Abstract
Cochlear implants electrically stimulate residual spiral ganglion neurons (SGNs) to provide auditory cues for the severe-profoundly deaf. However, SGNs gradually degenerate following cochlear hair cell loss, leaving fewer neurons available for stimulation. Providing an exogenous supply of neurotrophins (NTs) has been shown to prevent SGN degeneration, and when combined with chronic intracochlear electrical stimulation (ES) following a short period of deafness (5 days), may also promote the formation of new neurons. The present study assessed the histopathological response of guinea pig cochleae treated with NTs (brain-derived neurotrophic factor and neurotrophin-3) with and without ES over a four week period, initiated two-weeks after deafening. Results were compared to both NT alone and artificial perilymph (AP) treated animals. AP/ES treated animals exhibited no evidence of SGN rescue compared with untreated deafened controls. In contrast, NT administration showed a significant SGN rescue effect in the lower and middle cochlear turns (two-way ANOVA, p < 0.05) compared with AP-treated control animals. ES in combination with NT did not enhance SGN survival compared with NT alone. SGN function was assessed by measuring electrically-evoked auditory brainstem response (EABR) thresholds. EABR thresholds following NT treatment were significantly lower than animals treated with AP (two-way ANOVA, p = 0.033). Finally, the potential for induced neurogenesis following the combined treatment was investigated using a marker of DNA synthesis. However, no evidence of neurogenesis was observed in the SGN population. The results indicate that chronic NT delivery to the cochlea may be beneficial to cochlear implant patients by increasing the number of viable SGNs and decreasing activation thresholds compared to chronic ES alone.
Keywords: deafness, neurotrophins, electrical stimulation, cochlear implant, spiral ganglion neurons, neurogenesis
1 Introduction
Hearing loss affects over 278 million people worldwide (World Health Organization, 2005), and has significant detrimental impacts economically and emotionally on individuals and society (de Graaf and Bijl, 2002; Fellinger et al., 2005; Mohr et al., 2000). Treatment with a cochlear implant can dramatically improve speech perception and production, as well as quality of life for patients with a severe-profound sensorineural hearing loss (SNHL) (Bond et al., 2009; Harris et al., 1995). Cochlear implants bypass damaged sensory hair cells to directly stimulate spiral ganglion neurons (SGNs). However, SGNs degenerate following hair cell loss in both humans (Miura et al., 2002; Nadol et al., 1989; Zimmermann et al., 1995) and animal models of SNHL (Leake and Hradek, 1988; Spoendlin, 1984; Wise et al., 2005; Gillespie et al., 2003). This is thought to be largely caused by the loss of the endogenous supply of the pro-survival neurotrophin (NT) peptides brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), which are normally produced by inner hair cells and support cells of the organ of Corti (Fritzsch et al., 2004; Stankovic et al., 2004; Tan and Shepherd, 2006; Ylikoski et al., 1993). Severe SGN degeneration may limit the efficacy of hearing rehabilitation by a cochlear implant. The prevention of SGN degeneration following a SNHL may therefore improve the clinical outcome for implant patients.
The supply of an exogenous source of NTs can lead to significant anatomical and functional changes to the deafened cochlea. SGN density within Rosenthal’s canal is greater in NT-treated deaf cochleae (Agterberg et al., 2008; Gillespie et al., 2003; McGuinness and Shepherd, 2005; Miller et al., 1997; Shepherd et al., 2008; Shepherd et al., 2005; Staecker et al., 1996), SGN soma size is increased (Agterberg et al., 2008; Richardson et al., 2005; Shepherd et al., 2005; McGuinness and Shepherd, 2005), and peripheral neural fibers are thicker and more numerous compared to deaf controls (Wise et al., 2005; Wise et al., 2010). Furthermore, the electrically-evoked auditory brainstem response (EABR) thresholds were found to be lower in NT-treated cochleae compared to untreated deafened controls (Shepherd et al., 2005; Shinohara et al., 2002).
Previous guinea pig studies also suggest interactive effects of combined chronic electrical stimulation (ES) and NT treatment, with SGN density observed to be greater following the combined treatment compared to both BDNF treatment alone and normal hearing controls (Shepherd et al., 2008; Shepherd et al., 2005). In contrast, ES alone showed no evidence of SGN rescue in these studies, in agreement with some reports (Agterberg et al., 2010; Li et al., 1999), but at odds with others showing a trophic effect of ES (Hartshorn et al., 1991; Kanzaki et al., 2002).
SGN density measures greater than normal hearing controls have also been reported following a combined BDNF and acidic fibroblast growth factor treatment in deafened animals (Glueckert et al., 2008; Miller et al., 2007). An increase in SGN density to values greater than normal suggests that the formation of new SGNs (i.e. neurogenesis) may be induced by these treatments. In vitro studies of spiral ganglion explants also suggest that trophic/growth factors can induce neurogenesis (Rask-Andersen et al., 2005; Wei et al., 2007), although these studies did not investigate the effects of NTs specifically.
In the present study deafened guinea pigs were chronically treated with intracochlear NT infusion and/or ES in order to assess how these treatments affect the anatomical and physiological response of the cochlea following hair cell loss. A longer deafness period of two weeks prior to the onset of treatment was used here compared to previous studies in our laboratory (Shepherd et al., 2008; Shepherd et al., 2005) to ensure a level of SGN degeneration at the onset of treatment (Versnel et al., 2007). This is considered a more clinically realistic model of cochlear implantation.
2 Materials and methods
2.1 Experimental animals
Twenty-six (N = 26) young adult male and female pigmented guinea pigs (300-600g) were used in this study. Animals were housed together in small groups (2-5), but segregated by gender. All procedures were approved by the Royal Victorian Eye and Ear Hospital Animal Research & Ethics Committee. Prior to any experimental manipulation the external ears were examined to ensure they were otoscopically normal, and the hearing status assessed under anesthesia (ketamine, 60mg/kg (Parnell Australia) and xylazine, 4mg/kg (Ilium, Australia); intramuscular) by measuring the auditory brainstem response (ABR) to acoustic clicks to each ear (Coco et al., 2007). Briefly, the differential voltage between the skull vertex and the back of the neck was recorded to 100μs square pulse clicks, and the response threshold for the PIII-NIII wave of the ABR was visually determined (Fig.1). Ears were defined as normal hearing if the threshold was ≤50dB peak equivalent sound pressure level (p.e. SPL). Five animals served as normal hearing controls, and twenty-one animals were deafened and randomly assigned to a treatment group. The group numbers are shown in Table 1.
Figure 1.
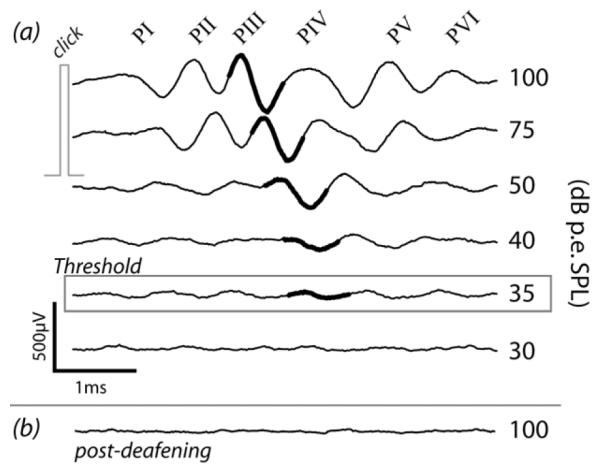
Auditory brainstem response traces (averaged from 200 trials) to free-field click stimuli presented to the left ear of a guinea pig (a) one week prior to deafening, and (b) two weeks after deafening. The PIII-NIII wave (heavy black) was used to determine threshold. Large threshold increases (>50dB) occurred following deafening, usually to thresholds >100dB p.e. SPL.
