A patient with chronic moderate neutropenia, acute hemolysis, and pyrexia was found to be infected with a novel hemoplasma species. A clinical response to doxycyline was noted, and moxifloxacin was added subsequently to aid infection clearance. This represents the first report of hemolysis in association with confirmed hemoplasma infection in a human.
Abstract
A patient with chronic moderate neutropenia, acute hemolysis, and pyrexia was found to be infected with a novel hemoplasma species. A clinical response to doxycyline was noted, and moxifloxacin was added subsequently to aid infection clearance. This represents the first report of hemolysis in association with confirmed hemoplasma infection in a human.
A 62-year-old white woman from Devon, England, presented with a 3-week history of pyrexia, abdominal pain, joint pain, weight loss, and night sweats, starting when she returned home from vacation in Australia and Singapore. In Australia she had swum in a freshwater tropical pond and had fed kangaroos with other tourists. She recalled no tick, insect, or animal bites and kept a dog at home. She had a 3-year history of moderate neutropenia (cell count, 0.7–1.2 × 109 cells/L) and distal symmetrical polyarthralgia but was otherwise fit and well.
On examination she was pyrexial (temperature, 39.4°C) with hepatosplenomegaly. Full blood count showed anemia (hemoglobin concentration, 97 g/L), a reduced white blood cell (WBC) count (WBC count, 1.8 × 109 cells/L; neutrophil count, 0.5 × 109 cells/L), and thrombocytopenia (platelet count, 81 × 109 cells/L). A direct antiglobulin (Coombs') test was strongly positive for immunoglobulin G (IgG) and complement C3d, and low haptoglobin concentrations (<0.06 g/L; reference interval, 0.3–2.0 g/L) were found, confirming hemolytic anemia. A clotting screen was within the reference interval, and no hemoglobinuria was detected. The C-reactive protein (CRP) level (94 mg/L; reference interval, <5 mg/L) and lactate dehydrogenase level (794 U/L; reference interval, 240–480 U/L) were raised. Liver and renal parameters were within the reference intervals except for a mildly elevated aspartate transaminase level (43 U/L; reference interval, 0–31 U/L). Serological tests for hepatitis B and C virus were negative. Antinuclear and smooth muscle antibody tests were positive (concentrations, 1:160 and 1:80, respectively), whereas mitochondrial antibody testing was negative. Blood smears showed no evidence of micro-organisms. Blood cultures were negative and a transthoracic echocardiogram was normal.
Ultrasound-guided liver biopsy to investigate persistent pyrexia and hepatosplenomegaly resulted in a hepatic arterial bleed, which was refractory to radiological embolization and required laparotomy to achieve hemostasis. Liver histology revealed no specific etiological features. Continuing postoperative pyrexia was then attributed to infection of a hepatic subcapsular hematoma, but was refractory to co-amoxiclav and metronidazole, and subsequently to vancomycin and piperacillin-tazobactam, given to broaden coverage against possible infection with hospital-acquired pathogens.
During the postoperative period the patient developed renal failure. Renal biopsy revealed acute tubular interstitial nephritis with focal granuloma formation and was negative for acid-fast bacilli and fungi. Tests for antineutrophil cytoplasmic antibody (concentration, 1:1280) and proteinase 3 antibody (weak) were positive. Systemic lupus erythematosus was considered, but insufficient features were present to make a diagnosis; although the patient was positive for antinuclear antibody (ANA), cytopenias, and arthralgia, both double-stranded DNA and lupus anticoagulant tests were negative, and there was no serositis, oral ulcers, photosensitivity, pre-existing renal disorder, neurological disorder, malar, or discoid rash. Nonetheless, prednisolone (40 mg once daily) was started in view of possible sarcoidosis or other immune disorder. Piperacillin-tazobactam and doxycycline (100 mg once daily orally) for 5 days were also started at this time as empirical treatment for hospital-acquired pneumonia, diagnosed due to fever, inspiratory crepitations, and shadowing in the right base on chest radiography. Blood and sputum cultures were negative. The pyrexia resolved within 24 hours and on resolution of the pneumonia, recovery of the platelet count, and no immediate need for further transfusion over 5 days, the patient was discharged and continued taking prednisolone.
