Abstract
The pleiotropic sphingolipid mediator, sphingosine-1-phosphate, produced in cells by two sphingosine kinase isoenzymes, SphK1 and SphK2, regulates many cellular and physiological processes important for homeostasis and development and pathophysiology. Many of the actions of S1P are mediated by a family of five specific cell surface receptors that are ubiquitously and specifically expressed, although important direct intracellular targets of S1P have also recently been identified. S1P, SphK1, and or S1P receptors have been linked to onset and progression of numerous diseases, including many types of cancer, and especially inflammatory disorders, such as multiple sclerosis, asthma, rheumatoid arthritis, inflammatory bowel disease, and sepsis. S1P formation and signaling are attractive targets for development of new therapeutics. The effects of a number of inhibitors of SphKs and S1PRs have been examined in animal models of human diseases. The effectiveness of the immunosuppressant FTY720 (known as Fingolomod or Gilenya), recently approved for the treatment of multiple sclerosis, whose actions are mediated by downregulation of S1PR1, has become the gold standard for S1P-centric drugs. Here, we review S1P biology and signaling with an emphasis on potential therapeutic benefits of specific interventions and discuss recent development of small molecule antagonists and agonists that target specific subtypes of S1P receptors as well as inhibitors of SphKs.
Keywords: sphingosine-1-phosphate, sphingosine kinase, inhibitors, antagonists, cancer, multiple sclerosis, asthma, rheumatoid arthritis, inflammatory bowel disease, sepsis, apoptosis, S1P receptors
1. Introduction
Sphingosine-1-phosphate (S1P), once regarded primarily as an intermediate of sphingolipid metabolism, is now recognized as a powerful mediator of many vital cellular processes. Since the discovery of its positive effects on cell proliferation 20 years ago (Zhang et al, 1991), a plethora of roles for S1P have emerged in the regulation of such diverse phenomena as inflammation, cell death and survival, angiogenesis, and immunity and lymphocyte trafficking. Many actions of S1P have been shown to originate from its now well-defined function as a specific ligand for five specific G protein-coupled receptors. Autocrine or paracrine binding of S1P to these receptors, now designated S1PR1-5, activates a variety of G proteins whose downstream signaling accounts for many of its important functions in cancer and inflammation (Fig. 1).
Fig. 1.
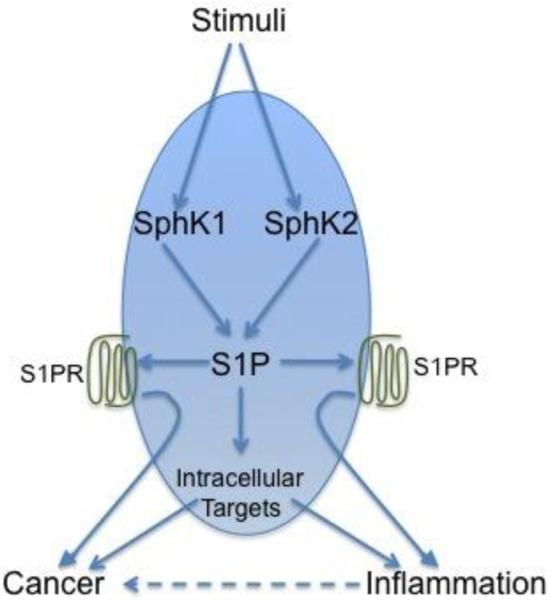
S1P is a regulator of many processes important for cancer and inflammatory disorders. Many stimuli increase S1P by increasing the activity of SphK1 and SphK2. S1P secreted from cells activates specific S1P receptors, either on the same cell or on neighboring cells, and downstream signaling mediates disease progression. S1P also has direct intracellular targets that are likely to be important.
2. Intracellular S1P targets
Intracellular roles for this bioactive sphingolipid metabolite have also long been suggested (Van Brocklyn et al., 1998). Indeed, this notion was confirmed recently with the identification of several direct intracellular targets of S1P (Hait et al., 2009; Alvarez et al., 2010; Puneet et al., 2010; Strub et al., 2011; Takasugi et al., 2011). The discovery that the histone deacetylases HDAC1 and HDAC2 specifically bind S1P that inhibits their activity (Hait et al, 2009), identified the first bona fide intracellular target of S1P and also established an intracellular function for S1P in the nucleus in the epigenetic regulation of gene expression. Subsequent studies demonstrated that binding of S1P also binds to and is required for the E3 ubiquitin ligase activity of TRAF2, an essential mediator of the NF-κB pathway initiated by the major inflammatory signaling molecule TNF-α (Alvarez et al, 2010). It is thus now clear that the effects of S1P are mediated by both extracellular and intracellular signaling mechanisms; additional direct intracellular targets will likely be uncovered as research in this area progresses.
