Abstract
Histamine is a bioactive amine that exerts immunomodulatory functions, including many allergic symptoms. It is preformed and stored in mast cells and basophils but recent evidence suggests that other cell types produce histamine in an inducible fashion. During infection, it has been suggested that neutrophils may produce histamine. We also observed that histamine is released in a neutrophil-mediated LPS-induced model of acute lung injury. Therefore, we sought to examine whether innate signals promote histamine production by neutrophils. Bone marrow-derived neutrophils stimulated with a range of TLR agonists secreted histamine in response to LPS or R837, suggesting TLR4 or TLR7 are important. LPS-driven histamine was enhanced by coculture with GM-CSF and led to a transient release of histamine that peaked at 8 hours post stimulation. This was dependent upon de novo synthesis of histamine, since cells derived from histidine decarboxylase (HDC) deficient mice were unable to produce histamine but did generate reactive oxygen species upon stimulation. Using pharmacological inhibitors, we show that histamine production requires PI3 kinase, which has been shown to regulate other neutrophil functions, including activation and selective granule release. However, unlike mast cells, HDC deficiency did not alter the granule structure of neutrophils, suggesting that histamine does not participate in granule integrity in these cells. Consequently, our findings establish that neutrophils generate histamine in response to a select panel of innate immune triggers and that this might contribute to acute lung injury responses.
Keywords: Histamine, neutrophil, histidine decarboxylase, TLR4, TLR7, granule
INTRODUCTION
Histamine is best known for its potent effects on vasodilation and smooth muscle contraction during immediate hypersensitivity responses such as anaphylaxis and insect sting reactions [1]. Consequently, antihistamines are a mainstay therapy in allergic diseases, particularly allergic rhinitis. The immediacy of the histamine effect is due largely to it being preformed and stored within the granules of mast cells and basophils, allowing for rapid release on their activation. However, in recent years the role of histamine in biology has been revised due to the findings that it can exert potent immunomodulatory effects that are outside of its influence on allergic effector responses [2] and that other cell types may also be capable of generating histamine. Accumulating evidence has demonstrated that cells which lack the storage ability of mast cells or basophils may still synthesize histamine in an inducible fashion through transient expression of the histamine synthesis enzyme histidine decarboxylase (HDC). For example, Dunford et al. demonstrated that dendritic cells can generate low levels of histamine and that this acts in an autocrine manner on H4R to enhance CD4+ T cell priming [3]. Similarly, Yokoyama et al. demonstrated protection from P. acnes-primed/LPS-induced hepatitis that was mediated via histamine production from Kupffer cells that then prevented hepatocyte apoptosis [4]. Additionally, while HDC is critical for the de novo synthesis of histamine from histidine, extracellular histamine can also be sequestered by cells through the effects of vesicular monoamine transporters, as has been shown to occur in endometrial cells and enterochromaffin cells [5,6]. Consequently, some cells may possess histamine without actively synthesizing it.
In addition to its functional role in the responses mediated by histamine receptors, histamine also plays a necessary role in stabilizing the granules that contain the preformed mediators in mast cells via linking proteoglycans, glycosaminoglycans and heparin or chondroitin sulphate [7,8]. Consequently, mast cells derived from histamine deficient HDC−/− animals are severely impaired in their granule formation and growth [9].
Neutrophils are critically important cells in innate immune responses due to their capacity for killing microbes via release of their own preformed granule products, such as elastase, as well as reactive oxygen species (ROS) [10]. However, while best recognized for their tissue-destructive abilities during acute infection, they are also being increasingly explored for their capacity to modulate immunity [11] and have recently been shown to influence anaphylaxis [12]. A connection between neutrophils and histamine was initially shown by Tanaka et al., who described HDC expression in neutrophils recovered from the peritoneal cavity using a casein-induced peritonitis model [13]. Later, the levels of histamine itself was shown to correlate with neutrophil infiltration into the lung during the course of a mycoplasma pneumonia infection, even in mast cell deficient mice [14]; these authors demonstrated in vitro histamine production from neutrophils stimulated by either live or dead mycoplasma. Therefore, it seems likely that neutrophils have the capacity to upregulate HDC and to produce histamine during innate immune insults. However, the specific signals required for neutrophil production of histamine or the mechanisms behind this remain unknown.
Here, we investigated whether TLR signals are sufficient for neutrophils to produce histamine in vitro. We show that LPS (TLR4) and R837 (TLR7) are potent inducers of histamine from bone marrow-isolated neutrophils and also observed in vivo neutrophil-associated histamine during LPS-induced acute lung injury. The mechanism of histamine production by LPS is dependent upon HDC and could be blocked using Ly294002, a pharmacological inhibitor of PI3 kinase. However, unlike mast cells, histamine was not important for granule integrity in neutrophils, since HDC−/− neutrophils were indistinguishable from WT cells. Therefore, our findings suggest that neutrophils are another cell capable of generating inducible histamine and that this occurs specifically in response to TLR4 and TLR7 signals but not the other TLR signals studied.
MATERIAL AND METHODS
Animals
C57/BL6 mice (4–8 weeks old) were obtained from Taconic Farms (Hudson, NY, USA). HDC−/− mice were obtained from Dr Hiroshi Ohtsu and are previously described [9]. Mice were housed under specific pathogen-free conditions and all experiments were approved by the Northwestern University Animal Care and Use Committee.
