Abstract
Background
CD4+ T cells are responsible for the progressive lung damage seen in patients with chronic beryllium disease (CBD), a granulomatous lung disorder in which antigen-specific, Th1-type cytokine-secreting T cells have been characterized. Compared to beryllium (Be)-sensitized subjects, an increased number of Be-responsive T cells are present in the blood of CBD patients.
Objective
The aim of this study was to determine whether the number of Be-specific T cells in blood predicted the development of CBD in a cohort of Be-exposed subjects.
Methods
Using IFN-γ ELISPOT and proliferation-based assays, we determined the frequency and proliferative capacity of Be-responsive T cells in blood.
Results
Compared with the Be lymphocyte proliferation test which detected an abnormal Be-induced proliferative response in 11 of 260 (4.2%) workers from a Be-machining facility, IFN-γ ELISPOT detected a sensitization rate of 10% (χ2 = 55.7; P < 0.0001). A significant positive correlation was also noted between the number of Be-responsive CD4+ T cells in blood and lung of CBD patients. Importantly, the transition from Be sensitization to CBD was associated with an increased number of antigen-specific T cells in blood.
Conclusion
These findings have important implications for Be-induced disease and potentially other immune-mediated disorders, suggesting that the frequency of antigen-specific T cells in blood can serve as a noninvasive biomarker to predict disease development and severity of the Be-specific CD4+ T cell alveolitis.
Clinical implications
These findings suggest that the number of Be-responsive T cells in the circulation can serve as a biomarker of disease progression and as an estimate of the severity of Be-induced lung inflammation.
Keywords: Human, Lung, CD4-Positive T-Lymphocytes, Beryllium, Cytokines, Granuloma, ELISPOT
INTRODUCTION
Chronic beryllium disease (CBD) is an organ-specific immune-mediated disorder characterized by the presence of granulomatous inflammation in the lung.1 Depending on an individual’s exposure and genetic susceptibility, the disorder occurs in 1–18% of beryllium (Be)-exposed workers.2–5 Sensitization to Be is detected by the presence of Be-specific CD4+ T cells in blood which proliferate upon exposure to Be salts in vitro. This proliferative response forms the basis of the beryllium lymphocyte proliferation test (BeLPT), which is the standard assay for the detection of Be sensitization in the workplace.6 Importantly, the BeLPT is incapable of distinguishing between Be sensitization and CBD.7 While sensitization precedes disease, not all Be-sensitized (BeS) subjects progress to CBD, and predicting which individuals will advance is currently not possible.8 Thus, the identification of markers of disease progression would greatly advance our understanding of the immunopathogenesis of Be-induced disease as well as impact patient care.
Despite similar levels of Be-induced proliferation of blood T cells, significantly increased numbers of Be-specific T cells are present in the blood of CBD patients compared to BeS subjects.9 These findings suggest the presence of a proliferative defect in blood T cells of CBD patients. We have previously shown that certain Be-specific CD4+ T cells proliferate poorly after antigen exposure while maintaining the ability to secrete Th1-type cytokines.9, 10 Using ELISPOT analysis, IFN-γ-secreting CD4+ T cells were present in the blood of some CBD subjects at a time when Be-induced T cell proliferation was undetectable.9 In addition, the ability of Be-responsive T cells to divide was closely linked to the memory maturation state of the T cell as well as expression of the coinhibitory receptor, programmed death-1 (PD-1).10, 11 Thus, the lack of Be-induced proliferation seen in certain individuals was not due to the absence of circulating Be-specific CD4+ T cells but rather to a high fraction of PD1-expressing effector memory T (TEM) cells that were incapable of vigorous proliferation.10, 11 Thus, we hypothesized that proliferation-based assays may underestimate the frequency of Be sensitization in the workplace.
In the present study, we show that IFN-γ ELISPOT has a greater ability to detect a Be-specific immune response in blood compared to the BeLPT and identify a significant positive correlation between the number of Be-responsive T cells in blood and in the target organ. In addition, the transition from Be sensitization to CBD is associated with an increased number of Be-specific T cells in blood. Our findings suggest that an antigen-specific T cell subset in blood can be used as a biomarker to predict disease development in Be-exposed workers.
METHODS
Study population
We prospectively enrolled 150 current and 110 former workers of a Be-machining facility,12, 13 with current workers being enrolled during biannual workplace surveillance and former workers enrolled during a one-time screening program. The demographics of the study population are shown in Table 1. An additional cohort of 27 non-Be-exposed control subjects, 139 BeS subjects and 31 CBD patients were separately enrolled. CBD was diagnosed based on a history of Be exposure, presence of a Be-specific immune response as measured by an abnormal blood and/or BAL BeLPT, and the presence of granulomatous inflammation and/or mononuclear cell infiltration on lung biopsy.6, 14 Diagnosis of a BeS subject was based on a history of Be exposure, a positive proliferative response of PBMCs to BeSO4 in vitro, and the absence of granulomatous inflammation or other abnormalities on lung biopsy.7, 15 Progressors are defined as those subjects who advanced from BeS to CBD at the time of the current clinical evaluation, based on the development of a Be-specific immune response in BAL and granulomatous inflammation and/or mononuclear cell infiltration on lung biopsy.8 The demographics of these study subjects are shown in Table E1 in the Online Repository.