Table 1.
Experimental cohorts and group numbers. Treatment details are given in the next sections.
| Unstimulated (US) | Chronically Stimulated (ES) | |
|---|---|---|
| Artificial perilymph (AP) | US/AP n = 6 | ES/AP n = 4 |
| BDNF & NT-3 (NT) | US/NT n = 5 | ES/NT n = 6 |
| Normal hearing | n = 5 |
2.2 Deafening
Animals were systemically deafened using a procedure which has been previously shown to produce a bilaterally symmetrical hearing loss (Gillespie et al., 2003). The jugular vein was exposed under gaseous anesthesia (1-2% isoflurane in O2, 1L/min), cannulated, and frusemide (130mg/kg; Ilium, Australia) diluted in warm Hartmann’s solution was slowly injected. The vein was tied off and the incision sealed with cyanoacrylate. Kanamycin sulfate (420mg/kg; Sigma-Aldrich, USA) dissolved in 3ml Hartmann’s solution was then injected subcutaneously (s.c.).
2.3 Cochlear implantation: Implant specifications and neurotrophin delivery
The electrode arrays were similar to the electrode array/drug delivery system described previously (Shepherd and Xu, 2002). Each electrode array had six Pt rings (0.2mm ring width; 0.4mm diameter; 0.3mm inter-electrode spacing) on a medical grade silicone carrier. The most apical electrode is designated “E1” and the most basal electrode is “E6” (Fig.2). For unstimulated (US) animals, the implant was identical but without any active electrode rings. A marker was placed in the same location as E6 of stimulating arrays to ensure a similar insertion depth. A polyimide drug delivery cannula, open at the apical implant tip, was located in the core of the implants. A miniosmotic pump (200μL capacity; 28 day delivery period; 0.25μL/hr; Alzet, USA) was attached to the distal end of the drug delivery cannula. The pump was filled with artificial perilymph (AP; 126mM NaCl, 3mM KHCO3, 24mM NaHCO3, 0.7mM CaCl2, 5mM NH2C(CH2OH)3; pH = 7.4) with or without the NTs BDNF and NT-3 (30μg/ml of each; human recombinant; PeproTech, USA). A 20% absorption rate of NTs by the pump and tubing was assumed (Gillespie et al., 2003), giving a total delivery of 5μg each of BDNF and NT-3 over 28 days.
Figure 2.
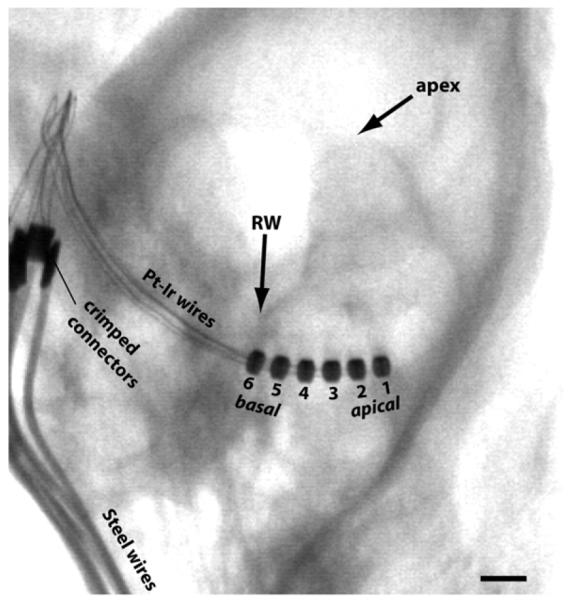
Micro-focus x-ray from an ES/AP animal taken 28 days after implantation showing the six electrode rings of the implant in the basal turn of the left cochlea, and the lead wires exiting the bulla. Intracochlear electrode numbering is shown. The site of neurotrophin infusion was just apical to E1. RW = round window. Scale bar = 1mm.
2.4 Cochlear implantation: Surgical details
Two weeks after deafening, guinea pigs were anesthetized (ketamine and xylazine, as above) and deafness was confirmed by ABR measurement, with a threshold increase >50dB indicating a severe-to-profound SNHL (Fig. 1). Threshold was usually >100dB p.e. SPL, the maximum intensity presented. The left cochlea was then exposed and implanted under aseptic conditions as follows. Supplementary local anesthesia with lignocaine (Ilium, Australia) was applied above the left pinna where an incision was made. A hole was drilled in the dorsolateral temporal bone and the malleoincudal ossicle was removed to access the middle ear cavity and expose the round window. A small perforation was created in the round window membrane and the implant was inserted into the scala tympani until E6 was just inside the round window (~3mm; Fig.2). Crushed muscle pieces were packed around the round window to minimize perilymph leakage and the risk of infection spreading to the cochlea. The bulla cavity was filled with Durelon dental cement (3M, USA) to secure the implant in place. The lead wire/delivery cannula was fixed to the skull by threading a strip of Dacron mesh (Invista, USA) through two small craniotomies and tying the lead against the skull. The osmotic pump was housed between the shoulders in a subcutaneous tissue pocket. In ES cohorts, the electrode lead wire exited the skin between the scapulae. The supra-auricular wound was sutured and stapled. Hartmann’s solution (10ml/kg; s.c.), antibiotic (Baytril; Bayer, Germany; 0.10mg/kg; s.c.), and an analgesic (Temgesic; Reckitt-Benckiser, UK; 50 mg/kg; s.c.) were given after surgery and the following day to aid recovery. Normal hearing controls were not chronically implanted. Animals were housed individually following implantation to prevent disruption to the surgical wounds and implanted devices by other guinea pigs.
2.5 Electrically-evoked auditory brainstem response recording
Immediately following cochlear implantation in ES animals, EABRs were recorded to bipolar ES using the same arrangement as ABRs (Coco et al., 2007). Bipolar stimuli consisted of single biphasic current pulses (100μs phase duration, 50μs interphase gap), and are identified as, for example, “E1-2” to indicate an anodic lead phase on E1 with an E2 cathodic return. The threshold for myogenic activity via unintended facial nerve stimulation (Shepherd et al., 1994) was determined for each bipolar configuration by monitoring the evoked activity on an oscilloscope. EABR recordings were then made to stimulation at different intensities from 0-2mA or just below myogenic threshold for all adjacent bipolar electrodes (E1-2, E2-3, etc.). EABRs were averaged across 100 trials and at least two set of recordings were obtained at each current level. EABR threshold was visually determined within 25μA for the PIII-NIII wave (a clear, consistent response at 1-2ms). At 16 and 28 days post-implantation (the end of the treatment period) animals were anesthetized as above and EABRs again recorded to all bipolar configurations, including wider inter-electrode separations.
For US animals, EABRs were recorded at the end of the treatment period following acute implantation with a stimulating array identical to chronic ES arrays and using the same surgical approach. Normal hearing controls also underwent acute implantation and EABR recording following cochlear exposure. To prevent hair cell mediated electrophonic activity contaminating the EABR, these animals were acutely deafened by replacing the perilymph with 10% neomycin sulfate in normal saline. This was repeated at least six times to ensure thorough ototoxin exposure.