LPS-induced Lung Injury Model
Mice were anesthetized with isoflurane before intranasal administration of 50µg of Escherichia coli LPS (strain 0127:B8, Sigma Chemical Co., St Louis, MO, USA) in 50µl volume. PBS was administered as a control. After 8 hours, lung tissue was collected or brochial alveolar lavage (BAL) performed.
Bronchial Alveolar Lavage
Airway inflammatory cells were collected by flushing lungs with 0.8 ml BAL fluid (10% FCS, 1mM EDTA, 1X PBS). BAL fluid was counted and the cells cytospun onto slides and differential cell counts performed after staining with DiffQuik reagent (Dade Behring, Neward, DE, USA).
Bone Marrow Neutrophil Isolation
Bone marrow neutrophils were isolated according to the methods described by Boxio et al. [15], with minor modifications. Briefly, dispersed bone marrow was filtered through a 70 µM nylon mesh, red blood cells lysed with PharmLyse solution (BD Pharmingen, San Diego, CA, USA) and overlaid onto an 82/62/51% discontinuous Percoll-HBSS gradient. These were centrifuged at 1000 × g for 30 minutes and the cell fraction at the 82%–62% interface collected. Cells were washed, then stained with biotin labeled anti-Ly6G antibody and anti-biotin immunomagnetic beads (Miltenyi Biotec, Auburn, CA, USA). Bead-bound cells were isolated by positive selection using an AutoMACS system (Miltenyi Biotec, Auburn, CA, USA), washed and resuspended at 106/mL in RPMI 1640/10% FCS for further culture or analysis. The purity of neutrophils was confirmed using flow cytometry against Ly-6C and Ly-6G and/or through cytometric analysis of cytospins stained with DiffQuik reagent and was greater than 95% neutrophils in all experiments.
In Vitro Neutrophil Culture
1 × 105 neutrophils/well were plated in 100µL RPMI 1640 (containing 0.015g/L of L-histidine) (Sigma Adrich, St Louis, MO, USA)/10% FCS in 96-well plates. Indicated treatments were applied in triplicate and cells cultured from 1 to 24 hours. Following culture, plates were spun down at 300 × g for 5 minutes and supernatant and cell fractions collected for further analysis. TLR ligands were LPS (Sigma Chemical Co., St Louis, MO, USA), Peptidoglycan from S. aureus, FSL-1, Poly IC, Flagellin from S. Typhimurium, R837, M362 (all from Invivogen, San Diego, CA, USA). The concentrations for each were: Peptidoglycan from S. aureus (PGN) 5µg/ml, FSL-1 500ng/ml, Poly IC 1 µg/ml, LPS 1 µg/ml, Flagellin from S. Typhimurium (FLA) 5 µg/ml, R837 5 µg/ml, M362 10 µg/ml.
Real-time RT-PCR
Total RNA was isolated from cells using a RNeasy kit (Qiagen, Valencia, CA). cDNA was prepared using qScript cDNA Super Mix (Quanta, Gaithersburg, MD). Gene expression was determined as previously described [16,17].
Histamine Quantification
Histamine concentrations were determined using an ELISA kit (Becton Dickinson, NJ, USA) as per the manufacturers instructions.
Reactive Oxygen Species Quantification
Bone Marrow isolated neutrophils (2.5 × 105/sample) from wildtype or HDC−/− mice were treated for 12 minutes with 50µM dihydrorhodamine 123 (DHR 123) (Sigma Chemical Co., St Louis, MO, USA). The cells were subsequently stimulated with PMA (500ng/mL) for 20 minutes at 37°C and analyzed by flow cytometry for DHR 123 oxidation.
Electron Microscopy
Cells were fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer and transmission electron microscopy performed by the Northwestern University Cell Imaging Facility core service.
Statistics
Data are represented as mean ± SEM. Statistical significance was determined using Student t test. All analysis was done using GraphPad Prism Software (La Jolla, CA, USA).
RESULTS
Histamine is upregulated during LPS-induced acute lung injury
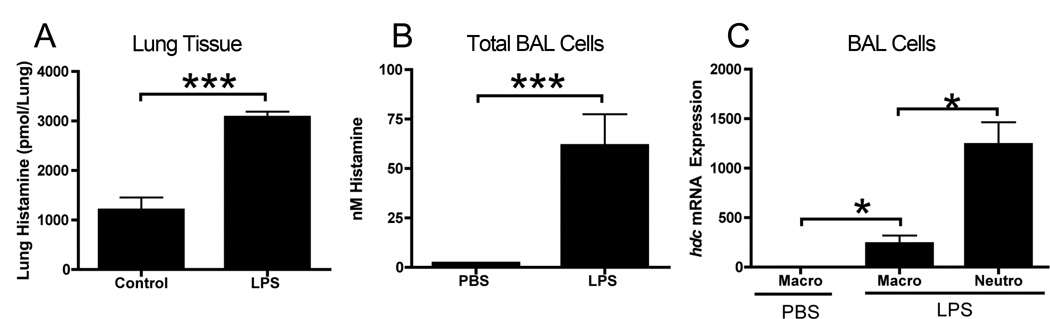
Using an LPS-induced model of acute lung injury, we observed that the levels of histamine were significantly elevated in the homogenates from the lungs of WT mice 24 hours after intratracheal instillation of 50µg LPS (Figure 1A). While lung tissue contained some constitutive histamine in sham-treated mice, isolation of the inflammatory cells from the airway by BAL showed this was significantly elevated in cells from LPS-treated animals compared to those from PBS-treated (Figure 1B). Cytological examination confirmed that the cells consisted exclusively of macrophage (approximately 10–20%) and neutrophils (approximately 80–90%) in LPS-treated mice, while PBS-treated mice contained macrophage alone. Separation of these subtypes by plating on plastic culture plates, to facilitate macrophage adherence, showed that gene expression for HDC was predominately in the neutrophil population, although there was significant upregulation in the macrophage population from LPS-treated mice versus those from PBS-treated mice (Figure 1C). The relatively higher levels of histamine observed after cell isolation, compared to the whole lung homogenates, might reflect greater degradation in the presence of the lung epithelium, which has high expression of the histamine-degrading enzymes diamine oxidase and histamine N-methyltransferase [18,19].
