Abstract
Atrial fibrillation (AF) is a growing clinical problem associated with increased morbidity and mortality. Development of safe and effective pharmacological treatments for AF is one of the greatest unmet medical needs facing our society. In spite of significant progress in non-pharmacological AF treatments (largely due to the use of catheter ablation techniques), anti-arrhythmic agents (AADs) remain first line therapy for rhythm control management of AF for most AF patients. When considering efficacy, safety and tolerability, currently available AADs for rhythm control of AF are less than optimal. Ion channel inhibition remains the principal strategy for termination of AF and prevention of its recurrence. Practical clinical experience indicates that multi-ion channel blockers are generally more optimal for rhythm control of AF compared to ion channel-selective blockers. Recent studies suggest that atrial-selective sodium channel block can lead to safe and effective suppression of AF and that concurrent inhibition of potassium ion channels may potentiate this effect. An important limitation of the ion channel block approach for AF treatment is that non-electrical factors (largely structural remodeling) may importantly determine the generation of AF, so that “upstream therapy”, aimed at preventing or reversing structural remodeling, may be required for effective rhythm control management. This review focuses on novel pharmacological targets for the rhythm control management of AF.
Keywords: Cardiac arrhythmias, atrial fibrillation, antiarrhythmic therapy, pharmacological treatment
1.0 Introduction
Atrial fibrillation (AF) is a growing clinical problem associated with increased morbidity and mortality. It affects approximately 2.7 million individuals in the USA and about 4.5 million people in the European Union The prevalence of AF is expected to achieve epidemic proportions increasing to up to 15.1 million affected people by 2050 in the USA alone (ranging from 5.6 to 15.1 millions, by different estimates) (Miyasaka et al., 2006; Go et al., 2001). This increase is largely due to aging of general population and a prolongation of life-span AF prevalence is significantly increased with aging (from less than 0.5% in individuals < 50 years old to up to 15% in > 80 year old individuals) (Camm et al., 2010). The prolongation of life-span is in part due to a better treatment of many life-threatening diseases, including those that promote AF (i.e., heart failure, acute coronary syndrome, hypertension, etc). It seems that ever - improving treatment of these co-morbidities may act to reduce AF prevalence, but the prolongation of life-span may increase AF prevalence. Anti-arrhythmic agents (AADs) remain the mainstay for the management of AF patients. Because currently available AADs are far less than optimal, novel effective, safe, and well-tolerated anti-AF AADs are highly desirable. The current review focuses on investigational targets and approaches for the rhythm control managements of AF.
2.0. Current medical treatments of AF and the unmet need
AF may occur in individuals with undetectable heart disease, so called “lone or idiopathic AF”, which accounts for up to 30% of all AF occurrences (Fuster et al., 2011). Ion channel genetic abnormalities (channelopathies) appear to be a rare primary cause of lone AF (Lubitz et al., 2009). It is increasingly recognized that many “lone AF” patients also have some degree of structural abnormalities in the atria (not readily detectable with gross methods) (Frustaci et al., 1997; Stiles et al., 2009), which may contribute in the generation of AF. Other forms of AF are typically associated with significant electrical and structural remodeling as well as with a number of, often overlapping, medical conditions, such as hypertension, heart failure, coronary artery disease, valvular heart disease, myocardial infarction, etc (Camm et al., 2010; Fuster et al., 2011). Because these diseases may cause/promote AF and AF may cause or aggravate many of the diseases, their long-term cause-affect interactions are very complex and remains poorly understood. The presence and severity of co-existing diseases in AF patients can significantly modulate the efficacy and safety of AADs.
AF is commonly defined as paroxysmal, persistent (lasting < 7 and ≥ 7 days, respectively) or permanent. (Camm et al., 2010; Fuster et al., 2011). AF is often a progressive disease, tending to recur progressively more often, last longer, and transition to a persistent or permanent form. It is now well established that the longer the duration of AF, the more difficult it is to terminate AF and prevent its recurrence. The development and/or augmentation of electrical and structural remodeling secondary to AF contributes to the tendency of AF to progress from paroxysmal to persistent and permanent (Wijffels et al., 1995). Other factors causing or contributing to structural remodeling of the atria can be aging and disease (hypertension, heart failure, ischemia).
According to the current guidelines (Fuster et al., 2006; Camm et al., 2010), rarely occurring paroxysmal asymptomatic AF (lasting < 48 hours) should not be treated in most cases. This is because serious threat from such AF is believed to be insignificant in most of the cases and the risk of adverse effects from the treatments may exceed the elusive benefit. The need for early interventions in some patients who start to develop paroxysmal AF (with the intention of stopping/delaying/reversing potential electrical, structural, and contractile remodeling in atria) is under discussion and the underlying hypothesis is being tested in a clinical trial (EAST; results are expected to be reported in 2015) (Cosio et al., 2008; Kirchhof et al., 2009; Anter & Callans, 2009; Camm et al., 2010). This idea stems from the realization that many AF patients, who are started on treatment under the current guidelines, have already established largely irreversible electrical and structural changes in atria (Cosio et al., 2008; Kirchhof et al., 2009; Anter & Callans, 2009; Camm et al., 2010). There appears to be a “window of opportunity” for early interventions in some of the “AF newcomers” (Kirchhof et al., 2009; Anter & Callans, 2009; Camm et al., 2010), but among the challenges is identification of patients who will develop AF, which could lead to symptoms and measurable health problems in future years. In many people who present with AF for the first time, AF may not recur for a long time (years) or may recur only rarely (Kerr et al., 2005).
Most AF patients need some management aimed at preventing AF-induced complications (primarily stroke and cardiomyopathy) and, if AF is symptomatic, at reducing symptoms (chest pain, dyspnea, palpitation, fatigue, dizziness, etc). These AF-related adverse symptoms are mainly the consequences of hemodynamic derangements caused by rapid and irregular ventricular rate, so that decreasing ventricular rate by any means may reduce/eliminate the symptoms. Broadly, the management of AF patients includes two major approaches: 1) to restore and maintain sinus rhythm or 2) to control ventricular rate without making any specific attempts to suppress or prevent AF. These strategies are referred to as rhythm control and rate control, respectively. For reduction of the risk of stroke, anticoagulation is recommended for most rate and rhythm control patients. For the latter it is because rhythm control with currently available tools does not guarantee the maintenance of sinus rhythm (Camm et al., 2010). Stroke is a major adverse consequence of AF; the risk of stroke is increased fivefold in the patients who have AF vs. those who do not.
Several large clinical trials (i.e., AFFIRM, RACE, PIAF, HOT CAFÉ, STAF, AF-CHF) have demonstrated that the rhythm control approach with AADs is not superior to the rate control in terms of morbidity and mortality (Wyse et al., 2002; Roy et al., 2008; Van Gelder et al., 2002; Hohnloser et al., 2000; Opolski et al., 2004). It is important to recognize that these clinical trials compared the rate vs. rhythm intention-to-treat approaches were based on AADs available at that time, and that the study was not necessarily conducted exclusively in patients with AF for rate control or patients in sinus rhythm for rhythm control. Many patients initially assigned to the rhythm control arm were in AF (e.g., 37.4% had AF at 5 years of follow-up in AFFIRM) and many patients initially assigned to the rate control arm were in sinus rhythm (e.g., 34.6% at 5 years in AFFIRM). A greater use of β-adrenergic receptor blockers in the rate- vs. rhythm-control arms in some of the clinical trials (applied to control ventricular rate) (Wyse et al., 2002; Roy et al., 2008) might have acted to reduce mortality in the rate control group. β-adrenergic receptor blockers reduce mortality in patients with CHF and ischemic heart diseases (Doughty et al., 1997). Several post-hoc or sub-study analyses of the large clinical trials (such as the DIAMOND, AFFIRM, CHF-STAF trials), directly comparing “sinus rhythm” vs. “AF” regardless of the initial rate or rhythm control assignment, indicate that AF patients maintained in sinus rhythm (with or without AADs) have better survival rate and better quality of life than those in whom AF persists (Corley et al., 2004; Pedersen et al., 2001; Guglin et al., 2010; Deedwania et al., 1998), although this may not always be the case, e.g., RACE and AF-CHF trials (Rienstra et al., 2006); (Talajic et al., 2010; Wyse, 2009). Because morbidity and mortality in patients with AF are commonly associated with other diseases (often more serious than AF) and because many of these diseases and AF may mutually promote each other, the relative contribution of AF to the morbidity and mortality (apart from AF-related stroke) is very difficult to determine. Therefore, it is recognized that, “on average”, a greater mortality and morbidity in patients with AF might be attributable to the fact that these patients are generally sicker than those who are in sinus rhythm (Wyse, 2009; Roy et al., 2009; Anter & Callans, 2009; Reiffel, 2011).
It appears obvious that normality (i.e., sinus rhythm) is better than abnormality (i.e., AF), but the currently available therapeutic rhythm control options (with entailed benefits/risks/cost) are limited. Rate control is often a preferable strategy in older asymptomatic AF patients (≥65 years old). In cases in which AF is thought to contribute to mortality and morbidity, more of an effort should be made to restore and maintain sinus rhythm, particularly in relatively young patients (< 65 years old). It is noteworthy that the above-mentioned large clinical AF trials (i.e., AFFIRM, RACE, PIAF, HOT CAFÉ, STAF, AF-CHF) (Wyse et t al 2002; Roy et al., 2008; Van Gelder et al., 2002; Hohnloser et al., 2000; Opolski et al., 2004) were conducted in a relatively old population (typically ≥ 65 years old), in which the percent of patients benefiting from rate vs. rhythm control is greater than that of relatively younger patients (< 65 years old).
Rhythm control can be achieved with AADs, catheter ablation, electrical cardioversion, or, surgical techniques, although surgical approaches are rarely used nowadays. Despite significant progress in non-pharmacological AF treatments (largely due to the use of catheter ablation techniques), AADs remain first line therapy for rhythm control management of AF in the majority of patients (Fuster et al., 2011; Camm et al., 2010) and are expected to play a prominent role in the foreseeable future. The recent Guidelines indicate that left atrial ablation may be considered as frontline therapy in select patients with paroxysmal symptomatic AF (i.e., relatively young individuals with minimal to no heart disease) (Camm et al., 2010; Fuster et al., 2011). AADs are also commonly used post-ablation to improve the overall success rate (Roux et al., 2009).
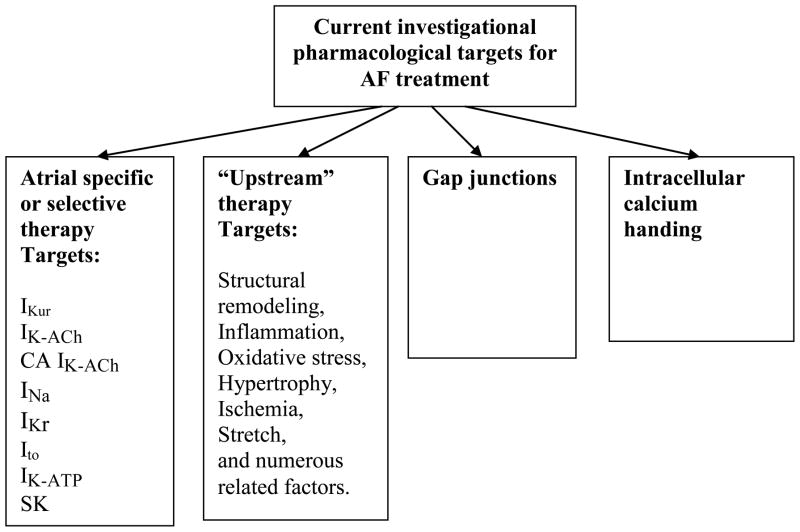
The present reality is that available AADs, particularly over the long-term, do not confer reliable maintenance of sinus rhythm and the use of these agents is associated with a substantial risk of intra- or extracardiac adverse effects, such as ventricular pro-arrhythmias, extra-cardiac toxicity, and aggravation of pre-existing diseases (Camm et al., 2010; Fuster et al., 2011). These adverse effects of the AADs may balance or even exceed the beneficial effects. Consequently, there is a great need for safer and more effective anti-AF drugs. Because drug-related adverse cardiac effects commonly occur in AF patients with specific cardiac pathologies (particularly heart failure, ischemic heart disease, etc.), an improvement in treatment outcomes may be achieved with optimization of existing or novel drug use (i.e., appropriately matching patients with different pathologies with appropriate AADs). Cardiac ion channel activity remains the primary target of most AADs under development (Fig. 1). Due to involvement of non-electrical factors in the generation of AF (primarily structural remodeling), the current search for anti-AF agents is focused on both modulation of ion channel activity as well as on upstream therapies, which reduce/prevent structural remodeling. Among other approaches are “gap junction” and “normalization of intracellular calcium” therapies (Fig. 1).
