Abstract
Chronic social disruption stress (SDR) exacerbates acute and chronic phase Theiler’s murine encephalomyelitis virus (TMEV) infection, a mouse model of multiple sclerosis. However, the precise mechanism by which this occurs remains unknown. The present study suggests SDR exacerbates TMEV disease course by priming virus-induced neuroinflammation. It was demonstrated that IL-1β mRNA expression increases following acute SDR; however, IL-6 mRNA expression, but not IL-1β, is upregulated in response to chronic SDR. Furthermore, this study demonstrated SDR prior to infection increases infection related central IL-6 and IL-1β mRNA expression, and administration of IL-6 neutralizing antibody during SDR reverses this increase in neuroinflammation.
Keywords: Multiple sclerosis, TMEV, SDR, stress, IL-6, neuroinflammation
1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune demyelinating disease of the central nervous system (CNS) affecting approximately 2 million people worldwide (Anderson et al., 1992; Noonan et al., 2002; Sospedra and Martin, 2005; WHO and MSIF, 2008). Despite the prevalence of MS, the etiology of this disease is uncertain. Research suggests that it arises from complex interactions between environmental and genetic factors (Kurtzke and Hyllested, 1987; Monteyne et al., 1997, 1998; Noseworthy et al., 2000; Sospedra and Martin, 2005). Viral infection (e.g., herpes, rubella, mumps) is one environmental factor likely to be associated with later disease development (Acheson, 1977; Challoner et al., 1995; Gilden, 2005; Hernan et al., 2001; Jilek et al., 2008; Kurtzke and Hyllested, 1987; Sospedra and Martin, 2005). Stress appears to be another environmental trigger.
Periods of psychological stress have been associated with the development and exacerbation of MS (Ackerman et al., 2000, 2002; Brown et al., 2005, 2006; Grant et al., 1998; Li et al., 2004; Morh et al., 2004; Warren et al., 1982). For example, a study of bereaved parents showed that the loss of a child resulted in an increased risk for developing MS and the risk was further increased for those who unexpectedly lost a child (Li et al., 2004). Furthermore, a meta-analysis of 14 studies evaluating stress and MS conclude that stressful life events significantly increase the risk of disease exacerbation (Mohr, 2007). This effect, however, may depend on the type of stress. While mild to moderate chronic stressors have repeatedly been associated with disease risk, the threat of missile attack during the Persian Gulf War, a severe life-threatening stressor, was found to reduce the rate of relapse (Nisipeanu and Korczyn, 1993). These differential effects may occur due to the complex interaction between stress and immune function.
The mechanisms by which stress alters susceptibility to and disease course of multiple sclerosis have yet to be fully elucidated. Due to the complexity of this relationship and the ethical limitations of human studies, this area of research benefits from the investigation of animal models of MS, such as Theiler’s murine encephalomyelitis virus (TMEV) infection. Intracerebral inoculation of TMEV induces a biphasic disease of the CNS in susceptible strains of mice (e.g., SJL and Balb/cJ). The acute phase is characterized by viral-mediated encephalomyelitis, and the chronic phase is characterized by a demyelinating disease that is similar to MS. During the acute phase mice exhibit sickness behavior and polio-like hindlimb impairment. This phase remits in approximately 4 weeks. Susceptible strains fail to mount an effective immune response to early infection (Lipton and Melvold, 1984; Rodriguez et al., 1983; Oleszak et al., 2004) and, approximately 3-5 months after infection, develop chronic phase TMEV, characterized by demyelination (Aubert et al., 1987; Brahic et al., 1981; Campbell et al., 2001; Fiette et al., 1995; McGavern et al., 2000; Rodriguez et al., 1996; Welsh et al., 1987).
Our laboratory has previously demonstrated that chronic social disruption (SDR) prior to TMEV infection exacerbates both the acute and chronic phases of the disease (Johnson et al., 2004, 2006; Meagher et al., 2007; Young et al., 2008). Specifically, SDR has been shown to enhance functional impairment and increase CNS inflammatory lesions during the acute phase of disease. Furthermore, SDR has been shown to reduce viral clearance. During the chronic phase of disease SDR has been shown to result in increased motor impairment and lead to higher circulating levels of antibodies to virus and myelin. The interaction been stress and TMEV appears to be mediated by SDR-induced increases in the proinflammatory cytokine interleukin-6 (IL-6), which is up-regulated by both TMEV infection (Chang et al., 2000; Mi et al., 2006; Sato et al., 1997; So et al., 2006; Theil et al., 2000) and SDR (Avistur et al., 2002; Johnson et al., 2006; Meagher et al., 2007;Merlot et al., 2003, 2004; Stark et al., 2002). Central administration of a neutralizing antibody to IL-6 during the stress exposure period attenuated and/or reversed stress-induced exacerbations of acute TMEV, including enhanced sickness behavior, increased CNS inflammatory lesions, and reduced viral clearance (Meagher et al., 2007). This suggests that increases in IL-6 during the stress exposure period lead to a disrupted immune response to TMEV infection.
Infection results in a robust up-regulation of proinflammatory cytokines, such as IL-6, which are critically involved in the orchestration of the early immune response to TMEV infection. Given that susceptible strains of mice exhibit a higher level of proinflammatory cytokines than resistant mice (Chang et al., 2000; Mi et al., 2006; Sato et al., 1997; Theil et al., 2000) and that SDR has been shown to enhance inflammation and exacerbate disease course (Johnson et al., 2004, 2006, Meagher et al., 2007), we suggest that the adverse effects of SDR on TMEV infection are mediated by the sensitization of virus-induced cytokine expression. Specifically, we hypothesize that social stress-induced increases in IL-6 enhance the CNS proinflammatory cytokine (i.e., IL-1β and IL-6) response to TMEV infection. Furthermore, given prior research suggests stress-induced increases in inflammation are mediated by these cells (Frank et al., 2007; Nair and Bonneau, 2006; Sugama et al., 2007), we hypothesize that stress sensitizes the inflammatory response by priming microglia.
The present study began by demonstrating that social disruption stress can exacerbate sickness behaviors and motor impairment following Theiler’s virus infection (Experiment 1) and modulate central inflammatory processes (Experiment 2). Then we demonstrated that stress prior to infection enhances the central inflammatory response to viral infection and that the protective effects exerted by IL-6 neutralizing antibody treatment (nAbTx) during the stress exposure period acts by reversing this enhancement in inflammatory cytokine expression (Experiment 3). This study supports the hypothesis that SDR exacerbates TMEV infection by priming the neuroinflammatory response to disease.
2. Methods
2.1 Animals
Male BALB/cJ mice (22-24-days-old; Jackson Labs, Bar Harbor, ME) were maintained on a 12-h light/dark cycle (lights off at 5pm) with continuous white noise and ad libitum access to food and water. Subjects were individually housed until they recovered from cannulation surgery, at which time they were housed 2-3 per cage. Intruder mice were retired BALB/cJ breeders (Jackson Labs, Bar Harbor, ME). All animal care protocols were in accordance with the Texas A&M University Laboratory Animal Care and Use Committee (ULACC).
