Abstract
The Ebf transcription factors play important roles in the developmental processes of many tissues. We have shown previously that four members of the Ebf family are expressed during mouse retinal development and are both necessary and sufficient to specify multiple retinal cell fates. Here we describe the changes in cell differentiation and retinal ganglion cell (RGC) projection in Ebf1 knockout mice. Analysis of marker expression in Ebf1 null mutant retinas reveals that loss of Ebf1 function causes a significant increase of Müller cells. Moreover, there is an obvious decrease of ipsilateral and retinoretinal projections of RGC axons at the optic chiasm, whereas the contralateral projection significantly increases in the mutant mice. These data together suggests that Ebf1 is required for suppressing the Müller cell fate during retinogenesis and important for the correct topographic projection of RGC axons at the optic chiasm.
Keywords: Ebf1, Early B-cell Factor, Knockout, Retinal Development, Retinal Ganglion Cell, Axon Projection, Optic Chiasm
Introduction
The mouse retina is composed of six classes of neuronal cells, including the ganglion, amacrine, horizontal, bipolar, rod and cone cells, and one class of glia cells, the Müller cells. The seven classes of cells can be further divided into more than 50 subgroups with distinct morphologies and functions [1,2]. All retinal cell types arise from the same group of multipotent progenitors during retinogenesis. The process of retinal development is under the tight and delicate controls of both intrinsic and extrinsic factors [3,4]. The rod and cone photoreceptors reside in the outer nuclear layer and are responsible for collecting the light signal from the environment. The horizontal, bipolar and amacrine cells are interneurons located in the inner nuclear layer and are responsible for integrating and relaying the signal from the photoreceptors. The retinal ganglion cells (RGCs) integrate the signals from amacrine and bipolar cells, and then transmit them through their long exons, which bundle into the optic nerve and cross the optic chiasm before finally projecting into the brain.
Visual signals from each retina are transmitted to the thalamus and cerebral cortex on both sides of the brain. One of the crucial steps to establish the binocular and 3-dimensional vision is the proper RGC axon projection at the optic chiasm region. In the wild type mouse, there are about 3–5% RGC axons projecting ipsilaterally at the optic chiasm, while more than 95% RGC axons project contralaterally to the other side of the brain [5,6]. Interestingly, there is a small portion of RGC axons originated from the nasal part of the retina that project through the chiasm into the contralateral optic nerve, which is called the retinoretinal projection [7,8,9,10]. The retinoretinal projection normally disappears soon after birth, and its mechanism and developmental significance is still not clear. Whether RGC axons would extend to cross the midline at the optic chiasm, and at which direction they will further project into the brain, are under the dynamic control of interactions between a group of factors, such as EphB1/Ephrin B2 [11,12], NrCAM [13], Slit1/Slit2 [10], and transcription factors Zic2 [14,15], Foxd1 [16], etc.
The Ebf/Olf (Early B-Cell Factors) family of proteins are helix-loop-helix (HLH) transcription factors including four members, Ebf1 through Ebf4, that are important for neural development [7,17,18,19], adipogenesis [20,21], and lymphocyte development [22,23]. Previously we reported that all four members of the Ebf family are involved in retinal cell specification [24]. In the retinas, Ebfs are expressed in horizontal, ganglion, type 2 OFF-cone bipolar, and non-All glycinergic amacrine cells [24]. However, the loss-of-function study with the knockout mice has not been done yet. Here we report the increase of Müller cell in the retina and abnormal RGC axon projection at the optic chiasm in the Ebf1 null mouse, and reveal an essential role of Ebf1 in regulating these developmental events.
Materials and Methods
Animals
All experiments with mice were performed in compliance with the IACUC protocols approved by the University of Medicine and Dentistry of New Jersey. The animals were housed and bred at the university facility. The C57BL/6J mice were obtained from the Jackson Laboratory. The Ebf1 knockout mice were reported previously [22] and maintained by breeding with C57BL/6J mice.