Twenty-one days after cessation of the piperacillin-tazobactam and doxycycline, the patient represented with light-headedness, nausea, petechiae on the legs, and pyrexia. Anemia (hemoglobin concentration, 89 g/L) and thrombocytopenia (platelet count, 31 × 109 cells/L) were present but the WBC count was within the reference interval (WBC count, 7.3 × 109 cells/L; neutrophil count, 4.3 × 109 cells/L). The prednisolone dose was increased (60 mg once daily) and azathioprine (50 mg once daily) was initiated, but continued hemolysis (positive Coombs' test positive and low haptoglobin level) necessitated multiple transfusions (14 units over 14 days). Treatment with IgG (1 g/kg) was ineffective. Bone marrow cellularity was normal with brown pigment (likely hemosiderin) and an absence of hemophagocytosis. A Gallium scan showed prominent tracer uptake throughout the axial skeleton but no hepatosplenic uptake. Magnetic resonance imaging showed diffuse low signal intensity of the bone marrow on T1-weighted images, suggesting myeloproliferative disease. A further bone marrow analysis revealed hemophagocytosis only. Testing for acid-fast bacilli, Epstein Barr virus, cytomegalovirus, and Leishmania species (spp) by means of polymerase chain reaction (PCR) and serology, were all negative.
Ongoing hemolysis and pyrexia with a temperature of up to 40°C resulted in initiation of doxycycline treatment (100 mg twice daily orally), this time as monotherapy for possible nonculturable intracellular pathogens. After starting this treatment, the pyrexia resolved within 24 hours, the thrombocytopenia resolved within 4 days, and the hemoglobin concentration (112 g/L) was within the reference interval within 1 week. Bone marrow, collected during the clinical relapse, was subjected to eubacterial 16S ribosomal RNA (rRNA) gene PCR to identify any yet unidentified bacterial infections. Sequence data of an amplified PCR product showed greatest identity (92.1% and 89.9%, respectively) to the veterinary hemotropic mycoplasma species (trivial name hemoplasma) Mycoplasma haemomuris (GenBank no. U82963) and “Candidatus Mycoplasma turicensis” (GenBank no. DQ464423).
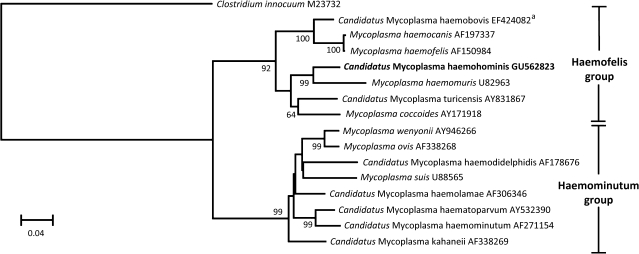
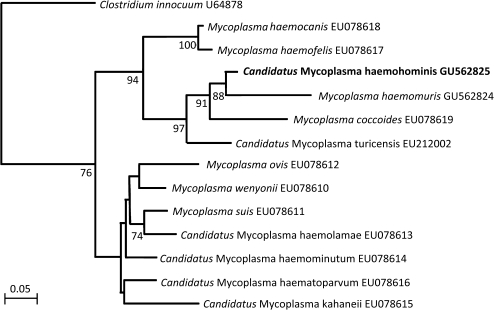
On the basis of treatment recommendations for veterinary hemoplasmosis, doxycycline was continued for 3 weeks. The patient remained well during this time, and the azathioprine was stopped and the prednisolone gradually withdrawn. However, 17 days after stopping doxycycline the patient deteriorated again with pyrexia, sweats, vomiting, and shortness of breath. Hemolysis was confirmed as before with painful splenomegaly. Repeat bone marrow samples were collected, which showed macrophage inclusions consistent with hemolysis. Doxycycline (100 mg twice daily orally) was restarted and the patient again improved immediately; temperature normalized within 24 hours and the hemogram normalized within a week. The bone marrow was negative for Mycoplasma and Ureaplasma spp by culture, but was again positive for hemoplasma DNA, this time demonstrated by panhemoplasma quantitative PCR (qPCR) [1]. PCR, sequencing, and phylogeny of the near-complete 16S rRNA and partial RNase P RNA genes (GU562823 and GU562825, respectively) confirmed the hemoplasma species to be novel (Figures 1 and 2).
Figure 1.
