Abstract
We showed that when IGF-II is highly expressed in breast tissues and cell lines the IGF-1 receptor signaling pathway is highly activated. Since IGF-II activates the insulin receptor (INSR), we propose that the INSR signaling is also activated in this system. We examined the expression of both INSR isoforms, INSR-A, INSR-B, and the downstream signaling pathways in breast cancer cells and in paired (normal/tumor) breast tissues from 100 patients. Analysis was performed by Real Time-PCR, Western blot, immunohistochemistry and Phospho-ELISA techniques. Tumor tissues and cell lines from African American patients (AA) expressed higher levels of INSR-A, but lower levels of INSR-B. In accordance, IRS-1 and FAK activation were significantly increased in these women. We conclude that higher INSR-A and lower INSR-B contributes to higher proliferation and lower metabolic response. Thus, differential expression of INSR isoforms represents a potential biological link between breast cancer and diabetes.
Keywords: Breast cancer, IGF, Insulin receptor A, Insulin receptor B, Cell signaling
Introduction
Mortality rates from breast cancer remain higher in African-American (AA) women despite a lower incidence when compared to Caucasian (CA) women. Multiple factors including differences in access to health care, disparate utilization of screening tools as well as biologic factors are being explored as causative agents. We are addressing this concern by studying the insulin receptor expression and the IGF-II activation of this receptor signaling pathway in breast cancer cell lines and in paired breast tissues from AA and CA patients.
IGF-II plays a key role in the regulation of growth and metabolism as well as in the initiation and maintenance of breast tumors (Belfiore, 2007). High IGF-II levels increases breast cancer (BC) susceptibility and stimulates tumor growth and progression by signaling through the IGF-I and Insulin receptors (Morrione et.al., 1997; Sciacca et.al., 1999). We have recently shown that tumor tissues and cell lines established from AA BC patients express significantly higher levels of IGF-II and higher IGF-I receptor activation as compared to CA (Kalla Singh et.al, 2010a; Kalla Singh et.al, 2010b). Since IGF-II also activates the insulin receptor (INSR), we propose that higher IGF-II levels among AA BC patients translates into higher INSR activation.
The insulin receptor (INSR) is a heterotetrameric protein consisting of two extracellular α-subunits (ligand binding) and two transmembrane β-subunits (tyrosine kinase domain) that can be activated by insulin or IGF-II (Avruch, 1998; Kao et.al., 1997; Louvi et.al., 1997; Myers and White et.al., 1996). Alternative splicing of exon 11 of the INSR gene results in two transcripts, INSR-A and INSR-B (Moller et.al., 1989). INSR-A is activated by IGF-II and insulin to promote mitogenic and antiapoptotic signals (Frasca et.al., 1999). In contrast, IGF-I does not bind the INSR-A. INSR-B activation induces cell metabolism and differentiation signals (Sciacca et.al.,, 2003).
The activated INSR tyrosine kinase phosphorylates several intracellular substrates including INSR substrates 1–4 (IRS-1–4) and the focal adhesion kinase (FAK). IRS-1 has been associated with cell proliferation, inhibition of differentiation and malignant transformation through activation of the PI3K pathway (D’Ambrosio et.al.,1995). FAK is involved in the integrin and growth factor receptor signaling pathway and when FAK is phosphorylated it activates the Ras/ERK pathway (Schlaepfer et.al., 1994). Therefore, the present study focuses on the differential expression of the INSR isoforms (A and B) and how IGF-II expression regulates the phosphorylation and activation of the INSRs as part of the mechanisms associated with the more aggressive breast tumors and increased mortality observed in AA women.
Materials and Methods
Breast tissue specimens (normal and malignant)
Paired frozen breast cancer and normal tissue specimens (M=breast cancer; N=normal adjacent) were obtained from the Cooperative Human Tissue Network (CHTN) from AA and CA women with ages ranging from 20–90 years (median age per group was AAM=60.2 years, CAM=60.1 years, AAN=51.9 years and CAN=62.7 years). All malignant samples were stage II or III (Bloom and Richardson’s) infiltrating ductal carcinomas (IDC) or papillary carcinomas (AAM stage II n=10, stage III n=17; CAM stage II n=12, stage III n=12). Total number of samples (n) per group is as follows: AAN= 23, CAN= 20, AAM= 27 and CAM= 24. Non-stained slides were also obtained with the tissue specimens as well as a pathology report containing information about patient age, macroscopic and microscopic characteristics of normal and tumor tissues, estrogen/progesterone/Her2 receptors status, and tumor grade/stage and metastases sites.