In Vitro Retinal Explant Culture and Immunostaining
In vitro retinal explant culture, immunohistochemisty, and whole-mount immunostaining were performed as described previously [24,25,26]. The following primary antibodies were used: mouse anti-Brn3a (Millipore); goat anti-Bhlhb5 (Santa Cruz Biotechnology); rabbit anti-calbindin D-28k (Swant); sheep anti-Chx10 (Exalpha); rabbit anti-GABA (Sigma); mouse anti-glutamine synthetase (Millipore); goat anti-GLYT1 (Millipore); rabbit anti-NF150 (Millipore); rabbit anti- Pax6, (Millipore); rabbit anti-recoverin (Millipore); and rabbit anti-RxRg/RxRγ (Santa Cruz Biotechnology). Images were captured with either the Nikon Eclipse 80i Microscope or Leica TCS-SP2 Confocal Microscope.
Dil Labeling and Tracing
DilC18(3) (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate) crystal was purchased from Invitrogen (Lot 454239). P0 mice were decapitated and fixed in fresh 4% PFA overnight. After the removal of lens and retina, a DilC18(3) crystal was placed onto the spot of the optic nerve The heads were kept at 37°C for 2 weeks in PBS with 0.05% sodium azide in a sealed cell culture plate. They were then dissected and the optic chiasm was exposed for imaging. All images were taken with the same parameters from the Nikon Eclipse 80i microscope.
Quantification
Retinas from the same litter were used for quantification analysis. Confocal images from matched regions of wild type and mutant retinas were captured with the same scanning thickness. At least 6 regions from each retina and 3 retinas from each genotype were captured. Marker-positive cells were scored from the images. All data were tested for significance using two sample Student’s t-test with unequal variances.
The Dil labeling images were quantified using the NIH ImageJ software. First, use the freehand tool to select the region (ipsilateral, contralateral, or retinoretinal), then choose plugins → analyze → measure RGB. Record the value of the area and the average intensity of the red signal in the area. The percentage of contralateral, ipsilateral and retinoretinal projections was calculated by the relative value of (area × intensity).