Figure 1. Histamine is upregulated during LPS-induced acute lung injury.
Mice were administered 50µg of LPS intranasally and 8 hours later the levels of histamine in (A) lung homogenate or (B) in cells recovered from the BAL fluid. Cells from the BAL fluid were separated and the relative expression of hdc determined by real-time RT-PCR (C). Data represents 3–4 mice per group. *=p<0.05 and ***=p<0.005 by Students t-test.
Since LPS appeared capable of promoting neutrophil-derived histamine in vivo, we sought to examine this directly using in vitro culture of bone marrow-derived neutrophils.
TLR4 and TLR7 agonists stimulate histamine production by neutrophils
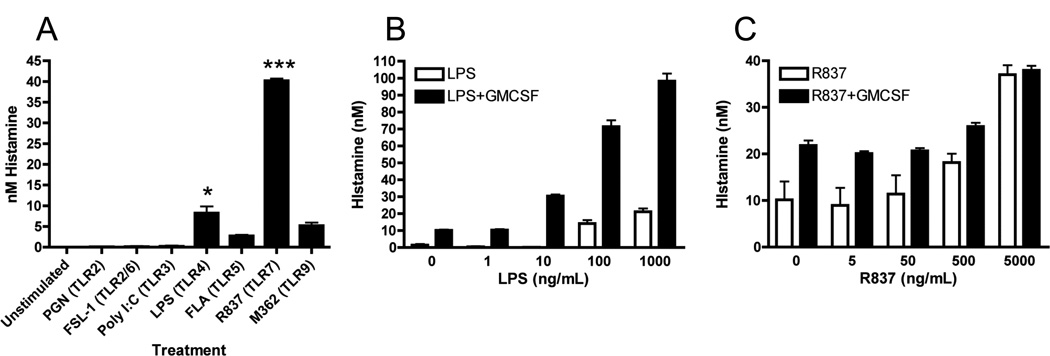
To determine which specific TLR signals were capable of inducing histamine, we cultured bone marrow-isolated neutrophils in vitro with various TLR agonists at concentrations we determined to promote expression of TNF (data not shown). Of the compounds tested, only LPS and R837 were capable of inducing significant increases in histamine (Figure 2A). Addition of recombinant murine GM-CSF (10ng/ml), in order to promote better neutrophil survival, led to a modest increase in histamine in unstimulated cells but promoted a striking enhancement in LPS-induced histamine which was highly dose-dependent (Figure 2B). Histamine production by R837 was also dose-dependent but, unlike LPS, this was unaltered by addition of GM-CSF (Figure 2C).
Figure 2. TLR4 and TLR7 agonists stimulate histamine production by neutrophils.
(A) Neutrophils were isolated from the bone marrow and stimulated in vitro (1 × 105/well) for 8 hours with various TLR agonists and histamine measured in the supernatant. The dose responsiveness production of histamine to (B) LPS and (C) R837 was then determined. Data represents 3–6 independent wells of neutrophils pooled from 5–10 mice from one (A) or three (B and C) independent experiments *=p<0.05 and ***=p<0.005 by Students t-test.
LPS promotes transient release of histamine
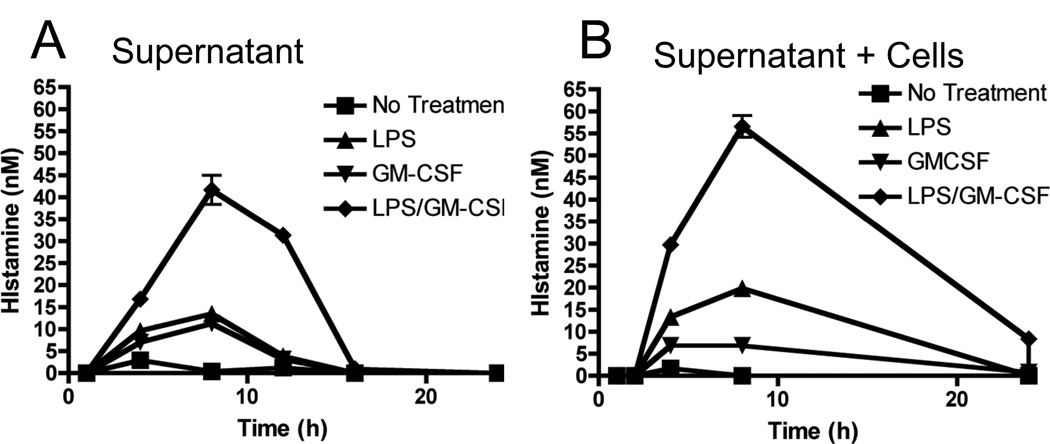
Since histamine can be generated and stored, we next wanted to determine the relative storage and release potential of neutrophils, as well as the temporal nature of LPS-induced histamine production by neutrophils. As shown in Figure 3A, LPS/GM-CSF treatment led to a transient increase in the levels of histamine secretion into the culture supernatant, peaking at 8 hours, where concentrations were around 40nM. Histamine was still present at 12 hours but undetectable at 18 or 24 hours. This was mirrored by the total histamine levels (determined by lyses of the cells into the supernatant), which also peaked at 8 hours and declined (Figure 3B). Interestingly, a low level of histamine remained detectable at 24 hours, which was not present in the supernatants (Figure 3A), suggesting some storage of histamine might occur.