Fig. 1.
Current investigational targets for rhythm control of atrial fibrillation. Modified from Burashnikov and Antzelevitch (Burashnikov & Antzelevitch, 2008c), with permission.
3.0 Ion channel targets
Most AADs in current clinical use exert their anti-AF actions exclusively or primarily via direct modulation of cardiac ion channel activity. Direct inhibition of calcium release from the sarcoplasmic reticulum may also be useful for anti-AF action. Chronic use of AADs may remodel the expression of ion channels (Le Bouter et al., 2004; Schumacher et al., 2009) and ameliorate structural remodeling (Ashikaga et al., 2006) Currently available agents used in the management of AF act largely via inhibition of the rapidly activating delayed rectified potassium current (IKr; e.g., d-sotalol, dofetilide, or ibutilide), the early sodium current (early or peak INa; e.g., flecainide or propafenone), or via inhibition of multiple ion channels (potassium, sodium, and calcium channels; e.g., amiodarone). It needs to be recognized that propafenone and flecainide inhibit potassium channels as well (particularly IKr). Vernakalant, a drug recently approved for conversion of recent-onset AF in Europe and is under regulatory review in the United States, is also a multichannel blocker (Bechard et al., 2011; Ehrlich & Nattel, 2009; Fedida, 2007). AADs can be very effective in acute termination of paroxysmal and some forms of persistent AF (up to 70–90% efficacy) (Alboni et al., 2004; Banchs et al., 2008; Cotiga et al., 2007; Aliot et al., 2011), but are generally only moderately effective in long-term maintenance of sinus rhythm. The efficacy of long-term maintenance of sinus rhythm with INa and IKr blockers (at 1 year) normally does not exceed 50%. Amiodarone remains the best available AAD the long-term maintenance of sinus rhythm with up to 65–80% patients remaining in sinus rhythm at 1 year (Roy et al., 2000; Roy et al., 2008). While relatively safe in patients without significant structural heart diseases, INa blockers (Class IC agents) can cause serious ventricular arrhythmias in patients with ischemic heart diseases, heart failure, hypertrophy or other structural disease (conditions encountered in the majority of AF patients) (Fuster et al., 2011). IKr blockers can induce acquired long QT syndrome (LQTS) and Torsades de Pointes (TdP) arrhythmias attended by increased mortality (Fuster et al., 2006; Waldo et al., 1996). It seems that IKr-block-associated TdP and/or mortality takes place more often in patients with significant heart diseases than without it (particular systolic heart failure) (Waldo et al., 1996; Pratt et al., 1998; Oral et el., 1999). This issue, however, remains poorly defined.
While the application of amiodarone is very rarely associated with ventricular pro-arrhythmia and this agent can be safely used in most patients with structural heart disease (with a potential exception of patients with severe heart failure (Bardy et al., 2005)), long-term use of amiodarone is often associated with multiple forms of extra-cardiac toxicity (Zimetbaum, 2007). Dronedarone is a derivative of amiodarone. Its anti-AF efficacy is inferior to amiodarone, but safety profile is better than that of amiodarone (Le Heuzey et al., 2010). The latest European and North American guidelines list dronedarone as an agent for the maintenance of sinus rhythm in AF patients (Camm et al., 2010; Fuster et al., 2011). Interestingly, dronedarone was approved by the US FDA for the reduction of hospitalization in AF patients (not for rhythm control), which may be because anti-AF efficacy of dronedarone appears to be relatively poor (Piccini et al., 2009; Singh et al., 2010). It is noteworthy that the PALLAS trial of dronedarone for permanent AF was stopped early in mid-2011 due to “a significant increase in cardiovascular events in the dronedarone arm”. PALLAS (Permanent Atrial fibriLLAtion outcome Study using Dronedarone on top of standard therapy) was a double-blind, placebo-controlled, phase IIIb trial comparing dronedarone to placebo in patients with permanent AF. Death rates were increased twofold in patients with permanent AF taking dronedarone compared with placebo (32 vs. 14), as were rates of stroke (17 vs. 7) and hospitalization for heart failure (118 vs. 81) (http://www.fda.gov/Drugs/DrugSafety/ucm264059.htm).
Improved rhythm control therapy with ion channel blockers can be achieved in several ways, including: 1) identification and characterization of atrial-specific/selective ion channels targets and development of AADs that can specifically/selectively modulate these targets; and 2) a better understanding of the actions of “old” AADs (particularly multiple channel blockers) in a variety of pathologic conditions associated with AF. It is important to define AF pathologies that may benefit from specific AADs as well as to identify AF-associated co-morbidities that may alter the actions of AADs or be aggravated with the use of AADs.
3.1. Atrial-specific ion channel targets
Atrial-specific strategies were first conceived in the early 1990s with the intention of avoiding the adverse effects of traditional anti-AF agents in the ventricles (Wang et al., 1993). Atrial-specific targets for AF treatment are those that are present exclusively or almost exclusively in the atria and include the ultrarapid delayed rectified potassium current (IKur), the conventional acetylcholine-regulated inward rectifying potassium current (IK-ACh), and the constitutively active IK-ACh (i.e., which does not require acetylcholine or muscarinic agents for activation) (Dobrev et al., 2005).
3.1.1. The ultrarapid delayed rectifier potassium current (IKur)
The atrial-specific current, IKur (carried by Kv1.5 channels encoded by KCNA5) was first described by Wang et al in 1993 (Wang et al., 1993). IKur is the most investigated atrial-specific ion channel and until recently was widely considered to be the most promising among atrial-specific targets for the treatment of AF (Ford & Milnes, 2008; Ravens, 2010). Enthusiasm for selective IKur blockers for AF management has diminished in recent years (Burashnikov & Antzelevitch, 2009; Ehrlich & Nattel, 2009; Ravens & Wettwer, 2011; Pandit et al., 2011). Block of IKur consistently prolongs only the upper phase of atrial action potential and may prolong, abbreviate, or causes no effect in APD70–90 (Fig. 2). Available data indicate that inhibition of IKur alone is ineffective against AF. At concentrations that effectively control AF, IKur blockers potently inhibit other currents (e.g., INa is inhibited by vernakalant, AZD7009, AZD1305 and Ito/IKACh/early INa are inhibited by AVE0118) (Fedida, 2007; Carlsson et al., 2006; Blaauw et al., 2004; Burashnikov & Antzelevitch, 2009; Pandit et al., 2011).
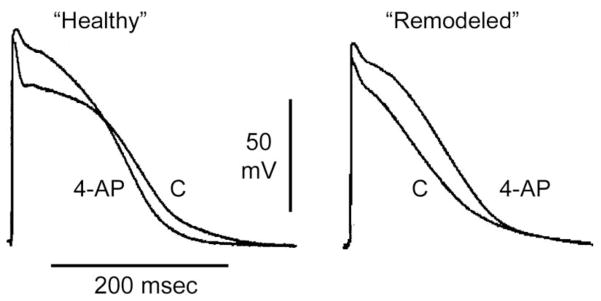
Fig. 2.
Block of IKur with 4-aminopyridine (4-AP, 50 μM) abbreviates APD90 in “healthy” (plateau-shaped action potential), but prolongs it in “acutely remodeled” (triangular-shaped action potential) canine coronary-perfused atrial preparations (pectinate muscles). Low flow ischemia was used to generate the “acutely remodeled” atria. Modified from Burashnikov et al. (Burashnikov et al., 2004; Burashnikov & Antzelevitch, 2008b), with permission.
Most IKur blockers also inhibit peak INa. Recent studies suggest that the atrial-selective action of many of the purported IKur blockers (e.g., AZD7009, AZD1305, vernakalant) to prolong ERP and exert anti-AF actions is due to their ability to block peak INa (Burashnikov & Antzelevitch, 2009; Burashnikov et al., 2010b). At concentrations that specifically inhibit IKur (≤ 50 μM), 4-aminopyridine (4-AP) abbreviates atrial APD and ERP, and promotes AF (Burashnikov & Antzelevitch, 2008b). 4-AP has also been reported to be incapable of terminating sustained AF or preventing its initiation in an experimental model of AF (Burashnikov & Antzelevitch, 2008b; Pandit et al., 2011).
The relatively poor ability of selective IKur inhibition to terminate AF may also be due to the fact that IKur density is reduced with acceleration of activation rate as occurs during AF (Feng et al., 1998) as well as to the fact that the contribution of IKur to atrial repolarization is reduced in AF (Van Wagoner et al., 1997; Christ et al., 2008).
There is an apparent inconsistency between significant prolongation of the effective refractory period (ERP) (Blaauw et al., 2004; Knobloch et al., 2004; Wirth et al., 2007b; Regan et al., 2007; Dorian et al., 2007; Goldstein et al., 2004; Seki et al., 2002; Matsuda et al., 2005; Li et al., 2008) and abbreviation of action potential duration (APD90) induced by IKur blockers in “healthy” atria or only a small prolongation of APD70–90 in remodeled atria (Fig. 2) (Burashnikov et al., 2004; Wettwer et al., 2004; Burashnikov & Antzelevitch, 2008b; Schotten et al., 2007). Prolongation of ERP without lengthening of APD90 is due to the development of post-repolarization refractoriness [PRR], which is secondary to inhibition of the channel underlying INa, but not IKur. Several prominent IKur blockers have been to shown to inhibit early INa (such as vernakalant, AZD7009, AZD1305, AVE0118) (Fedida, 2007; Carlsson et al., 2006; Burashnikov et al., 2010b; Burashnikov et al., 2009).
Vernakalant, ISQ1, and TAEA also slow conduction velocity in atria but ventricles in vivo (Bechard & Pourrier, 2011) (Regan et al., 2008), indicating that these agents block INa selectively in atria. Because INa blockers can selectively prolong atrial ERP (Burashnikov et al., 2007), atrial selectivity of these IKur blockers to prolong ERP can be explained by a concomitant inhibition of INa. Indeed, atrial selective prolongation of ERP with AZD1305 and AVE0118 is largely due to block of the sodium channel carrying peak INa (Burashnikov et al., 2010b; Burashnikov et al., 2009). It should be emphasized that many of the IKur blockers have not been tested for their ability to inhibit peak INa or have been tested under conditions that fail to uncover atrial selectivity (i.e., in ventricular myocytes, at slow pacing rates and negative holding potentials (Wirth et al., 2007a)).
Recent studies have shown that reduction of IKur may actually predispose to the development of AF (Burashnikov & Antzelevitch, 2008b; Olson et al., 2006; Yang et al., 2009; Yang et al., 2010). Loss-of-function mutations in KCNA5, the gene that encodes the Kv1.5 channels responsible for IKur was found to be associated with AF (Olson et al., 2006; Yang et al., 2009; Yang et al., 2010). Selective inhibition of IKur with low concentrations of 4-AP (25–50 μM) have been shown to abbreviate APD in healthy canine right atrium, permitting the induction of AF. Abbreviation of APD, whether due to 4-AP or other agents (Burashnikov et al., 2004; Wettwer et al., 2004; Burashnikov & Antzelevitch, 2008b), is well known to be associated with an increase in AF vulnerability (with exception of cases when early INa is depressed as well, which may cause the appearance of a prolonged PRR) (Nattel, 2002). It has been suggested that reduction of IKur may also lead to atrial arrhythmias due to prolongation of repolarization and induction of EAD (Olson et al., 2006; Yang et al., 2009; Yang et al., 2010). Of note, numerous studies testing the effect of IKur block on atrial repolarization have reported at best some APD prolongation (often an APD abbreviation), but never EAD (Wang et al., 1993; Fedida et al., 2003; Burashnikov & Antzelevitch, 2008b; Wettwer et al., 2004; Ford & Milnes, 2008; Schotten et al., 2007; Christ et al., 2008). Moreover, agents and interventions that consistently produce EAD in canine ventricular preparations (block of IKr and/or IKs, enhancement of late INa) (Burashnikov & Antzelevitch, 1998; Burashnikov & Antzelevitch, 2002) do not induce EADs in canine atrial preparations (Burashnikov & Antzelevitch, 2011a). This is consistent with clinical data showing that agents prolonging cardiac repolarization induce pro-arrhythmias (i.e., TdP) in ventricles, but not in atria.
