearly).
Table 5.
Estimated threshold values of variables given different societal willingness to pay per QALY
| Willingness to pay per QALY | ||||
|---|---|---|---|---|
| Variable | $25,000 | $50,000 | $100,000 | $150,000 |
| incidence | 0.0000149 | 0.0000086 | 0.0000051 | 0.0000039 |
| cost of screening test |
$3.62 | $8.46 | $18.14 | $27.82 |
| cost of diagnostic test |
$189.80 | $673.81 | $1641.80 | $2609.80 |
| specificity | 0.992 | 0.973 | 0.934 | 0.896 |
| sensitivity | 0.99 | 0.610 | 0.329 | 0.228 |
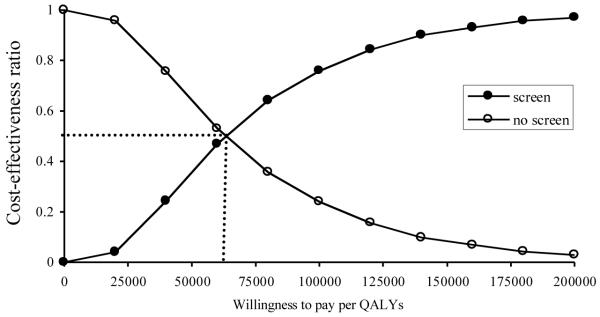
Finally, we conducted a Monte Carlo simulation with 10,000 runs to examine the likelihood of preferring screening as a function of a decision maker’s willingness to pay for the benefits, WTP/QALYs (Fig. 5). We found that $63,000 was the point of indifference where the likelihoods of preferring screening and non-screening were equal (where cost-effective proportion is 0.50). If society is willing to pay $100,000 per QALY, newborn screening for SCID would have a 78% likelihood of being preferred.
Figure 5.
Ranges of uncertainty given at a range of different willingness to pay per QALY is illustrated by a cost-effectiveness acceptability curve. Estimates for the variables were varied from a distribution at random for incidence, cost of screening test, sensitivity and specificity. Dotted line indicates the cross-point of the willingness to pay per QALY at 0.50 cost effective.
DISCUSSION
Our study shows that screening for SCID is likely to be cost-effective because the condition is rare, limiting the overall number of infants requiring treatment, and because of better health outcomes and lower costs associated with earlier HCT. Cost effectiveness of screening for SCID compares favorably with cost-effectiveness of other health interventions: $28,000 per QALY (based on the initial assumptions) would be considered moderately or highly favorable based on the scale proposed by Weinstein et al[27]. Screening appeared cost-effective for test costs up to $50, if all other variables are constant. Our estimates are similar to ICER between no screening and universal screening for other newborn metabolic diseases (ICER of $5,800 to $42,000/life years) or sickle cell anemia (ICER of $13,000/life years saved) [28, 29].
Incidence of disease and specificity of the screening test greatly influence cost-effectiveness. As expected, our model showed that higher incidence predicted greater cost-effectiveness of screening. Cost effectiveness was also sensitive to test specificity: ICER rose beyond $50,000 when specificity was less than 0.94. However, the range of acceptable test specificity was dependent on the underlying incidence of SCID. In summary, factors influential to the cost-effectiveness are the cost and specificity of the test and diagnostics, the incidence of disease (at extremes), and the improved health outcomes.
McGhee et al. previously examined potential costs and benefits of newborn screening for SCID using a hypothetical two-tiered testing protocol based on a proposed IL-7 assay (which to date does not have analytical validity) and a TREC assay [30]. They suggested an initial IL-7 assay followed by TRECs for the 4% of positive IL-7 specimens. This strategy was estimated to be 99.6% sensitive, 99.1% specific, and cost effective if test costs were less than $5. Our results create an even stronger case for SCID newborn screening by TREC assay alone, which we predict to be cost-effective with a test specificity as low as 94% with a similar test cost of $4.22.
Furthermore, because we modeled the natural history of SCID using stochastic probabilities to represent real-life case scenarios of late (Model A) vs. early (Model B) diagnosis, we could incorporate the reduced costs attributable to screening of needing less care in pre-diagnosis and pre-HCT states as well as avoiding the infections that increased hospitalization duration. Finally, unlike McGhee’s deterministic model based on an average life-span of the individual with SCID, our model considered the magnitude of reduced care costs over time. Both models reached the conclusion that screening for SCID is cost-effective.
Modeling requires simplifying assumptions about costs of testing and healthcare that could impact the validity of our results. We considered only laboratory costs of SCID screening integrated into an existing screening program without additional administrative services, sample collection or follow-up. Assumptions were also made in classifying the course of SCID using a limited number of health states and a single HCT. In reality, early and late HCT costs vary between institutions and depend on many patient and donor factors. However, our analysis tended to minimize the differences between early and late HCT, but still predicted newborn screening for SCID to be cost effective. When more specific actual data on costs and outcomes are available, these can be used to refine our model. Although our survey provided valuable insights and was in agreement with published outcome information from several studies [31-33], our set of subjects may not have represented SCID patients overall. To minimize these limitations we used conservative values for key variables affecting the cost effectiveness of screening and explored the impact of varying these estimates using sensitivity analyses.