Figure 3. LPS promotes release of histamine and not storage.
Bone marrow isolated neutrophils (1 × 105/well) were stimulated in vitro for the indicated times with LPS (100ng/ml) + GM-CSF (10ng/ml). At each time point, (A) supernatant or (B) total well content histamine levels (cell pellet plus supernatant) were determined. Data represents 3–6 independent wells of neutrophils pooled from 5–10 mice from two independent experiments.
HDC is required for histamine production by neutrophils
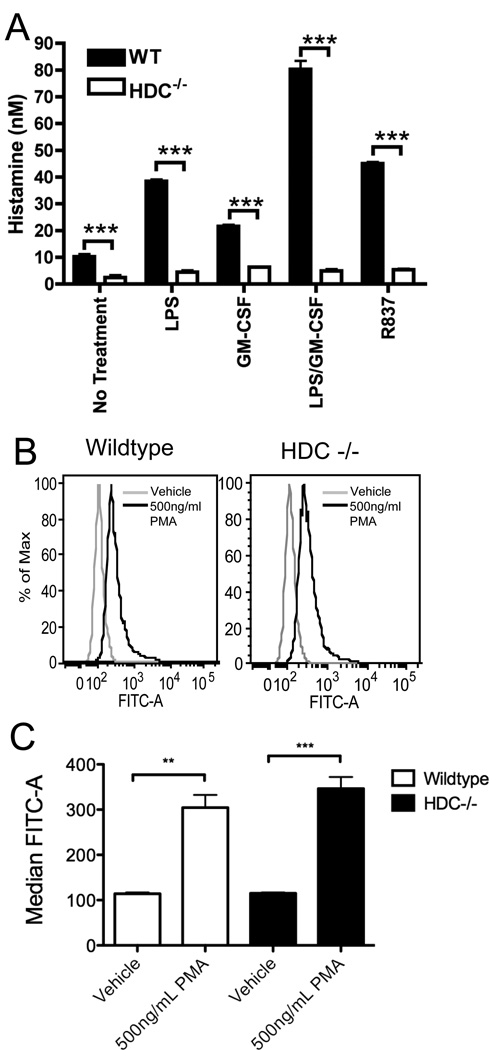
Since histamine can be sequestered by some cells [5,6] and has been described as being present in fetal bovine serum [20], we next investigated if neutrophil-derived histamine required de novo synthesis by using cells from HDC−/− mice. Under all conditions tested, the absence of HDC significantly abrogated the histamine release from cultured neutrophils (Figure 4A). This included the small amounts of histamine observed in unstimulated cultures, while the levels seen in HDC−/− cultures were at or below the limits of detection. This suggests that neutrophil-derived histamine occurs via the conversion of histidine into histamine by HDC. In contrast, the ability of HDC−/− neutrophils to generate reactive oxygen species was unaltered (Figure 4B and 4C), suggesting these cells retained their functional capacity to generate this important neutrophil product.
Figure 4. HDC is required for histamine but not reactive oxygen species production by neutrophils.