Phylogenetic analysis of nearly complete 16S ribosomal RNA (rRNA) gene sequences for the newly described hemoplasma species (shown in boldface) and other available hemoplasma species. The tree was constructed by the neighbor-joining method. Evolutionary distances are to the scales shown. The data set was resampled 1000 times to generate bootstrap percentage values, and values of >50% are given at the nodes of the tree. The phylogenetic tree was rooted to Clostridium innocuum (GenBank no. M23732). GenBank accession numbers are shown for all sequences. aThe bovine hemoplasma “Candidatus Mycoplasma haemobovis” 16S rRNA gene sequence (GenBank no. EF424082) shares 99.3% identity with the 1204 base pairs of the 16S rRNA gene sequence of the buffalo hemoplasma “Candidatus Mycoplasma haemobos” (GenBank no. EU367965).
Figure 2.
Phylogenetic analysis of partial RNase P RNA gene (rnp) gene sequences for the newly described hemoplasma species (shown in boldface) and other available hemoplasma species. The phylogenetic tree was rooted to Clostridium innocuum (GenBank no. U64878). The tree was constructed by the neighbor-joining method. Evolutionary distances are to the scales shown. GenBank accession numbers are shown for all sequences. The data set was resampled 1000 times to generate bootstrap percentage values, and values of >50% are given at the nodes of the tree.
Doxycycline treatment was continued and qPCR regularly used to monitor the amount of hemoplasma DNA in the blood (Table 1). Because blood was still qPCR positive after 8 weeks of doxycycline (albeit at low levels), moxifloxacin (400 mg once daily) was added as a quinolone with excellent activity against mycoplasmas. The patient remained taking dual doxycycline and moxifloxacin treatment for 6 months, during which time she had neutropenia (cell count, 0.6–1.6 × 109 cells/L) with normal hemoglobin concentration and reticulocyte count; low levels of hemoplasma DNA were intermittently detected by the panhemoplasma qPCR. Treatment was then stopped and the patient was monitored by means of blood panhemoplasma qPCR testing, which was negative throughout follow-up. The posttreatment ANA concentration was 1:80, ds DNA antibody testing was negative, and the CRP level was normal. One year following cessation of treatment the patient remains well, other than ongoing polyarthralgia and low WBC count. Blood from the patient’s husband and pet dog were both negative for hemoplasma DNA by qPCR.
Table 1.
Samples Analyzed, Quantitative Polymerase Chain Reaction Results, Clinical Details, and Treatment Details
| Time (months)relative to initialpresentation | Sample type | Panhemoplasma qPCR results:absolute no. of hemoplasma copies per PCR [GAPDH Ct value] | Clinical résumé and/or treatment |
| 2 | Serum | 5.8 x 103 [30.1] | Pyrexia, pancytopenia, hepatosplenomegaly |
| 5 | Bone marrow | 4.9 x 106 [21.8] | Pyrexia, hemolysis |
| 5.25 | Serum | 1.2 x 105 [27.8] | |
| 6 | Serum | Not detected [37.5] | DoxycyclineWithdrawal of prednisolone |
| 7 | Bone marrowa | 4.7 x 104 [26.2] 6.1 x 105 [19.1] |
Pyrexia, splenomegaly, hemolysis |
| 8 | Blood | <10 [21.7] | Doxycycline |
| 8.5 | Blood Bone marrow |
<10 [22.7] <10 [21.6] |
Doxycycline & moxifloxacin |
| 9.5 | Blood | <10 [22.1] | |
| 10.5 | Blood | Not detected [21.5] | |
| 11.5 |
Blood Bone marrow |
<10 [21.9] Not detected [19.7] |
|
| 12.5 | Blood | <10 [23.2] | |
| 13 | Blood | Not detected [24.5] | |
| 14.5 | Blood | Not detected [23.2] | Asymptomatic |
| 14.75 | Blood | Not detected [24.1] | |
| 15 | Blood | Not detected [21.8] | |
| 16 | Blood | Not detected [26.1] | |
| 18 | Blood | Not detected [22.3] | |
| 19 | Blood | Not detected [23.8] |
Abbreviations: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control [1]. The threshold cycle (Ct) number was obtained by quantitative polymerase chain reaction (qPCR) for the GAPDH internal control. The qPCR results for samples of different origin cannot be directly compared with each other due to differences in dilution, collection medium, and volume used for DNA extraction. However, the hemoplasma copy numbers shown represent those present per PCR, which equates to number of copies per 5 μL of blood, bone marrow, or serum, assuming complete efficiency of DNA purification and PCR amplification. Stored samples were analyzed retrospectively. Because hemoplasma organisms are erythrotropic, PCR analysis of samples containing erythrocytes (blood and bone marrow) is usually preferred over that of serum samples. However, some serum samples generated positive hemoplasma PCR results in the current case report. We believe this likely reflects the very high numbers of organisms present in the blood at the time of serum preparation, meaning that detachment of even a small number of organisms from erythrocytes allowed their detection in the serum by PCR.