2.6 Chronic intracochlear electrical stimulation
Five days after implantation, chronic intracochlear ES was initiated in the ES cohorts. Environmentally-derived stimuli were delivered to the electrode array via an ESPrit 3G speech processor and CI22 stimulator (Cochlear Ltd., Australia) housed in a jacket worn by ES animals (Fallon et al., 2009). The SPEAK sound processing strategy (McDermott et al., 1992) was used for stimulus delivery on E1-2, E3-4, and E5-6 (mapped to 150-300, 300-600, 600-1400Hz, respectively). The stimuli were interleaved current pulses (parameters same as EABR stimuli) delivered at 1200pps per electrode pair, giving a total rate of 3600pps for the three channels. The stimulus amplitude range was −3 to +6dB (re: EABR threshold) or to just below myogenic threshold, with the amplitude being reset relative to the new threshold values following the day 16 EABR recordings. Chronic ES was continuously delivered over the 28 day treatment period. The impedance of each electrode against common ground (all other electrodes) was recorded at least twice per week using clinical software (CustomSound EP 2.0; Cochlear, Australia) to monitor changes over time and to identify any malfunctioning electrodes (i.e. open or short circuits).
2.7 Chronic BrdU administration
To investigate potential ES/NT treatment-induced neurogenesis, 5-bromo-2′-deoxyuridine (BrdU), a label of newly synthesised DNA and therefore newly divided cells (Dolbeare, 1995), was injected over the treatment period. Two days after cochlear implantation, each animal received an injection of 1% (w/v) BrdU (50mg/kg, s.c.; Roche, Switzerland) dissolved in 0.007M NaOH/0.9% NaCl (Heine et al., 2004), and another injection was given the following day. Two injections per week were administered for the remaining three weeks. An injection was also given one day prior to sacrifice, and a final injection was given intraperitoneally 3h before sacrifice. Normal hearing control animals received daily BrdU injections for seven days prior to sacrifice.
2.8 Tissue collection and preparation
Following the treatment period and final EABR recording, animals were given an overdose of pentobarbital (150mg/kg; intraperitoneal) and transcardially perfused with 37°C 0.9% NaCl followed by 4°C 4% paraformaldehyde (PFA) in 0.1M phosphate buffered saline (PBS) (pH = 7.35). Each auditory nerve trunk was severed at the internal auditory meatus and the brain was removed. The temporal bones were dissected out and trimmed around the cochleae. The cochlear electrode array was verified to still be in place in ES animals and with no visible kinks in the drug delivery cannula. The array was then carefully removed from the left cochlea. To facilitate the diffusion of fixative through the cochleae, the left and right oval and round windows were perforated and the otic capsule was pierced at the apex. The cochleae were kept in 4% PFA for post-fixation overnight at 4°C, then placed in 10% ethylenediamine tetraacetic acid in PBS at room temperature for decalcification. The osmotic pumps were checked for residual fluid (all pumps contained less than 20μL) and the drug delivery line was checked for leaks. The brain and a short section of small intestine, which were to be used as positive control tissue for BrdU detection, were also collected and post-fixed in 4% PFA overnight.
Following decalcification, cochleae were cryoprotected in 30% sucrose and embedded in Tissue-Tek O.C.T. cryosectioning compound (Sakura, Japan) (Coleman et al., 2009), and stored at −80°C. Brain and gut tissue underwent the same embedding procedure. The tissues were sectioned at 12μm using a CM 1900 UV cryostat (Leica, Germany) at −22°C. The cochleae were sectioned in the modiolar plane up to midmodiolus (see Fig.5g), the brain was sectioned in the sagittal plane, and the gut was sectioned through intestinal lumen cross-sections. Three interleaved series of cochlear sections were collected. Frozen sections were used to facilitate the immunodetection of BrdU.
Figure 5.
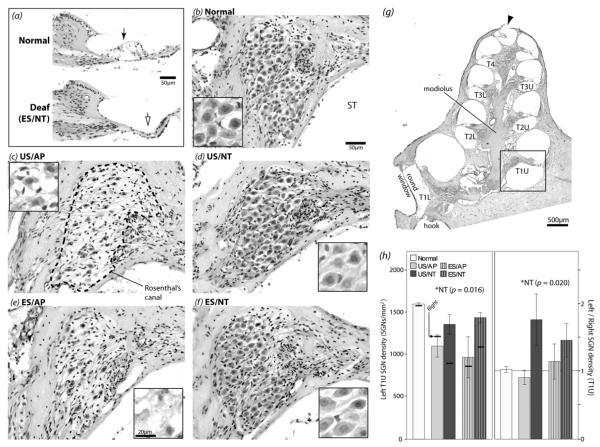
H&E stained cochlear histological samples and SGN density data for upper turn 1 (boxed area in g). (a) Representative midmodiolar sections showing an intact organ of Corti in a normal cochlea (solid arrow) compared to a degenerated organ of Corti in a chronically deaf ES/NT cochlea (open arrow). (b-f) Representative images of Rosenthal’s canal (e.g. dashed line in c) from the left (treated) cochlea for each group. SGN somata appear smaller and the packing density lower in AP cochleae compared to NT and normal controls. Higher magnification images of SGN somata from different cochleae are shown in the insets. Morphological degeneration of the somata was apparent in AP animals, unlike NT-treated cochleae. (g) Low magnification micrograph of a midmodiolar section from a normal hearing left cochlea. The upper (U) and lower (L) aspect of each turn (T) is shown (e.g. T1U = upper turn 1). The perforation created at the apex to facilitate fixative diffusion can be seen (arrowhead). (h) Left cochlea mean SGN density values (left panel) and left cochlea SGN density normalized to the contralateral control cochlea (right panel) for upper turn 1, one of the locations where significantly greater density was seen in NT animals (* two-way ANOVA). For comparison, the means for right deafened untreated control cochleae are illustrated in the left panel. The means for normal controls are shown for comparison but were not included in the ANOVAs. See Table 1 for group n values. Error bars ± 1 SEM. ST = scala tympani.
2.9 Cochlear examination and spiral ganglion neuron density measurement
One cochlear series was stained with Mayer’s haemotoxylin and Putt’s eosin (H&E) for general qualitative examination and SGN density measurements within Rosenthal’s canal. Slides were randomized and analyzed blindly. Sections viewed and imaged using an Axioplan 2 microscope and software (Zeiss, Germany). For one section per slide (4-5 analyzed sections per cochlea; at least 24μm separation for analyzed sections), a 20× magnification brightfield image was captured of Rosenthal’s canal for each half-turn (see Fig.5g). Using ImageJ (NIH, USA), Rosenthal’s canal was outlined, excluding the intraganglionic spiral bundle. The area of Rosenthal’s canal was measured and SGNs with a clear nucleus were counted to give the number of SGNs per mm2. This density measure is a standard method for assessing SGN survival (Agterberg et al., 2009; Glueckert et al., 2008; Shepherd et al., 1994; Li et al., 1999). Because sectioning ceased at midmodiolus, fewer data were available for more apical turns, and sufficient numbers of samples from turn 4 were not obtained for statistical analyses. There were also fewer midmodiolar sections of the lower turn 1 SGNs because Rosenthal’s canal was just appearing in this region at the midmodiolar plane (see Fig.5g). Statistical analyses of all data were performed using SPSS GradPack software (PASW, USA).