(A) Bone marrow isolated neutrophils (1 × 105/well) from WT or HDC−/− mice were stimulated in vitro for 8 hours with LPS (100ng/ml) + GM-CSF (10ng/ml) and histamine production measured in the supernatant. Data represents 3 independent wells of neutrophils pooled from 5 mice and is representative of 3 independent experiments. Bone Marrow isolated neutrophils (2.5 × 105/sample) were loaded with dihydrorhodamine 123 (DHR 123), stimulated with PMA and analyzed by flow cytometry for DHR 123 oxidation. Representative histogram plots (B) for wildtype and HDC−/− neutrophils and (C) median fluorescent intensity are shown. Data represents triplicate samples from 3 separate mice per group from 2 experiments. **=p<0.01 and ***=p<0.005 by Students t test
PI3-Kinase regulates histamine production by neutrophils
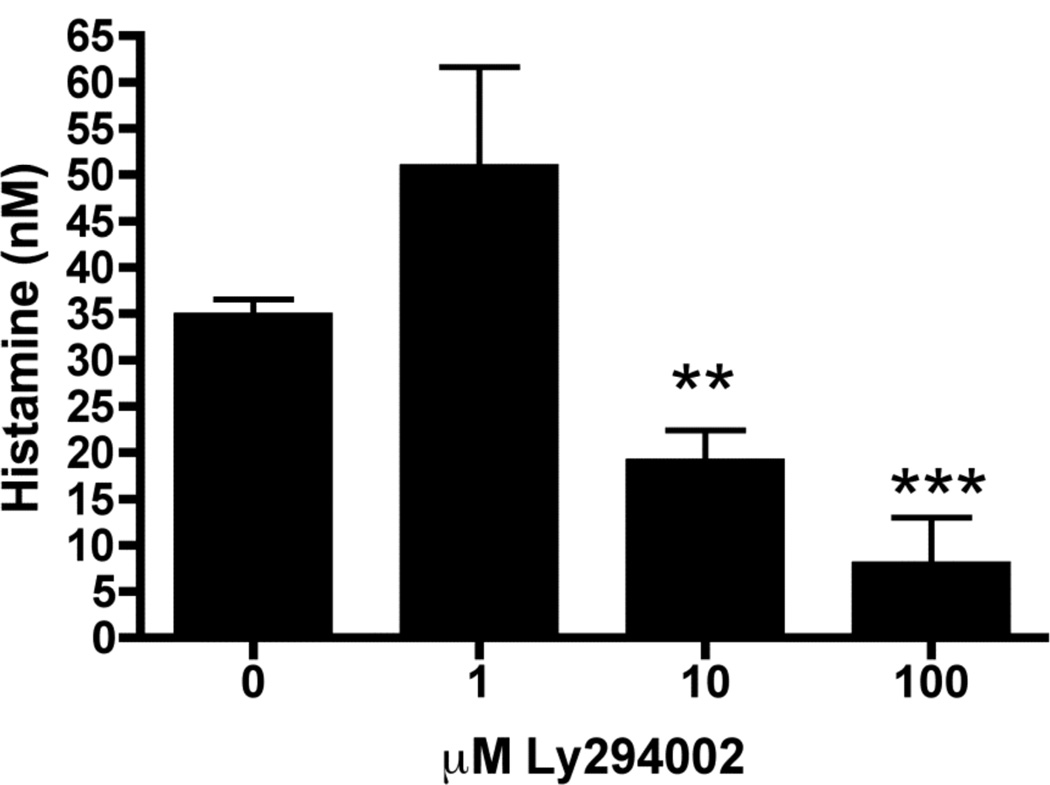
We next sought to examine possible mechanisms through which histamine might be generated in neutrophils. Recent studies have focused on PI3-kinase as an important signaling intermediate in the migration, activation and survival of neutrophils in response to LPS, including during acute injury [21–24]. We therefore examined the requirements for PI3-kinase activation in LPS-induced histamine production by neutrophils using Ly294002, a specific pharmacological inhibitor of PI3-kinase [25]. Indeed, the addition of Ly294002 led to a dose-dependent reduction in the quantity of histamine produced by 8 hours after LPS/GM-CSF treatment, supporting that this pathway is necessary for histamine generation (Figure 5).
Figure 5. Inhibition of PI3-kinase prevents LPS-induced histamine production.
Bone marrow isolated neutrophils (1 × 105/well) were pretreated with the indicated concentrations of Ly294002 for 30 minutes prior to in vitro stimulation for 8 hours with LPS (100ng/ml) + GM-CSF (10ng/ml) and histamine production measured in the supernatant. Data represents 3–6 independent wells of neutrophils pooled from 5–10 mice from 2 independent experiments **=p<0.01, ***=p<0.005 by Students t-test.
Histamine is not necessary for neutrophil granule integrity
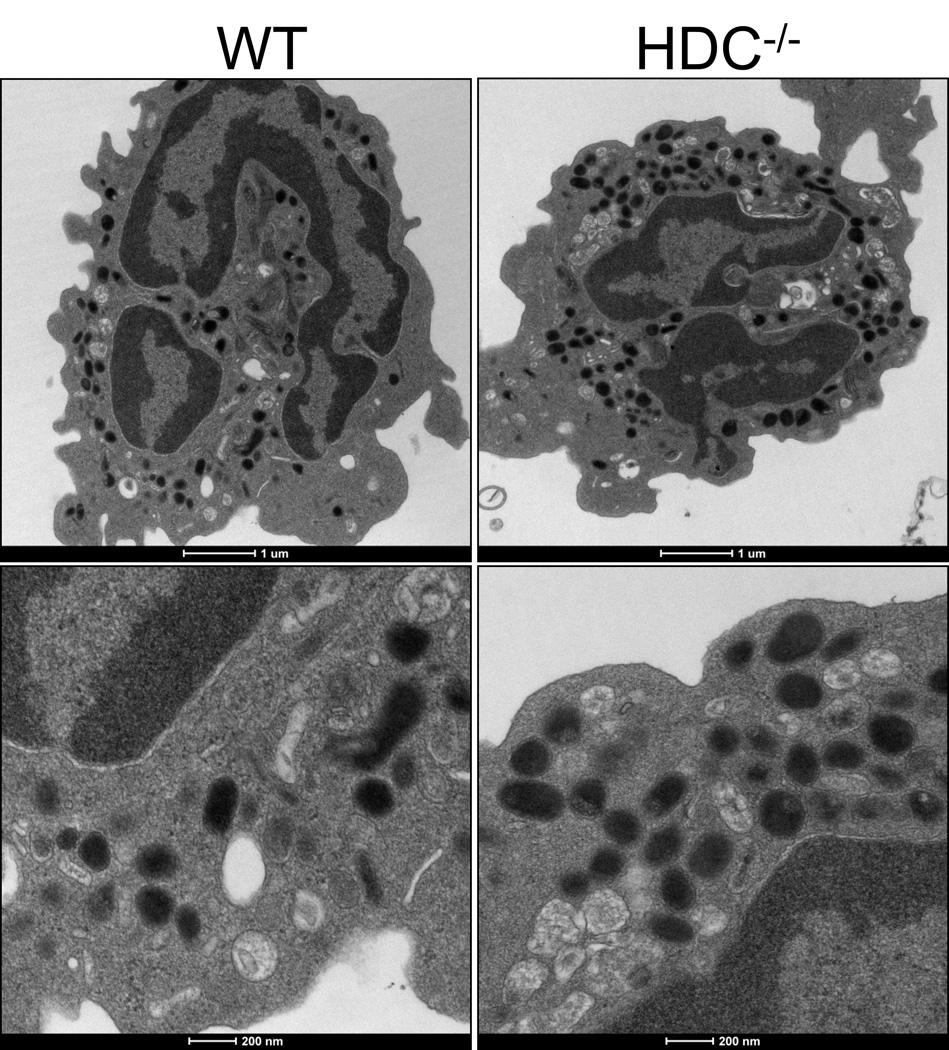
Release of specific neutrophil-derived granules has been shown to be PI3-kinase dependent [22] while the absence of histamine has been shown to cause loss of granule integrity in mast cells [9]. We hypothesized that histamine may also be required for formation of neutrophil granules. However, transmission electron microscopy showed that there were no obvious differences between the cellular structures of WT or HDC−/− neutrophils (Figure 6) and that granules were clearly present in both.
Figure 6. Histamine-deficient neutrophils exhibit normal granules.
Bone marrow isolated neutrophils were fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer and scanning transmission electron microscopy used to determine their granule structure and cellular morphology.
DISCUSSION
In this study, we sought to examine the specific innate immune triggers that might promote neutrophils to generate histamine. While mast cells and basophils are the dominant source of histamine in many inflammatory responses, the possibility that neutrophils might be an alternative source was first demonstrated in a respiratory infection model, wherein mast cell deficient mice infected with Mycoplasma pulmonis exhibited increases in lung histamine that correlated significantly with neutrophil influx [14]. Recent data from an hdc-EGFP reporter mouse also concluded that expression of HDC was important for differentiation of neutrophil precursors [26]. Our data now demonstrates that neutrophils are a likely source of histamine during LPS-induced lung injury and we provide the first direct evidence for how neutrophils are induced to produce histamine.