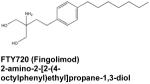
The rapid expansion in understanding of S1P’s modes of action has sparked intense investigations of S1P signaling as a possible therapeutic target. The great potential of this approach is illustrated by the discovery that the phosphorylated form of FTY-720, an immunosuppressive small molecule drug that was recently approved by the FDA for treatment of multiple sclerosis, functions at least in part by binding to and eventually inducing internalization and degradation of 1 out of the 5 known S1P cell surface receptors, which prevents its function as the sensor of S1P gradients (Graler, 2010). As autocrine and paracrine S1P signaling are involved in such a broad range of physiological and pathological processes, this finding raised the exciting possibility that FTY-720 and other modulators of “inside-out” signaling of S1P could prove useful in treating a myriad of conditions. Moreover, the emergent intracellular functions of S1P provide additional opportunities for therapeutic intervention that are only beginning to be explored. This review will highlight current efforts to establish therapeutic uses for small molecule inhibitors of S1P signaling, with an emphasis on approaches targeting sphingosine kinase 1 (SphK1), one of the two SphK isoenzymes whose expression has been correlated with severity and outcome of several diseases.
2. Enzymes of S1P metabolism
While S1P promotes cell growth and survival as discussed above, its two direct precursors, sphingosine and ceramide, promote growth arrest and apoptosis (Guillermet-Guibert et al., 2009). Regulation of these interconvertible signaling molecules is critical, as perturbation of the relative cellular levels of S1P vs. sphingosine and ceramide, known as the “sphingolipid rheostat”, are thought to alter normal control of cell fate and responses to environmental cues. S1P levels are thus tightly regulated via the equilibrium between its synthesis by sphingosine kinases (SphKs) and its degradation by sphingosine lyase and sphingosine phosphatases (Alvarez et al., 2007).
The two S1P-producing isoenzymes, SphK1 and SphK2, are highly homologous. However, whereas SphK1 generally has pro-survival effects, early studies associated SphK2 with inhibition of cell growth and promotion of apoptosis (Fyrst et al., 2010). SphK1 in particular is activated by diverse agonists and stimuli which upregulate its enzymatic activity acutely by phosphorylation and translocation to the plasma membrane where its substrate sphingosine resides, or longer term by transcriptional regulation, or by a combination of the these, leading to increases in S1P (Spiegel et al., 2003). SphK1 activity is therefore usually a major determinant of S1P levels. Indeed, SphK1 overexpression has been found in many types of cancer, suggesting that cancer cells may be able to escape normal controls against unchecked cell proliferation in part by harnessing the pro-survival power of SphK1-mediated S1P production. A growing body of research has further identified SphK1 expression and activity as essential for critical events in inflammation and immune response (Spiegel et al., 2011). SphK1 has accordingly been considered to be primary target for the development of therapeutic intervention strategies; however, recent studies suggest that inhibition of SphK2 may also be efficacious (French et al., 2010). Altogether, the sphingosine kinases – in conjunction with some of the other enzymes involved in sphingolipid metabolism – present untapped opportunities for therapeutic intervention in a wide range of pathological processes.
3. Targeting S1P signaling in diseases
Evidence linking S1P and SphK1 to inflammation and disease is broad and uncontested. As mentioned above, S1P is a critical modulator of TNF-α signaling, and as such, plays a role in the regulation of inflammatory events mediated by this cytokine. Moreover, SphK1 expression, enzymatic activity, and subsequent S1P production are stimulated by TNF-α as well as by a host of other inflammatory signaling molecules, including IL-1β, IFN-γ, IgE, and C5a (Snider et al., 2010). The SphK1/S1P axis is therefore both required for and upregulated by inflammatory signaling and regulates the trafficking and activity of various immune cells involved in both innate and adaptive immunity (Spiegel & Milstien, 2011). Considering these findings, it is unsurprising that abnormal upregulation of SphK1 and/or S1P has been implicated in many inflammatory and autoimmune pathologies, including asthma, rheumatoid arthritis, sepsis, and inflammatory bowel disease (reviewed in (Spiegel & Milstien, 2011)).
The relationship between SphK1/S1P signaling and inflammation becomes even more compelling in light of the fact that increasing evidence connects chronic inflammation to cancer development and progression (Karin, 2008). SphK1 is overexpressed in many human cancers (Shida et al., 2008), raising the possibility that it is this upregulation and concomitant S1P production, at least in part, which constitutes the link between pro-inflammation and pro-cancer environments. In any event, it is now clear that S1P can promote carcinogenesis by a variety of mechanisms including enhancing tumor growth, angiogenesis, and metastasis. In fact, SphK1 activity often correlates with higher tumor clinical grade, resistance to chemo- and radiotherapy, and poor patient prognosis (Pyne et al., 2010).