Cell culture
Cell lines established from tumors of CA women [MCF-7 (ER+PR+), Hs578t (ER-PR-)] and AA women [CRL-2335 (ER-PR-) and CRL-2329 (ER+PR+)] were obtained from the American Type Culture Collection (ATCC). Cells were maintained in a 5% CO2 incubator at 37°C, using DMEM/F12 or RPMI media (ATCC) supplemented with 10 ml of 5,000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro), 4 mM L-glutamine (Cellgro), 3 μg/ml β-amphotericin, and 10% fetal bovine serum (Hyclone). Recombinant human precursor IGF-II (proIGF-II, aa 1–156, non-glycosylated), recombinant mature IGF-II and bovine insulin were purchased from GroPep (Adelaide, Australia), PeproTech (Rocky Hill, NJ) and Sigma-Aldrich Corp. (St. Louis, MO) respectively. Cell lysates (CL) were collected, centrifuged (800 rpm for 5 min), and kept frozen (−20°C) until assayed.
Western blot analysis
Total protein (30 μg) of tissue cell lysates were prepared in RIPA buffer [1XTBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, 10 μl/ml PMSF (stock concentration of 10 mg/ml), 10 μl/ml protease inhibitor cocktail (stock concentration of 10 mg/ml), 10 μl/ml sodium orthovanadate(100mM)] and used to load polyacrylamide-SDS gradient gels (4–12%), transferred to a PVDF membrane (Invitrogen, Carlsbad, CA) using a X-Cell SureLockR electrophoretic Transfer module (Invitrogen, Carlsbad, CA). Protein concentration was measured using the Coomassie Plus Protein Assay Reagent™ (Pierce Biotechnology, Rockford, IL). PVDF membranes were blocked with 1% BSA IgG free (Sigma Chemical Co., St. Louis, MO) in PBS/0.05% Tween for 2 hrs. Membranes were then incubated overnight (4°C)with anti-INSR α-subunit (5D9) rabbit polyclonal antibody that recognizes INSR-A and INSR-B [Kindly provided by Dr. R. Roth (Stanford University, Stanford, CA)], and purified rabbit polyclonal anti-human INSR-B antibody (YenZym antibodies, LLC). The INSR-B antibody was produced by immunizing one rabbit (Y324) with an INSR aa peptide corresponding to the product of exon 11 (743–756, CPRKTSSGTGAEDPR-amide). The blots were also probed with cytokeratin 18 monoclonal antibody (tissue samples) or β-actin monoclonal antibody [(cell lines), (Santa Cruz Biotechnology, CA)], used as epithelial cell marker and loading control respectively. After 3 × 10 min washes in PBS/0.05% Tween, the corresponding biotinylated secondary antibodies (1:1000, Amersham, Arlington Heights, IL) were added to the membranes (1 hr at RT), followed by 3 × 10 min washes and incubation with HRP complexes (1:1000 Amersham, Arlington Heights, IL). Protein visualization was achieved by using enhanced chemiluminescence (ECL) and autoradiography with Hyperfilm ECL film (Amersham, Arlington Heights, IL). The signals on the x-ray films were quantified using ChemiImager™ 4000 (Alpha Innotech Corporation).
RNA extraction
Total RNA was extracted using Tri reagent (Molecular Research Center, Cincinnati, OH) according to manufacturer’s protocol. 40–70 mg of frozen tissue per sample was homogenized in the Tri reagent by using a hand-held homogenizer (Kontes Thomas Scientific, NJ). Total RNA was kept at −80° C until assayed.