2.2 Cannulation and IL-6 neutralizing antibody treatment
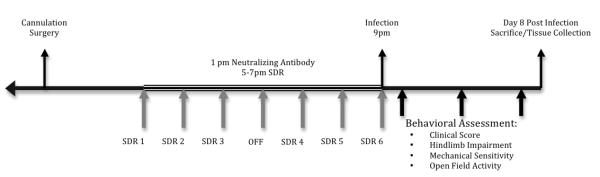
Mice underwent cannulation surgery the day after they arrived (see figure 1 for a general experimental design diagram). They were placed into an induction chamber and exposed to 5% isoflurane gas. After induction they were moved to a stereotaxic apparatus and maintained on 2% isoflurane. A 33-gauge guide cannula (PlasticsOne, Roanoke, VA, C315GS-2/SPC) was implanted into the left ventricle (+1.0 mm lateral and −0.4 mm rostral to bregma, and 1.75 mm from the top of the skull). They were provided with softened food and acetaminophen treated water (162.5 mg/L) for at least 48 hours prior to being group housed.
Figure 1.
This diagram presents a general experimental timeline of experimental manipulations used in this study.
IL-6 neutralizing antibody (polyclonal goat anti-mouse; 10 ng; R&D Systems, Madison, WI; AF-406-NA) or goat immunoglobulin G (IgG; Santa Cruz Biotechnology, Inc #SC-2342) was administered by intracerebroventricular (ICV) injection via the implanted guide cannula 4 hours prior to each SDR session. A volume of 2 μL was administered at a rate of 1 μL/min via a microinjection pump.
2.3 Social disruption stress
Cages were randomly assigned to either the control or SDR condition. SDR began at the onset of the dark cycle. During the SDR procedure, control mice remained undisturbed in their home cage while SDR mice were moved to another room and an aggressive male intruder was introduced to the cage for 2 hours. For chronic SDR (SDR6) this occurred for a total of 6 sessions (three consecutive nights, then one off, followed by three consecutive nights), for acute SDR (SDR1) only a single session occurred. The sessions were monitored by video camera to ensure that the intruder demonstrated dominant behavior and the residents demonstrated submissive behavior. If intruders failed to attack within 10 min they were replaced and the session was continued for the remaining 2 hours.
2.4 Virus and infection
The BeAn strain of Theiler’s virus was obtained from Dr. H.L. Lipton (Department of Microbiology-Immunology, University of Illinois, Chicago, IL) and was propagated in L2 cells (Welsh et al., 1987). Mice were anesthetized with isoflurane and inoculated with 5×104 pfu of TMEV in a 20 μL volume into the right mid-parietal cortex (Campbell et al., 2001; McGavern et al., 1999, 2000; Theil, 2000). In experiment 1 and 3, inoculation occurred 2 hours following the final session of SDR (21:00 h), animals in experiment 2 were uninfected.
2.5 Behavioral assessment
We have previously observed a variety of sickness behaviors in BALB/cJ mice in response to acute Theiler’s virus infection, including acute weight loss, reduced activity, mechanical allodynia, and hindlimb impairment (Johnson et al., 2004, 2006; Meagher, 2007). Given we generally observe a drop in body weight immediately following infection, we assessed body weight daily at 09:00 h using a scale sensitive to 0.01g (Scout® Pro Portable Balance, Ohaus, Pine Brook, NJ).
2.5.1 Mechanical sensitivity
We also generally observe a stress-induced enhancement of virus-induced sensitivity to typically non-noxious stimuli, also known as allodynia. Therefore, mechanical allodynia was assessed prior to infection and on day 1, 4, and 7 post-infection by placing the mice in transparent circular chambers positioned on a raised mesh screen (2 mm gauge); nylon filaments (von Frey monofilaments; Stoelting, Wooddale, IL; 0.008 – 2.0 grams) were applied to the hindpaw to determine their withdrawal threshold. Starting with the smallest filament, each filament was applied to the left and right hindpaws in an ABBA manner. Subjects received three trials: ascending, descending, and ascending (Dixon, 1980). Prior to testing they were habituated to the apparatus for 20 min.
2.5.2 Open field activity
SDR prior to infection has previously been shown to suppress horizontal activity in an open field (Meagher et al., 2007). Therefore, open field horizontal activity was assessed using six optical beam activity monitors (Model RXYZCM-16), equipped with two banks of eight photocells on each wall. The boxes were interfaced with a digital-multiplexor and Versamax software (Model DCM-4, Omnitech Electronics, Columbus, OH). Subjects were habituated to the chambers for 60 min prior to baseline data collection. Test sessions were conducted in the dark beginning at 15:00 h for 30 min with white noise to mask extraneous disturbances.
2.5.3 Hindlimb impairment and clinical scores
Furthermore, it has previously been shown that SDR prior to infection results in increased hindlimb impairment and clinical scores. Therefore, these measures were assessed at baseline and on days 1, 4, and 7 post-infection by a rater blind to experimental condition. Details on these scales have been presented elsewhere (Johnson et al., 2004). Briefly, the hindlimb impairment scale assesses weakness and paresis by observing alterations in locomotor function on an inverted grid. A separate score was given to each hindlimb (0=healthy; 1=slight weakness in grip; 2=clear weakness in grip; 3=slight paralysis; 4=moderate paralysis; 5=complete paralysis with muscle tone; 6=complete paralysis with no muscle tone). These scores were then summed to achieve the HLI score. Clinical scores were given on a 0-4 scale that considers the degree of hunched posture and piloerection.
2.5,4 Fur score
In experiment 1 degree of wounding was assessed using a fur score measure adapted from Merlot et al. (2003). Before each session of SDR mice were assigned a score from 1-4 (1=being well groomed and polished; 1.5=fur not well polished or a bit ruffled; 2=bristling of the fur; 2.5=one small bite; 3=numerous marks/bites with bristling of fur; 4=one or more visible wounds). Mice were also observed under red light immediately following each session of SDR for any visible wounds.
2.6 Tissue Collection and Preparation
At the termination of each experiment subjects were injected with 50-mg/kg pentobarbital and perfused. The brain and spleen were collected. A mouse brain matrix (PlasticsOne, Roanoke, VA) was used to assist with the microdissection of the hippocampus in experiment 2. Total RNA from CNS tissue was isolated and purified through the use of Qiagen lysis reagent and RNeasy kit (Qiagen, Valencia, CA) with on-column DNase digestion according to manufacturer’s instructions. cDNA was generated through the use of an RNA-to-cDNA kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. TaqMan probes and primers from Applied Biosystems were used. RT-PCR was run to assess mRNA expression levels on markers of inflammation, including IL-6 (Mm00446191_m1), IL-1β (Mm99999061_mH), IL-10 (Mm00439615_g1), TNF-α (Mm00443258_m1), CD11b/ITGAM (Mm01271259), and IFN-γ (Mm01168134_m1). β-actin served as a control and the 2(−ΔΔCT) method was used to determine fold difference in expression. Serum was collected at the termination of experiment 3 for analysis of IL-6 and IL-1β by ELISA (R&D Systems, Minneapolis, MN) per the manufacturer’s instructions.