TABLE 1.
CHARACTERISTICS OF FORMER AND CURRENT WORKERS*
| Characteristics | Former Workers (n = 110) | Current Workers (n = 150) |
|---|---|---|
| Age (years) | 56 (32 – 78)§ | 46 (23 – 65) |
| Gender (M/F) | 98/12 | 139/11 |
| Race (C/AF/H)† | 109/1/0 | 149/1/0 |
| Smoking status (CS/FS/NS)‡ | 23/41/43 | 13/51/84 |
| Time since first exposure (years) | 30 (2.4 – 48)§ | 14 (1.9 – 42) |
| Beryllium tenure (years) | 6.0 (0.4 – 35) | 14 (1.9 – 42)§ |
| HLA-DPB1 Glu69 (% positive) | 38% | 37% |
Data expressed as median (range).
C = Caucasian; AF = African-American; H = Hispanic.
CS = Current smoker; FS = Former smoker; NS = Never smoker.
p < 0.001.
Informed consent was obtained from each subject, and the protocol was approved by the Human Subject Institutional Review Boards at the University of Colorado Denver and National Jewish Health.
Lymphocyte proliferation assay
PBMCs were isolated from heparinized blood as previously described.16 Proliferation assays were performed in quadruplicate in the Clinical Immunology Laboratory at National Jewish Health as previously described.6, 14 Data are presented as a stimulation index (SI) with a positive response defined as SI ≥2.5.9
IFN-γ and IL-2 ELISPOT assay
ELISPOT assays were performed in triplicate using plates (ImmunoSpot M200, BD Biosciences Pharmingen) that were coated with IFN-γ or IL-2 capture mAb (BD Biosciences Pharmingen) and blocked with RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories Inc.) for 2 h at room temperature.9 PBMCs (5 × 105 cells/well) were added to wells and incubated overnight at 37°C with medium, PHA, 10 or 100 μM BeSO4. IFN-γ or IL-2 detection mAbs (BD Biosciences Pharmingen) were added, and spots were visualized as previously described.9 The ELISPOT plates were analyzed using a CTL Immunospot Analyzer (Cellular Technology Ltd.), and results are reported as mean ± SD spot-forming units (SFU) per well minus background SFU.
As a quality control measure, we assessed variability in the ELISPOT assay by splitting PBMCs from 13 CBD patients and determining the number of SFU in different plates. As shown in Figure E1 in the Online Repository, <10% variability in the number of IFN-γ SFU in response to 100 μM BeSO4 was seen (P = 0.94).
Immunofluorescence staining and analysis of intracellular cytokine expression
BAL was obtained as previously described.17, 18 For stimulation of cytokine expression, 1 × 106 BAL cells were exposed to either medium or 100 μM BeSO4 for 6 hours with 10 μg/ml brefeldin A added after the first hour of stimulation.19, 20 Cells were stained with mAbs directed against CD3 and CD4 (BD Biosciences Pharmingen) followed by fixation, permeabilization, and staining with mAbs directed against IFN-γ and IL-2 (Caltag).19, 20 The lymphocyte population was identified using forward and 90° light scatter patterns, and fluorescence intensity was analyzed using an LSR-II flow cytometer (BD Immunocytometry Systems).17–20 Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. Analysis was performed using FlowJo (Tree Star) software.
Statistical analysis
The Mann-Whitney U test and the Kruskal-Wallis ANOVA were used to determine significance of differences between subject groups. The comparison of the stability of IFN-γ SFU over time within subjects was performed using a Wilcoxon signed rank test. A Spearman correlation was performed to analyze the association between IFN-γ SFU in blood and the frequency of Th1-type cytokine-expressing CD4+ T cells in BAL. An ROC analysis was used to determine the optimal threshold value as measured by sensitivity and specificity. A P value <0.05 was considered statistically significant.