Real Time PCR
One Step SYBR real-time RT-PCR was performed to assess INSR-A mRNA expression (For- 5′-GCT GAA GCT GCC CTC GAG GA-3′ and Rev- 5′-CGA GAT GGC CTG GGG ACG AA-3′) INSR-B (For-5′-GCT GAA GCT GCC CTC GG GA-3′ and Rev- 5′-AGA TGG CCT AGG GTC CTC GG-3′), GAPDH was used as an internal control (For-5′-ACA ACT TTG GTA TCG TGG AAG GAC-3′ and Rev- 5′-CAG GGA TGA TGT TCT GGA GAG C-3′).
PCR amplifications were performed using the iCycler (BIO-RAD). Reactions were performed in a mixture consisting of a 50 μL volume solution containing 1X SYBR Green supermix PCR buffer (BIO-RAD), (100mM KCL, 6 mM MgCl2, 40mM Tris-HCL, PH 8.4, 0.4mM of each dNTP [dATP, dCTP, dGTP and dTTP], iTaq DNA Polymerase 50 U/mL, SYBR Green I, 20mM Fluorescein) 300 nM of each primer, 0.25U/mL MultiScribe Reverse Transcriptase (Promega) and 0.4U/mL RNAse Inhibitor (Promega). The RT-PCR protocol starts with 30 min at 42°C for the RT. Prior to the PCR step iTaq DNA polymerase activation at 95 °C for 10 min was performed. Followed by 30 sec denaturation at 95 °C, 15 sec annealing at 57 °C and 1.5 min elongation at 72 °C for 40 cycles. Fluorescence was detected at the end of every 72°C extension phase. To exclude the contamination of non-specific PCR products such as primer dimers, melting curve analysis was applied to all final PCR products after the cycling protocol.
Immunohistochemistry
INSR immunohistochemical analysis was performed using two different antibodies. Purified rabbit polyclonal anti-INSR α-subunit (5D9) [Kindly provided by Dr. R. Roth (Stanford University, Stanford, CA)], recognizes the α-subunit of INSR-A and INSR-B. Purified rabbit polyclonal anti-human INSR-B antibody recognizes the α-subunit C-terminal of INSR-B (Y324). The Y234 antibody was produced by immunizing one rabbit with the INSR aa peptide corresponding to the product of exon 11 present only in the INSR-B (743–756, CPRKTSSGTGAEDPR-amide (YenZym antibodies). 5 μm tick paraffin block sections were de-waxed, rehydrated and treated with 1X antigen retrieval solution (Reveal, Biocare Medical). After endogenous peroxidase blocking (H2O2 3%), slides were incubated in corresponding primary antibodies (1:10–1:50) with blocking serum (mouse or rabbit ABC staining Systems, Santa Cruz Biotechnology) overnight at 4°C. The antibodies were revealed with the corresponding anti-mouse or anti-rabbit biotinylated antibodies for 30 min at room temperature, followed by streptavidin/peroxidase label (30 min at room temperature) using diaminobenzidine (DAB) chromogen as a substrate. The samples were counterstained with hematoxylin, dehydrated and mounted. Tissue sections were washed with 1X PBS between each immunostaining step.
Phosphorylation studies
MCF-7, Hs578T, CRL-2335 and CRL-2329 cells were plated at a density of 2 X 106 cells per well in 10 mm petri dishes, and grown in serum free media (SFM). Cells were treated with IGF-II (precursor or mature forms, 100 ng/ml) and/or insulin (100 nM). Total cell lysates were prepared as previously described, 20 min post-treatment. PVDF membranes were incubated with antibodies against IRS-1/phosphoIRS-1 (Y632) and FAK/pFAK (Y925) (Santa Cruz Biotechnology). The blots were also probed with β-actin, used as a protein loading control.
INSR phosphorylation was assessed by ELISA. The PathScan® phospho-insulin receptor (Y 1150/1151) sandwich ELISA kit (Cell Signaling) was used. Tissue lysates were prepared using 50–80 mg of frozen tissue per sample and homogenized in 1X lysis buffer (20 mM Tris-HCL pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) by using a hand-held homogenizer (Kontes, Thomas Scientific, NJ). Total tissue lysates were kept at −80° C until assayed. 100 μl of tissue lysate (125 μg total protein concentration) were diluted in 100 μl of sample diluent and transferred to the INSR-coated microwells for overnight incubation at 4°C, followed by incubation in detection antibody. HRP-linked secondary antibody was added for 30 min. The reaction was visualized by addition of TMB substrate. Spectrophotometric determination was read at 450 nm. Wells were washed 4 times with 1X wash buffer in between steps. Western blots were performed using an anti-phosphoINSR/IGF1R antibody (R&R Systems) as previously described.