2.7 Statistical analysis
Data are presented as mean+/−SEM. Analysis of variance (ANOVA) was used to evaluate the difference across conditions. Repeated measures ANOVA were used as appropriate. These analyses were followed by post hoc mean comparisons.
3. Results
3.1 Experiment 1: SDR exacerbates sickness behavior in TMEV infection
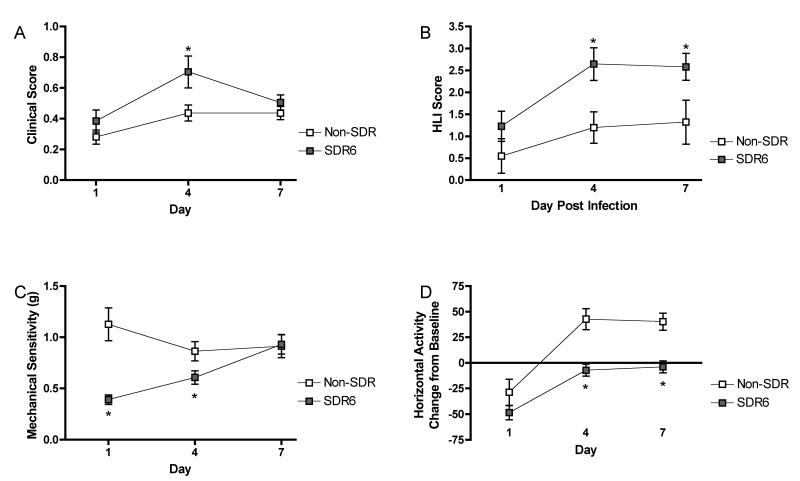
To verify that chronic SDR (SDR6) prior to infection exacerbates sickness behavior, subjects were exposed to either SDR6 or remained undisturbed in their home cage (n=10/group). All were infected 2 hours following the final SDR session. Behavioral data was collected prior to infection and for 7 days post infection. As previously described, SDR6 affected mechanical sensitivity, open field horizontal activity, clinical score, and hind limb impairment (fig 2). For mechanical sensitivity, there was a significant main effect of day and stress as well as a day by stress interaction, all Fs >3.9, p < .05. Post hoc analyses showed that stress resulted in increased mechanical sensitivity on day 1 and 4 post infection (fig 2A). Open field horizontal activity also showed a significant main effects of day and stress as well as a day by stress interaction, all Fs > 3.3, p < .05. Post hoc analyses showed that stress resulted in reduced activity on day 4 and 7 post infection (fig 2B).
Figure 2.
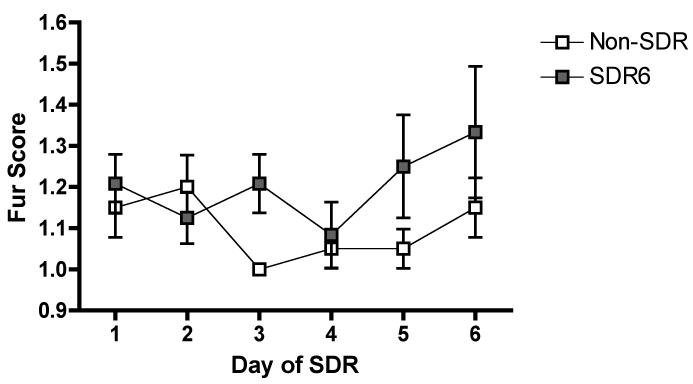
A repeated measures ANOVA showed no significant effect of social disruption stress on fur score.
Furthermore, a significant main effect of day and stress, Fs > 6.8, p <.05, was observed for hind limb impairment. Post hoc analysis revealed that stress increased hind limb impairment on day 4 and 7 post infection (fig 2C). Finally, there was a significant main effect of day, F(2,40)=12.8, p<.001, on clinical score. The effect of stress and the stress by day interaction failed to reach significance (p = .08 and .09, respectively) (fig 2D).
In addition to the replication of our previous findings, wounding and fur score data was evaluated in this experiment. Prior data suggest that wounding may be necessary for the adverse effects of SDR to be observed (Avistur et al., 2001; Merlot et al., 2003). However, personal observations suggest that little wounding is observed during SDR in BALB/cJ mice. This was confirmed by daily analysis of fur scores (see fig 3), which showed a non-significant effect of SDR6 (p>0.05). Furthermore, wounding was assessed immediately following SDR and only one tail bite was observed over the course of SDR. This level of wounding is significantly less than that observed in other laboratories and suggests that the effect of SDR on TMEV infection is not dependent upon wounding.
Figure 3.
SDR exacerbates sickness behaviors during acute TMEV infection. Repeated measures ANOVA indicated that SDR resulted in increased mechanical sensitivity (A), decreased open field horizontal activity (B), and increased hindlimb impairment (C) following infection. Furthermore, it showed a non-significant trend toward increased clinical scores (D). Asterisks denote significant differences between groups as indicated by post hoc means comparisons.
3.2 Experiment 2: SDR Modulate Central Inflammatory Processes
We hypothesized that SDR exacerbates TMEV infection by enhancing virus-induced proinflammatory cytokine release. Experiment 2 compared the impact of chronic (SDR6) and acute (SDR1) exposure to SDR on markers of CNS inflammation to home cage controls in uninfected subjects (n=15-18/group). Prior research has shown that SDR results in elevated IL-6 in sera and IL-1β in the lung (Curry et al., 2010); however less is understood about the impact of SDR on proinflammatory cytokines in the CNS. Therefore, this experiment focused on examining gene expression changes within the hippocampus. We focused on this brain region because it is highly sensitive to the effects of stress, due to its high levels of glucocorticoid receptors, and because it has been shown to be essential in the regulation of stress-induced inflammation (De Kloet et al., 1998; Frank et al., 2007). Uninfected subjects exposed to chronic SDR (SDR6), acute SDR (SDR-1), or non-SDR, were sacrificed at 0, 2, and 12 hours after SDR exposure. The hippocampus was micro-dissected for analysis of IL-6, IL-1β, and CD11b mRNA expression.