3.1.2. The acetylcholine-regulated inward rectifying potassium current (IK-ACh) and the constitutively active (CA) IK-ACh
Under normal conditions, IK-ACh is carried through the channels comprised of Kir3.1/Kir3.4 subunits and activated by the parasympathetic neurotransmitter acetylcholine through muscarinic (M2) receptors. There is another form of IK-ACh, the so called constitutively active IK-ACh (CA IK-ACh), that does not require cholinergic agonist stimulation or acetylcholine receptors for activation (Heidbuchel et al., 1992; Ehrlich et al., 2004; Dobrev et al., 2005; Voigt et al., 2007). This current is only marginally present in healthy human or canine atria and is significantly increased in atria of chronic AF patients and canine tachycardia-remodeled atria (Dobrev et al., 2005; Voigt et al., 2007; Cha et al., 2006; Ehrlich et al., 2004). The CA IK-ACh is thought to contribute importantly to abbreviation of atrial APD and to the maintenance of AF in remodeled atria (Dobrev et al., 2005; Cha et al., 2006; Voigt et al., 2007). The CA IK-ACh has recently been suggested to be an atrial-specific as well as pathology-specific target for AF treatment (Voigt et al., 2007; Ravens, 2008). Indeed, CA IK-ACh could be a valuable target for AF treatment, if it could be inhibited independently of conventional IK-Ach, which is present in organs other than the heart (e.g., in the central nerves system). However, there is currently no selective blocker of CA IK-ACh. Clinical studies indicate that a vagal component contributes to the initiation of some cases of paroxysmal AF in the clinic (Bettoni & Zimmermann, 2002; Pappone et al., 2004), suggesting that block of IKACh may exert an antiarrhythmic action in such cases (Nattel & Carlsson, 2006). In experimental models of AF, block of IK-ACh (presumably both conventional IKACh and CA IKACh) with tertiapin prolongs atrial APD and suppresses AF (Cha et al., 2006; Hashimoto et al., 2006). An atrial-pecific M2 receptor antagonist, cisatracurium, prevents vagally-mediated APD abbreviation and AF in dogs (Patterson et al., 2008). Note that many widely-used AADs (such as propafenone, flecainide, disopyramide, dofetilide) inhibit IK-ACh among their other actions (Ravens & Wettwer, 2011). An apparent limitation of the “IKACh approach” is that the channel, apart from the heart, is present in many organs of the human body, and that its modulation may cause some unwanted side effects.
3.2. Atrial-selective targets
Atrial-selective ionic channel targets are those that are present in both chambers of the heart, but inhibition of these targets produces greater effects in atria than in the ventricles.(Burashnikov & Antzelevitch, 2008c) Included among atrial-selective targets are early INa and IKr as well as potentially Ito and IK-ATP (Burashnikov et al., 2007; Burashnikov & Antzelevitch, 2008a; Burashnikov & Antzelevitch, 2008c). These AF targets are not newly discovered channels, but rather novel in the context of their potential use for atrial - selective AF strategies. Another potential atrial-selective target may be the small conductance calcium activated potassium channels (SK channels), found predominantly in atria (Tuteja et al., 2005; Diness et al., 2010).
3.2.1. The early sodium current (INa)
Some INa blockers (e.g., ranolazine, chronic amiodarone and AZD1305) have been shown to produce atrial-selective depression of sodium channel-dependent parameters and to suppress AF effectively in canine coronary-perfused atrial preparations at concentration that cause little to no effect in the ventricles (Fig. 3 and 4) (Burashnikov et al., 2007; Burashnikov et al., 2008a; Burashnikov et al., 2010b; Kumar et al., 2009; Goldstein et al., 2004; Szel et al., 2011). The atrial selectivity and anti-AF efficacy of ranolazine, AZD7009, and AZD1305 have been demonstrated in porcine and canine hearts in vivo and in vitro (Kumar et al., 2009; Goldstein et al., 2004; Burashnikov et al., 2010b). Vernakalant also appears to be an atrial-selective INa blocker since it slows conduction velocity in atria, but not in the ventricles.(Bechard & Pourrier, 2011) Our recent experimental studies conducted in canine atrial and ventricular preparations demonstrate that the combinations of chronic amiodarone and acute ranolazine as well as acute dronedarone and ranolazine (at a relatively low ranolazine’s concentration; 5 μM) cause a potent synergistic atrial-selective depression of sodium channel-mediated parameters (Fig. 4) (Sicouri et al., 2010; Burashnikov et al., 2010a). These combination of drugs exhibit high anti-AF efficacy in canine atria, while causing relatively minor electrophysiological change in the ventricles (Fig. 4) (Sicouri et al., 2010; Burashnikov et al., 2010a). The combination of dronedarone and ranolazine, like that of amiodarone and ranolazine, produces potent atrial-selective anti-AF effects, but is likely to be associated with fewer adverse effects and little to no extra-cardiac toxicity.
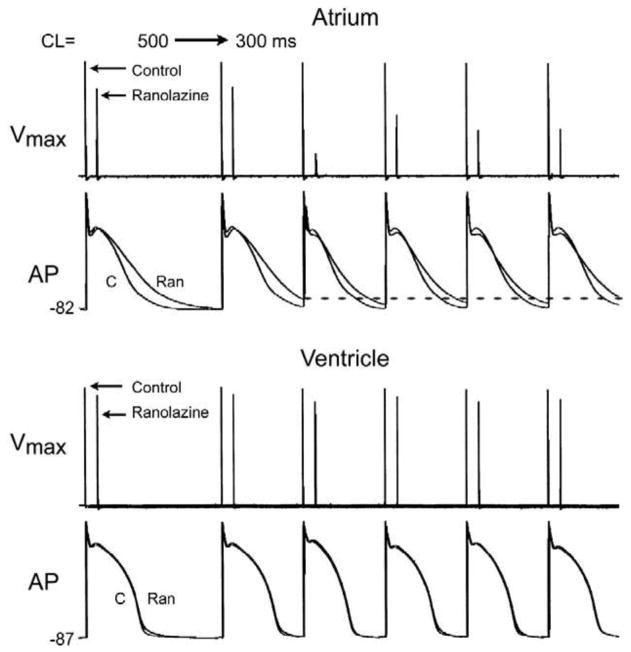
Fig. 3.
Ranolazine produces a much greater rate-dependent inhibition of the maximal action potential upstroke velocity (Vmax) in atria than in ventricles. Shown are Vmax and action potential (AP) recordings obtained from coronary-perfused canine right atrium and left ventricle before (C) and after ranolazine (10 μM). Ranolazine prolongs late repolarization in atria, but not ventricles (adue to IKr inhibition(Burashnikov et al., 2008a)) and acceleration of rate leads to elimination of the diastolic interval, during the recovery from sodium channel block largely occurs, which contributes to atrial selectivity of the drug. Reproduced from Antzelevitch and Burashnikov,(Antzelevitch & Burashnikov, 2009) with permission.
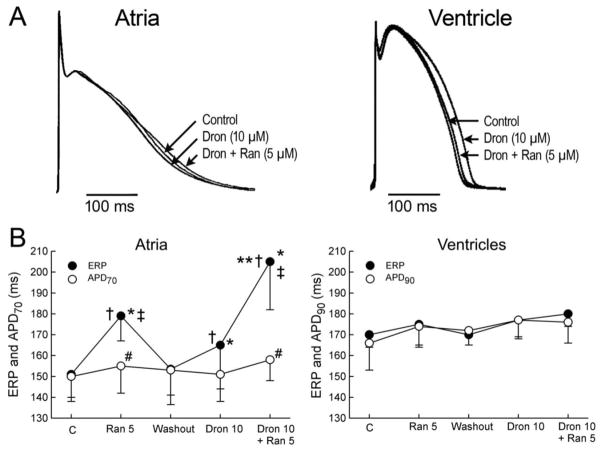
Fig. 4.
Atrial-selective induction of post-repolarization refractoriness (PRR) by ranolazine (Ran), dronedarone (Dron) alone and in combination (PRR was approximated by the difference between effective refractory period (ERP) and action potential duration measured at 70% repolarization (APD70) in atria and between ERP and APD measured at 90% repolarization (APD90) in ventricles; ERP corresponds to APD70–75 in atria and to APD90 in ventricles.
A: Shown are superimposed action potentials demonstrating relatively small changes with dronedarone and ranolazine and their combination. B: Summary data of atrial-selective induction of PRR. Ventricular data were obtained from epicardium and atrial data from endocardial pectinate muscle (PM). n=7–8. * p<0.05 vs. respective control (C). † p<0.05 vs. washout. ‡ p<0.05 vs. Dron 10. # p<0.05 vs. respective ERP. ** - p<0.05 - change in ERP induced by combination of Ran and Dron (from washout) vs. the sum of changes caused by Ran and Dron independently (both from washout). CL = 500 ms. From Burashnikov et al (Burashnikov et al., 2010a)
Dronedarone, a derivative of amiodarone, causes atrial but not ventricular PRR in canine and porcine hearts (Fig. 4) (Burashnikov et al., 2010a; Bogdan et al., 2011). The dronedarone-induced PRR is much less than that induced by amiodrone. Dronedarone’s inhibition of peak INa is very voltage-dependent in guinea pig ventricular myocytes (Bogdan et al., 2011), which may contribute to the dronedarone’s atrial-selective induction of PRR, owing to the fact that the resting membrane potential of atrial cells is more depoarlized than that of ventricular cells.
The factors underlying the atrial-selective effects of INa blockers include a more negative steady-state inactivation relationship, a more positive resting membrane potential (RMP), and a more gradual phase 3 of the action potential in atrial vs. ventricular cells (Burashnikov et al., 2007; Burashnikov & Antzelevitch, 2009; Burashnikov & Antzelevitch, 2008a; Burashnikov & Antzelevitch, 2010). The more negative half-inactivation voltage and more positive RMP importantly reduce the fraction of resting channels in atria vs. ventricles at RMP. Because recovery from sodium channel block occurs predominantly during the resting state of the channel, accumulation of sodium channel blockade is expected to be greater in atria vs. ventricles.
There appears significant variability in the degree to which sodium channel blockers are atrial-selective (Burashnikov & Antzelevitch, 2008a; Burashnikov & Antzelevitch, 2008c; Burashnikov & Antzelevitch, 2009). Available data suggest that binding affinity of the INa blocker for a given state of the channel (i.e., open, inactivated, or resting) does not determine the drug’s atrial-selectivity (afor review see (Antzelevitch & Burashnikov, 2009a; Burashnikov & Antzelevitch, 2009)). The rate of dissociation of the drug from the sodium channel, however, is likely to be key. INa blockers possessing rapid vs. slow unbinding kinetics tend to be highly atrial-selective (e.g., ranolazine, chronic amiodarone, but not propafenone) (Burashnikov & Antzelevitch, 2009; Burashnikov & Antzelevitch, 2010). Interestingly, a recent review indicates that flecainide, which dissociates relatively slowly form the sodium channel, causes a ventricular-predominant effect to slow conduction, a sodium channel-mediated parameter (Aliot et al., 2011).