Statistical analysis
Statistical differences between mean values were determined by using one-way ANOVA (for protein expression analysis between all groups), paired T test (for comparison between paired normal and tumor samples) and independent T test (for comparison between AA-CA samples) by using the SPSS 17.0 software (SPSS, Inc., Chicago, IL). *Values are expressed as the mean ± SEM of three or more replicate experiments. A level of P < 0.05 was considered significant.
Results
Insulin receptor (INSR) expression and phosphorylation in breast cancer cell lines
We analyzed INSR protein expression in breast cancer cell lines derived from African-American (AA) and Caucasian (CA) women (MCF-7, Hs578T, CRL-2329 and CRL-2335). Two INSR antibodies were used; antibody 5D9 (recognizes INSR-A and INSR-B) and Y324 (INSR-B only). As seen on figures 1A and 1B, estrogen receptor positive (ER+) cell lines (MCF-7 and CRL-2329) and the AA ER (−) cell line CRL-2335 expressed higher levels of INSR protein than the CA ER (−) Hs587T (*p<0.05). In contrast, CA cell lines expressed significantly higher levels of INSR isoform B (^p>0.05) as compared to AA breast cancer cell lines.
Figure 1.
INSR (5D9 antibody, anti-INSR-B and anti-phosphoINSR Y1162/Y1163) protein expression in African-American and Caucasian breast cancer cell lines [CRL-2329 (ER+), CRL-2335 (ER−), Hs578T (ER−) and MCF-7(ER+)] assessed by western blot analysis for comparison purposes (1A). Lower panel shows bar graph of INSR, INSR-B and phosphorylated INSR data normalized to β-actin and presented as the mean ± SE of all samples per group. Asterisks and Carets indicate values statistically different (*p<0.05, **p<0.01, ^p<0.03).
Next, we proceeded to analyze basal levels of INSR phosphorylation at tyrosine 1162/1163 which stimulates receptor intrinsic kinase activity. As seen on figure 1A, phosphorylation of the INSR was significantly higher in the AA ER(+) CRL-2329 cells as compared with the CA ER(+) MCF-7 cells (*p<0.05). Furthermore, no basal levels of INSR phosphorylation was detected in the CA ER (−) Hs578T cells. Figure 1B shows bar graph representation of three or more experiments conducted for each cell line studied. β-actin was used as loading control. Please notice that although only one western blot is depicted as a representative experiment, the bar graphs represent three separate experiments done at least in triplicate.
IRS-1and FAK phosphorylation in AA and CA breast cancer cell lines treated with precursor IGF-II (proIGF-II), mature IGF-II (mIGF-II) and insulin
We have previously shown that AA breast cancer cell lines and tissue samples express higher levels of precursor IGF-II than their CA counterparts. To gain further insights on the molecular mechanisms differentially activated by either IGF-II (mature and precursor) and insulin in the AA and CA breast cancer cell lines, we proceeded to analyze insulin receptor substrate-1 [(IRS-1 at Y632)] and focal adhesion kinase [(FAK at Y925)] phosphorylation at 0, 10 (data not shown) and 20 minutes after IGF-II or insulin treatment (100 ng/ml mature or precursor IGF-II and/or 10 nM insulin). As seen on figure 2A, MCF-7 cells expressed significantly higher IRS-1 protein levels (*p<0.05) than the other three cell lines studied, while, Hs578T cells expressed the lowest IRS-1 protein levels. Figures 2B-2E examine the effect of IGF-II and Insulin treatment on the IRS-1 and FAK protein levels and phosphorylation. No significant changes were seen in IRS-1 phosphorylation in the ER (−) cell lines Hs578T cells and CRL-2335 as compared to control groups (fig. 2D–E). In contrast, IRS-1 phosphorylation was significantly increased in MCF-7 cells treated with IGF-II (proIGF-II or mIGF-II) as compared to control and insulin treated groups (fig. 2B). Phosphorylation of IRS-1 in the AA ER (+) cell line CRL-2329 was increased in all treatment groups except with mIGF-II alone, as compared to control (fig. 2C). AA cell lines (CRL-2329 and CRL-2335) expressed higher levels of total FAK as compared with CA cell lines (MCF-7 and Hs578T *p<0.05). Among ER (−) cells, a significant increase in FAK phosphorylation was observed in the CRL-2335 with IGF-II (mature and precursor) and insulin co-treatment (fig. 2E). No changes in FAK phosphorylation was observed in Hs578T cells after IGF-II and/or insulin treatment as compared to control group (fig. 2D). On the contrary, no increased phosphorylation of FAK (Y925) occurred in ER (+) cells treated with insulin and or proIGF-II, while mIGF-II and insulin co-treatment induced a significant decrease in FAK phosphorylation (figures 2B–C).