3.2.1 IL-6 mRNA expression was increased following chronic SDR
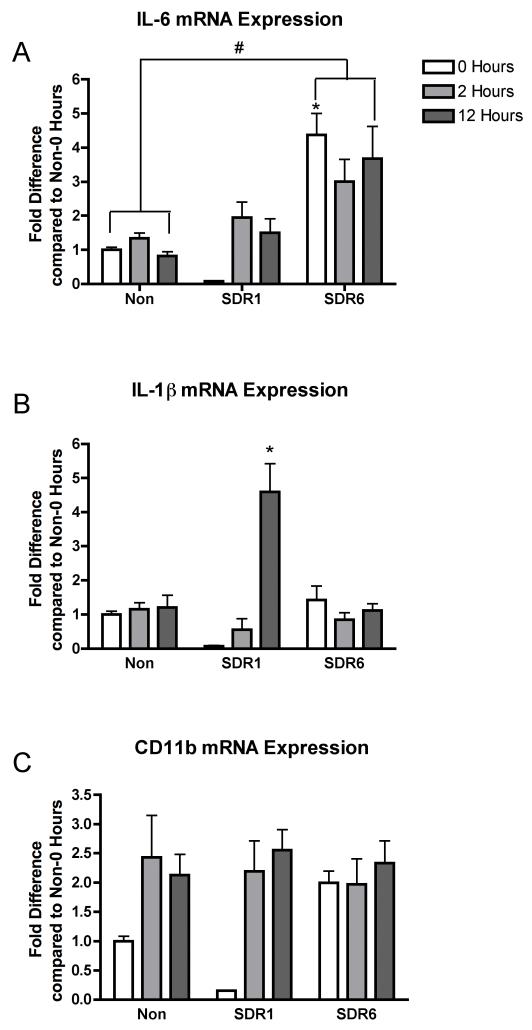
An ANOVA revealed a significant effect of SDR condition on IL-6 mRNA levels, F(2,50)=20.487, p < .001 (fig. 4A). Post hoc analyses reveal that the chronic SDR (6 sessions) condition was significantly different from home cage control condition. Further post hoc analyses revealed that SDR6 results in a significant increase in IL-6 mRNA, compared to the non-SDR and SDR1 conditions at 0 hours. Furthermore, IL-6 mRNA was increased at 12 h in the SDR6 condition compared to the non-SDR condition. This suggests that the IL-6 response was enhanced over the six SDR sessions and remained elevated for at least 12 hours following the last session of chronic SDR.
Figure 4.
SDR results in altered CNS inflammatory cytokine expression in uninfected subjects. An ANOVA followed by post hoc means comparisons indicated that IL-6 is elevated by exposure to chronic SDR (SDR6) (A), while a single session of SDR (SDR-1) resulted in an elevation of IL-1β (B). No significant change in CD11b, a cell surface marker increased in activated microglia, was observed (C). Asterisks indicate significant differences between groups.
3.2.2 IL-1β mRNA expression increased following acute SDR
A significant effect of time, F(2,49)=11.063 (p < .001), and a significant time by SDR condition interaction, F(4,49)=1.838, (p<.001), was observed for IL-1β mRNA levels (fig. 4B). Post hoc analyses reveal a significant elevation in IL-1β 12 h after SDR1 compared to all other groups at all other time points. Thus, while SDR induced a short-term increase in the IL-1β response after one SDR session, the response declines by the sixth session of SDR.
3.2.3 CD11b mRNA expression was not significantly altered by SDR
Furthermore, an ANOVA revealed a significant effect of time, F(2,49)=6.447 (p<.01) on CD11b, a marker of microglia activation. The time by SDR condition interaction failed to reach significance (p=.16), however, the data suggests that repeated exposure to SDR may lead to increased CD11b mRNA expression after six sessions when compared to the non-SDR condition (fig. 4C).
3.3 Experiment 3: Stress-Induced Priming of the CNS Inflammatory Response to TMEV is Reversed by IL-6 neutralizing antibody administered during SDR
This experiment was designed to determine whether exposure to SDR6 increases inflammation during acute TMEV infection and whether blocking the IL-6 response during the stress exposure period could reverse this inflammatory response. A 2 (SDR6 and Non-SDR) by 2 (nAbTx and control-IgG) experimental design was used (n=12/group). Subjects received microinjections of either IL-6 neutralizing antibody or IgG 4 hours prior to each SDR session. Two hours after the final SDR6 session, all subjects were intracranially infected with Theiler’s virus (Welsh et al., 1987). Body weight, clinical score, and hind limb impairment data were collected to examine the effect of SDR6 and antibody treatment on infection. On day 8 post-infection the brain and spleen were collected in order to determine if prior exposure to SDR enhances the inflammatory response to TMEV infection and if the protective effects of IL-6 neutralization may occur through reversing this effect.
3.3.1 SDR and IL-6 neutralizing antibody affect clinical score and hindlimb impairment
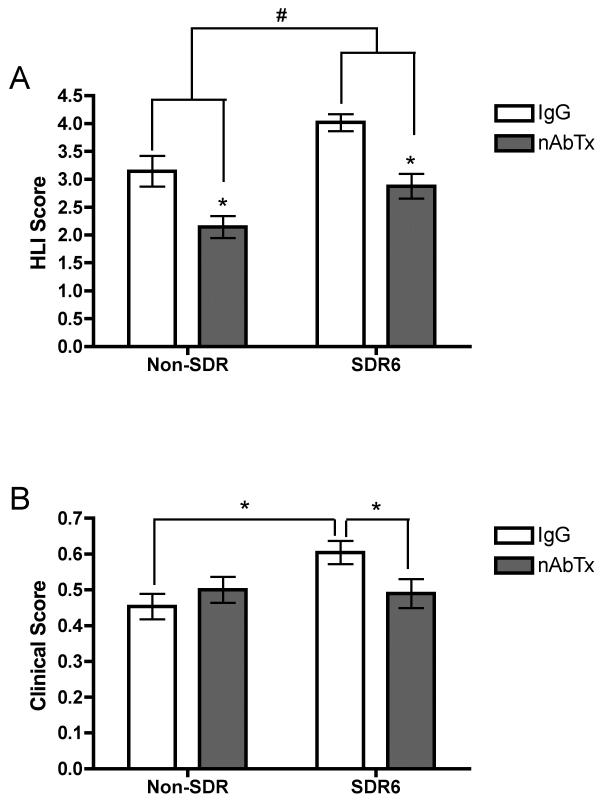
As anticipated, we saw a significant drop in body weight in all groups on day one post infection, t=−8.457, p<.001 (data not shown). A repeated measures ANOVA on hindlimb impairment revealed a significant main effect of time, nAbTx, and SDR6, all Fs > 4.8, ps< .01, and a significant time by nAbTx condition interaction, F(2,88) = 4.817, p ≤ .01 (fig 5A). For clinical score, a repeated measures ANOVA revealed significant main effects of time and SDR condition, Fs > 6.1, ps <.05, and a significant interaction between SDR condition and nAbTx condition, F(1,44)=7.488, p < .01 (fig 5B). Consistent with prior work from our laboratory, post hoc analysis indicate that SDR6 enhanced clinical score and hind limb impairment on day 4 post-infection and the IL-6 nAbTx reversed these effects
Figure 5.