As disruption of S1P signaling has the potential to curtail processes critical to both cancer and inflammatory disease, it has deservedly been the subject of intense research interest. The significant morbidity and mortality associated with these pathologies in conjunction with the limitations of presently available therapies – including the high toxicity of many anti-cancer drugs, the problem of drug resistance in tumor cells, and the deleterious side effects associated with common anti-inflammatory medications – only serve to underscore the urgency of this work. Recent efforts to identify and establish therapeutic uses for small molecule inhibitors of S1P signaling in cancer as well as various inflammatory and autoimmune diseases (Table 1) are highlighted below.
Table 1.
Small molecule inhibitors of S1P synthesis and metabolism
| Structure and name | Mechanism | Therapeutic Actions |
Applicatio ns |
Referenc es |
|---|---|---|---|---|
|
|
|
|
Tsunemi., 2010; Graler, 2010; Pchejetski, et al.,2010; Brinkmann, 2009; Idzko et al., 2006; Berdyshev et al., 2009; Snider et al., 2010; Snider et al., 2009 |
|
|
|
|
Kapitonov et al., 2009; Paugh et al., 2008 |
|
|
|
|
Dickson et al., 2011 |
|
|
|
|
Guillermet-Guibert et al., 2009; Ricci et al., 2009; Nishiuma et al., 2008; Ren et al., 2010 |
|
|
|
|
Antoon et al., 2010; French et al., 2010; Beljanski et al., 2010; Fitzpatric k et al., 2010; Maines et al., 2010; Chumanevich et al., 2010 |
|
|
|
|
Nishiuma et al., 2008;Lai et al., 2008 |
|
|
|
|
Chiba et al., 2010 |
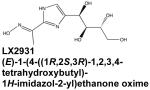
|
|
|
|
Bagdanoff et al., 2010 |
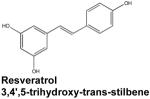
|
|
|
|
Issuree et al., 2009 |
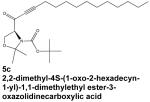
|
|
|
|
Jolly et al., 2004 |
3.1 Cancer
While cancer is often referred to as a monolithic entity, the term encompasses a group of diseases that, beyond sharing the common feature of uncontrolled cell growth, are incredibly varied in presentation and etiology. This diversity presents a significant obstacle to the development of widely applicable cancer therapies. For example, a drug demonstrating efficacy against one form of the disease may have no effect or even be deleterious in other forms. Thus, the ubiquitous SphK1/S1P axis, as discussed above, is a very attractive therapeutic target for cancer.
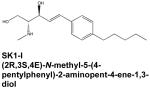
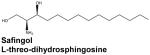
Treatment of head and neck cancer, in particular, is often limited by drug toxicity, resistance to chemotherapy and radiation, as well as the anatomical density of the region. Survival rates for these types of malignancy have not improved significantly over the past 20 years, indicating that new therapeutic options are desperately needed (Beckham et al., 2010). The results of many preclinical studies suggest that modulation of S1P signaling could prove a fruitful approach to this problem. For example, SphK1 overexpression is known to correlate with poor prognosis in patients with glioblastoma multiforme (GBM), the most common and lethal form of central nervous system malignancy (Van Brocklyn et al., 2005). Accordingly, a recent report demonstrated the efficacy of the potent SphK1 inhibitor (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol, referred to as SK1-I, in cultured human GBM cells as well as in glioblastoma xenografts (Kapitonov et al., 2009). SKI-1 attenuated growth, migration, and invasion of several GBM cell lines, and significantly reduced tumor growth and vascularization in mice bearing both subcutaneous and orthotopic glioblastoma xenografts. The profound effects of SK1-I were attributed to suppression of Akt activation and subsequent interruption of signaling through the Akt pathway, which is upregulated in the majority of glioblastomas (Kapitonov et al, 2009). It should also be noted that SK1-I was found to further enhance cell death when used in combination with inhibitors of other cancer-related signaling pathways. This type of combination therapy is emerging as an important strategy in the management of malignancies in general and is important for those in which highly efficacious therapeutic treatments are lacking, such as head and neck cancer. In that vein, a recently completed Phase I clinical trial examined the use of the SphK1 inhibitor safingol in combination with cisplatin to treat patients with advanced solid tumors. While this work is still in its early stages, the published findings indicate that the drug combination was very well tolerated and some degree of disease regression was observed (Dickson et al., 2011).