Interestingly, of the diverse range of TLR agonists that we explored, LPS and R837, activators of TLR4 and TLR7 respectively, were by far the most potent inducers of histamine. Since TLR4 is a membrane-bound TLR while TLR7 is an endosomal TLR [27], this suggests that neutrophils may generate histamine to a range of potential pathogens. Furthermore, while both TLR4 and TLR7 can be coupled to Myd88, the inability of other TLR agonists to induce histamine that are also coupled via this pathway might suggest histamine production is mediated via non-classical pathways. We were surprised to observe that the TLR2 agonists FSL-1 and PGN did not induce histamine, since is has been recently shown that TLR2 is critical for protection against Mycoplasma pulmonis in mice [28] and this organism was used in the study connecting neutrophils to histamine production [14]. Furthermore, systemic injection with lipid A, a TLR4 agonist, or FSL-1, a TLR2/6 agonist, has been shown to elevate the relative HDC activity in the lungs of mice [29], although the specific cell sources were not identified in that study. Despite this, our findings would suggest that bone marrow neutrophils produce histamine in response to TLR4 or TLR7 stimulation in vitro and that neutrophils in vivo also produce histamine in response to LPS.
Neutrophil activation, as determined by oxidative burst and release of primary and secondary granules, occurs within minutes of stimulation [15]. It is therefore intriguing that histamine generation requires several hours, although this time lapse is consistent with our conclusions that histamine is not stored by neutrophils and requires active synthesis by HDC. Our data prompts us to ask the question: what could the functional possibilities of this neutrophil-derived histamine be? Recently, is has been demonstrated that FoxP3+ regulatory T cells are required for the resolution of the neutrophil-mediated LPS-induced lung injury [30], with upregulation of apolipoprotein E (ApoE) and chemokine ligand 12 (CXCL12) being possible mechanisms through which this is mediated [31]. One intriguing possibility is that neutrophil-derived histamine serves to direct these cells into the site of inflammation after the initial proinflammatory events have occurred, since histamine 4 receptor (H4R) regulates recruitment of FoxP3+ regulatory T cells [32]. This histamine-mediated function would serve to limit the ongoing neutrophilic inflammation. In support of this concept, HDC−/− mice actually exhibit significant enhanced numbers of neutrophils in an air-pouch model of antigen-triggered inflammation [33] and HDC−/− mice elicit a significantly increased response during dinitrofluorobenzene-mediated contact sensitivity, in which the inflammatory response has a strong neutrophilic component [34]. Alternatively, histamine from neutrophils might provide a protective function during infection since histamine has been shown to prevent bacterial entry and translocation through intestinal epithelia [35] and to enhance innate inflammatory responses in endothelial cells by upregulating both TLR2 and TLR4 [36].