Results
Early Ebf1 Mutant Retinas Show No Obvious Cell Fate Changes
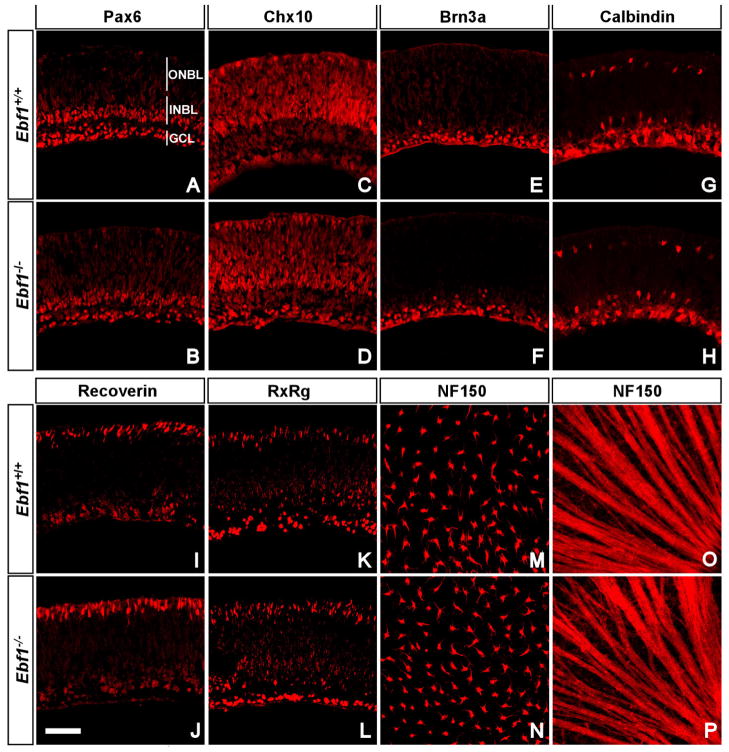
Ebf1 heterozygotes were crossed to obtain the homozygous mutant mice, which usually die soon after birth. To investigate whether loss of Ebf1 would affect cell fates in early stage retinas, E13.5 and P0 retinas were examined. At E13.5, the size and morphology between wild type and mutant retinas are indistinguishable, and ganglion cells are unchanged in the mutants (data not shown). At P0, retinas were examined for any defects by immunostaining with antibodies against several retina cell markers, which include Pax6 for RGCs in the ganglion cell layer, amacrine and horizontal cells in the neuroblastic layer(Fig. 1A, B), Chx10 for bipolar and progenitor cells (Fig. 1C, D), Brn3a for RGCs (Fig. 1E, F), calbindin for horizontal cells and starburst and other amacrine cells (Fig. 1G, H), recoverin and RxRg (also called RxRγ) for rod and cone photoreceptors, respectively, in the outer neuroblastic layer (Fig. 1I, J, K, L); NF150 for horizontal cells in the inner neuroblastic layer and RGC axon fibers (Fig. 1M, N, O, P). None of the markers exhibited any obvious difference between wild type and Ebf1 mutant retinas, suggesting that the absence of Ebf1 does not affect the fates and differentiation of early retinal cell types. The normal nerve fiber patterns revealed by NF150 labeling imply that there are no intraocular RGC axon projection defects in the Ebf1−/− retinas.
Fig. 1. Expression of cell markers in P0 Ebf1+/+ and Ebf1−/− retinas.