Figure 2.
Total and phosphorylated IRS-1 (Y632), FAK (Y925) western blots from CRL-2329, CRL-2335, Hs578T and MCF-7 breast cancer cells. (A) shows IRS-1 and FAK western blot in AA and CA breast cancer cells for comparison purposes. (B–E) shows total and phosphorylated IRS-1 and FAK 20 minutes without any treatment (control) or after treatment with precursor or mature IGF-II (pro- or mIGF-II, 100 ng/ml) or insulin (100 nM). β-actin was used as loading control.
Insulin receptor (INSR) mRNA expression in AA and CA breast cancer tissue samples
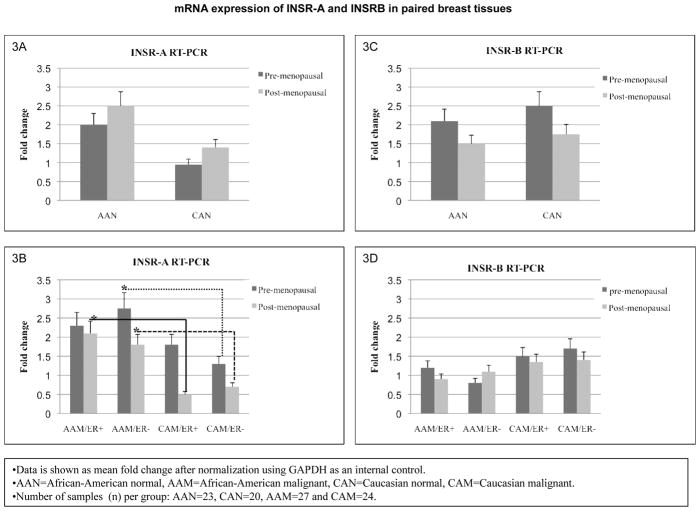
In order to compare INSR expression and phosphorylation in cell lines with breast tissues obtained from AA and CA women, we proceeded to analyze total INSR (isoforms A and B) expression by RT-PCR in breast tissues. As seen in figures 3A–B, pre- and post-menopausal AAN tissue samples expressed higher levels of INSR-A isoform, while INSR-B mRNA levels were slightly elevated in CAN tissues as compared to AA samples. These changes did not reach statistical significance. Post-menopausal ER (+) AAM tissues and pre- post-menopausal (ER−) AAM samples expressed significantly higher levels of INSR-A as compared to the corresponding CAM samples [(*p<0.03), (fig. 3C)]. Although CAM samples (pre- and postmenopausal) expressed more INSR-B mRNA, the changes did not reach statistical significance (fig. 3D).
Figure 3.