Our modeling indicates that the cost-effectiveness of TREC screening for SCID compares favorably with screening programs for other rare conditions as well as common diseases such as prostate cancer [34]. The benefits may be enhanced because the TREC assay detects non-SCID T cell lymphocytopenias in addition to SCID, such as DiGeorge syndrome, which are detected by low TRECs, as described in the first year of statewide TREC screening in Wisconsin [31]. These disorders would be differentiated from each other and from SCID through the confirmatory testing process and early intervention could lead to better outcomes. Indeed, immunologists agree that profound T lymphocytopenia from any cause places infants at risk for severe infections [35]; early diagnosis through screening could prevent infections through use of prophylactic antibiotics and immunoglobulin as well as avoidance of live attenuated rotavirus vaccine, a cause of infectious diarrhea in immunocompromised infants [32].
Our approach can be generalized for modeling natural histories of subject cohorts along multiple alternative paths in other health conditions. Markov models reflect different possible health states and incorporate information about cohort histories into decision analysis, making possible evaluation of cost effectiveness of interventions that have complex influences on disease course and healthcare costs.
Implementation of neonatal screening for SCID has evolved rapidly. In 2007, a SCID working group convened to discuss requirements for large-scale studies [36]. Wisconsin initiated the first state-wide trial of TREC screening, finding excellent sensitivity, while specificity, particularly in preterm infants, could benefit from refinement [37]. Recent Wisconsin TREC assay specificity has increased to 99.983% at an average cost of $6.00 per sample [38]. In 2009, Massachusetts began a state-wide pilot of TREC screening and has reported successful identification of a patient with SCID [9, 11]. Based on an evidence review [39], recommendation of an expert advisory committee, and the recommendation of the Department of Human and Health Serivces Secretary that SCID be added to the uniform panel of screened contitions, California, New York, Louisiana, Puerto Rico and other States are conducting SCID screening, and additional cases of SCID and T cell disorders have been found [J. Puck, unpublished data]. Follow up of the outcomes of these programs will provide direct information to evaluate cost effectiveness of SCID screening and to refine the model presented here.
Acknowledgement
Funds for this work came from the NHGRI, NIH, Division of Intramural Research, USIDNET grant NO1-AI-30070 to JMP and the Jeffrey Modell Foundation. We are grateful to the Immune Deficiency Foundation SCID Initiative and SCID.net for posting our survey online and to the parents of SCID children who recounted and re-lived their experiences for us in the hope that future SCID children would benefit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Buckley RH. Primary cellular immunodeficiencies. Journal of Allergy & Clinical Immunology. 2002;109:747–757. doi: 10.1067/mai.2002.123617. [DOI] [PubMed] [Google Scholar]
- [2].Campbell E, Ross LF. Parental attitudes regarding newborn screening of PKU and DMD. American Journal of Medical Genetics. Part A. 2003;120:209–214. doi: 10.1002/ajmg.a.20031. [DOI] [PubMed] [Google Scholar]
- [3].Howell RR. Introduction: newborn screening. Ment Retard Dev Disabil Res Rev. 2006;12:229. doi: 10.1002/mrdd.20128. [DOI] [PubMed] [Google Scholar]
- [4].Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, Veys P, Gennery AR, Gaspar HB. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117:3243–3246. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- [5].Buckley RH. Advances in the understanding and treatment of human severe combined immunodeficiency. Immunologic Research. 2000;22:237–251. doi: 10.1385/IR:22:2-3:237. [DOI] [PubMed] [Google Scholar]
- [6].Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–878. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- [7].Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–398. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [8].Baker MW, Laessig RH, Katcher ML, Routes JM, Grossman WJ, Verbsky J, Kurtycz DF, Brokopp CD. Implementing routine testing for severe combined immunodeficiency within Wisconsin’s newborn screening program. Public Health Rep. 2010 Nov;125(Suppl 2):88–95. doi: 10.1177/00333549101250S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Comeau HJ, Pai SY, Bonilla FA, Notarangelo LD, Pasternack MS, Meissner HC, Cooper ER, DeMaria A, Sahai I, Eaton RB. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. J Inherit Metab Dis. 2010;33:S273–281. doi: 10.1007/s10545-010-9103-9. AM. [DOI] [PubMed] [Google Scholar]
- [10].Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, Comeau AM. Identification of an infant with severe combined immunodeficiency by newborn screening. J Allergy Clin Immunol. 2010;126:1073–1074. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- [11].Gerstel-Thompson WJ, Baptiste JC, Navas JS, Pai SY, Pass KA, Eaton RB, Comeau AM. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem. 2010;56:1466–1474. doi: 10.1373/clinchem.2010.144915. JL. [DOI] [PubMed] [Google Scholar]