Atrial selective INa blockers have been shown to be effective in the management of AF in the clinic. A number of clinical studies have demonstrated the ability of ranolazine to prevent the induction of AF and terminate paroxysms of AF using a “pill-in-the-pocket” approach (Scirica et al., 2007; Murdock et al., 2008; Murdock et al., 2009; Murdock et al., 2010). AZD7009 and AZD1305 have been reported to suppress clinical AF effectively (Crijns et al., 2006; Geller et al., 2009; Ronaszeki et al., 2011). Amiodarone is the best available agent for the long-term maintenance of sinus rhythm in AF patients. All of these agents are atrial-selective INa blockers (ranolazine, amiodarone, AZD7009, and AZD1305), that also inhibit other ion channels (particularly IKr), which is likely to importantly contribute to their atrial selectivity and anti-AF efficacy (Fig. 3; and see 3.2.2 and 3.4). The degree to which the clinical efficacy of these AADs depends on block of INa remains to be determined. Of note, lidocaine, a “mild” atrial selective INa blocker (Burashnikov et al., 2007) is not particularly effective against clinical AF, presumably because it is a relatively “pure” INa blocker. The combination of INa and IKr block appears to be associated with more effective suppression of AF (see 3.4).
Recent experimental and mathematical modeling data demonstrate that mild hyperkalemia can effectively suppress AF, but not ventricular fibrillation, and that this atrioventricular difference is due to atrial-selective reduction in the availability of sodium channels by mild hyperkalemia (Pandit et al., 2011). A greater electrophysiological effect of hyperkalemia on INa-mediated parameters in atria vs. ventricles in animals and humans was previously reported (Arnsdorf et al., 1977; Barold et al., 1987). This atrial selective property of mild hyperkalemia may be useful for safe and effective termination of AF, perhaps in combination with atrial-selective sodium channel blockers (ranolazine, dronedarone, amiodarone or vernakalant).
3.2.2. The rapidly activating delayed rectifier potassium current (IK)
Both experimental and clinical studies indicate that specific block of IKr produces predominant prolongation of atrial vs. ventricular ERP at normal activation rates (Spinelli et al., 1992; Wiesfeld et al., 1996; Baskin & Lynch, Jr., 1998; Stump et al., 2005; Wang et al., 1994; Echt et al., 1982; Buchanan et al., 1996). These data can be explained on the basis of atrial-predominant prolongation of APD, since selective IKr block does not cause PRR. Indeed, a recent study has demonstrated that IKr block produces a much greater prolongation of APD and ERP in atria vs. ventricles at normal activation rates (CL = 500 ms) (Burashnikov et al., 2008a). In case of bradycardia and long pauses, however, it is the ventricle that develops greater APD prolongation, early afterdepolarization (EAD), and Torsades de Pointes when IKr is reduced (Antzelevitch et al., 1999; Burashnikov & Antzelevitch, 2006; Burashnikov & Antzelevitch, 2011a). This effect of IKr block limits the widespread use of many, but not all, drugs with IKr blocking properties in the treatment of AF (see 3.4).
3.2.3. The small conductance calcium activated potassium channels (SK channels)
SK channels have been identified in the hearts of mouse, rat, rabbit, and human (Tuteja et al., 2005; Diness et al., 2010). There are three SK channel subunits in the heart (i.e., SK1, SK2, and SK3), with SK1 and SK2 being selectively expressed in atria vs. ventricles. The interest in SK channels is fueled by the recent genome-wide associated studies, which have consistently demonstrated an association of variants in KCNN3 with lone AF (KCNN3 encodes the SK3 channel) (Ellinor et al., 2010).
Inhibition of the SK channels or genetic ablation of SK2 causes electrophysiological changes selectively in atria, particularly an impressive prolongation of atrial repolarization (Diness et al., 2010; et al., 2009b). Li Agents that block the SK channels have been reported to terminate AF effectively and to prevent the recurrence of the arrhythmia in experimental rat, guinea pig, and rabbit AF models (Diness et al., 2010). Thus, pilot experimental data suggest that the SK channels could be an atrial-selective pharmacological target for AF suppression. However, the functional consequences of modulation of SK channels, including anti- and pro-arrhythmic potential, are not well studied. For instance, it has been reported that genetic ablation of SK2 channels is associated with induction of AF in the mouse (Li et al., 2009b). Pathophysiological conditions may also modulate SK channel density or function, as has been recently reported in a rabbit heart failure model (Chua et al., 2011), and thus, may alter efficacy and safety of the SK blockers. The role of SK channels in large animal and human remain poorly investigated.
It needs to be recognized that the pharmacological ion channel specificity of SK modulators has been determined largely in expression systems and the specificity of the SK blockers in the native cardiac myocytes/preparations remains poorly defined. For instance, the potential atrial-selective effect of the SK blockers on peak INa or INa-mediated parameters, which may account for atrial selective ERP prolongation (see 3.2.1), has not been studied. Atrial selectivity of SK blockers should be tested in physiologically relevant conditions and cells/preparations, otherwise atrial selectivity may be missed or falsely-determined. For instance, the ability of an agent to block peak INa in atrial selective manner may not be evident even in atrial cells or preparations if tested at a holding potential −140 mV (Schram et al., 2004) or at relatively slow pacing rates, because atrial-selective INa blockers tends to have rapid unbinding kinetics (Burashnikov & Antzelevitch, 2009; Burashnikov & Antzelevitch, 2010). A recent example of this is the once promising selective IKur block approach. As it turned out the atrial selectivity of most prominent IKur blockers (i.e., vernakalant, AVE0118, AZD7009, etc) to prolong ERP is largely due to block of peak INa (see 3.1.1) and selective IKur inhibition appears not to be an effective strategy (see 3.1.1).
3.2.4. Other potential atrial-selective ion channel targets for the treatment of AF
The transient outward potassium current (Ito) is likely to be an atrial-selective target. Indeed, the predominant α-subunit of the Ito channel, Kv4.3, is expressed significantly more strongly in human atria vs. ventricles (Gaborit et al., 2007) and Ito is blocked much more effectively in atrial vs. ventricular myocytes. 4-AP IC50 values in atrial and ventricular myocytes are 471±97 and 1486±261 μM, respectively (Amos et al., 1996; Nattel et al., 2000). Ito inhibition likely contributes to the atrial-specific and anti-AF effects of IKur blockers since all agents that block IKur also inhibit Ito.
Another atrial selective target may be the adenosine triphosphate (ATP)-sensitive potassium current (IK-ATP). In rodents, the KATP channel in the heart is believed to be encoded by a co-assembly of Kir6.2 (the α-subunit) and SUR1 (a regulatory subunit) in atria and a co-assembly of KIR6 and SUR2 in ventricles (Flagg et al., 2008). It remains to be determined whether and to what extend this heterogeneity is present in other species. The expression of SUR2 is greater in human ventricles vs. atria and the expression of SUR1 is similar between the chambers (Gaborit et al., 2007). AF is encountered in patients diagnosed with the early repolarization syndrome, in which a gain of function in KCNJ8, encoding for Kir6.1, has been identified.(Haissaguerre et al., 2009; Medeiros-Domingo et al., 2010) There are other data indicating that IK-ATP may be involved in the generation of some forms of AF (Workman et al., 2008; Olson et al., 2007). Our recent data demonstrated that activation of IK-ATP with low therapeutic concentrations of pinacidil leads to preferential abbreviation of repolarization in atria and inducible arrhythmias in atria but not ventricles in the canine heart (Burashnikov & Antzelevitch, 2011b). Thus, in theory, atrial-selective block of IK-ATP may be of benefit in the management of IK-ATP–mediated forms of AF. Interestingly, propafenone, and potent sodium channel blocker used in the management of AF, has been reported to block IK-ATP with a 4-fold higher affinity in atrial than ventricular rabbit myocytes (Christe et al., 1999).
3.3 Late INa and AF
Augmented levels of late INa has been reported in atrial myocytes from patients with chronic AF (Sossalla et al., 2010) and prolonged atrial APD has been observed in a number of diseases associated with AF occurrence such as congestive HF (Li et al., 2000) atrial dilatation (Verheule et al., 2003; Roberts-Thomson et al., 2009) and LQTS (Kirchhof et al., 2003). These observations suggest that block of late INa may be useful in preventing the triggers that initiate AF in these cases.
3.4. Multiple-channel block for AF: efficacy and safety
Pure INa blockers (such as lidocaine and mexilitine) are generally not effective in suppressing AF in clinical and experimental studies (Fuster et al., 2011; Burashnikov et al., 2007), although at high concentrations, far beyond the therapeutic range, they can effectively suppress AF in experimental and theoretical studies (Kneller et al., 2005; Comtois et al., 2008). IKr block (with dofetilide and sotalol) is the only highly selective ion channel block approach that has proven to be effective against AF in clinic. Selective IKr blockers, however, are also associated with high risk for induction of TdP (Fuster et al., 2011).
Practical clinical experience indicates that with the exception of selective IKr blockers, currently available drugs showing anti-AF efficacy (amiodarone, dronedarone, flecainide, propafenone, vernakalant, etc) as well as promising investigational AADs (such as ranolazine), all inhibit multiple ion channels (particularly peak INa and IKr). Among these multiple channel blockers, those that inhibit INa with rapid dissociation kinetics (such as amiodarone, dronedarone, vernakalant, and ranolazine) are atrial selective and rarely if ever produce ventricular proarrhythmia. In contrast, multiple channel blockers inhibiting INa with slow/median dissociation kinetics (e.g., propafenone, flecainide, quinidine, procainamide etc) are far less atrial selective and are capable of inducing ventricular arrhythmias. Proarrhyhmia induced by these agents can be divided in two groups. The first type is associated with excessive and heterogeneous prolongation of ventricular repolarization (acquired long QT-mediated TdP; quinidine, procainamide, disopyramide) and the second type with conduction disturbances, commonly in structurally compromised hearts (propafenone, flecainide). Current understanding is that the probability of induction of TdP with multiple channel blockers depends largely on the balance of drug-induced inhibition on inward and outward currents, as well as on the pre-drug balance of these ion channel currents in the various cell types in the heart, which can be altered by disease, electrolyte abnormalities, hormonal imbalance. The likelihood of induction VT/VF with AADs depends on the degree to which they depress sodium channel activity and the degree to which they create heterogeneous depression of excitability. AAD-associated VT/VF commonly occurs in the structurally-compromised hearts, which are more responsive to INa block than healthy ventricles because sodium channels are already depressed.
The relative safety of multiple channel blockers with “rapid” unbinding from the sodium channel (ranolazine, amiodarone, vernakalant, etc; Class IB agents) is attributable to their actions to inhibit late INa and/or ICa and thus restrain the ability of outward current blockers to produce excessive prolongation of APD, thus preventing the development EAD and TdP.
A relatively high probability of induction of TdP by some multi-ion channel blockers such as quinidine and procainamide (Class IA agents) is due to the drugs’ effects to favor IKr inhibition, particularly at slow rates. Multiple ion channel blockers that exert the most potent INa inhibition possess the slowest unbinding kinetics from the sodium channel (flecainide and propafenone; Class IC agents) and their prime electrophysiological effect is significant conduction disturbances (i.e., conduction slowing and block). This accounts for their high proarrhythmic potential in the ventricles, particularly when significant structural abnormalities are present, including ischemic heart disease and advanced heart failure.