INSR gene expression in African-American and Caucasian paired breast tissue samples assessed by Real Time-PCR (RT-PCR). (A–D) shows INSR-A and INSR–B gene expression represented as fold change after normalization using GAPDH as an internal control. (A–B) show INSR-A and INSR-B mRNA fold change between AAN and CAN for pre- and post-menopausal samples. (C–D) show INSR-A and INSR-B mRNA fold change between ER (+) and ER (−) AAM and CAM pre- and post-menopausal samples. AAN = African-American normal tissue, AAM = African-American malignant tissue, CAN = Caucasian normal tissue and CAM = Caucasian malignant tissue. Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
Insulin receptor (INSR) protein expression in AA and CA breast tissue samples
We next proceeded to analyze total INSR (isoforms A and B) and INSR-B expression in breast tissue samples by western blot. Two distinct antibodies were used; the insulin receptor antibody 5D9 recognizes the α-subunit of INSR-A and INSR-B; while Y324 recognizes aa 743–756 of the INSR-B (corresponding to the exon 11 product). As seen on figure 4A (upper panel) pre-menopausal ER (−) AAM expressed significantly higher levels of INSR protein (p*<0.03) when compared to pre-menopausal ER(−) CAM samples. Furthermore, pre-menopausal CAN and (ER−) CAM samples expressed significantly higher levels of INSR-B isoform as compared to AAN samples (*p<0.03) and ER(−) AAM (*p<0.05) respectively. Figure 4B shows INSR expression in post-menopausal AA and CA women, were INSR-A expression was found to be increased in ER(+) AAM samples as compared to CAM tissues (p*<0.05). INSR-B protein levels were significantly higher in CAM samples as compared to AAM breast tissue samples [(ER+ and ER−), (p*<0.05)]. Cytokeratin 18 was used as an epithelial cell marker. Lower panels (figures 4A–B) show bar graphs of densitometry units on three separate experiments per each group (AAN, AAM, CAN and CAM).
Figure 4.
Representative Western blots analyses of INSR (incubated with 5D9 and anti-INSR-B antibodies) in paired tissue samples from African-American (AA) and Caucasian (CA) women. The samples were separated into pre-menopausal (figure 4A) and post-menopausal (figure 4B) groups. Immunoreactive bands for INSR and cytokeratin 18 were identified using ECL, scanned by densitometry and normalized to cytokeratin 18. The 135 kDa band represents INSR α-subunit. Cytokeratin 18 was used as an epithelial cell marker (45 kDa). Lower panels (4A–B) show bar graphs of INSR (total and B isoform) data normalized to cytokeratin 18 and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically different (*p<0.0, **p<0.03). Total number of patients (n) analyzed per group was as follows: AAN= 23, AAM=27, CAN=20 and CAM=24.
INSR, IRS-1 and FAK phosphorylation in AA and CA breast tissue samples
IGF-II and insulin effects through the INSR-A are mitogenic in contrast to insulin metabolic effects mediated by INSR-B. We proceeded to analyze INSR phosphorylation by using the PathScan® phospho-insulin receptor (Y 1150/1151) sandwich ELISA kit. As seen on figures 5A and 5B, INSR phosphorylation was significantly increased in ER(−) pre- and post-menopausal AAM samples as compared to ER(−) CAM samples. Similarly, a statistically significant higher INSR phosphorylation was observed in pre-menopausal AAN tissues in contrast to CAN tissue samples (fig. 5A). Thus, overall, INSR activation is significantly higher in breast tissues obtained from AA as compared to CA tissues. Similarly, INSR phosphorylation in ER(−) AAM tissues is significantly higher as compared to CAM tissues. In contrast, when the Elisa INSR activation results (Fig. 5A–B) are compared to the results obtained by Western blot analyses (Fig.5C) it shows that tissues from AA with the lowest INSR-B show the highest phorphorylation activity suggesting that the Elisa results predominantly represent the activation of INSR-A in this AA group. This implies that activation of INSR-A by insulin and IGF-II is predominantly mitogenic in AA women in contrast to more metabolic effects elicited in CA women that express higher levels of INSR-B.
Figure 5.
INSR phosphorylation study using the PathScan® phospho-INSR (Y1150/Y1151) ELISA kit. Figures 5A–B show a bar graph representation of INSR phosphorylation measured as OD 450 for all samples per group (AAN= 23, AAM=27, CAN=20 and CAM=24). Asterisks indicate values statistically significant (*p<0.05). Figure 5C shows representative western blot analyses of total and phosphorylated INSR, IRS-1 and FAK proteins. Figures 5D–G show bar graphs of phosphorylated IRS-1 and FAK normalized to total IRS-1 and FAK proteins in pre- and post-menopausal breast tissue samples, and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically significant (*p< 0.05).