(A–L) P0 retinal sections from wild type and mutant retinas were immunolabeled with the indicated antibodies. (A, B) Anti- Pax6 stains RGCs, amacrine and horizontal cells. (C, D) Anti-Chx10 stains progenitor and bipolar cells. (E, F) Anti-Brn3a stains RGCs. (G, H) Anti-calbindin stains horizontal and amacrine cells. (I, J) Anti-recoverin stains photoreceptors. (K, L) Anti-Rxrg stains cone photoreceptors and RGCs. (M–P) NF150 whole-mount staining shows horizontal cells and RGC axon fibers at different layers. There are no significant changes in these markers between wild type and mutant retinas. GCL, ganglion cell layer; INBL, inner neuroblastic layer; ONBL, outer neuroblastic layer. Scale bar: J (for A–P), 50μm.
Müller Cells Increase in the Ebf1−/− Retina
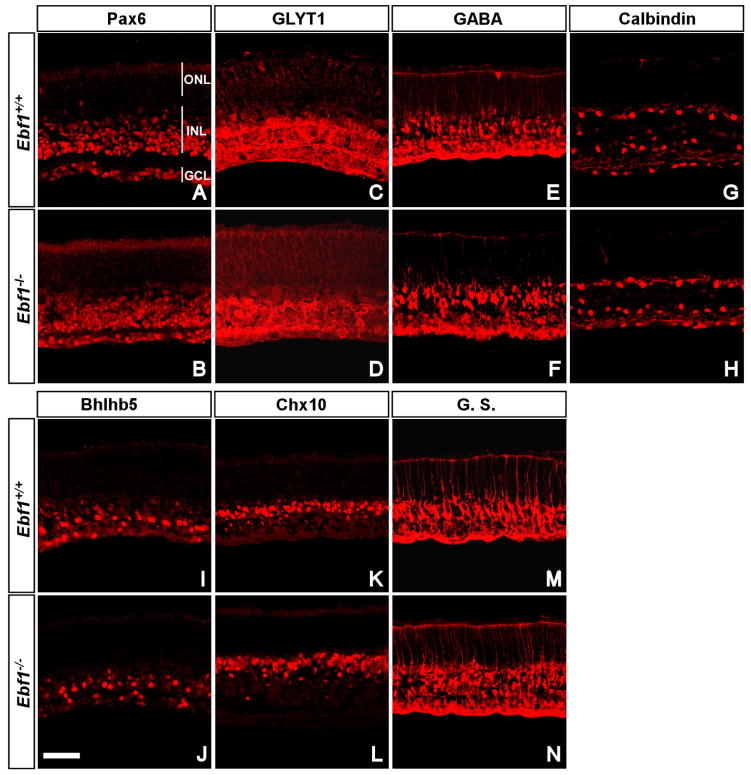
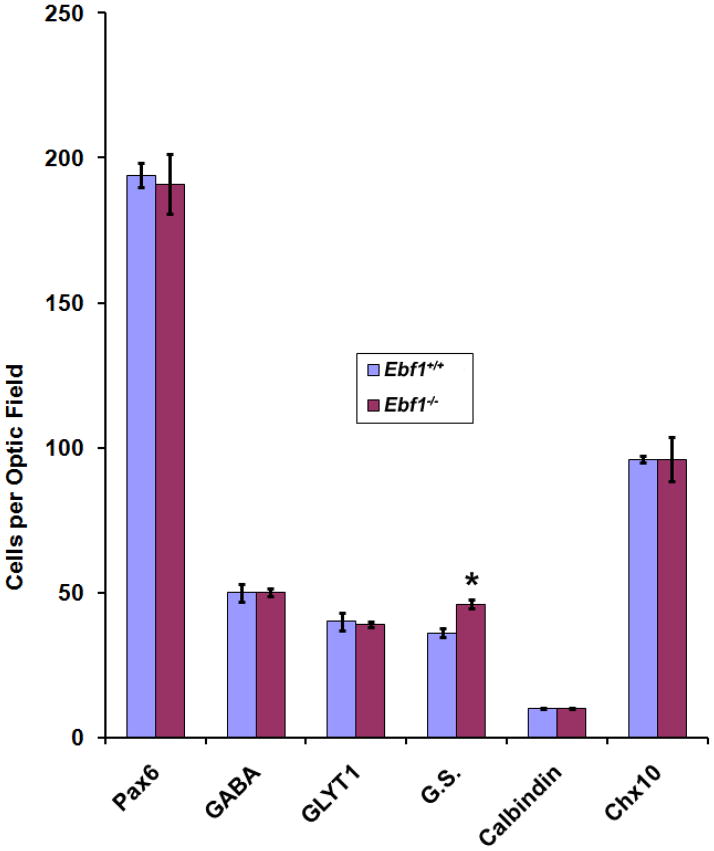
Since Ebf1 homozygous mutants die perinatally, we are unable to assess in vivo the development of late-born cell types in the mutant retina as most of them are not generated. To circumvent this problem, in vitro retinal explant culture was employed to examine any changes in cell fates and differentiation. P0 Ebf1+/+ and Ebf1−/− retinas were dissected and cultured on filters for 8 days. We examined markers for major cell types, for instances, Pax6 (Fig. 2A, B) for ganglion, horizontal and amacrine cells, GABA (Fig. 2E, F) for GABAergic amacrine cells, calbindin (Fig. 2G, H) for horizontal and some amacrine cells, and Chx10 (Fig. 2K, L) for bipolar cells. There was no significant difference in the number of cells immunoreactive for these markers between wild type and mutant retinas. We have shown previously that Ebf1 is expressed in the glycinergic amacrine cells and type 2 OFF-cone bipolar cells [24]. Using GLYT1 as a glycinergic amacrine cell marker and Bhlhb5 as a type 2 OFF-cone bipolar cell marker, we found that neither cell type was obviously affected in the mutant retina (Fig. 2C, D, I, J). However, the number of Müller cells labeled by glutamine synthetase increased by about 27% (Figs. 2M, N; 3), suggesting that Ebf1 normally suppresses the differentiation of Müller glial cells.
Fig. 2. Expression of cell markers in cultured P0 Ebf+/+and Ebf1−/− retinal explants.