Some agents causing atrial-predominant block of INa may induce TdP in the clinic apparently in cases in which repolarization reserve is reduced. The balance of current during the action potential plateau can be shifted in the inward direction by acquired or genetic factors that produce a loss of function of outward current or a gain of function of inward current. Amiodarone seems to strike the right balance in most diseases states, and only rarely produces TdP. The proarrhythmic potential of amiodarone can be unmasked by an augmentation of late INa (Wu et al., 2008). Available clinical and experimental data have demonstrated that ranolazine does not induce cardiac arrhythmias de novo, not even under a variety of pathological conditions (Antzelevitch et al., 2011). AZD1305 is a multiple channel blocker (inhibiting peak and late INa, IKr, IKur) that affects INa-mediated parameters in an atrial-selective manner (Burashnikov et al., 2010b). AZD1305 effectively terminates both experimental and clinical AF (Burashnikov et al., 2010b; Ronaszeki et al., 2011), but may cause significant QT prolongation and induce TdP in some individuals (in 2 out of 128 patients) (Ronaszeki et al., 2011). These two patients were relatively sick (one of them had heart failure and another hypertension) and were on a number of medications, which might have altered the balance of inward and outward currents, thereby “unmasking” the proarrhythmic potential of AZD1305. AZD1305-induced TdP is likely due to a potent and inadequately opposed block of IKr. In canine cardiac preparations, INa-mediated parameters (including Vmax and conduction time) were shown to be depressed by AZD1305 to a greater extent in atria vs. ventricles, but these parameters were significantly altered in ventricles (Burashnikov et al., 2010b), to a much greater extent than that caused by ranolazine or amiodarone (Burashnikov et al., 2007; Burashnikov et al., 2008a). The kinetics of AZD1305 in blocking INa remains uninvestigated.
It appears that the degree of atrial selectivity of the multiple channel blockers as well as their anti-AF efficacy are related to their ability to produce concomitant atrial-selective prolongation of APD secondary to block of IKr; see 3.2.2) (Burashnikov et al., 2008a; Burashnikov & Antzelevitch, 2009). Lidocaine, a Class IB agent, does not block IKr and manifests a modest atrial selectivity in blocking peak INa and is only modestly effective in AF suppression (Burashnikov et al., 2007). Atrial-selective APD prolongation potentiates drug-induced INa inhibition by reducing or eliminating the diastolic interval in atria (Fig. 3), thus potentiating the ability of a drug to suppress AF. Thus, inhibition of IKr exerts both direct and indirect anti-AF actions (by prolonging APD and promoting peak INa inhibition, respectively).
Sotalol has previously been demonstrated to inhibit peak INa in ventricular muscles and Purkinje fibers at high concentrations (>100 μM) (Carmeliet, 1985). While INa block of sotalol at therapeutic concentrations is not functionally detectable in the ventricles, it may be detectable in atria (considering the atrial selectivity of many INa blockers (Burashnikov & Antzelevitch, 2010)). Intra-atrial conduction time in human atria has been reported to be increased by dl-sotalol in a use-dependent fashion (Watanabe et al., 2001), consistent with block of peak INa. In theory, inhibition of peak INa may contribute to the anti-AF efficacy of sotalol.
AF patients typically display a relatively brief and triangular-shaped AP morphology. In such cases, reduction of IKur produces a small prolongation of APD70–90 and thus of ERP (Fig. 2) (Wettwer et al., 2004; Burashnikov & Antzelevitch, 2008b). While IKur inhibition alone may not be sufficient to prolong APD significantly and to suppress AF, block of this current in combination with other currents (such as INa, Ito and/or IKr) may importantly contribute to ERP prolongation in atria and to anti-AF efficacy. In a tachypacing-induced AF goat model, AVE0118 (an IKur/Ito and INa blocker) has been shown to restore the ability of IKr blockers to prolong atrial ERP (this ability is significantly reduced in remodeled atria (Duytschaever et al., 2005; Tse & Lau, 2002)), greatly improving anti-AF efficacy, compared to IKur/Ito/INa or IKr block alone (Blaauw et al., 2007) note, AVE0118 inhibits INa in an atrial-selective manner and AVE0118-induced prolongation of atrial ERP is due to the development of PRR (at least in the canine atrium) (Burashnikov et al., 2009).
The current search for novel AADs is focused on finding an optimal balance between anti-AF efficacy, safety, and tolerability, with safety being the first priority. A combination of atrial selective sodium and potassium channel blockade may be the most effective approach for safe and effective treatment of AF in the case of many, but all AF pathologies (Antzelevitch & Burashnikov, 2009a; Burashnikov & Antzelevitch, 2010). Indeed, clinical experience indicates that an optimal long-term risk-benefit ratio is best achieved with multiple channel blockers, which inhibit INa with rapid dissociation kinetics, late INa, and IKr, as the major ion channel targets. Addition of IKur block to the “cocktail” may to add to the atrial selectivity and anti-AF efficacy. Clearly, individualized therapy should be based on clinical presentations in conformance with recommended guidelines (Camm et al., 2010; Fuster et al., 2011) to optimize effectiveness and to reduce the risk of adverse instance, dronedarone and amiodrone, while generally safe agents, may increase mortality in patients with preexisting advanced heart failure (Kober et al., 2008; Bardy et al., 2005). The adverse effects may be related to worsening of heart failure rather than to ventricular pro-arrhythmias. Indeed, both amiodarone and dronedarone potently inhibit calcium channel current, that acts to reduce cardiac contractility, which is already significantly impaired in advanced heart failure patients.
3.5. Atrial selectivity in remodeled atria
It is important to recognize that the atrial selectivity of INa and IKr blockers has been demonstrated largely in “healthy” atria and ventricles (Burashnikov et al., 2007; Burashnikov et al., 2008b; Spinelli et al., 1992; Wiesfeld et al., 1996; Baskin & Lynch, Jr., 1998; Wang et al., 1994). Changes associated with AF (electrical and structural remodeling) can significantly modify pharmacologic response of the atria to INa and IKr blockers (Duytschaever et al., 2005; Wettwer et al., 2004; Linz et al., 2007), thereby modulating the atrial selectivity of these agents and their ability to suppress AF, an effect well recognized with IKur blockers (Wettwer et al., 2004). Triangulation of atrial action potential, typically observed in electrically remodeled atria, reduces the ability of IKr blockers to prolong atrial APD. In contrast, the ability of IKur blockers to prolong APD70–90 is augmented or even revealed in remodeled atria (Burashnikov et al., 2004; Wettwer et al., 2004; Burashnikov & Antzelevitch, 2008b).
3.6. Ventricular arrhythmias can be suppressed by atrial- selective agents
Like atrial arrhythmias, ventricular rhythm disturbances also commonly occur under pathophysiological conditions associated with ischemia, infarction, long QT syndrome, hypotrophy, etc. These conditions can importantly modulate the efficacy of sodium and potassium channel blockers in both atria and ventricles. For instance, the atrial-selective agent AVE0118 effectively suppresses ventricular ischemic arrhythmias in dogs (Billman & Kukielka, 2008). Ranolazine also reduces the incidence of ventricular arrhythmias associated with acute coronary and long QT syndromes, an effect attributed to the action of ranolazine to block late INa (Scirica et al., 2007; Antzelevitch, 2008; Antzelevitch et al., 2004). The ability of chronic amiodarone to prevent ventricular arrhythmias is also well recognized (Singh, 2006).
4.0 Upstream therapy targets for AF
An important limitation of the ion channel block approach for AF treatment is that non-electrical factors (largely structural remodeling) may primarily determine the generation of AF, so that interventions reducing/preventing structural remodeling (are referred to as “upstream therapies”) may be required for effective pharmacological management (Goette et al., 2007; Savelieva & Camm, 2008; Nattel et al., 2008). Structural remodeling is comprised of a number of pathological changes such as interstitial fibrosis, fibroblast proliferation, dilatation, hypertrophy, pathological collagen accumulation and its abnormal distribution or redistribution, and can be mediated by multiple often interrelated diseases/factors, such as AF itself, aging, heart failure, ischemia, hypertension, oxidative stress, and inflammation (Goette et al., 2007; Savelieva & Camm, 2008; Nattel et al., 2008; Van Wagoner, 2008).
In order to develop successful “upstream therapy” approaches, it is important to identify and to understand the factors and signaling pathways involved in various AF pathologies. There are a number of already identified factors and signaling pathways, the activities of which have been associated with atrial structural remodeling. Among these factors are angiotensin II, transforming growth factor- β1 (TGF-β1), mitogen-activated protein kinase (MAPK), platelet-derived growth factor (PDGF), matrix metalloproteinases, peroxisome proliferator-activated receptor-λ (PPAR- λ), Janus kinase (JAK), Rac1, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, signal transducers and activators of transcription (STAT), calcineurin, etc (Nakajima et al., 2000; Dudley, Jr. et al., 2005; Nattel et al., 2008; Burstein et al., 2008; Shimano et al., 2008; Goette et al., 2007; Tsai et al., 2008). Angiotensin II and its angiotensin II type 1 (AT1) receptors are critically involved in the initiation of most of the signaling cascades (Goette et al., 2007; Nattel et al., 2008). It is recognized that a cause-effect relationship of these factors with AF is poorly defined or unknown, so that many of the changes observed in the factors/signaling pathways could be mere consequences of AF (Nattel et al., 2008). Interestingly, atria can develop structural remodeling to a greater degree or even independently of the ventricles (Nakajima et al., 2000; Hanna et al., 2004; Xiao et al., 2004; Verheule et al., 2004; Adam et al., 2007; Tsai et al., 2008; Burstein et al., 2008), pointing to the presence of potential atrial-selective targets for “upstream” AF therapy (Burashnikov, 2008).
The precise contribution of structural remodeling, inflammation, oxidative injury, ischemia, and stretch (and numerous mediating factors/signaling pathways) in the development of AF remains poorly understood and is likely to vary significantly among different AF pathologies (Camm et al., 2010). The anti-AF mechanisms of the upstream interventions are not well established, and presumed to be largely due to their antihypertensive, anti-inflammatory, and anti-oxidative stress actions, reducing structural remodeling. Anti-AF actions of some “upstream therapy” agents may be in part due to a direct effect on the atrial electrical properties (Li et al., 2009a; Sicouri et al., 2011). On the other hand, chronic application of some AADs, like amiodarone (Ashikaga et al., 2006), can ameliorate atrial structural remodeling, so that long-term anti-AF action of some conventional AADs may be due to upstream actions.
Experimental and clinical evidence indicate that angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptors blockers (ARBs), statins, aldosterone antagonists, and omega-3 poly-unsaturated fatty acids (PUFAs) may or may not be beneficial for AF treatments (Goette et al., 2007; Savelieva & Camm, 2008; Ducharme et al., 2006; Salehian et al., 2007; Camm et al., 2010). Most of the current clinical data on upstream AF therapy are, however, derived from observational studies that were not sufficiently powered. Generally, while many clinical studies in the early - mid 2000th were very promising, revealing a significant AF reduction with various upstream therapy agents (i.e., ACEIs, ARBs, statins, PUFAs) in a number of AF pathologies, results of the recent studies, particularly from large randomized clinical trials, have been quite sobering (Camm et al., 2010; Disertori et al., 2009; Yusuf et al., 2011; Kowey et al., 2010; Almroth et al., 2009; Schwartz et al., 2011; Bianconi et al., 2011; Savelieva et al., 2011a; Savelieva et al., 2011b). These later data indicate that PUFAs do not significantly affect the incidence of new onset AF and AF recurrence (Camm et al., 2010; Kowey et al., 2010; Bianconi et al., 2011; Savelieva et al., 2011a; Savelieva et al., 2011b). ACEIs and ARBs may indeed reduce new onset AF (i.e., primary prevention) but this positive effect is limited only to patients with significant systolic dysfunction and hypertension (Camm et al., 2010; Healey et al., 2005; Maggioni et al., 2009; Disertori et al., 2009; Yusuf et al., 2011; Ducharme et al., 2006). Reasonably proven anti-AF efficacy of statins is largely limited to the prevention of new-onset AF post-operatively (Savelieva et al., 2011a; Camm et al., 2010). The effectiveness of ACEIs, ARBs, and statins to reduce AF recurrence (i.e., secondary prevention) appears to be poor. (Maggioni et al., 2009) (Disertori et al., 2009; Almroth et al., 2009; Schwartz et al., 2011; Savelieva et al., 2011b) (Salehian et al., 2007).
Available clinical data indicate that the success rate of upstream therapy is better in primary vs. secondary AF prevention (Savelieva et al., 2011a; Savelieva et al., 2011b). It seems that upstream therapy for AF may be useful in preventing the development of atrial structural remodeling (i.e., an AF substrate) than in reversing already established atrial structural remodeling (perhaps largely irreversible) (Savelieva et al., 2011a). Although recent studies of upstream therapy have been largely negative, additional investigations are needed to determine the effect of specific upstream therapy agents in specific AF pathologies, focusing on early stages of atrial structural remodeling.