INSR autophosphorylation induces activation of different downstream signaling pathways leading to metabolic or mitogenic effects. Thus, we analyzed the phosphorylation of insulin receptor substrate-1 (IRS-1) and the focal adhesion kinase (FAK). Figure 5C shows a representative Western blot of INSR downstream activation of IRS-1 and FAK in AA and CA breast tissue samples. First row (INSR) depicts the 130kDa INSR (non-phosphorylated) as assessed by using the 5D9 antibody that recognizes both INSR-A and INSR-B from pre-menopausal (left panel) and post-menopausal breast tissues as indicated. Second row (INSR) depicts the 130kDa INSR-B (non-phosphorylated) as assessed by using the antibody that specifically recognizes INSR-B developed from a peptide region absent in the INSR-A. Significantly increased IRS-1 phosphorylation is shown in pre-menopausal AAN and AAM (ER+ and ER−) samples as compared to CAN and CAM samples respectively. No difference in IRS-1 phosphorylation was seen in post-menopausal women.
Figure 5D–G show bar graphs of phosphorylated IRS-1 and FAK normalized to total IRS-1 and FAK proteins in pre- and post-menopausal breast tissue samples, and presented as the mean ± SE of all samples per group. Asterisks indicate values statistically significant (*p< 0.05). FAK, a ubiquitously expressed cytoplasmic tyrosine kinase, undergoes rapid phosphorylation in response to integrin and IGFs signaling. FAK phosphorylation at Y925 activates the Ras/ERK pathway. A significant increase in FAK phosphorylation was shown in pre-menopausal AA malignant tissue (ER+ and ER−) in comparison with CAM samples (fig. 5B). This effect was also seen in post-menopausal women ER (−) tissue samples.
To further confirm the expression of the insulin receptors identified by Western blot, we proceeded to assess their expression by immunohistochemistry. As seen on fig. 6, we stained paired normal and tumor breast tissues from African-American and Caucasian patients utilizing two distinct insulin receptor antibodies; INSR-A/B (5D9) which recognizes both insulin receptors A and B and INSR-B specific for insulin receptor B only. This picture is representative of 15 AA paired samples (normal/tumor) and 13 Caucasian paired samples (normal/tumor). Comparison of INSR-B staining between normal samples from African-American patients (panels C–D) and normal samples from Caucasian patients (panels K–L) demonstrated that expression of the INSR-B is higher in both, pre and post-menopausal Caucasian normal samples. Likewise, staining of the INSR-B was higher in the malignant pre and post-menopausal samples (panels O–P) from Caucasian patients (n=13) in contrast to the lower levels of INSR-B observed in the AA (n=15) malignant tissues (panels G–H). This indicates that AA breast cancer patients have a lower metabolic response to insulin and IGF-II in contrast to Caucasian patients. Interestingly, when the insulin receptor antibody 5D9 is used for immunostaining (recognizes both INSR-A and INSR-B) we detected stronger staining in the normal (panels A–B) and malignant (panels E–F) tissues of AA patients in contrast to Caucasian patients (panels I, J, M, N). Since specific staining for INSR-B (right panel) was lower for AA, we interpreted that higher staining obtained by the use of 5D9 antibody (INSR-A and INSR-B reflected higher level of INSR-A in AA breast tissues. These results suggest that AA breast cancer patients have a higher proliferative response to insulin and IGF-II in contrast to Caucasian patients whose tissues express lower levels of INSR-A.
Figure 6.
Immunohistochemistry of INSRs in paired human normal and malignant breast tissue samples. A comparison of the INSRs expression was assessed by utilizing an antibody that recognizes both receptors INSRA & INSRB (INSR α-subunit; (5D9) and a polyclonal antibody that only recognizes INSRB (see materials section). Malignant AA and CA samples correspond to ER (+) invasive ductal carcinomas (IDC) Bloom and Richardson’s grade III. Panels A–B corresponds to INSR immunostaining from AAN samples (pre- and post-menopausal). Panels E–H corresponds to INSR immunostaining from AAM samples. Panels I–L and M–P correspond to INSR immunostaining, in CAN and CAM samples respectively. Original magnifications 20X. Total number of patients (n) analyzed per group was as follows: AAN= 15, AAM=15, CAN=13 and CAM=13.