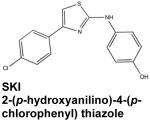
Modulation of SphK1/S1P signaling may also help overcome resistance to chemo- and radio-therapy in cancer cells. Resistance to therapeutic intervention is a serious problem in cancer management, contributing significantly to morbidity and mortality associated with the disease. In this regard, siRNA knockdown or chemical inhibition of SphK1 can sensitize cancer cell lines that are resistant to conventional treatments (Pyne & Pyne, 2010). Meanwhile, the mechanisms underlying these important observations are still being unraveled. The S1P/ceramide rheostat has been identified as one of the critical determinants of pancreatic cancer cell sensitivity (Guillermet-Guibert et al, 2009). Pancreatic cancer cells resistant to gemcitabine, a chemotherapeutic nucleoside analog, had high expression of SphK1 and an abnormally low ceramide/S1P ratio. Remarkably, these resistant cells developed gemcitabine sensitivity following upregulation of the ceramide/S1P ratio with the SphK inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (Guillermet-Guibert et al, 2009), referred to as SKI. Along these lines, it has been proposed that SphK inhibition by the immunosuppressive sphingosine analog FTY-720 is responsible for increased radiotherapeutic sensitivity of prostate cancer cells in culture as well as in subcutaneous and orthotopic murine xenografts (Pchejetski et al., 2010). These findings highlight the excellent potential of SphK inhibitors to serve as adjuvant treatments, diminishing the ability of resistant cancer cells to elude death mediated by conventional therapies.
SphK1 and S1P formation have also been implicated in the development and progression of hematopoietic malignancies (Stevenson et al., 2011). The demonstrated effects of SphK1 inhibition in leukemic cells are quite similar to those observed in solid tumors. Both SKI (Ricci et al., 2009) and SK1-I (Paugh et al., 2008) exhibited anti-proliferative and cytotoxic effects in a variety of leukemia cell lines as well as in mice with a xenograft tumor (Paugh et al, 2008). Moreover, SKI acted synergistically with imatinib mesylate, a tyrosine kinase inhibitor used in the treatment of chronic myelogenous leukemia (Ricci et al, 2009). In light of the data correlating SphK1 upregulation in leukemia with chemoresistance and poor patient prognosis (Sobue et al., 2006), these findings provide ample rationale for further testing of SphK inhibitors, both alone and as adjuvant treatments, for management of leukemia.
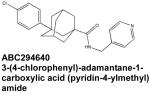
SphK2 expression, unlike that of SphK1, has not typically been correlated with cancer progression in humans. Nevertheless, the orally bioavailable specific SphK2 inhibitor known as ABC294640 has been reported to suppress cell proliferation and migration in a broad panel of tumor cell lines originating from skin, kidney, colon, prostate, and ovarian tissue (French et al, 2010). The potential in vivo applicability of ABC294640 has been explored in a murine model of breast cancer in which it attenuated the tumor growth in a dose-dependent manner while exhibiting a good pharmacological profile, low toxicity, and high target tissue specificity. Reduction in tumor size was correlated with S1P depletion and progressive tumor cell apoptosis (French et al, 2010). Moreover, co-treatment with sorafenib, a tyrosine protein kinase inhibitor approved for use in renal and hepatic cancer, revealed a synergistic anti-tumor effect (Beljanski et al., 2011).
Interestingly, subsequent in vitro studies of ABC294640 have demonstrated effects of this molecule that go beyond the scope of SphK2 inhibition. Surprisingly, ABC294640 has been recently identified as a partial agonist of the estrogen receptor (ER), attenuating ER-mediated transcription of proliferation-stimulating genes and reducing tumor size in vivo in a manner similar to that of the current anti-cancer drug tamoxifen (Antoon et al., 2010) These findings raise the possibility that ABC294640 could prove useful in the treatment of ER-sensitive breast cancer. The emerging off-target effects of ABC294640 may in part explain how this seemingly specific SphK2 inhibitor exhibits efficacy for treatment of mice bearing a variety of types of xenografts despite the fact that SphK2 is not the major isoenzyme responsible for cellular S1P synthesis.