5.0. Gap junction targets for AF treatment
Conduction disturbances may play a pivotal role in the generation of AF. Conduction abnormalities in the heart can occur due to 1) disturbances in the sodium and/or calcium channel activity; 2) structural changes in the myocardium; or 3) gap junction abnormalities (impairing cell-to-cell communications). Gap junctions are comprised of proteins called connexins (Cx), which connect myocardial cells through low-resistance pathways. There are Cx40, Cx43, and Cx45 in the human heart. Cx40 may be a potential target for atrial-specific treatment of AF because it is found in atrial but not ventricular myocardium (Ehrlich et al., 2008). Cx40 is, however, present in the ventricular conduction system.
There are diverse outcomes in the experimental studies testing the anti-AF efficacy of gap junction modifiers. Improving gap junction conductance using the gap junction modulator rotigaptide has been shown to suppress AF in a canine chronic mitral regurgitation model of AF (Guerra et al., 2006) and in the canine acute ischemia model of AF (Shiroshita-Takeshita et al., 2007). Rotigaptide, however, did not affect AF development in AF models associated with heart failure or atrial tachypacing (Guerra et al., 2006; Shiroshita-Takeshita et al., 2007). In a recent study involving a canine prolonged tachypacing-induced AF model, GAP-134 (a dipeptide compound similar to ratigaptide) exerted no anti-AF effect overall (Laurent et al., 2009). However, when the dogs were separated based on severity of atrial structural remodeling (a degree of dilation), GAP-134 did not change AF vulnerability in the dogs with significant structural remodeling, but somewhat reduced AF induction in the dogs with relatively mild structural remodeling (Laurent et al., 2009).
An apparent major limitation of gap junction therapy for AF is that conduction slowing in atria susceptible to AF occurs largely due to structural remodeling (Dhein et al., 2010). As a result, a practical applicability of the gap junction therapy seems to be limited to specific AF cases in which a malfunction in gap junction functions (such as channel conductance) is a principal cause of AF. Gap junction therapy may not be effective in AF cases associated with a reduction in the density of gap junction or their lateralization (Kostin et al., 2002).
6.0 Intracellular calcium handling and AF treatment
There is a growing body of evidence that abnormal intracellular calcium homeostasis, observed in experimental and clinical AF studies, may play a role in the generation of AF and that normalization of sarcoplasmic reticulum (SR) calcium release may be a potential therapeutic target (Burashnikov & Antzelevitch, 2003; Hove-Madsen et al., 2004; Vest et al., 2005; Dobrev & Nattel, 2008; Sood et al., 2008; Burashnikov & Antzelevitch, 2006). An increase in spontaneous SR Ca2+ release as well as a significant SR calcium leak have been observed in atrial myocytes isolated from AF patients and dogs with tachypacing-induced atrial remodeling (Hove-Madsen et al., 2004; Vest et al., 2005). This SR calcium leak may be mediated by PKA hyperphosphorylation and calstabin2 (a ryanodine receptor inhibitory subunit, FKBP12.6) (Vest et al., 2005). Calstabin2 deficient mice displaying an augmented SR calcium leak are prone to develop AF (Sood et al., 2008). A pharmacological normalization of SR calcium release with JTV519 and tetracaine has been shown to exert antiarrhythmic actions in experimental models of AF (Kumagai et al., 2003; Sood et al., 2008).
Electrophysiological mechanisms responsible for atrial arrhythmias caused by abnormal intracellular calcium handling could be delayed (DAD) and early (EAD) afterdepolarization-induced triggered activity, secondary to spontaneous SR calcium release. There is also a specific form of EAD, termed the late phase 3 EAD, that is caused by augmented normal SR calcium release under conditions of abbreviated APD, when the calcium transient extends beyond the end of final repolarization (Burashnikov & Antzelevitch, 2003; Burashnikov & Antzelevitch, 2006).
The “normalization of Cai” may be more important for prevention of the initiation of AF, rather than for termination of sustained AF. AF is often initiated by calcium-mediated triggered activity and commonly maintained by a reentrant mechanism. Some forms of AF generated in experimental studies are maintained by focal sources, specifically SR calcium release –mediated triggered activity, often originating from pulmonary vein muscle sleeves (Zhou et al., 2002; Fenelon et al., 2003; Chou et al., 2005; Hirose & Laurita, 2007).
Although pharmacological modulation of the Cai–dependent arrhythmogenic mechanisms might be of benefit, the challenge is to regulate calcium release and intracellular calcium loading, without compromising myocardial contractility. It has been shown recently that some INa blockers (such as flecainide and propafenone) can directly inhibit SR calcium release (Hwang et al., 2011), which may contribute to the anti-AF efficacy of these agents. Many multiple channel blockers (such as ranolazine, amiodarone, vernakalant etc) block ICa and/or late INa and, thus, reduce intracellular calcium loading, which may contribute to their anti-AF action (Maier, 2009).
7.0 “Old” drugs for improvement of AF therapy
Improved management of AF can be achieved not only with the development of novel agents, but also with optimization of the use of already available AADs (better knowing “where, when, and how to use or not to use an AAD”). There has been clear progress in this direction over the past two decades, chiefly due to “negative” results of the large clinical trials demonstrating adverse effects of agents in patients with specific cardiac diseases (such as myocardial infarction in the CAST and SWORD trials, and advanced systolic heart failure in the ANDROMEDA trial) (CAST Investigators, 1989; Waldo et al., 1996; Kober et al., 2008). This “negative experience” has provided physicians with valuable knowledge regarding use of specific AADs in specific AF populations. For instance, many AF patients can be successfully (in terms of safety, efficacy, and tolerability) treated with “dangerous” flecainide, provided that appropriate patients are selected, the treatment is correctly optimized and therapy is adjusted as needed (Aliot et al., 2011).
There are surprising and thought-provoking results of a large non-randomized study (including 141,500 AF patients, 40,823 of which are treated with AADs) (Andersen et al., 2009). This investigation demonstrated that the long term use of d-sotalol, flecainide, and propafenone is associated with significant reduction of total mortality in AF patients (Andersen et al., 2009). While having important limitations, such as being non-randomized, this trial has major strengths such as inclusion of all non-selected AF patients registered in Denmark from 1995–2004.
8.0 Some unresolved issues for improvement of pharmacological rhythm control
There are important atrioventricular differences in the development of proarrhythmia related to conduction slowing and prolongation of repolarization. It is well known that INa blockers (Class IC agents) readily induce ventricular arrhythmias, commonly in structurally compromised ventricles. In contrast, de novo AF is generally not observed in individuals treated with Class IC drugs (although de novo atrial flutter has been reported (Nabar et al., 2001)). Conduction slowing is believed to be principally involved in the generation of ventricular pro-arrhythmia induced by Class IC. Why do INa blockers slow down conduction velocity in both atria and ventricles, but produce VF but not AF? Is conduction disturbance not important for AF generation? Or are conduction disturbances giving rise to AF limited to those caused by structural remodeling and not by INa blockers. The use of IKr blockers is also often associated with the induction of ventricular proarrhythmia (i.e., TdP), but seems never with AF de novo. Why do IKr blockers that prolong atrial and ventricular repolarization cause TdP nd VF, but not AF? Consistent with clinical observations, AADs and conditions that consistently produce EAD and TdP in canine ventricular preparations (block of IKr and/or IKs, enhancement of late INa, etc) (Shimizu & Antzelevitch, 2000; Burashnikov & Antzelevitch, 1998; Burashnikov & Antzelevitch, 2002) do not induce EAD or arrhythmias in canine atrial preparations (Burashnikov & Antzelevitch, 2011a). Mechanisms underlying protection of atria from development of INa- and IKr block-induced proarrhythmia are poorly defined. Knowledge on these mechanisms/factors may help in developing safe and effective AADs.
9.0 Conclusion
The search for novel AADs is focused on achieving an optimal balance among anti-AF efficacy, safety, and tolerability, with safety being the first priority. Ongoing research aimed at development of new pharmacological strategies for the management of AF includes both ion channel and non-ion channel-mediated approaches to therapy. A better understanding of atrial- and pathology-selective targets and agents holds promise for the development of effective and safe new treatments.
Acknowledgments
Financial support: Supported by grant HL47678 from NHLBI (CA) and NYS and Florida Masons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- Aliot E, Capucci A, Crijns HJ, Goette A, Tamargo J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almroth H, Hoglund N, Boman K, Englund A, Jensen S, Kjellman B, et al. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo-controlled multicentre study. Eur Heart J. 2009;30:827–833. doi: 10.1093/eurheartj/ehp006. [DOI] [PubMed] [Google Scholar]
- Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491 (Pt 1):31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SS, Hansen ML, Gislason GH, Schramm TK, Folke F, Fosbol E, et al. Antiarrhythmic therapy and risk of death in patients with atrial fibrillation: a nationwide study. Europace. 2009;11:886–891. doi: 10.1093/europace/eup119. [DOI] [PubMed] [Google Scholar]
- Anter E, Callans DJ. Pharmacological and electrical conversion of atrial fibrillation to sinus rhythm is worth the effort. Circulation. 2009;120:1436–1443. doi: 10.1161/CIRCULATIONAHA.108.824847. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C. Ranolazine: a new antiarrhythmic agent for patients with non-ST-segment elevation acute coronary syndromes? Nat Clin Pract Cardiovasc Med. 2008;5:248–249. doi: 10.1038/ncpcardio1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, et al. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J Electrocardiol. 2009;42:543–548. doi: 10.1016/j.jelectrocard.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiological basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Arnsdorf MF, Schreiner E, Gambetta M, Friedlander I, Childers RW. Electrophysiological changes in the canine atrium and ventricle during progressive hyperkalaemia: electrocardiographical correlates and the in vivo validation of in vitro predictions. Cardiovasc Res. 1977;11:409–418. doi: 10.1093/cvr/11.5.409. [DOI] [PubMed] [Google Scholar]
- Ashikaga K, Kobayashi T, Kimura M, Owada S, Sasaki S, Iwasa A, et al. Effects of amiodarone on electrical and structural remodeling induced in a canine rapid pacing-induced persistent atrial fibrillation model. Eur J Pharmacol. 2006;536:148–153. doi: 10.1016/j.ejphar.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Banchs JE, Wolbrette DL, Samii SM, Penny-Peterson ED, Patel PP, et al. Efficacy and safety of dofetilide in patients with atrial fibrillation and atrial flutter. J Interv Card Electrophysiol. 2008 doi: 10.1007/s10840-008-9290-6. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Barold SS, Falkoff MD, Ong LS, Heinle RA. Hyperkalemia-induced failure of atrial capture during dual-chamber cardiac pacing. J Am Coll Cardiol. 1987;10:467–469. doi: 10.1016/s0735-1097(87)80034-7. [DOI] [PubMed] [Google Scholar]
- Baskin EP, Lynch JJ., Jr Differential atrial versus ventricular activities of class III potassium channel blockers. J Pharmacol Exp Ther. 1998;285:135–142. [PubMed] [Google Scholar]
- Bechard J, Gibson JK, Killingsworth CR, Wheeler JJ, Schneidkraut MJ, Huang J, et al. Vernakalant selectively prolongs atrial refractoriness with no effect on ventricular refractoriness or defibrillation threshold in pigs. J Cardiovasc Pharmacol. 2011;57:302–307. doi: 10.1097/FJC.0b013e3182073c94. [DOI] [PubMed] [Google Scholar]
- Bechard J, Pourrier M. Atrial selective effects of intravenously administrated vernakalant in conscious beagle dog. J Cardiovasc Pharmacol. 2011;58:49–55. doi: 10.1097/FJC.0b013e31821b8608. [DOI] [PubMed] [Google Scholar]
- Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- Bianconi L, Calo L, Mennuni M, Santini L, Morosetti P, Azzolini P, et al. n-3 polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011;13:174–181. doi: 10.1093/europace/euq386. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kukielka M. Novel transient outward and ultra-rapid delayed rectifier current antagonist, AVE0118, protects against ventricular fibrillation induced by myocardial ischemia. J Cardiovasc Pharmacol. 2008;51:352–358. doi: 10.1097/FJC.0b013e31816586bd. [DOI] [PubMed] [Google Scholar]