An intriguing possibility from our findings is that neutrophils might actively participate in anaphylactic reactions, especially since the dominant infiltrating cell seen in models of passive anaphylaxis are neutrophils [17,37,38]. Recently, this concept was demonstrated by Jönsson et al., who used an active systemic anaphylaxis model to show murine and human neutrophils regulate anaphylaxis [12] they concluded that this was mediated via production of platelet activating factor (PAF). Using cyproheptadine, a first generation antihistamine that possesses relatively poor selectivity, they also concluded that histamine did not play a role since treatment did not significantly alter the early response (within 60 minutes). However, survival was slightly improved by cyproheptadine treatment, indicating that histamine may actually play a role One possibility that can explain the seeming discrepancy between their study and ours is if neutrophil-derived PAF is important in the initiation of anaphylaxis while histamine production occurs later and influences the persistence of responses or generation of a late-phase response, which they did not specifically test for. Alternatively, the absence of innate signals in this systemic anaphylaxis model may have led to neutrophils that did not produce histamine. However, natural allergens that cause clinical anaphylaxis in humans, such as peanut, commonly possess innate activating properties [39,40] and it remains to be determined whether neutrophil-derived histamine might occur in response to such natural stimuli.
Our data demonstrates that expression of HDC is required for neutrophils to produce histamine and that this production is dependent on PI3-kinase activity. It has been shown that the activation and expression of histamine by mast cells and basophils is also dependent on PI3-kinase [41–45]. Interestingly, while several studies have described the importance of PI3-kinase in neutrophil survival and functions [21–24], various types of neutrophil granules were shown to be differentially regulated by the PI3-kinase pathway in response to LPS-activation, whereby the release of azurophilic granules was PI3-kinase dependent while the release of other granules was not [22]. Since Tanaka et al. described that HDC colocalizes with metalloproteinase-9 [13] in secondary granules, this might suggest that the synthesis of histamine occurs in a different cellular compartment from those involved in its release. However, our data demonstrates that histamine does not contribute to the overall granule integrity of the cell and so neutrophil histamine clearly plays a different role than is observed in mast cell granules [9].
In conclusion, our study demonstrates that neutrophils possess the capacity to generate histamine in response to specific TLR stimuli and that this is dependent upon HDC, supporting the conclusion that neutrophils require de novo synthesis of histamine after appropriate stimulation. While the level of histamine per cell is relatively low when compared to mast cells or basophils, neutrophils may possess the capacity to exert the histamine-dependent immunomodulatory influences by the cumulative effects of their massive influx and accumulation during inflammation. Consequently, neutrophil-derived histamine may have important roles to play in directing immune responses.
Research Highlights.
Histamine is upregulated during LPS-induced acute lung injury
Neutrophils release histamine upon activation by TLR agonists
Histidine decarboxylase is necessary for histamine production
LPS-induced histamine requires PI3 kinase
Histamine is not required for neutrophil granule integrity
ACKNOWLEDGEMENTS
We thank Dr Mendy L. Miller for editorial services, Dr Atushi Kato for TLR ligands and Lennell Reynolds, Jr. for performing electron microscopy. This work was supported by NIH/NIAID R01 AI072570 (PJB) and F30 ES017378 (CS & PJB), as well as the Northwestern University Cell Imaging Facility and a Cancer Center Support Grant (NCI CA060553).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39:1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 2.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nature reviews. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 3.Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Yokoyama A, Mori S, Takahashi HK, Yoshino T, Watanabe T, Watanabe T, Ohtsu H, Nishibori M. Inducible histamine protects mice from P. acnes-primed and LPS-induced hepatitis through H2-receptor stimulation. Gastroenterology. 2004;127:892–902. doi: 10.1053/j.gastro.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Noskova V, Bottalico B, Olsson H, Ehinger A, Pilka R, Casslen B, Hansson SR. Histamine uptake by human endometrial cells expressing the organic cation transporter EMT and the vesicular monoamine transporter-2. Molecular human reproduction. 2006;12:483–489. doi: 10.1093/molehr/gah259. [DOI] [PubMed] [Google Scholar]
- 6.Prinz C, Zanner R, Gerhard M, Mahr S, Neumayer N, Hohne-Zell B, Gratzl M. The mechanism of histamine secretion from gastric enterochromaffin-like cells. The American journal of physiology. 1999;277:C845–C855. doi: 10.1152/ajpcell.1999.277.5.C845. [DOI] [PubMed] [Google Scholar]