(A–N) P0 Ebf+/+and Ebf1−/− retinal explants were cultured in vitro for 8 days and their sections were then immunolabeled with the indicated antibodies. (A, B) Anti-Pax6 stains amacrine, horizontal cells and RGCs. (C, D) Anti-GLYT1 stains glycinergic amacrine cells. (E, F) Anti-GABA stains GABAergic amacrine cells. (G, H) Anti-calbindin stains horizontal and some amacrine cells. (I, J) Anti-Bhlhb5 stains type 2 OFF-cone bipolar cells and GABAergic amacrine cells. (K, L) Anti-Chx10 stains bipolar cells. (M, N) Anti-glutamine synthetase (G.S.) stains Müller cells. There are no obvious changes in these markers except the increase of G.S.-immunoreactive Müller cells labeled by G.S. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar: J (for A–N), 50μm.
Fig. 3. Quantification of marker-positive cells in P0 Ebf1+/+ and Ebf1−/− retinal explants.
Marker- positive cells were scored and analyzed. There are no significant changes in these immunoreactive cell types between wild type and mutant retinas, except that G.S. Müller cells increase in the mutant. * p<0.05.
Ebf1 Inactivation Causes Aberrant RGC Axon Projection at the Optic Chiasm
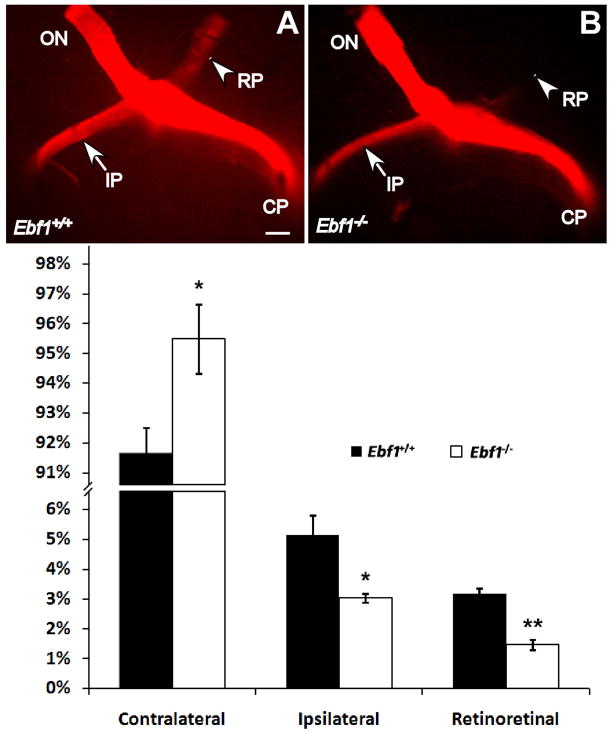
At the optic chiasm region, the majority of RGC axons cross the midline and project contralaterally to the other side of the brain; the remaining RGC axons (about 3–5% in the mouse) project ipsilaterally to the same side. Since Ebf1 and other Ebfs are involved in the axon guidance and projection in other tissues [27,28,29,30], [27,28,29,30], it’s possible that they may play similar roles in regulating the RGC axon projections in the retina and optic chiasm. As aforementioned, the intraocular projection is normal at P0 (Fig1O, P), indicating Ebf1 deficiency doesn’t affect RGC axon pathfinding and projection in the mutant retina. We then checked if the RGC axon projection was disturbed at the optic chiasm region in the P0 Ebf1−/− mice. At the optic chiasm of mutant mice, compared to the wild type, there are about 40% fewer RGC axons projecting ipsilaterally, and the RGC axons projecting retinoretinally to the contralateral optic nerve also decrease by more than 50% (Fig. 4); however, there is a significant increase of contralateral projection (Fig. 4). Thus, loss of Ebf1 function leads to abnormal topographic projection of RGC axons at the optic chiasm.
Fig. 4. RGC axon projection defects at the P0 Ebf1−/− optic chiasm.