Two different preservatives (Tryptic Soya Broth and Viral Transport Media) were used for bone marrow collection, and qPCR was performed on both, giving the 2 results shown.
During the prolonged hospitalization, serial blood and urine cultures were negative. Serological tests for human immunodeficiency virus (HIV), Toxoplasma gondii IgM, Coxiella burnetii, Brucella abortus, Burkholderia pseudomallei, Anaplasma phagocytophilum, Ehrlichia chaffeensis, Borrelia burgdorferi, Tropheryma whipplei, Histoplasma capsulatum, Rickettsia conorii, Rickettsia typhi, Rickettsia felis, and Francisella tularensis were all negative. Serological tests for Chlamydophila psittaci (concentration, 1:128) and Chlamydophila pneumoniae (concentration, 1:1500) were positive but remained unchanged on repeat sampling and were assumed to represent previous Chlamydophila infection. In particular, Bartonella henselae, Bartonella quintana, Bartonella genus-specific PCR, Mycoplasma pneumoniae serology, and mycoplasma agglutination tests were all negative.
Immune causes of the neutropenia in the years preceding presentation were considered. There were fluctuating titers of ANA levels (1:80–1:160), but the anti–ds DNA tests were consistently negative from 4 years prior to presentation and throughout the current febrile illness. Although the patient was neutropenic on initial presentation, during her hospital stay, her WBC count increased to within the reference range and, on occasions, above the reference range (as did the neutrophil count) until discharge. During and after treatment for hemoplasma infection, the WBC and neutrophil counts decreased to levels seen before infection.
Discussion
This case represents the first report of a novel hemoplasma species in a human in association with doxycycline-responsive pyrexia, hemolytic anemia, and a history of chronic moderate neutropenia. The name “Candidatus Mycoplasma haemohominis” is proposed for this novel organism should further characterization be possible. This case is unique in several ways: first in describing human infection with a novel hemoplasma species that is distinct from described veterinary hemoplasma species, second in associating human hemoplasma infection with clinically significant hemolysis, and third in the use of qPCR to quantify hemoplasma DNA in human diagnostic samples and thus monitor response to treatment. Diagnosis of hemoplasma infection in this patient was based on PCR analysis of bone marrow–derived DNA, but peripheral blood samples could have been an alternative source of DNA for diagnosis, as performed in veterinary practice [2].
Hemoplasmas infect many mammalian species and can induce life-threatening hemolytic anemia [2]. Human hemoplasma-like infections have been reported occasionally in immunocompromised patients by means of cytological diagnosis, which is known to be very unreliable [1, 3]. PCR methods are now used to investigate human hemoplasma infections. Limited human epidemiological studies have failed to detect infections [1, 4]. Although human hemoplasma infections have been reported in China [5, 6], these descriptions have not described clinical disease, PCR methodology, or infecting species, making interpretation difficult. Other studies have described the presence of existing veterinary hemoplasma species DNA in humans: Mycoplasma suis [7, 8], Mycoplasma haemofelis and/or Mycoplasma haemocanis [9, 10], and Mycoplasma ovis [11]. However, any association between hemoplasma infection and clinical signs in these reports is difficult to ascertain due to either lack of clinical information provided [7, 8, 10] or concurrent B. henselae infection [9, 11]. It is unlikely that co-infection with Bartonella spp was present in this case due to the negative serological and PCR results for Bartonella spp obtained and the fact that only hemoplasma DNA was detected by 16S rDNA PCR. It is possible that the patient’s neutropenia played a role in the initial development of clinical hemoplasmosis through immunocompromise. It is less likely that a chronic hemoplasma infection played a role in causing neutropenia, as evidenced by the long history of neutropenia prior to any illness and return to low WBC counts with effective treatment and clearance of infection on specific qPCR, but this remains a possibility.