- 7.Kolset SO, Prydz K, Pejler G. Intracellular proteoglycans. The Biochemical journal. 2004;379:217–227. doi: 10.1042/BJ20031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringvall M, Ronnberg E, Wernersson S, Duelli A, Henningsson F, Abrink M, Garcia-Faroldi G, Fajardo I, Pejler G. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008;121:1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS letters. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 10.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. International immunopharmacology. 2010;10:1325–1334. doi: 10.1016/j.intimp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, Shimizu T, Daeron M, Bruhns P. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Deai K, Konomi A, Takahashi K, Yamane H, Sugimoto Y, Ichikawa A. Expression of L-histidine decarboxylase in granules of elicited mouse polymorphonuclear leukocytes. Eur J Immunol. 2004;34:1472–1482. doi: 10.1002/eji.200324636. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. Journal of leukocyte biology. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 16.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–238. doi: 10.1016/j.jaci.2008.10.011. e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PloS one. 2010;5 doi: 10.1371/journal.pone.0011944. e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignesti G, Banchelli G, Raimondi L, Pirisino R, Buffoni F. Histaminase activity in rat lung and its comparison with intestinal mucosal diamine oxidase. Agents and actions. 1992;35:192–199. doi: 10.1007/BF01997499. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi K, Sekizawa K, Suzuki H, Nakazawa H, Ohkawara Y, Katayose D, Ohtsu H, Tamura G, Shibahara S, Takemura M, et al. Structure and function of human histamine N-methyltransferase: critical enzyme in histamine metabolism in airway. The American journal of physiology. 1994;267:L342–L349. doi: 10.1152/ajplung.1994.267.3.L342. [DOI] [PubMed] [Google Scholar]
- 20.Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reutershan J, Saprito MS, Wu D, Ruckle T, Ley K. Phosphoinositide 3-kinase gamma required for lipopolysaccharide-induced transepithelial neutrophil trafficking in the lung. Eur Respir J. 2010;35:1137–1147. doi: 10.1183/09031936.00085509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brzezinska AA, Johnson JL, Munafo DB, Ellis BA, Catz SD. Signalling mechanisms for Toll-like receptor-activated neutrophil exocytosis: key roles for interleukin-1-receptor-associated kinase-4 and phosphatidylinositol 3-kinase but not Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-beta (TRIF) Immunology. 2009;127:386–397. doi: 10.1111/j.1365-2567.2008.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 24.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding J, Vlahos CJ, Liu R, Brown RF, Badwey JA. Antagonists of phosphatidylinositol 3-kinase block activation of several novel protein kinases in neutrophils. The Journal of biological chemistry. 1995;270:11684–11691. doi: 10.1074/jbc.270.19.11684. [DOI] [PubMed] [Google Scholar]
- 26.Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A, Wang TC. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nature medicine. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Love W, Dobbs N, Tabor L, Simecka JW. Toll-like receptor 2 (TLR2) plays a major role in innate resistance in the lung against murine Mycoplasma. PloS one. 2010;5 doi: 10.1371/journal.pone.0010739. e10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funayama H, Huang L, Asada Y, Endo Y, Takada H. Enhanced induction of a histamine-forming enzyme, histidine decarboxylase, in mice primed with NOD1 or NOD2 ligand in response to various Toll-like receptor agonists. Innate immunity. 2010;16:265–272. doi: 10.1177/1753425909341070. [DOI] [PubMed] [Google Scholar]
- 30.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal NR, D'Alessio FR, Tsushima K, Sidhaye VK, Cheadle C, Grigoryev DN, Barnes KC, King LS. Regulatory T cell-mediated resolution of lung injury: Identification of potential target genes via expression profiling. Physiological genomics. 2009 doi: 10.1152/physiolgenomics.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan RK, McAllister B, Cross L, Green DS, Kornfeld H, Center DM, Cruikshank WW. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol. 2007;178:8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- 33.Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Enhancement of neutrophil infiltration in histidine decarboxylase-deficient mice. Immunology. 2002;107:217–221. doi: 10.1046/j.1365-2567.2002.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garaczi E, Szell M, Janossy T, Koreck A, Pivarcsi A, Buzas E, Pos Z, Falus A, Dobozy A, Kemeny L. Negative regulatory effect of histamine in DNFB-induced contact hypersensitivity. Int Immunol. 2004;16:1781–1788. doi: 10.1093/intimm/dxh179. [DOI] [PubMed] [Google Scholar]
- 35.Duan L, Chen X, Alexander JW. Regulatory effect of histamine on the barrier function of intestinal mucosal. J Gastrointest Surg. 2010;14:1180–1185. doi: 10.1007/s11605-010-1208-9. [DOI] [PubMed] [Google Scholar]
- 36.Talreja J, Kabir MH, M BF, Stechschulte DJ, Dileepan KN. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology. 2004;113:224–233. doi: 10.1111/j.1365-2567.2004.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko M, Schimming A, Gleich GJ, Kita H. Ligation of IgE receptors causes an anaphylactic response and neutrophil infiltration but does not induce eosinophilic inflammation in mice. J Allergy Clin Immunol. 2000;105:1202–1210. doi: 10.1067/mai.2000.106731. [DOI] [PubMed] [Google Scholar]
- 38.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, Kohl J, Finkelman FD. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 41.Aichberger KJ, Mayerhofer M, Vales A, Krauth MT, Gleixner KV, Bilban M, Esterbauer H, Sonneck K, Florian S, Derdak S, Pickl WF, Agis H, Falus A, Sillaber C, Valent P. The CML-related oncoprotein BCR/ABL induces expression of histidine decarboxylase (HDC) and the synthesis of histamine in leukemic cells. Blood. 2006;108:3538–3547. doi: 10.1182/blood-2005-12-028456. [DOI] [PubMed] [Google Scholar]
- 42.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 43.Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 44.Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochemical Society transactions. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- 45.Zemtsova IM, Heise N, Frohlich H, Qadri SM, Kucherenko Y, Boini KM, Pearce D, Shumilina E, Lang F. Blunted IgE-mediated activation of mast cells in mice lacking the serum- and glucocorticoid-inducible kinase SGK3. American journal of physiology. 2010;299:C1007–C1014. doi: 10.1152/ajpcell.00539.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]