Neutralizing antibody to IL-6 (nAbTx) prevents the adverse effects of SDR on acute TMEV. An ANOVA followed by post hoc mean comparisons indicated that SDR exacerbates both hindlimb impairment (A) and clinical score (B) and administration of IL-6 neutralizing antibody reversed these adverse effects. Asterisks indicate significant differences between groups.
3.3.2 SDR and IL-6 neutralizing antibody alter TMEV-induced central IL-6 and IL-1β mRNA expression
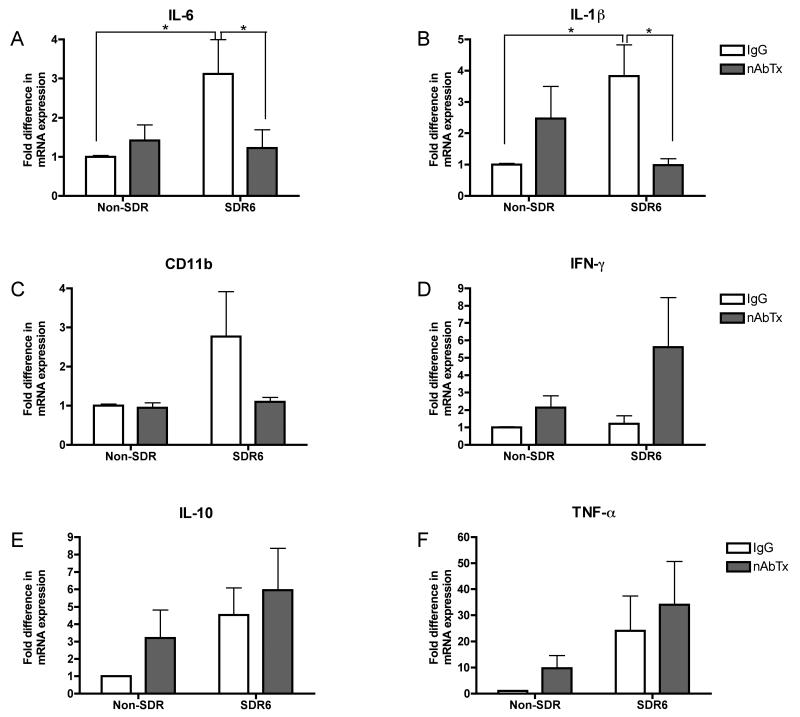
An ANOVA conducted on IL-6 mRNA expression levels in the brain revealed a significant interaction between SDR condition and neutralizing antibody condition, F(1,43)=4.135, p<.05 (fig. 4A). Post hoc tests revealed that the SDR6-IgG group has a significantly higher level of IL-6 mRNA expression than all other groups. Similarly, analysis of IL-1β mRNA expression in the brain revealed a significant SDR condition by neutralizing antibody condition interaction, F(1,42)=7.586, p<.01 (fig 6B). Post hoc analyses reveal that the SDR6-IgG group had significantly higher levels of IL-1β mRNA than the NonSDR-IgG group and the SDR6-nAbTx group. This suggests that administration of IL-6 neutralizing antibody reverses the sensitizing effect of stress. Analysis of the CD11b mRNA expression levels in the brain revealed no significant main effects or interactions (p>.05), however the pattern is similar to that of IL-6 and IL-1β, with an elevation in the SDR6-IgG group compared to all other groups (fig 6C). Brain TNF-α, INF-γ, and IL-10 mRNA levels were also examined, however, no significant main effects or interactions were observed.
Figure 6.
Neutralizing antibody to IL-6 (nAbTx) prevents the SDR-induced enhancement in virus induced CNS inflammation on day 8 post infection as measured by real time RT-PCR. An ANOVA followed by post hoc mean comparisons indicated that SDR-induced increases in IL-6 (A) and IL-1 β (B) mRNA expression were reversed by IL-6 nAbTx. A similar, but non-significant pattern was observed for CD11b mRNA expression (C), a cell surface marker increased in activated microglia. Asterisks indicate significant differences between groups.
3.3.3 SDR enhances TMEV-induced peripheral IL-1β and TNF-α mRNA
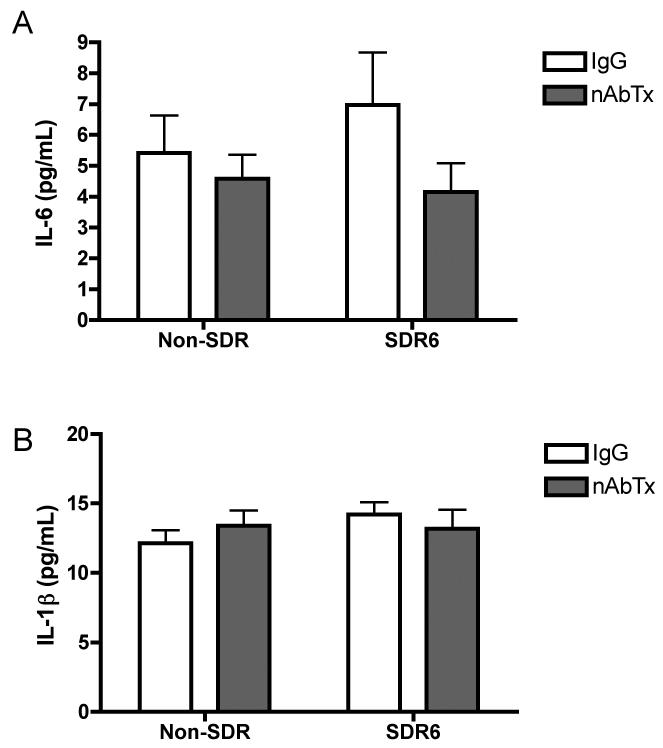
These same markers were evaluated in the spleen. Contrary to the interactions observed with IL-6 and IL-1β in the brain, no significant interactions between SDR6 and neutralizing antibody were observed in the periphery, suggesting that the protective effect of IL-6 neutralizing antibody treatment is centrally mediated. We did however observe a significant main effect of SDR6 on IL-1β, F(1,40)=8.663, p<.01, and TNF-α, F(1,38)=8.83, p<.01, such that SDR6 prior to infection resulted in increased mRNA expression of both cytokines. Furthermore, we observed a significant main effect of IL-6 neutralizing antibody treatment on INF-γ mRNA expression levels, F(1,38)=5.242, p<.05, such that neutralizing antibody resulted in increased levels. We also conducted ELISAs to measure serum level of IL-6 and IL-1β (fig. 7). No significant main effects or interactions were observed at 8 days post infection.
Figure 7.
On day 8 post infection with TMEV, an ANOVA revealed no significant difference in level of IL-6 (A) or IL-1β (B) in serum as determined by ELISA.