Cochlear features examined qualitatively in the H&E sections were SGN soma size/shape, organ of Corti degeneration, and the fibrous tissue response in the scala tympani to chronic implantation. To compare the extent of the tissue response between treatment groups, one representative image was chosen from each cochlea and the images were placed in ordered response severity ranks (i.e. most severe to least severe; blinded to treatment). The response severity ranks were subjectively determined using both the proportion of the scala tympani occupied by fibrous tissue and the optical density of the fibrosis (denser fibrosis taken to be a more severe response).
2.10 BrdU immunolabeling
Immunodetection of BrdU was performed using a protocol adapted from Shimada et al. (2008) and Kass et al. (2000) to assess whether neurogenesis occurred in the cochlea. Sections were immersed in a bath of 0.01M citric acid (pH adjusted to 6.0) and heated with a 700W microwave oven at 100% power until boiling (~3min), then at 40% power to maintain a gentle boil for 5min. The slides were then incubated in 2M HCl for 1h at 37°C, then in 0.1M boric acid for 10min at room temperature to neutralize the tissue. Sections were covered in 0.2% Triton-X (T-X, Sigma-Aldrich, USA) and 10% goat serum in PBS for 1h, followed by mouse anti-BrdU (1:100; Sigma-Aldrich, USA) and rabbit anti-neurofilament antibodies (200kD heavy chain; 1:400; Millipore, USA) in 2% goat serum/T-X/PBS. The sections were left overnight at 4°C. Following several PBS washes the tissue was exposed for 2h to TRITC-conjugated goat anti-mouse (1:200; Sigma-Aldrich, USA) and Alexa 488-conjugated goat anti-rabbit (1:200; Sigma-Aldrich, USA) diluted in 2% goat serum/T-X/PBS. The slides were washed in PBS and mounted in Fluoromount (Sigma-Aldrich, USA).
The sections were examined and imaged using an Axioplan 2 fluorescence microscope and software (Zeiss, Germany). Brain and gut sections underwent the same procedures as cochlear sections. Brain structures in which post-mitotic neurons have been previously detected (Abrous et al., 2005; Garcia-Verdugo et al., 1998; Guidi et al., 2005) were examined. In gut tissue, the stem cell region in the small intestine crypts were examined (O’Hara and Sharkey, 2007). The treatment group with the greatest potential for cochlear neurogenesis is ES/NT, based on the results of a previous study (Shepherd et al., 2005). At least three sections per cochlea were examined for the left and right cochleae of all ES/NT animals (n = 6) and the left cochlea of one animal (n = 1) in every other group.
3. Results
3.1 Electrically-evoked auditory brainstem response thresholds
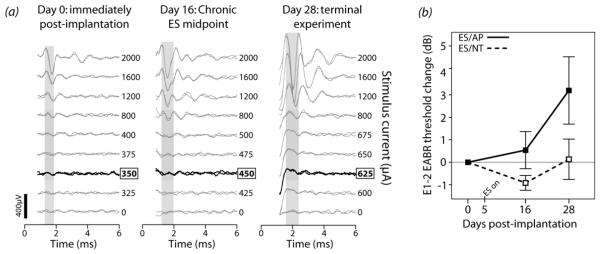
Examples of EABR traces to E1-2 stimulation at the beginning and end of the treatment period are shown in Figure 3 for an ES/AP animal. Analysis was focused upon E1-2 because they were the most apical electrodes located in the basal turn and nearest to the source of NTs; therefore, treatment effects would be expected to be most prevalent in this region. Changes in threshold from post-implantation (day 0) were compared between ES/NT and ES/AP. Threshold changes in US animals could not be examined as stimulating arrays were only implanted at the time of the terminal experiment. The mean change in E1-2 threshold over time (Fig.3b) shows an increase in threshold for ES/AP animals, but little change for ES/NT animals. An independent samples t-test on day 0 threshold current values confirmed that there was no significant group difference between groups post-implantation (p > 0.05). To determine if changes in E1-2 threshold over time were significant, and whether NT treatment had any effect, a repeated measures one-way analysis of variance (ANOVA) was performed on EABR threshold data (within-subjects factor: day 0-day 16-day 28 EABR threshold; between-subjects factor: ES/AP-ES/NT treatment). There was a significant change in EABR threshold over time (F (df 12) = 9.614, p = 0.003) that was driven primarily by a threshold increase is ES/AP animals, as indicated by a significant interaction between day and treatment (F = 5.367, p = 0.022). The treatment main effect was not significant (p > 0.05). Therefore, changes in EABR threshold over time were dependent upon the treatment received.
Figure 3.
(a) Bipolar (E1-2) EABRs evoked by biphasic current pulses recorded at three points during the treatment period in an ES/AP animal. Threshold (indicated by the bold traces) increased over time. Grey windows indicate the wave used to determine threshold (1-2ms post-stimulus, presumably corresponding to PIII-NIII of ABR; see Fig.1). Note that EABR waveform shape and amplitude were different between days. This may be due to changes over the treatment period, but is more likely related to slight differences in recording electrode placement. Threshold should not be significantly altered by electrode placement differences alone. Stimulus artifacts have been blanked out (0-1ms). (b) Mean EABR threshold change for E1-2 from day 0 to day 28 in ES animals. An increase occurred in ES/AP animals (n = 3) whereas little change occurred in ES/NT animals (n = 5). Error bars ± 1 SEM.
EABR thresholds were examined across all cohorts at the end of the treatment period. The group means for E1-2 are shown in Figure 4. E1-2 group means were compared using a two-way ANOVA (US-ES and AP-NT factors; normals excluded). NT animals had a significantly lower threshold than AP (F (df 15) = 5.508, p = 0.033). Higher thresholds were observed in ES compared to US animals, although this effect was only just significant (F = 4.559, p = 0.050) (Fig.4). There was no significant interaction between the two treatments (p > 0.05). An a posteriori independent samples t-test between normal controls and US/NT E1-2 thresholds showed that the means were not significantly different. The above trend – lower thresholds in NT animals and higher in ES animals – was observed across other electrodes following the treatment period, including at wider bipolar inter-electrode separations (e.g. E1-3, data not shown).
Figure 4.
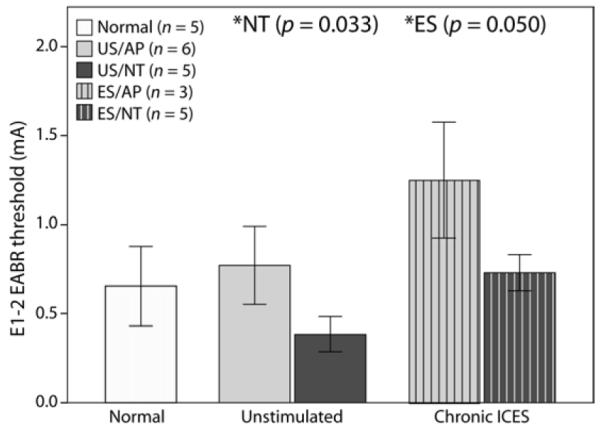
(a) Mean E1-2 EABR threshold for each group at the conclusion of the treatment period. Thresholds were significantly lower in NT animals compared to AP, and thresholds were significantly higher in ES compared to US (* two-way ANOVA). Data from acutely deafened normal animals are shown for comparison but were not included in the ANOVA. Data from one ES/AP and one ES/NT animal were not included as electrode 1-2 for these two animals was not available at the time of the experiment. Error bars ± 1 SEM.