RESULTS
Delineation of a positive ELISPOT response
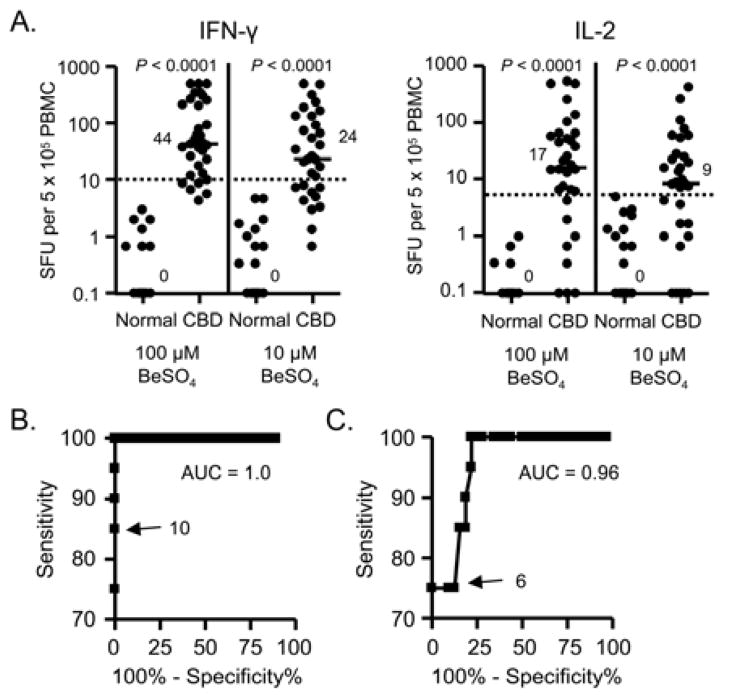
In order to determine a cut-off for a positive IFN-γ and IL-2 ELISPOT assay, we prospectively enrolled 27 non-Be-exposed normal control subjects and 31 CBD patients. Demographics of these subjects are shown in Table E1 in the Online Repository. In response to 100 μM BeSO4, the median number of IFN-γ-producing cells in the blood of CBD patients (44 spot-forming units (SFU)/5 × 105 cells; range, 1.3 – 500) was significantly higher compared to normal control subjects (0 SFU; range, 0 – 3; P < 0.0001) (Figure 1A). Similar findings were seen at the lower concentration of Be (Figure 1A). In addition, the median number of IL-2-producing cells in response to 100 μM BeSO4 was significantly higher in CBD patients (17 SFU; range, 0 to 500) compared to normal control subjects (0 SFU; range, 0 – 1; P < 0.0001) (Figure 1A). Similar to our previous study,9 100 μM BeSO4 was the optimal antigen concentration for the induction of IFN-γ and IL-2 secretion.
Figure 1.
Frequency of Be-specific T cells in blood of normal control subjects and chronic beryllium disease (CBD) patients. A, IFN-γ and IL-2 ELISPOT analysis. Data are expressed as the mean SFU with median values indicated with solid lines. Above the dotted line represents a positive cytokine response. B and C, Receiver Operator Characteristic (ROC) curves for IFN-γ and IL-2 SFU in blood of CBD (true positives) and control subjects (true negatives). The area under the curve (AUC) and the chosen cut-off value for each ROC curve is shown.
Using a Receiver Operator Characteristic (ROC) curve to distinguish normal control subjects from CBD patients, a threshold of ≥ 10 IFN-γ and ≥ 6 IL-2 SFU was chosen as an abnormal response (Figure 1B and C). With this cut-point, IFN-γ ELISPOT had a sensitivity of 85% (95% confidence interval, 67% to 95%) and a specificity of 100% (95% confidence interval, 83% to 100%), with a positive and negative predictive value of 100% and 81%, respectively. Of the five subjects with a negative ELISPOT, four had a negative BeLPT. For IL-2, there were eight CBD patients who had below 6 SFU in response to 100 μM BeSO4, resulting in a sensitivity of 76% and a specificity of 100% for the IL-2 ELISPOT. Based on the ROC analyses, an abnormal IFN-γ and IL-2 ELISPOT assay for this study will be defined as ≥10 and ≥6 SFU, respectively.
Quantitation of Be-specific, IFN-γ- and IL-2-producing PBMCs
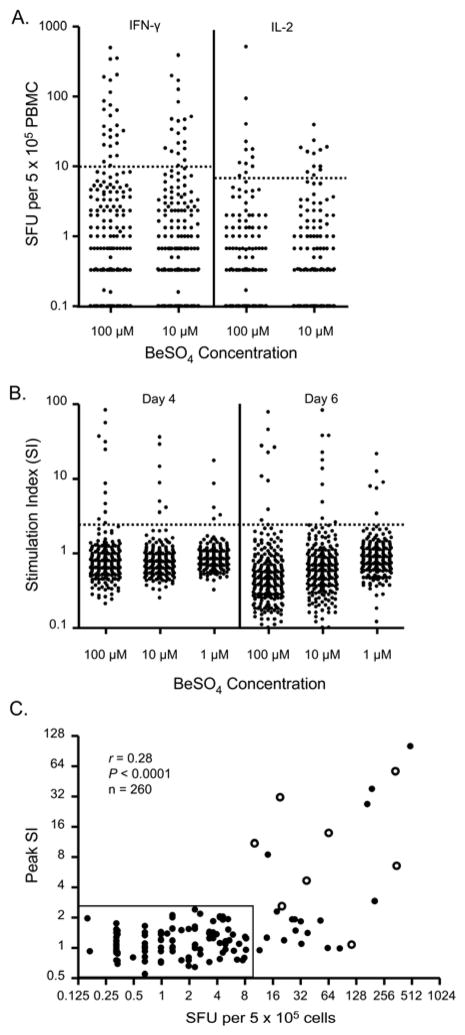
In order to compare the ability of the ELISPOT assay and the BeLPT to detect Be sensitization in the workplace, we prospectively enrolled 150 current and 110 former workers from a single Be-machining facility. None of these subjects had previously been identified as Be-sensitized. The majority of Be-exposed workers did not demonstrate an adaptive immune response to Be (Figure 2A and 2B). However, an abnormal IFN-γ ELISPOT to 100 μM BeSO4 was detected in 15 current and 11 former workers, resulting in rates of Be sensitization of 10% and 9.9%, respectively. This rate of sensitization is similar to the 9.4% sensitization rate that was previously reported for this facility.13 Due to the identical rates of sensitization detected in current and former workers, we have grouped the workers together (Figure 2A). In response to 10 μM BeSO4, 20 subjects had an abnormal IFN-γ ELISPOT, and all of these individuals displayed a positive response to the higher concentration of Be.