(A, B) In P0 Ebf1−/− m ice, there are fewer RGC axons projecting to the ipsilateral direction at the optic chiasm (pointed by the arrow), and even less axons projecting retinoretinally to the contralateral optic nerve (pointed by the arrowhead). (C) Percentage of axon projections. *P<0.05, **p<0.01. CP, contralateral projection; IP, ipsilateral projection; ON, optic nerve; RP, retinoretinal projection. Scale bar: A (for A–B), 500μm.
Discussion
Ebf1 Influences Cell Specification and Differentiation in the Mouse Retina
Ebfs have been reported to influence cell differentiation and cell fates in lymphocyte genesis [22,23,31], in adipogenesis [20,21,32] and in neuronal differentiation [7,17,29,33], including specification of multiple retinal cell types and subtypes in the retinal development [24]. In the adult mouse retinas, Ebfs are expressed strongly in the RGCs, glycinergic amacrine cells, type 2 OFF-cone bipolar cells, and weakly in the horizontal cells; but not in GABAergic amacrine cells, photoreceptors, or Müller cells. Overexpression of Ebf1, Ebf2, or Ebf3 is sufficient to induce the glycinergic amacrine, type 2 OFF-cone bipolar, and horizontal cells in the mouse retina. Such effect can be successfully suppressed by the overexpression of a dominant-negative form Ebf1EnR [24].
In our current work, however, we found no obvious changes in glycinergic amacrine, bipolar and horizontal cells in Ebf1−/− mutants, which is not surprising. First, Ebf1, Ebf2, Ebf3 and Ebf4 have a near identical expression pattern and act redundantly in the retina [24], and it is highly possible that other Ebfs could compensate for the complete loss of Ebf1 in the Ebf1 null retina. Such a redundant role is evidenced by the observations in the brain. While there are olfactory neuron projection defects in Ebf2 and Ebf3 double heterozygous mice, the Ebf2 or Ebf3 single heterozygous mouse shows no such defects [27], since partial loss of Ebf2 can be compensated by Ebf3, and vice versa. Another evidence from the opposite angle is that, Ebf1−/− embryos show specific defects in the embryonic striatum, where Ebf1 is the only Ebf gene expressed [34]. Therefore, the loss of Ebf1 function in homozygous mutant retinas is very likely to be partly compensated by other Ebf factors in the neuronal differentiation process. Second, due to the limitation of the in vitro explant culture, there are a lot of cell deaths that would easily cover the minor differences between wild type and mutants. Using conditional knockout mouse of Ebf1 should overcome the limitation. To avert the redundant effect and fully understand the role of Ebfs in retinal cell development, mouse with compound knockouts of two or more Ebfs should be examined for any possible specification defects.
In the cultured Ebf1−/− retinal explant, there is a 27% increase of Müller cells compared to the wild type, indicating that Ebf1 normally inhibits the Müller cell fate during retinogenesis. This is consistent with our previous gain-of-function analysis showing that misexpressed Ebf1 has a potent activity to suppress Müller cell differentiation [24]. The increase of Müller cells in the mutants should be a non-autonomous effect, since none of the Ebf factors is expressed in the Müller cells [24]. In addition, the role of Ebf1 to bias a neuronal versus a glial cell fate is also shared by many other bHLH factors, for exam ples, Mash1[25], NeuroD [35,36], Math3[35,36], Math5[37], Ngn1 & Ngn2 [38], etc.
Ebf1 Regulates RGC Axon Guidance and Projection
In various neural tissues, Ebfs have been shown to play an important role in controlling axon guidance and pathfinding. In the C. Elegans, mutants of the Ebf homolog unc-3 have motor neuron axon pathfinding deficiency and display moving abnormality [30]. In the mouse olfactory bulb, knockout of Ebf2 or Ebf3 causes defects in olfactory axon projection [27]. In the cerebellum, null mutation of Ebf2 leads to Purkinje cell migration defect [33]. Ebfs are expressed in newborn RGCs and continue their expression throughout the adult stage, suggesting that Ebfs may be involved in RGC axon guidance and projection, and other RGC routine functions. Our DiI tracing experiment demonstrated that Ebf1 does regulate the RGC axon projection.