3.2 Organ of Corti and spiral ganglion neuron survival
H&E-stained cochlear sections were examined to assess the status of the organ of Corti and SGNs. The organ of Corti was severely degenerated in the left and right cochleae of all deafened treatment groups, as indicated by the collapsed support cell structures (Fig.5a). Figure 5 also illustrates representative examples of SGNs in Rosenthal’s canal at upper turn 1 for each treatment group, as well as the peripheral region in a normal and a chronically deaf cochlea. The density of SGNs in Rosenthal’s canal was greater in NT cochleae compared to AP-treated and contralateral control cochleae, with no obvious rescue effect associated with ES. The cell bodies in NT cochleae also appeared closer to a normal morphology (i.e. large, round) compared to AP cochleae, which had shrunken and amorphous somata.
For each animal the mean SGN density value was calculated for each half-turn of the left (treated) and right (deafened control) cochlea from lower turn 1 to upper turn 3 and values compared across groups. No significant treatment group effects were seen at any turn in the right control cochleae (two-way ANOVA, AP-NT and US-ES factors, p > 0.05), indicating no significant differences in the degenerative response to deafening in any cohort. Significantly greater SGN density was seen in NT-treated cochleae compared to AP controls in upper turn 1 (Fig.5h), lower turn 2, upper turn 2, and lower turn 3 (not illustrated; p < 0.05). No other main or interaction effects, including ES effects, were found at other cochlear locations (p > 0.05). The same pattern of results was seen when comparing the means of left SGN density normalized to the contralateral control cochlea density (upper turn 1 shown Fig.5h), except a significant increase in NT animals above AP was not seen at lower turn 3 (not illustrated; p > 0.05). NT treatment therefore had a significant SGN preservation effect in the basal and second cochlear turns compared both to AP-treated controls and within-animal controls. An a posteriori independent samples t-test showed that left upper turn 1 SGN density for ES/NT (treatment group with the highest mean density) was significantly lower than normal (two-tailed t (df 9) = 3.264, p = 0.010).
3.3 Scala tympani tissue response and electrode impedance
Electrode impedances were periodically monitored over the treatment period and were compared to the scala tympani tissue response to chronic implantation. At the time of implantation, electrode impedances were found to be similar between electrodes for each implant (~2kΩ), with a tendency to increase over time (Fig.6). To determine whether significant differences occurred between treatment groups, a repeated measures two-way ANOVA was performed (within-subjects factor: day 0-day 28 impedance, between-subjects factors: implant electrode, ES/AP-ES/NT treatment). Open or short circuit electrode measurements were excluded. There was a significant increase in impedance over time (F (df 33) = 60.517, p < 0.0005), a significant effect of electrode number (F = 7.966, p < 0.0005), and a significant interaction between day and electrode number (F = 6.612, p < 0.0005). The significant interaction between day and electrode indicates that the impedance increase over time was dependent upon the electrode, as is evident from the data shown in Figure 6. Post-hoc analysis of the electrode effect showed that E6 – the electrode closest to the round window – exhibited significantly greater impedance over time compared with all other electrodes (Bonferroni, p < 0.03). The treatment main effect and all treatment interactions were not significant (p > 0.05), indicating that NT treatment did not have an effect on electrode impedance.
Figure 6.
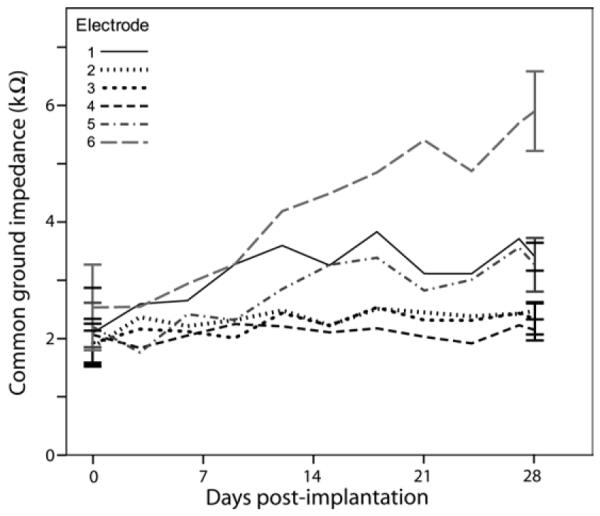
Common ground impedance means are shown for all implant electrodes over the treatment period. There was no significant treatment effect so data are shown pooled across the two ES groups (total n = 10). Measurements other than day 0 and day 28 have been pooled across 3-day bins. Impedances increased significantly over time. Error bars ± 1 SEM.
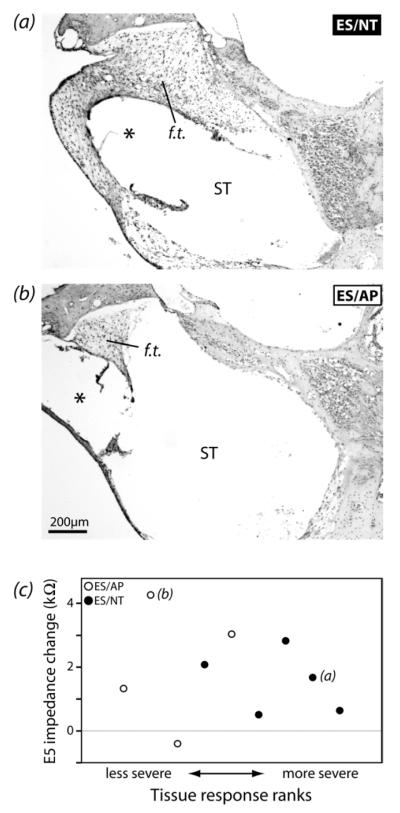
The tissue response in the ST of implanted cochleae was examined. Very little tissue response was observed in the majority of cochleae. No cochlea exhibited new bone growth in any turn, or fibrous tissue beyond upper turn 1. There appeared to be a greater volume of fibrous tissue present in ES/NT cochleae compared to ES/AP (Fig.7). When qualitatively ranked for lower turn 1 response severity, the difference between ES/AP and ES/NT groups was significant (Mann-Whitney U test, p = 0.027). There was not a significant relationship between the extent of the tissue response and the change in impedance of E5 (electrode near the tissue response region examined) over time either across all ES animals or within the ES/AP or ES/NT groups (Spearman’s rho, p > 0.05) (Fig.7c).
Figure 7.
(a) Representative examples of the fibrous tissue response (f.t.) in lower turn 1 scala tympani (ST) of ES/NT and ES/AP cochleae. ES/NT cochleae appeared to have a more extensive tissue reaction in the ST than ES/AP. The * show the likely chronic locations of the implants. (b) Qualitative ranks for tissue response severity plotted against the change in impedance from post-implantation to day 28 for E5 (electrode near cochlear region examined). There was no significant relationship between impedance change and rank across all ES animals or within either ES/AP or ES/NT groups (Spearman’s rho, p > 0.05). The letters in c indicate the data points for the micrographs in a and b.