3.2 Multiple sclerosis
As mentioned above, FTY-720 was recently approved by the FDA for the treatment of multiple sclerosis, an inflammatory autoimmune disorder causing demyelination and scarring in the brain and spinal cord. FTY-720 is a sphingosine analogue that is phosphorylated in vivo by SphK2 to generate a S1P mimetic that is able to bind to all of the S1PRs except S1PR2. With respect to treatment of multiple sclerosis, its most important action is believed to be the internalization of lymphocytic S1PR1 and its degradation, leading to prolonged attenuation of the ability of lymphocytes to sense the S1P gradient between the circulation and tissues, which is required for their egress from lymph nodes and lymphoid organs. The sequestration prevents autoreactive T cells from infiltrating the nervous system where they play an important role in the progression of this disease (Mehling et al., 2008). However, Phase II clinical trials of FTY-720 revealed that its peak effectiveness for multiple sclerosis treatment occurs at doses that are suboptimal for lymphopenia induction (Graler, 2010). This unexpected result, together with the finding that S1P receptors are expressed in several types of neuronal cells (Graler, 2010), suggests that FTY-720 could also exert direct neuroprotective effects in the brain. Indeed, a recent study reported that FTY-720 may be able to reduce astrogliosis via the downregulation of S1PR1 in astrocytes (Brinkmann, 2009), and subsequent work in a mouse model of multiple sclerosis demonstrated that the protective effect of FTY-720 was dependent upon astrocytic S1PR1 expression (Choi et al., 2011). Meanwhile, it is becoming clear that the functions of S1P in the nervous system are more complex than previously understood. For example, it was recently discovered that S1P is an essential player in the development of long-term potentiation in the CA3 region of the hippocampus, establishing a connection for the first time between S1P and memory (Kanno et al., 2010). Increasing understanding of the importance of S1P in the CNS and the multiple actions of FTY-720 and other drugs targeting sphingosine kinases suggests this approach deserves consideration for treatment of other neurodegenerative diseases.
3.3 Asthma
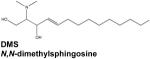
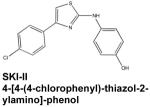
Asthma is a common chronic inflammatory disease characterized by hypercontraction of airway smooth muscle cells in response to inhaled or ingested antigens, accompanied by influx of inflammatory cells to the lungs. Specific roles for S1P have been identified in the hallmark features of this condition. Antigen crosslinking of the high affinity IgE receptor (FcεRI) on mast cells induces degranulation, which results in the release of many inflammatory mediators, as well as their migration. Both depend upon stimulation of SphK1 and formation, secretion of S1P, and autocrine/paracrine activation of S1PR2 (Jolly et al., 2004). Furthermore, S1P is known to also induce bronchial smooth muscle contraction (Chiba et al., 2011). Accordingly, inhibition of SphKs via inhalation of either N,N-dimethylsphingosine (DMS) or SKI reduced both airway hyperresponsiveness and eosinophil infiltration in a rodent model of asthma (Nishiuma et al., 2008). More recent work has established the similar efficacy of another SphK1 inhibitor, SKI-II (4-[4-(4-chlorophenyl)-thiazol-2-ylamino]-phenol). Injection of SKI-II prior to antigen challenge in sensitized mice significantly a meliorated airway hypercontraction (Chiba et al., 2010). However, no effects were noted on antigen-induced inflammatory events such as immune cell infiltration, upregulation of inflammatory cytokines, and elevation of antigen-specific IgE in serum (Chiba et al, 2010). These apparently discrepant observations might be due to non-specific actions of this pharmacological agent. For example, it was recently discovered that while SKI does inhibit SphK1 in vitro, it also induces both proteasomal and lysosomal degradation of SphK1 in cells (Loveridge et al., 2010; Ren et al., 2010), which was not an observed effect of SKI-II. In any case, the therapeutic benefit of SphK1 inhibition in human airway inflammation remains to be determined.
Inhibition of S1PR signaling also shows promise as a therapeutic intervention for asthma. In a murine model, inhalation of FTY-720 by sensitized mice prior to antigen challenge inhibited migration of lung dendritic cells to the lymph nodes and reduced both airway hypercontraction and eosinophilic infiltration (Idzko et al., 2006). Although immune cell trafficking was clearly impacted in this study, there was no systemic lymphopenia, demonstrating that inhibition of S1PR signaling by local application of receptor agonists could ameliorate pathology while avoiding undesirable systemic effects. Interestingly, a recent study suggested that FTY-720 acts not only by inducing S1PR1 internalization but also by inhibiting ceramide synthases, thereby decreasing cellular levels of ceramides, sphingosine, and pro-inflammatory S1P, while increasing levels of dihydrosphingosine and dihydro-S1P, effects reminiscent to those of the classical ceramide synthase inhibitor fumonisin B1 (Berdyshev et al., 2009). As fumonisin B1 has also been shown to improve symptoms in a murine asthma model (Masini et al., 2008), the use of agents targeting the sphingolipid rheostat is a potential avenue for treatment of allergic asthma.