Ebf1 null retina, the RGC axon fibers form normally, as seen by NF150 staining of P0 retinas (Fig1P). There are no obvious defects in the optic fissure or optic disk, and optic nerves exit the eyes normally. However, abnormal RGC axon projections do occur at the optic chiasm in Ebf1 null mice. Compared to the wild type, Ebf1 mutants exhibit more contralateral projection of RGC axons but less ipsilateral projection. The retinoretinal projection is also greatly reduced in the Ebf1 mutant, a phenotype contrary to Slit1/Slit2 double knockout mice[10], and EXT1 null mice [39], suggesting Ebf1 has an opposing role against Slit1/Slit2 and EXT1 in regulating RGC axon projection. On the other hand, transcription factors Zic2 [14,15], Foxd1 [16]and Foxg1 [40,41] are the crucial proteins in regulating the ipsilateral and contralateral projections. Microarray analysis should give clues how these factors are affected in the Ebf1 mutants.
HILIGHTS.
Müller cell increase
aberrant ipsilateral projection
aberrant retinoretinal crossing
Acknowledgments
We thank members of the Xiang lab for valuable discussion of the project and thoughtful comments on the manuscript. This work was supported by the National Institutes of Health Grants EY012020 and EY020849 (to M.X.).
Footnotes
Competing Interests statement
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 2.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 3.Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 4.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 5.Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci. 2008;31:295–315. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- 6.Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Pratt T, Conway CD, Tian NM, Price DJ, Mason JO. Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. J Neurosci. 2006;26:6911–6923. doi: 10.1523/JNEUROSCI.0505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunt SM, Lund RD. Development of a transient retino-retinal pathway in hooded and albino rats. Brain Res. 1981;211:399–404. doi: 10.1016/0006-8993(81)90712-5. [DOI] [PubMed] [Google Scholar]
- 9.McLoon SC, Lund RD. Transient retinofugal pathways in the developing chick. Exp Brain Res. 1982;45:277–284. doi: 10.1007/BF00235788. [DOI] [PubMed] [Google Scholar]
- 10.Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slitl and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 11.Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphBl mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25:599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelina P, Avci HX, Thelen K, Pollerberg GE. The cell adhesion molecule NrCAM is crucial for growth cone behaviour and pathfinding of retinal ganglion cell axons. Development. 2005;132:3609–3618. doi: 10.1242/dev.01934. [DOI] [PubMed] [Google Scholar]
- 14.Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114:545–557. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee R, Petros TJ, Mason CA. Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphBl. J Neurosci. 2008;28:5910–5919. doi: 10.1523/JNEUROSCI.0632-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera E, Marcus R, Li S, Williams SE, Erskine L, Lai E, Mason C. Foxdl is required for proper formation of the optic chiasm. Development. 2004;131:5727–5739. doi: 10.1242/dev.01431. [DOI] [PubMed] [Google Scholar]
- 17.Garel S, Marin F, Mattei MG, Vesque C, Vincent A, Charnay P. Family of Ebf/Olf-l-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210:191–205. doi: 10.1002/(SICI)1097-0177(199711)210:3<191::AID-AJA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Bally-Cuif L, Dubois L, Vincent A. Molecular cloning of Zcoe2, the zebrafish homolog of Xenopus Xcoe2 and mouse EBF-2, and its expression during primary neurogenesis. Mech Dev. 1998;77:85–90. doi: 10.1016/s0925-4773(98)00144-0. [DOI] [PubMed] [Google Scholar]