Figure 2.
Identification of a Be-induced adaptive immune response. A, Frequency of Be-specific T cells in blood using IFN-γ and IL-2 ELISPOT. Data are expressed as the mean SFU for each subject. Cytokine values above the dotted line represent a positive cytokine response. B, Be-induced proliferative response. A stimulation index indicative of a positive response is defined as ≥2.5 (dotted line). C, Correlation between the number of Be-responsive T cells in blood and peak SI. Subjects with confirmed CBD upon clinical evaluation are shown as open circles. Rectangle surrounds subjects with a negative IFN-γ ELISPOT and BeLPT.
Twelve subjects demonstrated an abnormal IL-2 ELISPOT to 100 and 10 μM BeSO4 (Figure 2A). All subjects with an abnormal IL-2 ELISPOT also had ≥10 IFN-γ SFU in response to 100 μM BeSO4. Since IFN-γ is the predominant Th1-type cytokine secreted by Be-specific CD4+ T cells derived from either blood or lung,19 we have chosen to focus on the IFN-γ response of PBMCs to 100 μM BeSO4 for our comparison of the ability of ELISPOT versus BeLPT to detect an adaptive immune response in Be-exposed workers.
Beryllium-induced proliferation of PBMCs
PBMCs from the same 260 Be-exposed workers were examined for Be-induced proliferation at the same time as the ELISPOT assays were performed. As shown in Figure 2B, 11 of 260 (4.2%) subjects demonstrated a significant proliferative response (denoted as an SI ≥2.5) to 100 μM BeSO4 after 4 days of culture. Although similar findings were seen in response to different BeSO4 concentrations and days of culture, 100 μM BeSO4 after 4 days induced the greatest proliferative response (Figure 2B). In addition, all subjects with a positive BeLPT to 1 and 10 μM BeSO4 had a significant proliferative response to the higher dose of Be. As compared to ELISPOT where similar rates of sensitization were seen in current and former workers, 9 former and 2 current workers had abnormal BeLPTs, resulting in rates of Be sensitization of 8.1% and 1.3%, respectively. Overall, 10% of the workers had an abnormal IFN-γ ELISPOT compared to 4.2% with a positive BeLPT (χ2 = 55.7; P < 0.0001).
To investigate the relationship between Be-specific T cell proliferation and cytokine secretion, we correlated peak SI as detected via the BeLPT and the number of IFN-γ SFU per 5 × 105 cells in the cohort of 260 subjects. Despite a low r value, a significant positive correlation was noted (r = 0.28; P < 0.0001) (Figure 2C). However, this correlation was lost when only those subjects with a detectable Be-specific immune response were included in the analysis (r = 0.27; P = 0.2). Figure 2C also shows the 15 subjects with >10 IFN-γ SFU who failed to proliferate in the presence of Be salts in vitro (SI <2.5). No subject had an abnormal BeLPT in the presence of <10 IFN-γ SFU as detected by ELISPOT (Figure 2C).
Assessment of IFN-γ ELISPOT in relation to HLA-DPB1 Glu69
Since HLA-DPB1 alleles with a glutamic acid at position 69 (HLA-DPB1 Glu69) are the most important genetic susceptibility marker for the development of Be-induced disease,5, 21, 22 we analyzed the IFN-γ ELISPOT data based on the expression of HLA-DPB1 Glu69 in this cohort of subjects. HLA-DP genetic analysis was available for 212 subjects, including 23 subjects with >10 IFN-γ SFU. As shown in Figure E2 in the Online Repository, a significantly greater number of SFU were present in the Glu69-positive subjects (median, 2.3; range 0 – 500) compared to Glu69-negative individuals (median, 0; range 0 – 8.3; P < 0.0001). More importantly, all subjects with a positive IFN-γ ELISPOT possessed at least one HLA-DPB1 Glu69-containing allele.