3.5 BrdU labeling
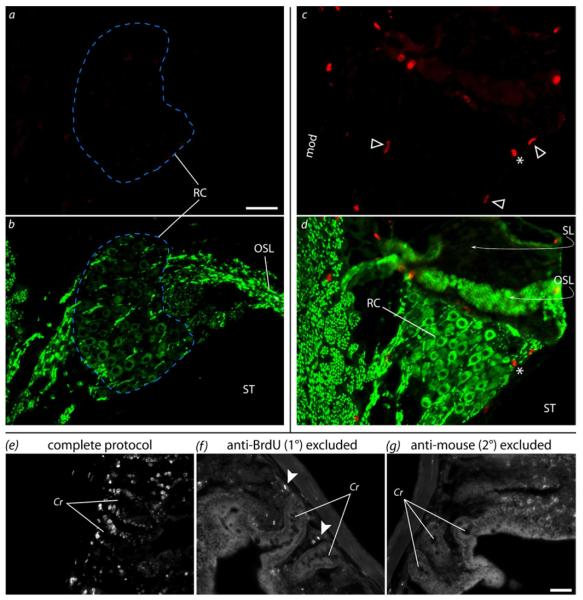
The potential for neurogenesis in the cochlea was assessed using BrdU. Positive BrdU labeling was observed in brain and gut control tissue in locations previously shown to exhibit mitosis (Garcia-Verdugo et al., 1998; Guidi et al., 2005; O’Hara and Sharkey, 2007; Abrous et al., 2005). In the present study, labeling in the brain was seen in the subgranular zone of the hippocampus, the subventricular zone, along the rostral migratory stream, and in the olfactory bulbs. In the gut, labeling was seen in the intestinal crypts, along the villi, and in the external wall tissue (Fig.8). Immunolabeling negative controls were obtained by excluding the primary and/or secondary antibodies. No nuclear labeling was seen in any of these control sections, indicating specific labeling of nuclear BrdU, although some non-specific binding of the secondary antibody was seen. However, the non-specific fluorescence signal was observed as globular and intense puncta, whereas positive BrdU labeling had a more uneven and sharp-edged appearance to the labeling, allowing positive signal to be distinguished in experimental tissue. In contrast to the positive control tissue, there was no evidence of BrdU labeling in SGNs within Rosenthal’s canal (Fig.8). Identification of SGNs in Rosenthal’s canal was possible by neurofilament labeling.
Figure 8.
Immunodetection of BrdU in cochlear and control tissue. (a,b) Representative example showing the lack of BrdU labeling in Rosenthal’s canal (RC; blue dashed line) of an ES/NT cochlea. (a) BrdU (red) and (b) neurofilament (green) in upper turn 1. SGN somata can be seen in the neurofilament channel, but no BrdU labeling was seen in SGN nuclei. (c,d) In one ES/NT cochlea, positive BrdU labeling was seen in what appear to be Schwann cell nuclei (arrowheads) in the modiolus (mod, not shown) and RC . An intense globular object (*) that was likely artifactual – possibly non-specific secondary antibody binding as was seen in negative controls (arrowheads in f) – can also be seen. No BrdU-labeled nuclei in cochlear tissue were surrounded by a neurofilament-labeled soma. The peripheral structures (i.e. OSL and SL) in c-d can be seen to be detached from the slide and folded back (curved arrows), obscuring part of RC. This illustrates the difficulties the DNA denaturation steps can create for analysis of sectioned cochlear tissue. (e-g) Positive and negative BrdU labeling controls (gut tissue). The complete labeling protocol (e) yielded positive labeling (white) in stem cell nuclei in the intestinal crypts (Cr), whereas exclusion of primary (f) or secondary antibodies (g) did not result in labeling. The image brightness has been increased in f and g to better view the gut anatomy. OSL = osseous spiral lamina, SL = spiral limbus, ST = scala tympani. Scale bars = 50μm (same scale a-d and e-g).
Some BrdU-positive nuclei were observed in other cochlear tissues, although the degree of labeling was quite variable. Labeled nuclei were occasionally seen in the medial wall of the scala vestibuli, in bone, around blood vessels, or associated with a fibrous tissue response within the scala tympani. In one ES/NT cochlea, BrdU labeling was present in nuclei in the modiolus and upper basal turn Rosenthal’s canal, but these nuclei were not from neurofilament-labeled cells (Fig.8d). These were likely Schwann cells, as judged by the distinct spindle-shaped nuclei and apparent association with neural processes (compare with H&E images, Fig.5). However, Schwann cell BrdU labeling was only observed in this one cochlea, and therefore Schwann cell genesis cannot be presumed to be a treatment effect.
4 Discussion
This study examined the response of the cochlea to chronic NT and/or ES treatment following an initial two-week period of deafness in guinea pigs. NT treatment increased SGN survival and reduced EABR thresholds compared to AP treated controls. Moreover, there was no significant interaction between ES and NT treatments for either EABR threshold or SGN density, although fibrous tissue response was greater following ES/NT compared to ES/AP treatment. Finally, greater-than-normal SGN densities reported following treatment with several neurotrophic factors including NTs (Glueckert et al., 2008; Miller et al., 2007; Shepherd et al., 2005), implying that neurogenesis may be induced by ES/NT treatment, however no evidence of neurogenesis was evident in the present study.
4.1 Effects of treatments on spiral ganglion neurons
In the basal and middle turns of all left (treated) and right (untreated) deafened cochleae, the organ of Corti had undergone significant degeneration, although some organ of Corti structure was usually present in the apical turn. Increases in ABR threshold of >50dB were also observed, indicating a severe-profound SNHL. These deafness induced changes were also associated with lower SGN density than normal, in agreement with previous observations (Leake and Hradek, 1988; Sugawara et al., 2005; Takeno et al., 1998). The SGN density values measured in the present study tended to be higher than in previous guinea pig studies (Agterberg et al., 2008; Dodson and Mohuiddin, 2000; Shepherd et al., 2005; Gillespie et al., 2004; Wise et al., 2005), due to the use of thicker (12μm) frozen sections in the present study compared to thin sections (e.g. 2μm, resin-embedded) in most previous studies, resulting in more SGNs captured in each section. Frozen sections were used in the present study to facilitate immunohistochemistry.
Our results show that chronic intracochlear ES in the deafened guinea pig does not provide trophic support of SGNs, in that ES/AP-treated cochleae exhibited no evidence of increased SGN survival compared with deafened, unstimulated control cochleae. These results are consistent with previous chronic ES studies obtained from a number of species (Araki et al., 1998; Shepherd et al., 2005; Shepherd et al., 1994; Agterberg et al., 2010; Li et al., 1999), although other groups have reported a trophic influence on SGNs from chronic ES alone (Hartshorn et al., 1991; Kanzaki et al., 2002; Leake et al., 1991; Leake et al., 1999; Leake et al., 1992; Mitchell et al., 1997). These differences may be due to methodological differences including the technique and duration of deafness prior to treatment and stimulation parameters (Miller, 2001; Shepherd et al., 2006).
SGN survival was significantly greater in NT cochleae compared to controls, in agreement with previous studies on the application of exogenous NTs to deaf cochleae (Agterberg et al., 2009; Ernfors et al., 1996; Gillespie et al., 2003; Miller et al., 1997; Richardson et al., 2005; Wise et al., 2005; McGuinness and Shepherd, 2005). Furthermore, SGN cell bodies appeared shrunken in both US/AP and ES/AP compared to normal controls and NT-treated cochleae, although this was not quantified in the present study. Previous quantitative studies have reported significantly greater soma area (Richardson et al., 2005; Shepherd et al., 2005; McGuinness and Shepherd, 2005) and circularity (Agterberg et al., 2008) following NT treatment compared to deafened control cochleae. Changes in soma size can be associated with corresponding diameter changes in peripheral and central SGN processes (Agterberg et al., 2008; Dodson and Mohuiddin, 2000; Glueckert et al., 2008; Wise et al., 2005), which could have a direct effect upon SGN responsiveness to ES.