3.4 Rheumatoid arthritis
Rheumatoid arthritis (RA), a systemic autoimmune disorder primarily impacting the synovial joints, is characterized by immune cell infiltration of the synovium followed by upregulation of inflammatory cytokines and tissue destruction. Expression of SphK2 is elevated in synovial fibroblasts (Kamada et al., 2009) and S1PR1 is more strongly expressed in synovial lining cells, vascular endothelial cells, and inflammatory mononuclear cells of RA synovium compared with osteoarthritis synovium (Kitano et al., 2006a). Moreover, S1P enhanced expression of cyclooxygenase 2 (COX-2) and production of PGE2 induced by stimulation with TNF-α or IL-1β in RA synoviocytes (Kitano et al, 2006a). SphK1 and SphK2 expression are also increased in RA synovium. Moreover, inflammatory events such as hypertrophy, COX-2 expression, and prostaglandin production in affected joint tissue are dependent on S1PR1 signaling (Kamada et al, 2009; Kitano et al., 2006b). These results suggest that SphK1 inhibition as well as S1PR antagonism could be viable therapeutic strategies for RA.
Indeed, the pan SphK inhibitor DMS significantly reduced inflammatory cytokine levels in cultured peripheral blood mononuclear cells isolated from RA patients and also attenuated synovial inflammation, inflammatory cytokine production, and tissue destruction in a collagen-induced murine model of the disease (Lai et al., 2008). In collagen-induced arthritis in mice as well as in adjuvant-induced arthritis in rats, the SphK2 inhibitor ABC294640 was reported to reduce joint pathology, inflammatory infiltration, and synovial hyperplasia (Fitzpatrick et al., 2011). ABC294640 also displayed additive effects when used in combination therapy with methotrexate (MTX), the current anti-RA drug of choice. It is still not clear how inhibition of SphK2 ameliorates the symptoms of allergic arthritis in these models.
Modulation of S1PR1 signaling with FTY-720 has also demonstrated efficacy in arthritis models, reducing hindpaw edema, joint destruction, and lymphocyte invasion in two different rat models of the disease (Snider et al, 2010). Studies with SKG mice, which spontaneously develop T cell-mediated chronic autoimmune arthritis due to a mutation of the T cell signal transduction molecule ZAP-70, suggest that FT Y-720 inhibits a rthritis development by sequestering CD4+ T cells in the thymus, enhancing Th2 rather than Th1 immune responses, and inhibiting synovial prostaglandin production (Tsunemi et al., 2010). Both the shift toward Th2 differentiation and the downregulation of prostaglandin production represent new modes of action for the observed immunosuppressive and anti-inflammatory effects of FTY-720.
Targeting of S1P lyase, a cellular S1P-degrading enzyme, is alternative approach to regulate inflammatory processes. Inhibition of S1P lyase by tetrahydroxybutylimidazole (THI) increased circulating S1P levels and induced lymphopenia similar to the effect of FTY-720 (Schwab et al., 2005). This has sparked interest in the investigation of S1P lyase inhibitors as therapeutic agents in autoimmune disease (Graler, 2010). A THI-related SPL inhibitor, designated LX2931, was subsequently developed and shown to reduce synovial inflammation and tissue destruction in mouse models of arthritis (Bagdanoff et al, 2010). LX2931 demonstrated a favorable safety and efficacy profile in a Phase 1 trial and is now in Phase II clinical trials for RA (Bagdanoff et al., 2010).
3.5 Inflammatory bowel disease
Ulcerative colitis and Crohn’s disease, collectively referred to as inflammatory bowel disease (IBD), feature uncontrolled immune cell invasion of the intestinal tract accompanied by overproduction of inflammatory cytokines (Snider et al, 2010). The importance of the SphK/S1P axis in the pathophysiology of these conditions has been confirmed by studies with SphK1 knockout mice. A body of work has accumulated documenting the efficacy of both modulation of S1PR signaling and pharmacological and genetic approaches to inhibit SphKs in several different murine models of IBD. For example, in dextran sulfate sodium (DSS)-induced colitis, SphK1−/− mice had significantly decreased systemic inflammation and localized immune cell infiltration compared to wild type animals (Snider et al, 2010). The SphK2 inhibitor ABC294640 has been reported to attenuate trinitrobenzene sulfonic acid-mediated Crohn’s disease progression in mice and rats (Maines et al., 2010), although there is no evidence that SphK2 contributes to increased S1P levels in RA. Strikingly, in this study ABC294640 had a similar spectrum of actions to prednisolone, a corticosteroid notoriously known for its side effects that is currently used in treatment of human IBD patients.
Resveratrol, a polyphenol found in the skin of red fruits, is a relatively new player with regard to S1P generation and IBD. This molecule, which initially attracted attention as a possible mediator of the health benefits of red wine consumption, has since been shown to possess antioxidant, anti-proliferative, and anti-inflammatory properties that have been largely attributed to the downregulation of both COX-2 and NF-κB signaling (Das et al., 2007). Recent studies indicate SphK1 inhibition may also contribute to the potent anti-inflammatory activity of resveratrol. In primary neutrophils stimulated with C5 anaphylatoxin (C5a), resveratrol inhibited SphK1 activity within 5 minutes, preventing its translocation to the plasma membrane. Furthermore, resveratrol abrogated C5a-mediated pathology in a mouse peritonitis model by attenuating leukocyte migration, mast cell degranulation, and inflammatory cytokine production (Issuree et al., 2009).