4. Discussion
Evidence indicates that a prior history of social stress is linked to onset and exacerbation of neurodegenerative diseases, such as multiple sclerosis (Ackerman et al., 2002; Grant et al., 1989; Mohr et al., 2000, 2004; Uno et al., 1989; Warren et al., 1982). Our laboratory has sought to understand the mechanisms by which social stress exerts these effects using TMEV infection, an animal model of MS (Johnson et al., 2004, 2006; Meagher, 2007). Prior research demonstrated that social disruption stress exacerbates both acute and chronic phases of TMEV infection (Johnson et al., 2004, 2006). Furthermore, IL-6 neutralization during the stress exposure period can reverse the adverse effects of SDR on acute phase TMEV infection (Meagher et al.,, 2007).
To extend these findings the present study examined the impact of SDR exposure on stress-induced sensitization of the neuroinflammatory response to TMEV infection. Our results suggest that the adverse effects of social stress are mediated by stress-induced enhancement of CNS cytokines. It was demonstrated that chronic SDR results in an up-regulation of IL-6 mRNA expression and sensitizes infection-related increases in central and peripheral proinflammatory cytokines. Furthermore, the protective effects of IL-6 neutralizing antibody appear to be mediated, in part, by reversing central increases in proinflammatory cytokine expression.
4.1 Social disruption induces CNS inflammation in Uninfected Subjects
Prior studies have shown that social stress results in peripheral increases in proinflammatory cytokines and other markers of inflammation (Curry et al., 2010; Engler et al., 2008; Gaab et al., 2005; Merlot et al., 2003, 2004; Stark et al., 2002). For example, Stark and colleagues (2002) showed that social disruption stress results in an increase in IL-6 level within plasma and liver. Additionally, it has been demonstrated that SDR results in an up-regulation of IL-1β in the spleen, liver, and lungs (Engler et al., 2008; Curry et al., 2010). The current study extends this research by showing that social stress also increases proinflammatory cytokine expression within the CNS. Specifically, we found a significant up-regulation of IL-6 mRNA expression in the hippocampus following chronic exposure to SDR. Furthermore, it was demonstrated that there is a robust increase of hippocampal IL-1β following a single session of SDR. However, this effect appears to habituate, such that by the sixth session of SDR no significant up-regulation of IL-1β was observed.
Prior research has also demonstrated that SDR is capable of enhancing and activating immune cells (Bailey et al., 2007; Curry et al., 2010; Engler et al., 2008; Powell et al., 2009). For example, Curry and colleagues (2010) demonstrated that SDR results in increased CD11b+ cells in the lungs (e.g., neutrophils and monocytes), as well as an increase in the activational state of neutrophils that is concurrent with an increase in proinflammatory cytokines. CD11b is a subunit of the Mac-1, complement receptor 3 and is expressed my many cells of the innate immune system, including microglia, macrophage, and neutrophils. Microglia are the innate immune cell CNS and are though to be the primary producer of CNS inflammation (Krakowski and Owens, 1996; Van Dam et al., 1995). To begin to evaluate their role in SDR-induced CNS inflammation, we measured hippocampal CD11b+ mRNA expression. This method failed to confirm a significant up-regulation of CD11b+ mRNA; however, because a trend was observed, we suggest further research is necessary to elucidate this relationship.
The effect of SDR on CNS inflammation may be driven by the development of glucocorticoid resistance (Avitsur et al., 2001; Miller et al., 2002; Quan et al., 2001; Sheridan et al., 2000; Stark et al., 2002). It has been demonstrated that chronic exposure to SDR results in a blunting of the anti-inflammatory effects of glucocorticoids on splenocytes (Avitsur et al., 2001; Johnson et al., 2004; Meagher et al., 2007; Miller and Chen, 2006; Quan, 2001). Data from Quan and colleagues (2001) suggest that the blunting of this effect may occur through a down-regulation of GC receptors. The effect of GCR may not be limited to the spleen, as it has been demonstrated that there is also a reduction of GC receptor mRNA in the brain following SDR (Quan et al., 2001).
Prior research suggests that GCR depends upon the presence of IL-1β (Engler et al., 2008). Mice lacking the IL-1 type 1 receptor have shown an elevation in serum corticosterone, but did not show an accumulation of CD11b+ cells in the spleen or the development of GCR. Despite research indicating that SDR increases IL-6 release, it is not essential in the development of SDR-induced GCR within the spleen (Stark et al., 2002). Prior research from our laboratory is in line with this, showing the development of GCR even when IL-6 neutralizing antibody is administered (Meagher et al., 2007).
The development of GCR does not occur following all stressors, but appears to develop uniquely in response to social stress (Avitsur et al., 2001; Quan et al., 2001). Chronic exposure to restraint stress does not alter the responsivity of cells to the anti-inflammatory effects of glucocorticoids (Quan et al., 2001). This may explain why social disruption stress has proinflammatory effects (Gaab et al., 2005; Merlot et al., 2003, 2004; Stark et al., 2002), in contrast to the anti-inflammatory effects generally observed in response to restraint stress (Mi et al.,, 2004, 2006).
4.2 Social disruption sensitizes TMEV-induced inflammatory responses
Furthermore, this study sought to determine if SDR sensitized Theiler’s virus-induced proinflammatory cytokine release and if the protection conferred by IL-6 neutralizing antibody is through reversal of this stress-induced sensitization. This hypothesis is supported by a growing body of evidence suggesting prior exposure to social stress can sensitize or prime the inflammatory pathway, such that a subsequent inflammatory stimulus results in an exaggerated inflammatory response (Bailey et al., 2009; Dong-Newsom et al., 2010; Mays et al., 2010; Powell et al., 2011; Quan et al., 2001). For example, repeated social disruption stress was shown to result in increased HSV-1 infection-related CD11b+ macrophages and proinflammatoy cytokines, IFN-α and TNF-α (Dong-Newsom et al., 2010). Furthermore, SDR enhances allergen-induced airway inflammation (Bailey et al., 2009) and LPS-induced proinflammatory cytokines (Quan et al., 2001).
It has also been shown that other inflammatory-stimuli can sensitize the innate immune response to a challenge (Cunningham et al., 2005; Deak et al., 2005; Johnson et al., 2002; Matsumoto et al., 2006; Perry et al., 2007). For example, exposure to inescapable shock prior to administration of LPS results in enhanced plasma IL-1β (Johnson et al., 2002). Additionally, administration of IL-6 or exposure to stress has been shown to sensitize the inflammatory response to a stressor (Deak et al., 2005; Matsumoto et al., 2006). Furthermore, exposure to LPS can exacerbate local brain inflammation in prion disease infected mice (Cunningham et al., 2005).
Previous research suggests that SDR may also enhance inflammatory response to TMEV infection (Meagher et al., 2007). It was demonstrated that SDR results in increased IL-6 in sera and increased inflammatory lesions within the CNS that depend upon stress-induced IL-6. This study extended this line of research by demonstrating that chronic SDR sensitizes the inflammatory response to TMEV infection. Exposure to SDR prior to TMEV infection resulted in increased infection related central IL-6 and IL-1β mRNA expression and increased peripheral IL-1β and TNF-α mRNA expression at day 8 post-infection. Furthermore, the protective effect of IL-6 neutralizing antibody administration during the stress exposure period reversed the enhancement of central inflammation, but not peripheral inflammation. This suggests that SDR-induced IL-6 serves to sensitize the CNS inflammatory pathway. Furthermore, given that administration of IL-6 neutralizing antibody has been shown to reverse the adverse behavioral effects of IL-6 (Meagher et al., 2007), this data suggests that the sensitization of neuroinflammatory processes mediate many of the adverse effects of social stress on TMEV infection, including enhanced sickness behavior, increased histological markers of CNS inflammatory lesions, and reduced CNS viral clearance (Meagher et al., 2007).