It has been previously reported that NT-mediated SGN survival was enhanced by chronic ES (Shepherd et al., 2005), although this effect was not evident in the present study. There were, however, some methodological variations between these studies - including the use of two neurotrophins in the present study (NT-3 and BDNF) versus BDNF alone in Shepherd et al. (2005) - that may have contributed to these different findings. However, perhaps the most important difference is that a longer deafness period before treatment used in the present study. Longer deafness periods result in differences in SGN degenerative state at the time of treatment onset. A previous study using deafening procedures almost identical to the present study, reported that one week after deafening there was no change in SGN density or soma circularity (Versnel et al., 2007). At two weeks after deafening, there was still little decrease in SGN density compared to normal (Versnel et al., 2007; Agterberg et al., 2008; Gillespie et al., 2004), morphological changes to SGNs were evident, including reduced soma area and circularity. Therefore, we expect that in the present study most SGNs were available for rescue at the time of implantation; however – unlike the Shepherd et al., (2005) study - the degenerative process was well underway, perhaps irreversibly for some neurons. We note that the rate of SGN degeneration in this animal model is very rapid compared with other deaf animal models and experience with clinical material. It is possible that there is a post-deafening “critical period” during which the potentially beneficial interactive effects between ES and NT treatments are maximally effective. After this period, ongoing SGN degeneration may be accompanied by significant changes in gene expression which affect the SGN response to the combined treatment. For example, changes in the expression of Trk receptors – the receptors to which NTs bind and initiate pro-survival signaling (Skaper, 2008) – would affect the amount or type of NT binding. Also, changes in voltage-gated ion channel expression could affect modulations in intracellular levels of ions (e.g. Ca2+) in response to ES. Such effects would be likely to influence the treatment outcome by altering the activation of molecular signaling pathways.
4.2 Effects of treatments on EABR threshold
Significantly lower EABR threshold was observed in NT treated animals compared to AP animals in the present study, as well as a barely significant increase in EABR threshold in ES compared to US animals. Previous studies have also observed reductions in EABR threshold following treatment with neurotrophic factors (Shepherd et al., 2005; Agterberg et al., 2009; Shinohara et al., 2002). This effect could be due to changes in SGN morphology, including increased SGN soma size (Agterberg et al., 2008; Richardson et al., 2005; Shepherd et al., 2005) or peripheral afferent fiber diameter (Wise et al., 2005), which could directly cause reductions in activation threshold (McNeal, 1976; Rushton, 1951). The lower EABR thresholds could also be related to changes in the expression levels of different voltage-gated ion channels (Adamson et al., 2002). Reduced thresholds are regarded as a functional improvement for cochlear implants, including decreased battery consumption and the potential for the use of smaller stimulating electrodes (Seligman and Shepherd, 2004).
Because of the experimental design, EABR threshold change could not be monitored over time in US animals, making it difficult to compare electrophysiological data between US and ES cohorts. Therefore, it is unclear to what extent the threshold increase over time in ES/AP animals is related to SNHL-induced degeneration of SGNs and their peripheral fibers. However, previous studies in which EABRs are monitored longitudinally typically report an increase in threshold over time, with no significant difference between chronically-implanted unstimulated or electrically stimulated cohorts (Shepherd et al., 2005; Coco et al., 2007; Shinohara et al., 2002; Vollmer et al., 2007). In the present study, the significantly lower EABR threshold observed longitudinally in the ES/NT cohort compared with ES/AP animals indicates that combined ES and NT treatment can effectively decrease EABR thresholds, and is consistent with previous observations showing threshold reductions associated with chronic NT delivery with and without chronic ES (Shepherd et al., 2005; Shinohara et al., 2002).
4.3 Effects on scala tympani tissue response and electrode impedance
Electrode impedances increased significantly over time, although the increases were small in magnitude, with the possible exception of E6, which showed a larger increase. This was likely related to the position of E6 proximal to the round window. Fibrous tissue growth was seen in most cochleae to varying degrees. The tissue response appeared to be more extensive in ES/NT compared to ES/AP, indicating that NTs may increase the severity of the tissue response. This was supported by statistical analysis of qualitatively ranked response severity. A more extensive tissue response following NT treatment has also been observed previously (Shepherd et al., 2005), and may be an immune response to the foreign (human recombinant) peptides, rather than a reaction to NTs per se. In the long term, the growth of fibrous and osseous tissue around the implant and throughout the ST could interfere with implant functionality (Xu et al., 1997), as well as reduce residual hearing function (Choi and Oghalai, 2005). With increasing numbers of patients with residual hearing receiving cochlear implants (Kim et al., 2010), any factor contributing to fibrous tissue growth would be of clinical concern. Severe tissue response is also typically related to significant SGN degeneration (Xu et al., 1997). However, the present results indicate that the tissue response did not affect SGN survival or implant function, presumably due to the relatively short treatment period. Longer treatment periods may result in a more significant impact of the NT-enhanced tissue response. The increased tissue response may be able to be mitigated by NT co-treatment with other drugs, such as anti-inflammatory agents.
4.4 Investigating neurogenesis in the cochlea
Previous studies reported SGN densities that were greater than normal following treatment with neurotrophic factors (Glueckert et al., 2008; Miller et al., 2007; Shepherd et al., 2005), suggesting that neurogenesis may be induced by these treatments. However, no evidence of neurogenesis as a result of cell division was evident in the present study. The appropriate control measures were taken to ensure that the application and detection was successful, and evidence of this success was apparent in positive and negative control tissues, as well as positive labeling in non-neuronal cochlear cells. Although there were no indications that new neurons were present in the cochlea from newly divided cells, this does not rule out the possibility that new neurons were present, but had differentiated from pre-existing neural progenitor cells. However, in vitro studies showed neurogenesis in pharmacologically treated spiral ganglion explants by induced mitosis (Rask-Andersen et al., 2005; Wei et al., 2007), supporting a mitotic route for SGN neurogenesis. Those studies also suggest that other trophic factors such as glial-derived neurotrophic factor may more readily produce spiral ganglion neurogenesis than NTs.
There were some methodological differences between the previous studies reporting greater-than-normal SGN density and the present study. As discussed above, the present study used a longer pre-treatment deafness period of two weeks compared to five days by Shepherd et al. (2005). The lack of neurogenesis detection in the present study could be indicative of a “critical period” following deafness onset after which neurogenesis cannot be induced, at least by treatment with BDNF/NT-3 and chronic ES. Another explanation for the greater-than-normal SGN density in the previous studies (Glueckert et al., 2008; Miller et al., 2007; Shepherd et al., 2005) is that there were systematic differences in ratios of counting error types between treatment groups, due to treatment related changes in SGN morphology (although appropriate corrections were applied in some studies). While the potential for induced neurogenesis in the spiral ganglion requires further study prior to any clinical application of NTs to the cochlea, the present results suggest that neurogenesis does not occur.