As disorders of intestinal inflammation have been correlated with the development of colon cancer (Langholz et al., 1992), it should be noted that suppression of intestinal inflammation by inhibiting SphKs has the potential to prevent subsequent colon carcinogenesis. The SphK2 inhibitor ABC294640 has recently been found to ameliorate not only DSS-induced murine colitis but also colitis-associated colon cancer (Chumanevich et al., 2010). While the mechanism underlying this anti-cancer activity of ABC294640 may stem from effects on inflammation, it is also possible that it has protective effects in intestinal epithelial cells.
Although decreasing S1P with SphK inhibitors can have beneficial effects, it is possible that this might be a counter-indicated approach in specific situation where increased S1P may actually contribute to normal anti-inflammatory activity. For example, SphK1 upregulation was recently identified as the mechanism by which isoflurane, a volatile anesthetic, prevents intestinal inflammation and dysfunction after acute kidney injury (AKI) (Kim et al., 2011). In a bilateral nephrectomy model of AKI, isoflurane significantly attenuated multiple hallmarks of intestinal injury, including epithelial necrosis, villous swelling, apoptosis, vascular permeability, and expression of inflammatory cytokines (Kim et al, 2011). These protective effects were accompanied by increased SphK1 expression and S1P in small intestinal crypts, and were abrogated in mice receiving a SphK1 inhibitor prior to surgery (Kim et al, 2011). However, a mechanism for the observed suppression of inflammatory signaling by isoflurane remains elusive.
3.6 Sepsis
Given the well-established role of S1P in inflammatory signaling, it is unsurprising that SphK1 is involved in sepsis, an often fatal condition in which overproduction of inflammatory cytokines leads to systemic inflammation, vascular leakage, and multiple organ failure. A specific inhibitor of SphK1, called compound 5c, prevented NF-κB activation and inflammatory cytokine release from human macrophages activated with lipopolysaccharide or bacterial lipoprotein (Puneet et al, 2010). Moreover, treating mice with 5c prior to induction of endotoxic shock or sepsis protected against systemic inflammation, tissue damage, inflammatory cytokine production, and increased mortality (Puneet et al, 2010), indicating the therapeutic potential of SphK1 inhibitors in sepsis.
4. Conclusion
The development of pharmacological agents targeting enzymes that produce and metabolize S1P as well as its receptors has potentially wide-reaching benefits due to the diverse cellular functions of this lipid mediator. Some of the S1P-centric compounds investigated thus far exhibit qualities amenable to clinical use, such as good oral bioavailability, relatively low toxicity, and acceptable side effect profiles when compared to currently favored treatments. Moreover, such inhibitors have excellent promise for use as adjuvant therapies, as their molecular targets are distinct from those of conventional drugs. In addition to enhancing our overall understanding of the complex actions of S1P, advancements in the development of agents targeting this powerful signaling metabolite represent exciting progress toward effective clinical management of cancer, inflammatory disorders, and autoimmune disease.
Acknowledgements
We apologize to authors whose work has not been cited here owing to space limitations. This work was supported by grants from the US National Institutes of Health R01CA61774, R37GM043880, R01AI50094, 1U19AI077435 (to S.S.).
Abbreviations
- AKI
acute kidney injury
- COX-2
cyclooxygenase-2
- DMS
N,N-dimethylsphingosine
- ER
estrogen receptor
- GBM
glioblastoma multiforme
- HDAC
histone deacetylase
- IBS
inflammatory bowel disease
- IgE
immunoglobulin E
- MTX
methotrexate
- NF-κB
nuclear factor kappa B
- PGE2
prostaglandin E2
- RA
rheumatoid arthritis
- S1P
sphingosine-1-phosphate
- S1PR
sphingosine-1-phosphate receptor
- SphK
sphingosine kinase
- siRNA
small interfering RNA
- THI
tetrahydoxybutylimidazole
- TRAF2
TNF receptor associated factor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
The authors declare no competing financial interests.
