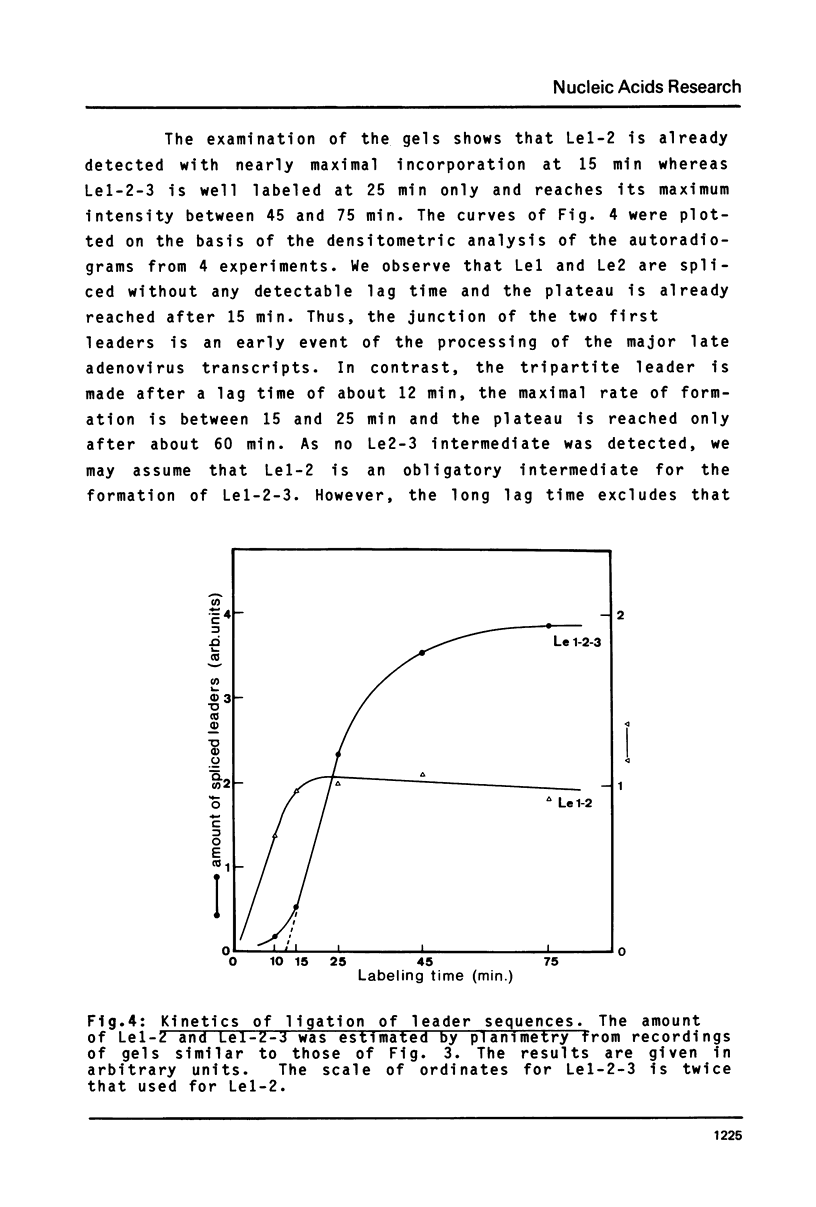
Abstract
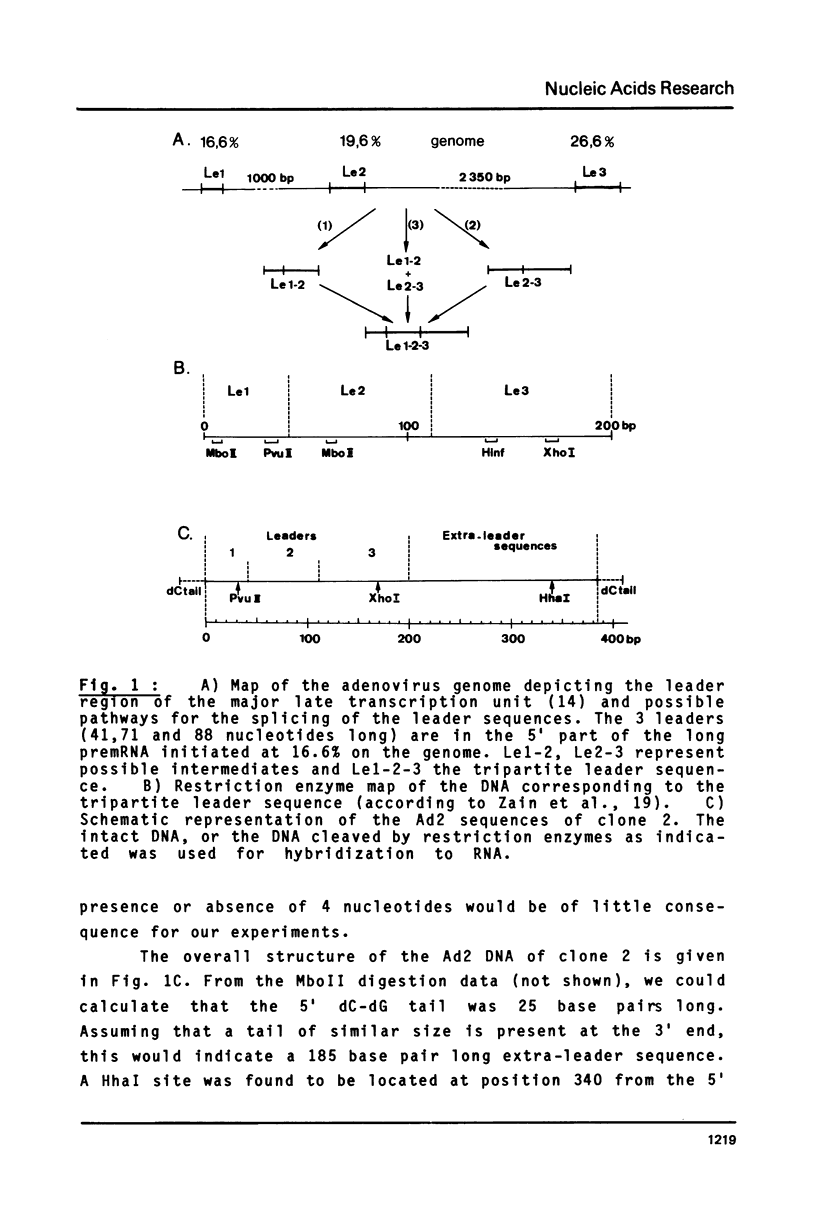
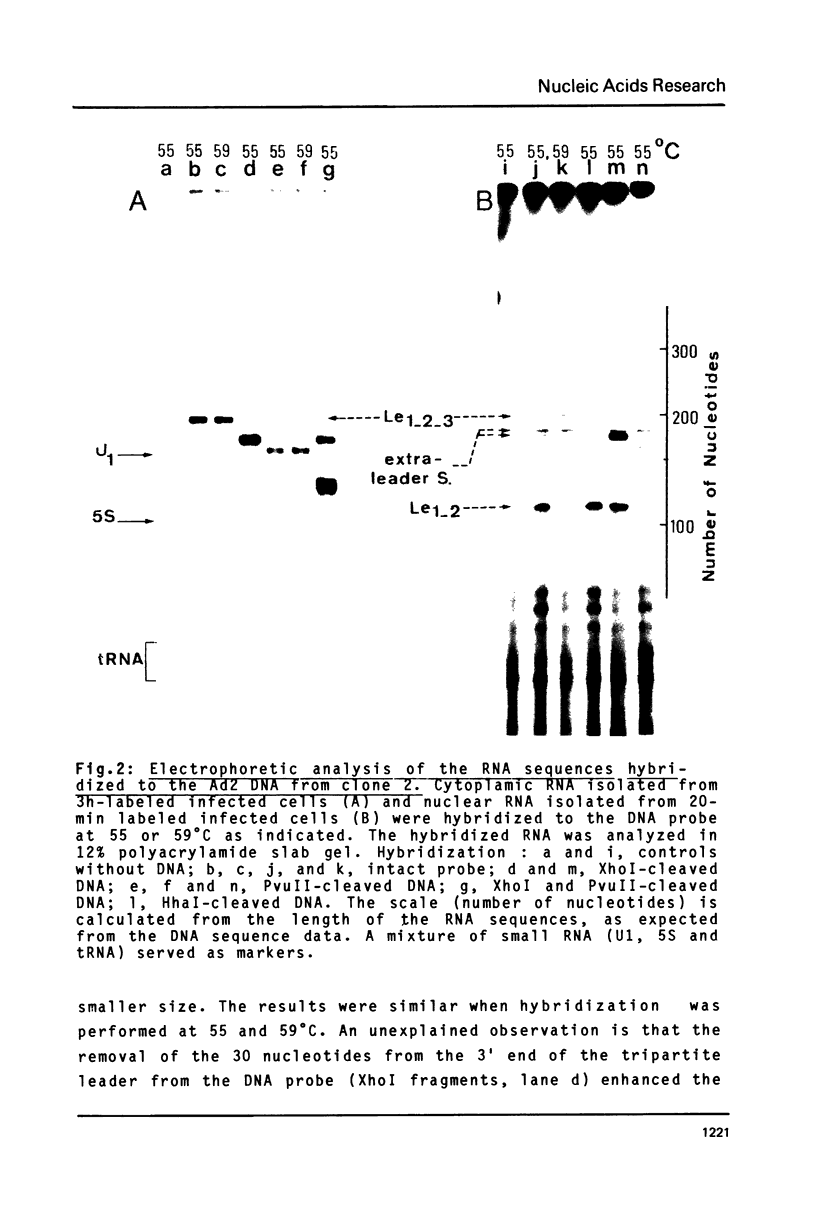
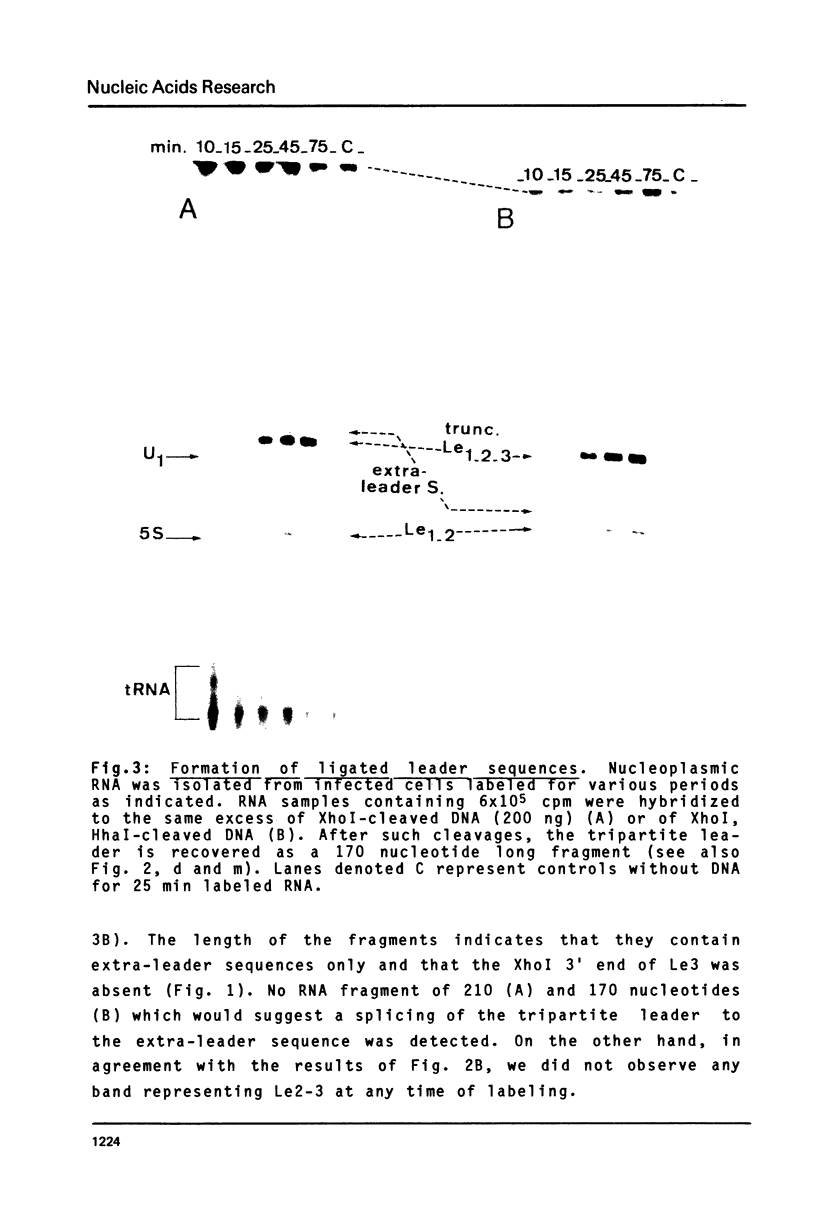
A strategy based on the hybridization of labeled nucleoplasmic RNA to a short cloned cDNA probe was devised to study the ligation of the three first leader sequences (Le1, Le2, Le3) of the major late adenovirus-2 transcript. The hybridized RNA was subsequently fractionated by electrophoresis and identified with the aid of restriction fragments of the DNA probe. The ligations were shown to occur stepwise and in an orderly fashion. Le1 and Le2 were first ligated without detectable lag time. The tripartite leader was formed after a lag time of 10-15 min probably due, for a large part, to the stepwise excision of the intervening sequence between Le2 and Le3. The possible processing intermediate Le2-Le3 was not detected.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Pettersson U. Sequence analysis of adenovirus DNA: complete nucleotide sequence of the spliced 5' noncoding region of adenovirus 2 hexon messenger RNA. Cell. 1979 Apr;16(4):841–850. doi: 10.1016/0092-8674(79)90099-0. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Vogeli G., Yamada Y., Maizel J. V., Jr, Pastan I., de Crombrugghe B. Correlation between splicing sites within an intron and their sequence complementarity with U1 RNA. Cell. 1980 Oct;21(3):689–696. doi: 10.1016/0092-8674(80)90432-8. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Sharp P. A. Structure of late adenovirus 2 heterogeneous nuclear RNA. J Mol Biol. 1979 Apr 25;129(4):547–565. doi: 10.1016/0022-2836(79)90468-6. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Weber J. Nuclear ribonucleoprotein particles from adenovirus infected Hela cells. Mol Biol Rep. 1981 May 22;7(1-3):107–113. doi: 10.1007/BF00778740. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Comparison of the nuclear ribonucleoproteins containing the transcripts of adenovirus-2 and HeLa cell dna. Eur J Biochem. 1980;108(1):203–211. doi: 10.1111/j.1432-1033.1980.tb04713.x. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Varshavsky A. J., Ryskov A. P., Church R. B. On the structural organization of the transcriptional unit in animal chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:869–884. doi: 10.1101/sqb.1974.038.01.089. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Raskas H. J. Splicing patterns of nuclear precursors to the mRNA for adenovirus 2 DNA binding protein. Cell. 1979 Jan;16(1):131–138. doi: 10.1016/0092-8674(79)90194-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Galibert F. Nucleotide sequence of the EcoRI E fragment of adenovirus 2 genome. Nucleic Acids Res. 1981 Mar 11;9(5):1229–1240. doi: 10.1093/nar/9.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Mollov G., Salditt M., Wall R., Sheiness D., Darnell J. E., Jr Origin of mRNA in HeLa cells and the implications for chromosome structure. Cold Spring Harb Symp Quant Biol. 1974;38:891–898. doi: 10.1101/sqb.1974.038.01.091. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Ross J. Processing of the mouse beta-globin mRNA precursor: at least two cleavage-ligation reactions are necessary to excise the larger intervening sequence. Cell. 1979 Aug;17(4):915–921. doi: 10.1016/0092-8674(79)90331-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mory Y. Y., Gefter M. L. Synthesis of messenger RNA-like molecules in isolated myeloma nuclei. Nucleic Acids Res. 1977 Jun;4(6):1739–1757. doi: 10.1093/nar/4.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Processing of late adenovirus nuclear RNA to mRNA. Kinetics of formation of intermediates and demonstration that all events are nuclear. J Mol Biol. 1979 Jun 5;130(4):493–506. doi: 10.1016/0022-2836(79)90436-4. [DOI] [PubMed] [Google Scholar]
- Roskam W. G., Rougeon F. Molecular cloning and nucleotide sequence of the human growth hormone structural gene. Nucleic Acids Res. 1979 Sep 25;7(2):305–320. doi: 10.1093/nar/7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. J., Ting A. C., Nordstrom J. L., Zimmer W., O'Malley B. W. Processing of high molecular weight ovalbumin and ovomucoid precursor RNAs to messenger RNA. Cell. 1980 Nov;22(1 Pt 1):219–230. doi: 10.1016/0092-8674(80)90170-1. [DOI] [PubMed] [Google Scholar]
- Weber J., Blanchard J. M., Ginsberg H., Darnell J. E., Jr Order of polyadenylic acid addition and splicing events in early adenovirus mRNA formation. J Virol. 1980 Jan;33(1):286–291. doi: 10.1128/jvi.33.1.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Gingeras T. R., Bullock P., Wong G., Gelinas R. E. Determination and analysis of adenovirus-2 DNA sequences which may include signals for late messenger RNA processing. J Mol Biol. 1979 Dec 5;135(2):413–433. doi: 10.1016/0022-2836(79)90444-3. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]