- [12].Baker GW, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, Cogley MF, Litsheim TJ, Katcher ML, Routes JM. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:522–527. doi: 10.1016/j.jaci.2009.04.007. MW. [DOI] [PubMed] [Google Scholar]
- [13].McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr. 2005;147:603–608. doi: 10.1016/j.jpeds.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [14].Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- [15].Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, Myers LA, Ward FE. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- [16].Griffith LM, Cowan MJ, Kohn DB, Notarangelo LD, Puck JM, Schultz KR, Buckley RH, Eapen M, Kamani NR, O’Reilly RJ, Parkman R, Roifman CM, Sullivan KE, Filipovich AH, Fleisher TA, Shearer WT. Allogeneic hematopoietic cell transplantation for primary immune deficiency diseases: current status and critical needs. J Allergy Clin Immunol. 2008;122:1087–1096. doi: 10.1016/j.jaci.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- [18].U.S.N.S.S.R.A.T. 2004 [Google Scholar]
- [19].US Census Bureau . Statistical Abstract of the United States. US Census Bureau; Washington DC: 2006. [Google Scholar]
- [20].Venditti LN, Venditti CP, Berry GT, Kaplan PB, Kaye EM, Glick H, Stanley CA. Newborn screening by tandem mass spectrometry for medium-chain Acyl-CoA dehydrogenase deficiency: a cost-effectiveness analysis. Pediatrics. 2003;112:1005–1015. doi: 10.1542/peds.112.5.1005. [DOI] [PubMed] [Google Scholar]
- [21].Panepinto JA, O’Mahar KM, DeBaun MR, Loberiza FR, Scott JP. Health-related quality of life in children with sickle cell disease: child and parent perception. Br J Haematol. 2005;130:437–444. doi: 10.1111/j.1365-2141.2005.05622.x. [DOI] [PubMed] [Google Scholar]
- [22].Haberman M, Bush N, Young K, Sullivan KM. Quality of life of adult long-term survivors of bone marrow transplantation: a qualitative analysis of narrative data. Oncol Nurs Forum. 1993;20:1545–1553. [PubMed] [Google Scholar]
- [23].Koscik RL, Douglas JA, Zaremba K, Rock MJ, Splaingard ML, Laxova A, Farrell PM. Quality of life of children with cystic fibrosis. J Pediatr. 2005;147:S64–68. doi: 10.1016/j.jpeds.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [24].Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- [25].Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- [26].Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- [27].Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- [28].Tsevat J, Wong JB, Pauker SG, Steinberg MH. Neonatal screening for sickle cell disease: a cost-effectiveness analysis. J Pediatr. 1991;118:546–554. doi: 10.1016/s0022-3476(05)83375-x. [DOI] [PubMed] [Google Scholar]
- [29].Panepinto JA, Magid D, Rewers MJ, Lane PA. Universal versus targeted screening of infants for sickle cell disease: a cost-effectiveness analysis. J Pediatr. 2000;136:201–208. doi: 10.1016/s0022-3476(00)70102-8. [DOI] [PubMed] [Google Scholar]
- [30].McGhee SA, Stiehm ER, Cowan M, Krogstad P, McCabe ER. Two-tiered universal newborn screening strategy for severe combined immunodeficiency. Mol Genet Metab. 2005;86:427–430. doi: 10.1016/j.ymgme.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [31].Roifman CM, Somech R, Grunebaum E. Matched unrelated bone marrow transplant for T+ combined immunodeficiency. Bone Marrow Transplant. 2008;41:947–952. doi: 10.1038/bmt.2008.11. [DOI] [PubMed] [Google Scholar]
- [32].Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, Sempowski GD, Buckley RH. Thymic output, T cell diversity and T cell function in long-term human SCID chimeras. Blood. 2009;114:1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Titman P, Pink E, Skucek E, O’Hanlon K, Cole TJ, Gaspar J, Xu-Bayford J, Jones A, Thrasher AJ, Davies EG, Veys PA, Gaspar HB. Cognitive and behavioral abnormalities in children after hematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–3913. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
- [34].Benoit RM, Gronberg H, Naslund MJ. A quantitative analysis of the costs and benefits of prostate cancer screening. Prostate Cancer Prostatic Dis. 2001;4:138–145. doi: 10.1038/sj.pcan.4500510. [DOI] [PubMed] [Google Scholar]
- [35].Routes GW, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, Baker MW. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302:2465–2470. doi: 10.1001/jama.2009.1806. JM. [DOI] [PubMed] [Google Scholar]
- [36].Puck JM. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120:760–768. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- [37].Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS. From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117:923–929. doi: 10.1542/peds.2005-0553. [DOI] [PubMed] [Google Scholar]
- [38].Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, Cogley MF, Litsheim TJ, Katcher ML, Routes JM. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [39].Lipstein VS, Browning MF, Green NS, Kemper AR, Knapp AA, Prosser LA, Perrin JM. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125:1226–1235. doi: 10.1542/peds.2009-1567. EA. [DOI] [PubMed] [Google Scholar]