References
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, Rhodes LV, Ashe HB, Wiese TE, Smith CD, Burow ME, Beckman BS. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdanoff JT, Donoviel MS, Nouraldeen A, Carlsen M, Jessop TC, Tarver J, Aleem S, Dong L, Zhang H, Boteju L, Hazelwood J, Yan J, Bednarz M, Layek S, Owusu IB, Gopinathan S, Moran L, Lai Z, Kramer J, Kimball SD, et al. Inhibition of sphingosine 1-Phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone Oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J. Med. Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- Beckham TH, Elojeimy S, Cheng JC, Turner LS, Hoffman SR, Norris JS, Liu X. Targeting sphingolipid metabolism in head and neck cancer: rational therapeutic potentials. Expert Opin. Ther. Targets. 2010;14:529–539. doi: 10.1517/14728221003752768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest. New Drugs. 2011 doi: 10.1007/s10637-010-9452-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Takeuchi H, Sakai H, Misawa M. SKI-II, an inhibitor of sphingosine kinase, ameliorates antigen-induced bronchial smooth muscle hyperresponsiveness, but not airway inflammation, in mice. J. Pharmacol. Sci. 2010;114:304–310. doi: 10.1254/jphs.10202fp. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Takeuchi H, Sakai H, Misawa M. Sphingosine-1-phosphate augments agonist-mediated contraction in the bronchial smooth muscles of mice. Pharmacol. Rep. 2011;63:544–547. doi: 10.1016/s1734-1140(11)70521-7. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Carvajal RD, Merrill AH, Jr., Gonen M, Cane LM, Schwartz GK. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin. Cancer Res. 2011;17:2484–2492. doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LR, Green C, Frauenhoffer EE, French KJ, Zhuang Y, Maines LW, Upson JJ, Paul E, Donahue H, Mosher TJ, Smith CD. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology. 2011;19:75–87. doi: 10.1007/s10787-010-0060-6. [DOI] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell Physiol. Biochem. 2010;26:79–86. doi: 10.1159/000315108. [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol. Cancer Ther. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issuree PD, Pushparaj PN, Pervaiz S, Melendez AJ. Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009;23:2412–2424. doi: 10.1096/fj.09-130542. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by Fc{epsilon}RI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada K, Arita N, Tsubaki T, Takubo N, Fujino T, Soga Y, Miyazaki T, Yamamoto H, Nose M. Expression of sphingosine kinase 2 in synovial fibroblasts of rheumatoid arthritis contributing to apoptosis by a sphingosine analogue, FTY720. Pathol. Int. 2009;59:382–389. doi: 10.1111/j.1440-1827.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T, Nakamura S. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience. 2010;171:973–980. doi: 10.1016/j.neuroscience.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- Kim M, Park SW, D’Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against bilateral nephrectomy-induced liver and intestine dysfunction. Am. J. Physiol. Renal Physiol. 2011;300:F167–F176. doi: 10.1152/ajprenal.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: Regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006a;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006b;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- Loveridge C, Tonelli F, Leclercq T, Lim KG, Long JS, Berdyshev E, Tate RJ, Natarajan V, Pitson SM, Pyne NJ, Pyne S. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol. Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology. 2010;18:73–85. doi: 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, Comhair SA, Li D, Cuzzocrea S, Matuschak GM, Salvemini D. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J. Pharmacol. Exp. Ther. 2008;324:548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RL, Kappos L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, Salunkhe V, Teissie J, Malavaud B, Waxman J, Cuvillier O. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010;70:8651–8661. doi: 10.1158/0008-5472.CAN-10-1388. [DOI] [PubMed] [Google Scholar]
- Puneet P, Yap CT, Wong L, Lam Y, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell Physiol. Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- Ricci C, Onida F, Servida F, Radaelli F, Saporiti G, Todoerti K, Deliliers GL, Ghidoni R. In vitro anti-leukaemia activity of sphingosine kinase inhibitor. Br. J. Haematol. 2009;144:350–357. doi: 10.1111/j.1365-2141.2008.07474.x. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92:707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue S, Iwasaki T, Sugisaki C, Nagata K, Kikuchi R, Murakami M, Takagi A, Kojima T, Banno Y, Akao Y, Nozawa Y, Kannagi R, Suzuki M, Abe A, Naoe T, Murate T. Quantitative RT-PCR analysis of sphingolipid metabolic enzymes in acute leukemia and myelodysplastic syndromes. Leukemia. 2006;20:2042–2046. doi: 10.1038/sj.leu.2404386. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CE, Takabe K, Nagahashi M, Milstien S, Spiegel S. Targeting Sphingosine-1-Phosphate in Hematologic Malignancies. Anticancer Agents Med. Chem. 2011 doi: 10.2174/187152011797655122. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Suzuki K, Osawa S, Isshiki H, Hori Y, Shimada N, Higo T, Yokoshima S, Fukuyama T, Lee VM, Trojanowski JQ, Tomita T, Iwatsubo T. BACE1 Activity Is Modulated by Cell-Associated Sphingosine-1-Phosphate. J. Neurosci. 2011;31:6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 2010;136:197–204. doi: 10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J. Neuropathol. Exp. Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJP, Thangada S, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]