Other studies also support the detrimental role of enhanced levels of IL-6. For example, susceptible strains have higher levels of IL-6 (Jin et al., 2007) and higher levels of IL-6 have been associated with disease progression (Trottier, 2004). Furthermore, IL-6 levels lead to a shift toward a Th-17 immune response, which is associated with viral persistence and enhanced disease course (Hou et al., 2009). However, there is other data to suggest a protective role of IL-6 in TMEV disease progression. Notably, it has been demonstrated that IL-6 deficient mice show a worse disease course, increased mortality, and increased anterior horn neural damage (Pavelko et al, 2003). This finding is not necessarily contradictory to our results. IL-6 is a complex, pleiotropic cytokine, in which there is likely an optimal level of IL-6. Another possibility is that IL-6 can be protective as it can act in an anti-inflammatory capacity. We suggest this may be why supplemental hrIL-6 reduced demyelination and inflammation TMEV infected mice (Rubio & Sierra, 1993). Given that IL-1β is elevated concurrently with IL-6 in TMEV infected mice pre-exposed to chronic SDR, we have confidence that IL-6 is acting as a proinflammatory cytokine in our model.
Research suggests that microglia are important mediators of stress-induced neuroinflammation. For example, it has been demonstrated that administration of minocycline, a glial cell inhibitor, reverses shock-induced increases in central proinflammatory cytokine expression (Blandino et al., 2006). It has also been demonstrated that stress can induce a hyperinflammatory microglial phenotype (Frank et al., 2007; Nair and Bonneau, 2006; Perry, 2007; Sugama et al., 2007, 2011), which may underlie stress-induced exacerbations of neuroinflammation in various diseases, including MS. Therefore, CD11b mRNA expression was measured in experiment 3. Although the interaction between SDR and IL-6 neutralizing antibody treatment for CD11b failed to reach statistically significant, there were trends suggesting (1) SDR may increase TMEV-induced CD11b mRNA expression and (2) that this effect may be reversed by IL-6 neutralization during the stress exposure period. However, given the inconclusive nature of this finding, future research is needed to determine if priming of microglia is the cellular mechanism underlying stress-induced sensitization of TMEV infection-induced inflammation.
4.3 Potential interaction between innate and adaptive immune systems
It has been demonstrated that the initial innate immune response to infection shapes the later adaptive immune response (Biron, 1999; Biron et al., 1998). Furthermore, IL-6 neutralizing antibody treatment during the stress exposure period reverses the adverse effect of SDR on viral clearance (Meagher et al., 2007). This suggests that exaggerated infection-related neuroinflammation might have cascading effects, including a dysregulation of T cell and antiviral responses during early infection. Furthermore, a poorer antiviral response during the acute phase is likely to result in increased severity of the later Theiler virus induced demyelination (Lipton et al., 2005; Sieve et al., 2004; Trottier et al., 2004).
In support of this, it has been demonstrated that stress can modify T cell responses (Sommershof et al., 2010; Steelman et al., 2009). For example, Young and colleagues (submitted) demonstrate that infection related increases in virus-specific CD4+ and CD8+ T cells within the CNS are significantly attenuated due to SDR exposure. There are a variety of mechanisms by which SDR-induced inflammation may be able to exert this effect, including shifting the T cell response (e.g., from Th2 to Th1/Th17), inhibiting trafficking of T cells into the CNS, and/or by altering efficacy of antigen presenting cells.
4.4 Summary and implications
In summary, this study demonstrates that pre-exposure to SDR sensitizes the inflammatory response to Theiler’s murine encephalomyelitis virus infection. Specifically, chronic SDR-induced release of IL-6 sensitizes virus-initiated CNS proinflammatory cytokine release. This line of work may have broad implications for understanding the role of stress and cytokine expression in altering vulnerability to MS and other inflammatory neurodegenerative diseases. Elucidating the mechanisms by which social stress exacerbates the severity of a virally initiated autoimmune disease may lead to the development of new interventions. Specifically, this line of research suggests that early anti-inflammatory interventions may improve neurodegenerative and inflammatory disease course.
Acknowledgements
This research was supported by NIH/NINDS R01-NS060822 awarded to MWM and CJW. The authors would like to thank Thomas Prentice for his technical assistance and Nicole Reusser for her assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson ED. Epidemiology of multiple sclerosis. Br Med Bull. 1977;33:9–14. doi: 10.1093/oxfordjournals.bmb.a071407. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, Baum A. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64:916–920. doi: 10.1097/01.psy.0000038941.33335.40. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Heyman R, Rabin BS, Baum A. Stress and its relationship to disease activity in multiple sclerosis. Int J Multiple Sclerosis. 2000;7:20–29. [Google Scholar]
- Anderson DW, Ellenberg JH, Leventhal CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31:333–336. doi: 10.1002/ana.410310317. [DOI] [PubMed] [Google Scholar]
- Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Avistur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–57. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor dependent pathway. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1180–1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, Haczku A. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J Immunol. 2009;182:7888–7896. doi: 10.4049/jimmunol.0800891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- Biron CA, Cousens LP, Ruzek MC, Su HC, Salazar-Mather TP. Early cytokine responses to viral infections and their roles in shaping endogenous cellular immunity. Adv Exp Med Biol. 1998;452:143–149. doi: 10.1007/978-1-4615-5355-7_15. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr., Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Brahic M, Stroop WG, Baringer JR. Theiler’s virus persists in glial cells during demyelinating disease. Cell. 1981;26:123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJ. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JR, Zaczynska E, Katsetos CD, Platsoucas CD, Oleszak EL. Differential expression of TGF-beta, IL-2, and other cytokines in the CNS of Theiler’s murine encephalomyelitis virus-infected suseptible and resistant strains of mice. Virology. 2000;278:364–360. doi: 10.1006/viro.2000.0646. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr., Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dong-Newsom P, Powell ND, Bailey MT, Padgett DA, Sheridan JF. Repeated social stress enhances the innate immune response to a primary HSV-1 infection in the cornea and trigeminal ganglia of Balb/c mice. Brain Behav Immun. 2010;24:273–280. doi: 10.1016/j.bbi.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau JF. Theiler’s virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Brown GW, Harris T, McDonald WI, Patterson T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Zhang SM, Lipworth L, Olek MJ, Ascherio A. Multiple sclerosis and age at infection with common viruses. Epidemiology. 2001;12:301–306. doi: 10.1097/00001648-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–28. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A, Kleeberg J, Le Goff G, Pantaleo G, Du Pasquier RA. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–1721. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- Jin YH, Mohindru M, Kang MH, Fuller AC, Kang B, Gallo D, Kim BS. Differential virus replicaiton, cytokine produciton, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler’s virus. J Virol. 2007;81:11690–702. doi: 10.1128/JVI.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Prentice TW, Bridegam P, Young CR, Steelman AJ, Welsh TH, Welsh CJ, Meagher MW. Social stress alters the severity and onset of the chronic phase of Theiler’s virus infection. J Neuroimmunol. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Storts R, Welsh TH, Jr., Welsh CJ, Meagher MW. Social stress alters the severity of acute Theiler’s virus infection. J Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Hyllested K. MS epidemiology in Faroe Islands. Riv Neurol. 1987;57:77–87. [PubMed] [Google Scholar]