Frequency of Be-responsive T cells in blood as a biomarker of disease progression
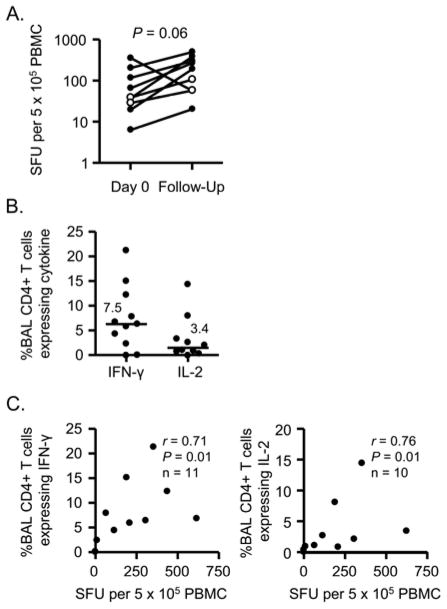
Eight of 26 subjects who demonstrated evidence of Be sensitization in the current study have been clinically evaluated for the presence of granulomatous lung inflammation, and all have been diagnosed with CBD. Of the eight subjects (shown as open circles in Figure 2C), seven had both an abnormal IFN-γ ELISPOT and BeLPT and one had an abnormal IFN-γ ELISPOT and a negative BeLPT. That subject had 116 IFN-γ SFU and a peak SI of 1.06. IFN-γ ELISPOTs were repeated on seven subjects at the time of clinical evaluation and two of the 15 subjects with an abnormal ELISPOT and a negative BeLPT (denoted by open circles in Figure 3A). Although a trend was noted for an increase in the number of circulating Be-responsive T cells at the second time point, no statistically significant differences were seen (P = 0.06). Importantly, the two individuals with an abnormal ELISPOT in the absence of a Be-induced proliferation retained a Be-specific immune response in blood.
Figure 3.
Frequency of Be-responsive CD4+ T cells in blood and BAL of CBD subjects. A, Be-specific T cells in blood over time. Data are expressed as the mean spot-forming units (SFU) per 5 × 105 cells per well. Open circles show two subjects who had a positive ELISPOT and a negative BeLPT at first evaluation. B, Percentage of BAL CD4+ T cells that express IFN-γ (n = 11) and IL-2 (n = 10) after stimulation with 100 μM BeSO4. Mean percentage of Th1 cytokines produced by BAL cells from CBD patients is shown as a solid line. C, Correlation between the number of Be-responsive T cells in blood and the frequency of Be-responsive BAL CD4+ T cells expressing either IFN-γ (left panel) or IL-2 (right panel).
Since Be-responsive T cells present in blood are likely trafficking to lung, we queried whether the frequency of IFN-γ-secreting T cells in blood could serve as a biomarker of T cell alveolitis severity. We obtained simultaneous blood and BAL samples from 11 CBD patients (six individuals who were from the initial study and enrolled at the time of their clinical evaluation and five previously diagnosed subjects from the same Be-machining facility). In the representative examples shown in Figure E3 in the Online Repository, 7.9% and 2.7% of BAL CD4+ T cells expressed IFN-γ and IL-2, respectively, after short-term culture with BeSO4. Overall, the frequency of IFN-γ- and IL-2-expressing, Be-specific CD4+ T cells in BAL of the CBD subjects was 7.5 ± 2.0% and 3.4 ± 1.4% (mean ± SEM), respectively (Figure 3B). Comparing the frequency of Be-specific T cells in blood and lung, a significant positive correlation was observed between the number of IFN-γ-secreting T cells in blood and the frequency of IFN-γ expressing CD4+ T cells in lung (r = 0.71; P = 0.01) (Figure 3C). A positive correlation was also seen between Be-responsive T cells in blood and IL-2-expressing CD4+ T cells in lung (r = 0.76; P = 0.01) (Figure 3C).
Based on the association between the frequency of Be-specific T cells in blood and lung shown here and the CD4+ T cell alveolitis which characterizes CBD, we queried whether the IFN-γ ELISPOT could predict the development of granulomatous inflammation in BeS subjects who were returning for follow-up evaluation. We consecutively enrolled 139 BeS subjects. The median number of IFN-γ-producing cells in blood of BeS subjects was 3.7 SFU (range, 0 to 97) in response to 100 μM BeSO4 (Figure 4A). More importantly, 14 BeS subjects progressed to CBD at the time of that clinical evaluation based on the development of a Be-specific immune response in BAL and/or granulomatous inflammation on lung biopsy, and these progressors had a significantly increased number of Be-responsive, IFN-γ-secreting cells in blood (median, 27 SFU; range, 4.7 to 500) compared to those BeS subjects whose disease status remained unchanged (P < 0.0001) (Figure 4A). In addition, progressors had a significantly greater number of lymphocytes in the BAL compared to stable BeS subjects (P < 0.01) (Table S1).
Figure 4.