- Blaauw Y, Gogelein H, Tieleman RG, van HA, Schotten U, Allessie MA. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- Blaauw Y, Schotten U, van HA, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc Res. 2007;75:89–98. doi: 10.1016/j.cardiores.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Goegelein H, Ruetten H. Effect of dronedarone on Na(+), Ca (2+) and HCN channels. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:347–356. doi: 10.1007/s00210-011-0599-9. [DOI] [PubMed] [Google Scholar]
- Buchanan LV, LeMay RJ, Walters RR, Hsu CY, Brunden MN, Gibson JK. Antiarrhythmic and electrophysiologic effects of intravenous ibutilide and sotalol in the canine sterile pericarditis model. J Cardiovasc Electrophysiol. 1996;7:113–119. doi: 10.1111/j.1540-8167.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Burashnikov A. Are there atrial selective/predominant targets for “upstream” atrial fibrillation therapy? Heart Rhythm. 2008;5:1294–1295. doi: 10.1016/j.hrthm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Prominent IKs in epicardium and endocardium contributes to development of transmural dispersion of repolarization but protects against development of early afterdepolarizations. J Cardiovasc Electrophysiol. 2002;13:172–177. doi: 10.1046/j.1540-8167.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers: do they exist? J Cardiovasc Pharmacol. 2008a;52:121–128. doi: 10.1097/FJC.0b013e31817618eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008b;5:1304–1309. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? J Atrial Fibrillation. 2008c;1:98–107. doi: 10.4022/jafib.v1i1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs. 2009;14:233–249. doi: 10.1517/14728210902997939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. New development in atrial antiarrhythmic drug therapy. Nat Rev Cardiol. 2010;7:139–148. doi: 10.1038/nrcardio.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Absence of early afterdepolarizations in canine atria under long QT syndrome. Heart Rhythm. 2011a;8(5S):S328. Ref Type: Abstract. [Google Scholar]
- Burashnikov A, Antzelevitch C. Activation of IK-ATP with low therapeutic concetrations of pinacidil leads to preferential abbreviation of repolarization in atria and inducible arrhythmias in atria but not ventricles. Heart Rhythm. 2011b;8(5S):S466. Ref Type: Abstract. [Google Scholar]
- Burashnikov A, Barajas-Martinez H, Hu D, Nof E, Blazek J, Antzelevitch C. The atrial-selective potassium channel blocker AVE0118 prolongs effective refractory period in canine atria by inhibiting sodium channels. Heart Rhythm. 2009;6:S98. (Abstract) [Google Scholar]
- Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008a;5:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block as a strategy for suppression of atrial fibrillation. Ann N Y Acad Sci. 2008b;1123:105–112. doi: 10.1196/annals.1420.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of dronedarone and ranolazine to suppress atrial fibrillation. J Am Coll Cardiol. 2010a;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Zygmunt AC, Di Diego JM, Linhardt G, Carlsson L, Antzelevitch C. AZD1305 exerts atrial-predominant electrophysiological actions and is effective in suppressing atrial fibrillation and preventing its re-induction in the dog. J Cardiovasc Pharmacol. 2010b;56:80–90. doi: 10.1097/FJC.0b013e3181e0bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;12:1360–1420. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J Cardiovasc Pharmacol. 2006;47:123–132. doi: 10.1097/01.fjc.0000196242.04384.c3. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Electrophysiologic and voltage clamp analysis of the effects of sotalol on isolated cardiac muscle and Purkinje fibers. J Pharmacol Exp Ther. 1985;232:817–825. [PubMed] [Google Scholar]
- CAST Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–1737. doi: 10.1161/CIRCULATIONAHA.105.561738. [DOI] [PubMed] [Google Scholar]
- Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, Fishbein MC, Lin SF, Chen PS. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, et al. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe G, Tebbakh H, Simurdova M, Forrat R, Simurda J. Propafenone blocks ATP-sensitive K+ channels in rabbit atrial and ventricular cardiomyocytes. Eur J Pharmacol. 1999;373:223–232. doi: 10.1016/s0014-2999(99)00217-4. [DOI] [PubMed] [Google Scholar]
- Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois P, Sakabe M, Vigmond EJ, Munoz MA, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers. Insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295:H1489–H1504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- Cosio FG, Aliot E, Botto GL, Heidbuchel H, Geller CJ, Kirchhof P, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–27. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- Cotiga D, Arshad A, Aziz E, Joshi S, Koneru JN, Steinberg JS. Acute conversion of persistent atrial fibrillation during dofetilide initiation. PACE. 2007;30:1527–1530. doi: 10.1111/j.1540-8159.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- Crijns HJ, Van GI, Walfridsson H, Kulakowski P, Ronaszeki A, Dedek V, Malm A, Almgren O. Safe and effective conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. Heart Rhythm. 2006;3:1321–1331. doi: 10.1016/j.hrthm.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98:2574–2579. doi: 10.1161/01.cir.98.23.2574. [DOI] [PubMed] [Google Scholar]
- Dhein S, Hagen A, Jozwiak J, Dietze A, Garbade J, Barten M, et al. Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:221–234. doi: 10.1007/s00210-009-0473-1. [DOI] [PubMed] [Google Scholar]
- Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, Lucci D, Di PG, Tognoni G. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, et al. The G protein-gated potassium Gprotein current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008 doi: 10.1097/FJC.0b013e318171924d. [DOI] [PubMed] [Google Scholar]
- Dorian P, Pinter A, Mangat I, Korley V, Cvitkovic SS, Beatch GN. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J Cardiovasc Pharmacol. 2007;50:35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- Doughty RN, Rodgers A, Sharpe N, MacMahon S. Effects of beta-blocker therapy on mortality in patients with heart failure. A systematic overview of randomized controlled trials. Eur Heart J. 1997;18:560–565. doi: 10.1093/oxfordjournals.eurheartj.a015297. [DOI] [PubMed] [Google Scholar]
- Ducharme A, Swedberg K, Pfeffer MA, Cohen-Solal A, Granger CB, Maggioni AP, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006;152:86–92. [PubMed] [Google Scholar]
- Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- Duytschaever M, Blaauw Y, Allessie M. Consequences of atrial electrical remodeling for the anti-arrhythmic action of class IC and class III drugs. Cardiovasc Res. 2005;67:69–76. doi: 10.1016/j.cardiores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Echt DS, Berte LE, Clusin WT, Samuelson RG, Harrison DC, Mason JW. Prolongation of the human monophasic action potential by sotalol. Am J Cardiol. 1982;50:1082–1086. doi: 10.1016/0002-9149(82)90421-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008;51:787–792. doi: 10.1016/j.jacc.2007.08.067. [DOI] [PubMed] [Google Scholar]
- Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JR, Nattel S. Atrial-selective pharmacological therapy for atrial fibrillation: hype or hope? Curr Opin Cardiol. 2009;24:50–55. doi: 10.1097/HCO.0b013e32831bc336. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16:519–532. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Shepard RK, Stambler BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:1093–1102. doi: 10.1046/j.1540-8167.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am J Physiol. 1998;275:H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP Channels Require SUR1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (IKur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52:105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Gaborit N, Le BS, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller JC, Egstrup K, Kulakowski P, Rosenqvist M, Jansson MA, Berggren A, Edvardsson N, Sager P, Crijns HJ. Rapid conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. J Clin Pharmacol. 2009;49:312–322. doi: 10.1177/0091270008329549. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Goette A, Bukowska A, Lendeckel U. Non-ion channel blockers as anti-arrhythmic drugs (reversal of structural remodeling) Curr Opin Pharmacol. 2007;7:219–224. doi: 10.1016/j.coph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Goldstein RN, Khrestian C, Carlsson L, Waldo AL. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J Cardiovasc Electrophysiol. 2004;15:1444–1450. doi: 10.1046/j.1540-8167.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- Guerra JM, Everett TH, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- Guglin M, Chen R, Curtis AB. Sinus rhythm is associated with fewer heart failure symptoms: insights from the AFFIRM trial. Heart Rhythm. 2010;7:596–601. doi: 10.1016/j.hrthm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Yamashita T, Tsuruzoe N. Tertiapin, a selective IK,ACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol Res. 2006;54:136–141. doi: 10.1016/j.phrs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Heidbuchel H, Callewaert G, Vereecke J, Carmeliet E. Membrane-bound nucleoside diphosphate kinase activity in atrial cells of frog, guinea pig, and human. Circ Res. 1992;71:808–820. doi: 10.1161/01.res.71.4.808. [DOI] [PubMed] [Google Scholar]
- Hirose M, Laurita KR. Calcium-mediated triggered activity is an underlying cellular mechanism of ectopy originating from the pulmonary vein in dogs. Am J Physiol Heart Circ Physiol. 2007;292:H1861–H1867. doi: 10.1152/ajpheart.00826.2006. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez FE, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, et al. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of llass I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R, et al. Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA consensus conference on atrial fibrillation entitled ‘research perspectives in atrial fibrillation’. Europace. 2009;11:860–885. doi: 10.1093/europace/eup124. [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Eckardt L, Franz MR, Monnig G, Loh P, Wedekind H, et al. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, et al. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res. 2005;96:e35–e47. doi: 10.1161/01.RES.0000160709.49633.2b. [DOI] [PubMed] [Google Scholar]
- Knobloch K, Brendel J, Rosenstein B, Bleich M, Busch AE, Wirth KJ. Atrial-selective antiarrhythmic actions of novel Ikur vs. Ikr, Iks, and IKAch class Ic drugs and beta blockers in pigs. Med Sci Monit. 2004;10:BR221–BR228. [PubMed] [Google Scholar]
- Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Kumar K, Nearing BD, Carvas M, Nascimento BC, Acar M, Belardinelli L, Verrier RL. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. doi: 10.1111/j.1540-8167.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- Laurent G, Leong-Poi H, Mangat I, Moe GW, Hu X, So PP, et al. Effects of chronic gap junction conduction–enhancing antiarrhythmic peptide GAP-134 administration on experimental atrial fibrillation in dogs. Circ Arrhythm Electrophysiol. 2009;2:171–178. doi: 10.1161/CIRCEP.108.790212. [DOI] [PubMed] [Google Scholar]
- Le Bouter S, El Harchi A, Marionneau C, Bellocq C, Chambellan A, van Veen T, et al. Long-term amiodarone administration remodels expression of ion channel transcripts in the mouse heart. Circulation. 2004;110:3028–3035. doi: 10.1161/01.CIR.0000147187.78162.AC. [DOI] [PubMed] [Google Scholar]
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597–605. doi: 10.1111/j.1540-8167.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- Li GR, Sun HY, Zhang XH, Cheng LC, Chiu SW, Tse HF, Lau CP. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc Res. 2009a;81:286–293. doi: 10.1093/cvr/cvn322. [DOI] [PubMed] [Google Scholar]
- Li GR, Wang HB, Qin GW, Jin MW, Tang Q, et al. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation. 2008;117:2449–2457. doi: 10.1161/CIRCULATIONAHA.108.769554. [DOI] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009b;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz DK, Afkham F, Itter G, Rutten H, Wirth KJ. Effect of atrial electrical remodeling on the efficacy of antiarrhythmic drugs: comparison of amiodarone with IKr- and Ito/IKur-blockade in vivo strial electrical remodeling and antiarrhythmic drugs. J Cardiovasc Electrophysiol. 2007;18:1313–1320. doi: 10.1111/j.1540-8167.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- Lubitz SA, Yi BA, Ellinor PT. Genetics of atrial fibrillation. Cardiol Clin. 2009;27:25–33. doi: 10.1016/j.ccl.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, et al. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- Maier LS. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late INa using ranolazine. J Cardiovasc Pharmacol. 2009;54:279–286. doi: 10.1097/FJC.0b013e3181a1b9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Takeda K, Ito M, Yamagishi R, Tamura M, Nakamura H, et al. Atria selective prolongation by NIP-142, an antiarrhythmic agent, of refractory period and action potential duration in guinea pig myocardium. J Pharmacol Sci. 2005;98:33–40. doi: 10.1254/jphs.fpj04045x. [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, et al. G -of-function ain mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- Murdock DK, Kersten M, Kaliebe J, Larrian G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a reveiw of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–267. [PMC free article] [PubMed] [Google Scholar]
- Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8:175–181. [PMC free article] [PubMed] [Google Scholar]
- Murdock DK, Reiffel JA, Kaliebe JW, Larrian G. The conversion of paroxysmal of initial onset of atrial fibrillation with oral ranolazine: implications for “pill in the pocket” approach in structural heart disease. J Am Coll Cardiol. 2010;55:A6.E58. (Abstract) [Google Scholar]
- Nabar A, Rodriguez LM, Timmermans C, van MR, Wellens HJ. Class IC antiarrhythmic drug induced atrial flutter: electrocardiographic and electrophysiological findings and their importance for long term outcome after right atrial isthmus ablation. Heart. 2001;85:424–429. doi: 10.1136/heart.85.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-β1 transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- Nattel S, Matthews C, De Blasio E, Han W, Li D, Yue L. Dose-dependence of 4-aminopyridine plasma concentrations and electrophysiological effects in dogs: potential relevance to ionic mechanisms in vivo. Circulation. 2000;101:1179–1184. doi: 10.1161/01.cir.101.10.1179. [DOI] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Liu XK, Park SJ, Zingman LV, Bienengraeber M, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kaplon B, Kolodziej P, Achremczyk P. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- Oral H, Souza JJ, Michaud GF, Knight BP, Goyal R, Strickberger SA, Morady F. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–1854. doi: 10.1056/NEJM199906173402401. [DOI] [PubMed] [Google Scholar]
- Pandit SV, Zlochiver S, Filgueiras-Rama D, Mironov S, Yamazaki M, Ennis SR, et al. Targeting atrio-ventricular differences in ion channel properties for terminating acute atrial fibrillation in pigs. Cardiovasc Res. 2011;89:843–851. doi: 10.1093/cvr/cvq359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- Patterson E, Scherlag BJ, Zhou J, Jackman WM, Lazzara R, Coscia D, Po S. Antifibrillatory actions of cisatracurium: an atrial specific m receptor antagonist. J Cardiovasc Electrophysiol. 2008;19:861–868. doi: 10.1111/j.1540-8167.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–296. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM, Kong DF. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol. 2009;54:1089–1095. doi: 10.1016/j.jacc.2009.04.085. [DOI] [PubMed] [Google Scholar]
- Pratt CM, Camm AJ, Cooper W, Friedman PL, Macneil DJ, Moulton KM, et al. Mortality in the Survival With ORal D-sotalol (SWORD) trial: why did patients die? Am J Cardiol. 1998;81:869–876. doi: 10.1016/s0002-9149(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Ravens U. Potassium channels in atrial fibrillation: targets for atrial and pathology-specific therapy? Heart Rhythm. 2008;5:758–759. doi: 10.1016/j.hrthm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Ravens U. Antiarrhythmic therapy in atrial fibrillation. Pharmacol Ther. 2010;128:129–145. doi: 10.1016/j.pharmthera.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Ravens U, Wettwer E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res. 2011;89:843–851. doi: 10.1093/cvr/cvq398. [DOI] [PubMed] [Google Scholar]
- Regan CP, Kiss L, Stump GL, McIntyre CJ, Beshore DC, Liverton NJ, et al. Atrial antifibrillatory effects of structurally distinct IKur blockers 3-[(dimethylamino)methyl]-6-methoxy-2-methyl-4-phenylisoquinolin-1(2H)-one and 2-phenyl-1,1-dipyridin-3-yl-2-pyrrolidin-1-yl-ethanol in dogs with underlying heart failure. J Pharmacol Exp Ther. 2008;324:322–330. doi: 10.1124/jpet.107.127654. [DOI] [PubMed] [Google Scholar]
- Regan CP, Stump GL, Wallace AA, Anderson KD, McIntyre CJ, Liverton NJ, Lynch JJ., Jr In vivo cardiac electrophysiologic and antiarrhythmic effects of an isoquinoline IKur blocker, ISQ-1, in rat, dog, and nonhuman primate. J Cardiovasc Pharmacol. 2007;49:236–245. doi: 10.1097/FJC.0b013e3180325b2a. [DOI] [PubMed] [Google Scholar]
- Reiffel JA. Atrial fibrillation: what have recent trials taught us regarding pharmacologic management of rate and rhythm control? Pacing Clin Electrophysiol. 2011;34:247–259. doi: 10.1111/j.1540-8159.2010.02967.x. [DOI] [PubMed] [Google Scholar]
- Rienstra M, Van GI, Hagens VE, Veeger NJ, Van Veldhuisen DJ, Crijns HJ. Mending the rhythm does not improve prognosis in patients with persistent atrial fibrillation: a subanalysis of the RACE study. Eur Heart J. 2006;27:357–364. doi: 10.1093/eurheartj/ehi637. [DOI] [PubMed] [Google Scholar]
- Roberts-Thomson KC, Stevenson I, Kistler PM, Haqqani HM, Spence SJ, Goldblatt JC, Sanders P, Kalman JM. The role of chronic atrial stretch and atrial fibrillation on posterior left atrial wall conduction. Heart Rhythm. 2009;6:1109–1117. doi: 10.1016/j.hrthm.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Ronaszeki A, Alings M, Egstrup K, Gaciong Z, Hranai M, Kiraly C, et al. Pharmacological cardioversion of atrial fibrillation--a double-blind, randomized, placebo-controlled, multicentre, dose-escalation study of AZD1305 given intravenously. Europace. 2011 doi: 10.1093/europace/eur120. (in press) [DOI] [PubMed] [Google Scholar]
- Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study) Circulation. 2009;120:1036–1040. doi: 10.1161/CIRCULATIONAHA.108.839639. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dubuc M, Thibault B, Guerra P, Macle L, Khairy P. Atrial fibrillation and congestive heart failure. Curr Opin Cardiol. 2009;24:29–34. doi: 10.1097/hco.0b013e32831c8c58. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- Salehian O, Healey J, Stambler B, Alnemer K, Almerri K, Grover J, et al. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am Heart J. 2007;154:448–453. doi: 10.1016/j.ahj.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:30–41. doi: 10.1038/ncpcardio1038. [DOI] [PubMed] [Google Scholar]
- Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace. 2011a;13:308–328. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]
- Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011b;13:610–625. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- Schotten U, de Haan S, Verheule S, Harks EG, Frechen D, Bodewig E, et al. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007;73:37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Schram G, Zhang L, Derakhchan K, Ehrlich JR, Belardinelli L, Nattel S. Ranolazine: Ion-channel-blocking actions and in vivo electrophysiological effects. Br J Pharmacol. 2004;142:1300–1308. doi: 10.1038/sj.bjp.0705879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ Res. 2009;104:1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: Analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- Seki A, Hagiwara N, Kasanuki H. Effects of NIP-141 on K currents in human atrial myocytes. J Cardiovasc Pharmacol. 2002;39:29–38. doi: 10.1097/00005344-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Shimano M, Tsuji Y, Inden Y, Kitamura K, Uchikawa T, Harata S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5:451–459. doi: 10.1016/j.hrthm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Gianetti B, Zygmunt AC, Cordeiro JM, Antzelevitch C. Antiarrhythmic effects of simvastatin in canine pulmonary vein sleeve preparations. J Am Coll Cardiol. 2011;57:986–993. doi: 10.1016/j.jacc.2010.08.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN. Amiodarone: a multifaceted antiarrhythmic drug. Curr Cardiol Rep. 2006;8:349–355. doi: 10.1007/s11886-006-0074-2. [DOI] [PubMed] [Google Scholar]
- Singh D, Cingolani E, Diamond GA, Kaul S. Dronedarone for atrial fibrillation: have we expanded the antiarrhythmic armamentarium? J Am Coll Cardiol. 2010;55:1569–1576. doi: 10.1016/j.jacc.2009.10.071. [DOI] [PubMed] [Google Scholar]
- Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, et al. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, et al. Altered Na+ currents in atrial fibrillation: effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Spinelli W, Parsons RW, Colatsky TJ. Effects of WAY-123,398, a new class III antiarrhythmic agent, on cardiac refractoriness and ventricular fibrillation threshold in anesthetized dogs: a comparison with UK-68798, E-4031, and dl-sotalol. J Cardiovasc Pharmacol. 1992;20:913–922. doi: 10.1097/00005344-199212000-00011. [DOI] [PubMed] [Google Scholar]
- Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol. 2009;53:1182–1191. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Stump GL, Wallace AA, Regan CP, Lynch JJ., Jr In vivo antiarrhythmic and cardiac electrophysiologic effects of a novel diphenylphosphine oxide IKur blocker (2-isopropyl-5-methylcyclohexyl) diphenylphosphine oxide. J Pharmacol Exp Ther. 2005;315:1362–1367. doi: 10.1124/jpet.105.092197. [DOI] [PubMed] [Google Scholar]
- Szel T, Koncz I, Jost N, Baczko I, Husti Z, Virag L, et al. Class I/B antiarrhythmic property of ranolazine, a novel antianginal agent, in dog and human cardiac preparations. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc M, et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;55:1796–1802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Lai LP, Kuo KT, Hwang JJ, Hsieh CS, Hsu KL, et al. Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodeling. Circulation. 2008;117:344–355. doi: 10.1161/CIRCULATIONAHA.107.695346. [DOI] [PubMed] [Google Scholar]
- Tse HF, Lau CP. Electrophysiologic actions of dl-sotalol in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2002;40:2150–2155. doi: 10.1016/s0735-1097(02)02592-5. [DOI] [PubMed] [Google Scholar]
- Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- Van Gelder I, Hagens VE, Bosker HA, Kingma JH, Kamp O, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der LM, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheule S, Wilson E, Everett T, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, et al. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Waldo AL, Camm AJ, DeRuyter H, Friedman PL, Macneil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Feng J, Nattel S. Class III antiarrhythmic drug action in experimental atrial fibrillation. Differences in reverse use dependence and effectiveness between d-sotalol and the new antiarrhythmic drug ambasilide. Circulation. 1994;90:2032–2040. doi: 10.1161/01.cir.90.4.2032. [DOI] [PubMed] [Google Scholar]
- Wang ZG, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes: Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Watanabe I, Nakai T, Oshikawa N, Kunimoto S, et al. Frequency-dependent electrophysiological effects of flecainide, nifekalant and d,l-sotalol on the human atrium. Jpn Circ J. 2001;65:1–6. doi: 10.1253/jcj.65.1. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- Wiesfeld AC, De Langen CD, Crijns HJ, Bel KJ, Hillege HL, Wesseling H, Lie KI. Rate-dependent effects of the class III antiarrhythmic drug almokalant on refractoriness in the pig. J Cardiovasc Pharmacol. 1996;27:594–600. doi: 10.1097/00005344-199604000-00021. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Wirth KJ, Brendel J, Steinmeyer K, Linz DK, Rutten H, Gogelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2007a;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- Wirth KJ, Brendel J, Steinmeyer K, Linz DK, Rutten H, Gogelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2007b;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- Workman AJ, Kane KA, Rankin AC. Cellular bases for human atrial fibrillation. Heart Rhythm. 2008;5:S1–S6. doi: 10.1016/j.hrthm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Rajamani S, Shryock JC, Li H, Ruskin J, Antzelevitch C, Belardinelli L. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008;77:481–488. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse DG. Cardioversion of atrial fibrillation for maintenance of sinus rhythm: a road to nowhere. Circulation. 2009;120:1444–1452. doi: 10.1161/CIRCULATIONAHA.109.884387. [DOI] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–1252. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li J, Lin X, Yang Y, Hong K, Wang L, et al. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Hum Genet. 2009;54:277–283. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, Hart RG, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928–938. doi: 10.1056/NEJMoa1008816. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Park AM, et al. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283:H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]