- Li J, Johansen C, Bronnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark. Neurology. 2004;62:726–729. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Kumar AS, Trottier M. Theiler’s virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 2005;111:214–223. doi: 10.1016/j.virusres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- Matsumoto T, Komori T, Yamamoto M, Shimada Y, Nakagawa M, Shiroyama T, Inui K, Okazaki Y. Prior intraperitoneal injection of rat recombinant IL-6 increases hypothalamic IL-6 contents in subsequent forced swim stressor in rats. Neuropsychobiology. 2006;54:186–194. doi: 10.1159/000099946. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Padgett DA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J Immunol. 2010;184:2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123(Pt 3):519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J Neurosci Res. 1999;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Johnson RR, Young EE, Vichaya EG, Lunt S, Hardin EA, Connor MA, Welsh CJ. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler’s virus infection. Brain Behav Immun. 2007;21:1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Importance of fighting in the immune effects of social defeat. Physiol Behav. 2003;80:351–357. doi: 10.1016/j.physbeh.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice: activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004;7:55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- Mi W, Belyavskyi M, Johnson RR, Sieve AN, Storts R, Meagher MW, Welsh CJ. Alterations in chemokine expression following Theiler’s virus infection and restraint stress. J Neuroimmunol. 2004;151:103–115. doi: 10.1016/j.jneuroim.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJ. Restraint stress decreases virus-induced pro-inflammatory cytokine mRNA expression during acute Theiler’s virus infection. J Neuroimmunol. 2006;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Bacchetti P, Boudewyn AC, Huang L, Marrietta P, Cheuk W, Dee B. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55:55–61. doi: 10.1212/wnl.55.1.55. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. Bmj. 2004;328:731. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteyne P, Bureau JF, Brahic M. The infection of mouse by Theiler’s virus: from genetics to immunology. Immunol Rev. 1997;159:163–176. doi: 10.1111/j.1600-065x.1997.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Monteyne P, Bureau JF, Brahic M. Viruses and multiple sclerosis. Curr Opin Neurol. 1998;11:287–291. doi: 10.1097/00019052-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nispeanu P, Korczyn AD. Psychological stress as risk factor for exacerbations in multiple sclerosis. Neurology. 1993;43:1311–2. doi: 10.1212/wnl.43.7.1311. [DOI] [PubMed] [Google Scholar]
- Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002;58:136–138. doi: 10.1212/wnl.58.1.136. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelko KD, Howe CL, Dresher KM, Gamez JD, Johnson AJ, Wei T, Ransohoff RM, Rodriguez M. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J Neurosci. 2003;23:481–92. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun. 2007;21:45–46. doi: 10.1016/j.bbi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Powell ND, Mays JW, Bailey MT, Hanke ML, Sheridan JF. Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behav Immun. 2011;25:46–52. doi: 10.1016/j.bbi.2010.07.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, Sheridan JF. Repeated social defeat activites dendritic cells and enhance Toll-like receptor dependent cytokine secretion. Brain Behav Immun. 2009;23:225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, Njenga MK, Logan WC, Wettstein PJ. The balance between persistent virus infection and immune cells determines demyelination. J Immunol. 1996;157:5699–5709. [PubMed] [Google Scholar]
- Rubio N, Sierra A. Interleukin-6 produciton by brain tissue and cultured astrocytes infected with Theiler’s murine encephalomyelitis virus. Glia. 1993;9:41–7. doi: 10.1002/glia.440090106. [DOI] [PubMed] [Google Scholar]
- Sato S, Reiner SL, Jensen MA, Roos RP. Central nervous system cytokine mRNA expression following Theiler’s murine encephalomyelitis virus infection. J Neuroimmunol. 1997;76:213–223. doi: 10.1016/s0165-5728(97)00059-3. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJ, Meagher MW. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- So EY, Kang MH, Kim BS. Inducition of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- Sommershof A, Basler M, Riether C, Engler H, Groettrup M. Attenuation of the cytotoxic T lymphocyte response to lymphocytic choriomeningitis virus in mice subjected to chronic social stress. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Steelman AJ, Dean DD, Young CR, Smith R, 3rd, Prentice TW, Meagher MW, Welsh CJ. Restraint stress modulates virus specific adaptive immunity during acute Theiler’s virus infection. Brain Behav Immun. 2009;23:830–843. doi: 10.1016/j.bbi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146:1388–1399. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, Hashimoto M. Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J Neuroimmunol. 2011;233:29–36. doi: 10.1016/j.jneuroim.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Theil DJ, Tsunoda I, Libbey JE, Derfuss TJ, Fujinami RS. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler’s virus infections. J Neuroimmunol. 2000;104:22–30. doi: 10.1016/s0165-5728(99)00251-9. [DOI] [PubMed] [Google Scholar]
- Trottier M, Schlitt BP, Kung AY, Lipton HL. Transition from acute to persistent Theiler’s virus infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease. J Virol. 2004;78:12480–12488. doi: 10.1128/JVI.78.22.12480-12488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- Warren SA, Warren KG, Greenhill S, Paterson M. How multiple sclerosis is related to animal illness, stress and diabetes. Can Med Assoc J. 1982;126:377–382. 385. [PMC free article] [PubMed] [Google Scholar]
- Welsh CJ, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68(Pt 6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- WHO. MSIF . Atlas: Multiple Sclerosis Resourses in the World. Multiple Sclerosis International Federation; London: 2008. Atlas: Multiple Sclerosis Resources in the World. [Google Scholar]
- Young EE, Sieve AN, Vichaya EG, Carcoba LM, Young CR, Ambrus A, Storts R, Welsh CJ, Meagher MW. Chronic restaint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice: II. CNS disease severity. J Neuroimmunol. 2010;220:79–89. doi: 10.1016/j.jneuroim.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Vichaya EG, Reusser NM, Cook JL, Steelman AR, Welsh CJR, Meagher MW. Chronic social stress impairs virus specific adaptive immunity during acute Theiler’s virus infection. submitted. [DOI] [PMC free article] [PubMed]