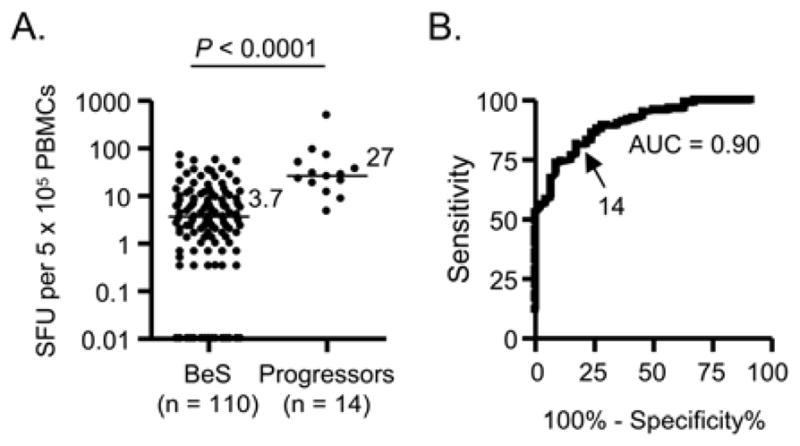
Frequency of Be-specific T cells in the blood of subjects with Be sensitization (BeS) and individuals whose disease status changed at the time of the clinical evaluation (Progressors). Data are expressed as the mean number of IFN-γ SFU in response to 100 μMBeSO4, and median values are indicated with solid lines. B, Receiver Operator Characteristic (ROC) curves for IFN-γ SFU in blood of CBD and BeS subjects. The area under the curve (AUC) and the chosen cut-off value are shown.
To determine whether the frequency of Be-specific T cells in blood can distinguish between those subjects with CBD (combining the 14 Progressors in Figure 4A and the 31 CBD patients from Figure 1A) from those with stable Be sensitization, we generated an ROC curve (Figure 4B). Progressors and CBD patients were combined since these subjects all have CBD and possess a similar frequency of Be-responsive T cells in blood (compare Figures 1A and 5A). Using a threshold value of >14 IFN-γ SFU to differentiate CBD from BeS, a sensitivity and specificity of 78% and 86%, respectively, were seen, with a positive and negative predictive value of 67% and 91%, respectively. Taken together, these findings suggest that the frequency of Be-specific T cells in blood can be used as a biomarker of disease progression.
DISCUSSION
The onset of Be sensitization is heralded by the development of a Be-specific adaptive immune response that is typically detected by the presence of Be-induced proliferation of blood T cells. Our previous studies show that the majority of Be-responsive CD4+ T cells in blood and lung express an effector memory phenotype, and the ability of CD4+ T cells to proliferate after Be exposure in culture is linked to the maturation state of the memory T cell.10 The retention of Be in the lung and the resultant persistent antigen exposure are associated with the upregulation of coinhibitory receptors, such as PD-1, on Be-specific CD4+ T cells that is also associated with reduced proliferation.11 Collectively, these findings suggest that Be-responsive CD4+ T cells in blood and lung represent differentiated TEM cells that have sacrificed proliferative capacity while retaining the ability to express Th1-type cytokines. Thus, we hypothesized that a cytokine-based assay, such as IFN-γ ELISPOT, would be better able to detect Be sensitization in the workplace compared to the BeLPT. Using blood from current and former workers of a Be-machining facility, the current study confirms our hypothesis and shows that the number of Be-responsive T cells in blood correlates with the frequency of IFN-γ- and IL-2-expressing, Be-specific CD4+ T cells in lung. We found that those subjects who progressed to CBD had a significantly increased number of Be-responsive T cells in blood compared to those BeS subjects who disease status remained unchanged, suggesting that quantitation of these cells in blood is an effective means of predicting disease development.
Comparing the rates of abnormal IFN-γ ELISPOT and BeLPT, similar rates were seen in former workers (10% versus 8.1%, respectively). Conversely, the rates of sensitization in current workers were 9.9% for IFN-γ ELISPOT and 1.3% for BeLPT. One potential explanation for this observation is that the former workers are no longer actively exposed to Be, and their compressed Be-specific CD4+ memory T cell pool in blood has regained its full proliferative capacity in the absence of current antigenic exposure. In contrast, with continued exposure, the predominance of Be-specific CD4+ TEM cells expressing an exhausted or even senescent phenotype limits the ability of these cells to mount a proliferative response.11, 23 Similar findings have been seen in other diseases characterized by persistent antigen exposure such as HIV disease, where the inability of HIV-1-specific CD4+ T cells to proliferate was one of the earliest reported immunologic manifestations of HIV-1 infection.24
Eight of 26 subjects with an abnormal ELISPOT in our cohort of 260 Be-exposed workers have subsequently been diagnosed with CBD. Seven of those subjects also had an abnormal proliferative response to Be salts in culture. One caveat is that the one CBD subject with a positive ELISPOT and a normal BeLPT had an abnormal Be-induced proliferative response at another facility, which prompted initiation of the clinical evaluation. While the BeLPT was equivalent to the IFN-γ ELISPOT in the detection of Be sensitization in workers with >100 IFN-γ SFU per 5 × 105 cells (detecting seven of eight individuals), it has a decreased sensitivity in subjects whose ELISPOT response ranged between 10 - 100 SFU. It remains possible that some of the 15 subjects with a negative BeLPT and a positive ELISPOT represent false-positive test results. To date, two of these 15 individuals had repeat ELISPOT and BeLPT performed, and both had an abnormal ELISPOT in the setting of a negative BeLPT. Other evidence supporting the validity of our findings includes a subject with a negative BeLPT and a positive ELISPOT who has been diagnosed with CBD and the fact that all subjects with an abnormal ELISPOT possessed HLA-DPB1Glu69, the major genetic susceptibility marker in this disease. Thus, our findings show that the IFN-γ ELISPOT is more sensitive than the BeLPT for the detection of CBD in this workplace. Additional advantages of the ELISPOT include a shorter duration of the assay (24 hours versus 4 or 6 days), lack of radioactivity and use of fetal bovine serum as opposed to human serum.