- 19.Dubois L, Bally-Cuif L, Crozatier M, Moreau J, Paquereau L, Vincent A. XCoe2, a transcription factor of the Col/Olf-1/EBF family involved in the specification of primary neurons in Xenopus. Curr Biol. 1998;8:199–209. doi: 10.1016/s0960-9822(98)70084-3. [DOI] [PubMed] [Google Scholar]
- 20.Akerblad P, Mansson R, Lagergren A, Westerlund S, Basta B, Lind U, Thelin A, Gisler R, Liberg D, Nelander S, Bamberg K, Sigvardsson M. Gene expression analysis suggests that EBF-l and PPARgamma2 induce adipogenesis of NIH-3T3 cells with similar efficiency and kinetics. Physiol Genomics. 2005;23:206–216. doi: 10.1152/physiolgenomics.00015.2005. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 23.Romanow WJ, Langerak AW, Goebel P, Wolvers-Tettero IL, van Dongen JJ, Feeney AJ, Murre C. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell. 2000;5:343–353. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 24.Jin K, Jiang H, Mo Z, Xiang M. Early B-cell factors are required for specifying multiple retinal cell types and subtypes from postmitotic precursors. J Neurosci. 2010;30:11902–11916. doi: 10.1523/JNEUROSCI.2187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita K, Nakanishi S, Guillemot F, Kageyama R. Mash1 promotes neuronal differentiation in the retina. Genes Cells. 1996;1:765–774. doi: 10.1111/j.1365-2443.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Wang SS, Lewcock JW, Feinstein P, Mombaerts P, Reed RR. Genetic disruptions of O/E2 and O/E3 genes reveal involvement in olfactory receptor neuron projection. Development. 2004;131:1377–1388. doi: 10.1242/dev.01009. [DOI] [PubMed] [Google Scholar]
- 28.Garel S, Yun K, Grosschedl R, Rubenstein JL. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development. 2002;129:5621–5634. doi: 10.1242/dev.00166. [DOI] [PubMed] [Google Scholar]
- 29.Giacomini C, La Padula V, Schenone A, Leandri M, Contestabile A, Moruzzo D, Goutebroze L, Consalez GG, Benfenati F, Corradi A. Both Schwann cell and axonal defects cause motor peripheral neuropathy in Ebf2−/− mice. Neurobiol Dis. 2011;42:73–84. doi: 10.1016/j.nbd.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Prasad BC, Ye B, Zackhary R, Schrader K, Seydoux G, Reed RR. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development. 1998;125:1561–1568. doi: 10.1242/dev.125.8.1561. [DOI] [PubMed] [Google Scholar]
- 31.Nieminen P, Liippo J, Lassila O. Pax-5 and EBF are expressed in committed B-cell progenitors prior to the colonization of the embryonic bursa of fabricius. Scand J Immunol. 2000;52:465–469. doi: 10.1046/j.1365-3083.2000.00821.x. [DOI] [PubMed] [Google Scholar]
- 32.Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-cell factor (O/E-1) is a Promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol. 2002;22:8015–8025. doi: 10.1128/MCB.22.22.8015-8025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, Corradi A, Rossi F, Hawkes R, Consalez GG. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–2729. doi: 10.1242/dev.02437. [DOI] [PubMed] [Google Scholar]
- 34.Garel S, Marin F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126:5285–5294. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- 35.Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- 36.Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498. doi: 10.1074/jbc.M400871200. [DOI] [PubMed] [Google Scholar]
- 37.Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 38.Cai L, Morrow EM, Cepko CL. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- 39.Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 40.Pratt T, Tian NM, Simpson TI, Mason JO, Price DJ. The winged helix transcription factor Foxg1 facilitates retinal ganglion cell axon crossing of the ventral midline in the mouse. Development. 2004;131:3773–3784. doi: 10.1242/dev.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian NM, Pratt T, Price DJ. Foxgl regulates retinal axon pathfinding by repressing an ipsilateral program in nasal retina and by causing optic chiasm cells to exert a net axonal growth-promoting activity. Development. 2008;135:4081–4089. doi: 10.1242/dev.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]