The origin of the hemoplasma infection in this patient is not known. It is possible that it represents a zoonotic infection acquired, for example, from blood-sucking arthropods such as ticks, fleas, and lice [2] or by direct transmission from another mammalian host through parenteral or oral inoculation of blood [3], but no such transmission events were recalled by the patient before onset of disease. It is possible that this novel hemoplasma represents a species of which the primary host is humans. Hemoplasma infections exist in veterinary species in both the United Kingdom and Australia so transmission could have occurred before, during, or after the patient’s vacation. Besides the patient’s husband and pet dog (both of which were tested for hemoplasma DNA) there was no other significant animal or mammal contact reported.
As described elsewhere for veterinary hemoplasma infections [3], the patient described here showed a remarkable clinical response to doxycycline. Subsequently, a 6-month course of doxycycline and moxifloxacin was associated with negative qPCR results for hemoplasma DNA, and the patient then remained qPCR negative following cessation of treatment. The low levels of hemoplasma DNA detected by qPCR at some time points during doxycycline and moxifloxacin treatment could reflect amplification of nonviable hemoplasma DNA that had not been cleared from the circulation, although in animal studies rapid clearance of hemoplasma DNA from the circulation is usually reported following effective antibiotic treatment.
In conclusion, hemoplasmosis should be considered as a differential diagnosis in patients with hemolytic anemia and pyrexia. PCR testing for hemoplasma DNA should be included in the investigation of such patients to enable the rapid detection of this infection, which may be more common than previously realized.
Notes
Acknowledgments.
The patient gave consent to all tests and procedures. Ethics approval from the University of Bristol was obtained for analysis of the human samples. We thank Hal Neimark and Chris Helps for helpful discussions during the preparation of this manuscript, and Sarinder Day, Daniel Thomas, and Andre Buckeridge for technical support.
Financial support.
This work was supported by The Wellcome Trust (grant 077718); the University of Bristol (Postgraduate Research Scholarship to E. N. B.); and Pfizer Animal Health (support for E. N. B.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tasker S, Peters IR, Mumford AD, et al. Investigation of human haemotropic Mycoplasma infections using a novel generic haemoplasma qPCR assay on blood samples and blood smears. J Med Microbiol. 2010;59:1285–92. doi: 10.1099/jmm.0.021691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messick JB. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet Clin Pathol. 2004;33:2–13. doi: 10.1111/j.1939-165x.2004.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 3.Tasker S. Haemotropic mycoplasmas: what’s the real significance in cats? J Feline Med Surg. 2010;12:369–81. doi: 10.1016/j.jfms.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willi B, Meli ML, Luthy R, et al. Development and application of a universal hemoplasma screening assay based on the SYBR Green PCR principle. J Clin Microbiol. 2009;47:4049–54. doi: 10.1128/JCM.01478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Z, Yin J, Shen K, Kang W, Chen Q. Outbreaks of hemotrophic mycoplasma infections in China. Emerg Infect Dis. 2009;15:1139–40. doi: 10.3201/eid1507.090174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang A, Congli Y, Yu F, Hua X. Haemotrophic mycoplasma: review of aetiology and prevalence. Rev Med Microbiol. 2007;18:1–3. [Google Scholar]
- 7.Yuan C, Liang A, Yu F, et al. Eperythrozoon infection identified in an unknown aetiology anaemic patient. Ann Microbiol. 2007;57:467–9. [Google Scholar]
- 8.Yuan CL, Liang AB, Yao CB, et al. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai, China. Am J Vet Res. 2009;70:890–4. doi: 10.2460/ajvr.70.7.890. [DOI] [PubMed] [Google Scholar]
- 9.Santos AP, Santos RP, Biondo AW, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis. 2008;14:1922–4. doi: 10.3201/eid1412.080964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallick CA inventor; Sphingomonas Research Partners, LP, assignee. Specific bacterial inclusions in bone marrow cells indicate systemic lupus erythematosus, and treatment for lupus. US patent: 2007. WO 2007/019415 A2, 15 February 2007. [Google Scholar]
- 11.Sykes JE, Lindsay LL, Maggi RG, Breitschwerdt EB. Human co-infection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J Clin Microbiol. 2010;48:3782–5. doi: 10.1128/JCM.01029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]