One uncertainty of the current study is whether our results will translate to other Be facilities where the level of exposure is not as significant and rates of Be sensitization are lower. It is well-established that machinists have the greatest levels of Be exposure and the highest rates of Be sensitization.12, 25 Workplace surveillance in the Be-machining facility tested in the current study has shown that the majority of subjects with Be sensitization had already progressed to CBD at the time of evaluation. In industries with less or intermittent Be exposure, it is possible that the two assays will perform in a similar manner or detect Be-induced proliferation in the absence of a cytokine response, which was not the case in this highly exposed cohort. Since the IFN-γ ELISPOT and the BeLPT are measuring different T cell functions (i.e., IFN-γ secretion and proliferation), it is also possible that these assays may have complimentary roles in workplace surveillance.
Our findings suggest that the Be-specific adaptive immune response in blood is maintained over time in CBD patients. We have previously shown that the BAL T cell receptor Vβ expansions and the Be-responsive T cell clones that comprise those expansions in CBD patients persist in serial lavages.17, 18 However, it remains unknown whether the same is true for Be sensitization. In this regard, we failed to demonstrate either a Be-induced cytokine or proliferative response in >70% of the BeS subjects in this study, raising the possibility that in some BeS subjects the Be-induced immune response may be a transient phenomenon. Conversely, of the 95 BeS subjects who underwent BAL, seven demonstrated a Be-specific proliferative response in BAL but did not show evidence of granulomatous inflammation or BAL lymphocytosis, thus not fulfilling the criteria for CBD. Of those subjects, 86% possessed >10 IFN-γ SFU, suggesting that these subjects may be at high-risk to progress to CBD or have already progressed despite the absence of granulomas on lung biopsy. These subjects also contribute to the overlap seen between the number of IFN-γ-secreting T cells in the progressors compared to those with stable Be sensitization.
A biomarker that can predict disease progression has important clinical implications, especially in Be-induced disease where uncertainty exists regarding who will progress to disease and when progression will occur. In the present study, we identified a cut-off of >14 SFU to differentiate CBD from BeS subjects. This is the identical value identified in our previous study investigating a different patient cohort,9 adding further validity to its use in separating stages of disease. However, due to its low positive predictive value, this cut-off may not be clinically useful in determining who has CBD or who needs to undergo an invasive procedure for diagnostic purposes. In this regard, our data suggest that the use of a lower and an upper cut-off may be clinically more useful. For example, in BeS subjects with <10 SFU, the probability of having progressed to CBD would be <7% (Table E2 in the Online Repository) and may not warrant the risk of bronchoscopy unless dictated by clinical, radiographic or physiologic abnormalities. Conversely, individuals with >40 SFU have an 81% probability of having progressed to CBD (Table E2). BeS subjects with between 10 and 40 SFU will likely warrant bronchoscopy as well as close clinical follow-up. Thus, the addition of the IFN-γ ELISPOT to the diagnostic algorithm of beryllium-induced disease is a significant advancement over that provided by the BeLPT, which only detects whether beryllium sensitization is present or not.
In summary, we have shown that the number of Be-responsive T cells in blood can serve as a biomarker of disease development and as an estimate of the severity of the Be-induced CD4+ T cell alveolitis. Thus, finding an increased quantity of Be-specific CD4+ T cells in blood of workers may signify a sentinel event, heralding the development of a Be-induced T cell alveolitis and the subsequent development of granulomatous inflammation.
Acknowledgments
Supported by the following NIH grants: HL062410, ES011810, HL102245 (to APF), and the Clinical Translational Research Center (UL1 RR025780) from the National Center for Research Resources.