4.5 Conclusions
The present study show increased SGN survival and reduced EABR thresholds in long-term deafened NT-treated cochleae compared to AP controls. There was no enhancement of NT-mediated SGN survival by chronic ES. Moreover, there was no evidence of neurogenesis in the spiral ganglion. However, the present results indicate that in cases with more advanced SGN degeneration, exogenous NT delivery with or without ES results in greater SGN survival and lower EABR thresholds compared to chronic ES alone. Thus, exogenous NT treatment has the potential to benefit implant patients, with the maximal benefit likely to be obtained with early intervention.
Highlights.
Chronic neurotrophins and electrical stimulation used in deaf guinea pig cochleae
Spiral ganglion neuron density and auditory brainstem response threshold assessed
Greater neuron survival with neurotrophin treatment compared to controls
Lower response thresholds with neurotrophin treatment compared to controls
No evidence of post-mitotic neurogenesis found in the spiral ganglion
Acknowledgements
This study was funded by the National Institute on Deafness and Other Communication disorders (National Institutes of Health contract HHS-N-263-2007-00053-C), The Bartholomew Reardon PhD Scholarship (The Bionics Institute), and The Mabel Kent Scholarship. The Bionics Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program. Many thanks to Mrs. M. Clarke and Ms. P. Nielsen for H&E staining, Mrs. H. Feng for implant manufacturing and contributions to cochlear implant design, Mr. R. Millard for technical support, Mrs. A. Neil for surgical assistance, and Dr. J. Xu for x-ray photography and contributions to cochlear implant design.
Abbreviations
- ABR
auditory brainstem response
- ANOVA
analysis of variance
- AP
artificial perilymph
- BDNF
brain-derived neurotrophic factor
- BrdU
5-bromo-2′-deoxyuridine
- DNA
deoxyribonucleic acid
- Cr
intestinal crypt
- EABR
electrically-evoked auditory brainstem response
- ES
electrical stimulation
- f.t.
fibrous tissue response
- H&E
haemotoxylin and eosin
- mod
modiolus
- NT
neurotrophin
- NT-3
neurotrophin-3
- OSL
osseous spiral lamina
- PBS
phosphate buffered saline
- p.e. SPL
peak equivalent sound pressure level
- PFA
paraformaldehyde
- RC
Rosenthal’s canal
- RW
round window
- s.c.
subcutaneous
- SEM
standard error of the mean
- SGN
spiral ganglion neuron
- SL
spiral limbus
- SNHL
sensorineural hearing loss
- ST
scala tympani
- T-X
Triton-X
- US
unstimulated cohort
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear Res. 2010;269:169–179. doi: 10.1016/j.heares.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced Survival of Spiral Ganglion Cells After Cessation of Treatment with Brain-Derived Neurotrophic Factor in Deafened Guinea Pigs. J Assoc Res Otolaryngol. 2009;10:355–367. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, Hoyle M, Liu Z, Price A, Stein K. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess. 2009;13:1–330. doi: 10.3310/hta13440. [DOI] [PubMed] [Google Scholar]
- Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Rickard NA, de Silva MG, Shepherd RK. A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods. 2009;176:144–151. doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R, Bijl RV. Determinants of mental distress in adults with a severe auditory impairment: differences between prelingual and postlingual deafness. Psychosom Med. 2002;64:61–70. doi: 10.1097/00006842-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29:525–537. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Dolbeare F. Bromodeoxyuridine: a diagnostic tool in biology and medicine, Part I: Historical perspectives, histochemical methods and cell kinetics. Histochem J. 1995;27:339–369. [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DR, Shepherd RK. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. J Comp Neurol. 2009;512:101–114. doi: 10.1002/cne.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellinger J, Holzinger D, Dobner U, Gerich J, Lehner R, Lenz G, Goldberg D. Mental distress and quality of life in a deaf population. Soc Psychiatry Psychiatr Epidemiol. 2005;40:737–742. doi: 10.1007/s00127-005-0936-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Guidi S, Ciani E, Severi S, Contestabile A, Bartesaghi R. Postnatal neurogenesis in the dentate gyrus of the guinea pig. Hippocampus. 2005;15:285–301. doi: 10.1002/hipo.20050. [DOI] [PubMed] [Google Scholar]
- Harris JP, Anderson JP, Novak R. An outcomes study of cochlear implants in deaf patients. Audiologic, economic, and quality-of-life changes. Arch Otolaryngol Head Neck Surg. 1995;121:398–404. doi: 10.1001/archotol.1995.01890040024004. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104:311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kass L, Varayoud J, Ortega H, Munoz de Toro M, Luque EH. Detection of bromodeoxyuridine in formalin-fixed tissue. DNA denaturation following microwave or enzymatic digestion pretreatment is required. Eur J Histochem. 2000;44:185–191. [PubMed] [Google Scholar]
- Kim LS, Jeong SW, Lee YM, Kim JS. Cochlear implantation in children. Auris Nasus Larynx. 2010;37:6–17. doi: 10.1016/j.anl.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res. 1991;54:251–271. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999;133:27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM, Vandali AE. A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant. J Acoust Soc Am. 1992;91:3367–3371. doi: 10.1121/1.402826. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976;23:329–337. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85:1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Miura M, Sando I, Hirsch BE, Orita Y. Analysis of spiral ganglion cell populations in children with normal and pathological ears. Ann Otol Rhinol Laryngol. 2002;111:1059–1065. doi: 10.1177/000348940211101201. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Feldman JJ, Dunbar JL, McConkey-Robbins A, Niparko JK, Rittenhouse RK, Skinner MW. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2000;16:1120–1135. doi: 10.1017/s0266462300103162. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- O’Hara JR, Sharkey KA. Proliferative capacity of enterochromaffin cells in guinea-pigs with experimental ileitis. Cell Tissue Res. 2007;329:433–441. doi: 10.1007/s00441-007-0430-6. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, Lindholm D. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–191. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O’Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rushton WA. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman PM, Shepherd RK. Cochlear implants. In: Horch KW, Dhillon G, editors. Neuroprosthetics: theory and practice. World Scientific Publishing; Singapore: 2004. pp. 878–904. [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–109. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Meltzer NE, Fallon JB, Ryugo DK. Consequences of deafness and electrical stimulation on the peripheral and central auditory system. In: Waltzman S, Roland T, editors. Cochlear Implants. Thieme medical Publishers Inc.; New York: 2006. pp. 25–39. [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shimada A, Shibata T, Komatsu K, Nifuji A. Improved methods for immunohistochemical detection of BrdU in hard tissue. J Immunol Methods. 2008;339:11–16. doi: 10.1016/j.jim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol Neurootol. 1998;3:281–290. doi: 10.1159/000013800. [DOI] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–543. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF, Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231:1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Vollmer M, Beitel RE, Snyder RL, Leake PA. Spatial selectivity to intracochlear electrical stimulation in the inferior colliculus is degraded after long-term deafness in cats. J Neurophysiol. 2007;98:2588–2603. doi: 10.1152/jn.00011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Jin Z, Jarlebark L, Scarfone E, Ulfendahl M. Survival, synaptogenesis, and regeneration of adult mouse spiral ganglion neurons in vitro. Dev Neurobiol. 2007;67:108–122. doi: 10.1002/dneu.20336. [DOI] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O’Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB., Jr. Patterns of degeneration in the human cochlear nerve. Hear Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]