ABBREVIATIONS
- BAL
Bronchoalveolar lavage
- Be
Beryllium
- BeLPT
Beryllium lymphocyte proliferation test
- BeS
Beryllium-sensitized
- BeSO4
Beryllium sulfate
- CBD
Chronic beryllium disease
- ROC
Receiver Operator Characteristic
- TEM cells
Effector memory T cells
- SFU
Spot-forming units
- SI
Stimulation index
- WBC
White blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontenot AP, Maier LA. Genetic susceptibility and immune-mediated destruction in beryllium-induced disease. Trends Immunol. 2005;26:543–9. doi: 10.1016/j.it.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kreiss K, Mroz MM, Newman LS, Martyny J, Zhen B. Machining risk of beryllium disease and sensitization with median exposures below 2 μg/m3. Am J Ind Med. 1996;30:16–25. doi: 10.1002/(SICI)1097-0274(199607)30:1<16::AID-AJIM3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Kreiss K, Mroz MM, Zhen B, Martyny JW, Newman LS. Epidemiology of beryllium sensitization and disease in nuclear workers. Am Rev Respir Dis. 1993;148:985–91. doi: 10.1164/ajrccm/148.4_Pt_1.985. [DOI] [PubMed] [Google Scholar]
- 4.Kreiss K, Wasserman S, Mroz MM, Newman LS. Beryllium disease screening in the ceramics industry: blood test performance and exposure-disease relations. J Occup Med. 1993;35:267–74. [PubMed] [Google Scholar]
- 5.Richeldi L, Sorrentino R, Saltini C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science. 1993;262:242–4. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- 6.Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, et al. Proliferative response of bronchoalveolar lymphocytes to beryllium. Ann Intern Med. 1988;108:687–93. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- 7.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- 8.Newman LS, Mroz MM, Balkissoon R, Maier LA. Beryllium sensitization progresses to chronic beryllium disease: a longitudinal study of disease risk. Am J Respir Crit Care Med. 2004;171:54–60. doi: 10.1164/rccm.200402-190OC. [DOI] [PubMed] [Google Scholar]
- 9.Pott GB, Palmer BE, Sullivan AK, Silviera L, Maier LA, Newman LS, et al. Frequency of beryllium-specific, TH1-type cytokine-expressing CD4+ T cells in patients with beryllium-induced disease. J Allergy Clin Immunol. 2005;115:1036–42. doi: 10.1016/j.jaci.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot AP, Palmer BE, Sullivan AK, Joslin FG, Wilson CC, Maier LA, et al. Frequency of beryllium-specific, central memory CD4+ T cells in blood determines proliferative response. J Clin Invest. 2005;115:2886–93. doi: 10.1172/JCI24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, et al. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 2008;180:2704–12. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelleher PC, Martyny JW, Mroz MM, Maier LA, Ruttenber AJ, Young DA, et al. Beryllium particulate exposure and disease relations in a beryllium machining plant. J Occup Environ Med. 2001;43:238–49. doi: 10.1097/00043764-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Newman LS, Mroz MM, Maier LA, Daniloff EM, Balkissoon R. Efficacy of serial medical surveillance for chronic beryllium disease in a beryllium machining plant. J Occup Environ Med. 2001;43:231–7. doi: 10.1097/00043764-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Newman LS, Kreiss K, King TE, Jr, Seay S, Campbell PA. Pathologic and immunologic alterations in early stages of beryllium disease: re-examination of disease definition and natural history. Am Rev Respir Dis. 1989;139:1479–86. doi: 10.1164/ajrccm/139.6.1479. [DOI] [PubMed] [Google Scholar]
- 15.Newman LS. Significance of the blood beryllium lymphocyte proliferation test. Environ Health Perspect. 1996;104:953–6. doi: 10.1289/ehp.96104s5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack DG, Lanham AM, Falta MT, Palmer BE, Maier LA, Fontenot AP. Deficient and dysfunctional regulatory T cells in the lungs of chronic beryllium disease subjects. Am J Respir Crit Care Med. 2010;181:1241–9. doi: 10.1164/rccm.201001-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontenot AP, Kotzin BL, Comment CE, Newman LS. Expansions of T-cell subsets expressing particular T cell receptor variable regions in chronic beryllium disease. Am J Respir Cell Mol Biol. 1998;18:581–9. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J Immunol. 1999;163:1019–26. [PubMed] [Google Scholar]
- 19.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4+ T cells in patients with chronic beryllium disease. J Clin Invest. 2002;110:1473–82. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot AP, Gharavi L, Bennett SR, Canavera SJ, Newman LS, Kotzin BL. CD28 costimulation independence of target organ versus circulating memory antigen-specific CD4+ T cells. J Clin Invest. 2003;112:776–84. doi: 10.1172/JCI18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossman MD, Stubbs J, Lee CW, Argyris E, Magira E, Monos D. Human leukocyte antigen Class II amino acid epitopes: susceptibility and progression markers for beryllium hypersensitivity. Am J Respir Crit Care Med. 2002;165:788–94. doi: 10.1164/ajrccm.165.6.2104002. [DOI] [PubMed] [Google Scholar]
- 22.Maier LA, McGrath DS, Sato H, Lympany P, Welsh K, Du Bois R, et al. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J Immunol. 2003;171:6910–8. doi: 10.4049/jimmunol.171.12.6910. [DOI] [PubMed] [Google Scholar]
- 23.Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium-induced disease. J Allergy Clin Immunol. 2007;120:184–91. doi: 10.1016/j.jaci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Shearer GM. HIV-induced immunopathogenesis. Immunity. 1998;9:587–93. doi: 10.1016/s1074-7613(00)80656-1. [DOI] [PubMed] [Google Scholar]
- 25.Henneberger PK, Cumro D, Deubner DD, Kent MS, McCawley M, Kreiss K. Beryllium sensitization and disease among long-term and short-term workers in a beryllium ceramics plant. Int Arch Occup Environ Health. 2001;74:167–76. doi: 10.1007/s004200100237. [DOI] [PubMed] [Google Scholar]