Discussion
Breast cancer etiology and epidemiology is complex and influenced by a myriad of environmental, lifestyle, genetic, socioeconomic and cultural factors. For African-American (AA) women these various elements converge to yield the paradoxical patterns of a relatively lower breast cancer incidence, higher mortality rate and younger age distribution as compared to Caucasian (CA) women. In spite of considerable efforts to identify the source for the survival disparities observed among AA breast cancer patients, there is limited knowledge that can be translated into effective interventions to reduce mortality. In fact, overall survival for AA breast cancer patients as compared to CA women is lower despite adjustments for uniform stage, treatment and follow-up in randomized phase III clinical trials (L.A. Carey et.al., 2006,K.S. Albain et.al., 2009). To address this challenging paradox, we have studied IGF-II and its signaling pathways to determine whether it plays a role in the increased breast cancer mortality observed among AA patients.
We successfully identified IGF-II as an important biological factor that may contribute to higher breast cancer mortality in AA women (Kalla Singh et.al., 2010a). Of significance, IGF-II expression and regulation of Bcl-XL and Survivin in cell lines correlated with their expression in paired breast tissues. We also demonstrated that IGF-II siRNA treatment or IGF-II stable antisense transfection completely inhibited Survivin expression in breast cancer cells (Kalla Singh et.al., 2008). Both, cell lines and paired breast tissues from AA patients expressed significantly higher levels of Survivin than their CA counterparts (Kalla Singh et.al., 2010a). Higher Survivin levels in breast cancer patients predicts poor clinical outcome and reduced survival (B. M. Ryan et.al., 2006). Similarly, we demonstrated that there is a significant differential expression of IGF1R and IGF2R as well as increased IGF-II signaling activation in both cells and tissues that may result in higher tumor growth rate and chemoresistance (Kalla Singh et.al, 2010a; Kalla Singh et.al, 2010b). Thus, IGF-II and its signaling pathways represent a novel therapeutic target to address the decreased breast cancer survival observed among AA breast cancer patients. IGF-II plays a critical role in differentiation and metabolism by signaling through the IGF-I and insulin receptors (INSR) (Abbas et.al., 2007). IGF-II is highly expressed in breast cancer and “free” circulating IGF-II level in humans is significantly correlated to breast cancer tumor size (Singer et.al., 2004). Transgenic animal models with increased IGF-II expression show a significant increase in breast cancer that develops at an early age and is more aggressive (Pravtcheva and Thomas, 1998; Pravtcheva, DD. and Wise, 2003; Moorehead et.al., 2003; Christofori et.al., 1994; Bates et.al., 1995).
Since IGF-II mitogenic effects are not only mediated by the IGF-IR but also by the INSRs, the present study focused on the INSR expression and activation in breast cancer cell lines and in paired breast tissues from CA and AA breast cancer patients. A significant differential expression of INSR-A and INSR-B was detected in the cell lines showing that cells developed from CA expressed significantly higher mRNA and protein levels of INSR-B than AA breast cancer cell lines. Likewise, significantly higher INSR-A mRNA and protein was detected in the normal and malignant breast samples tissues of AA women in contrast to the lower levels observed in CA tissue samples.
INSR-A up-regulation is associated with increased IGF-II signaling, whereas INSR-B up-regulation is predominantly associated with insulin-mediated metabolic effects (Belfiore et.al, 2009). Activation of the INSR-A elicit different biological effects and intracellular signaling upon insulin or IGF-II binding. Thus, INSR-A expressing cells undergo proliferation when stimulated by IGF-II, whereas they preferentially activate glucose uptake when stimulated with insulin (Frasca et.al, 1999, Morrione et.al, 1997). These differential effects coincide with quantitative and temporal differences in the phosphorylation of intracellular substrates IRS 1–4 and focal adhesion kinase (FAK) in response to either insulin or IGF-II.
In the present study, we observed that ER+PR+ cells showed high IRS-1 phosphorylation when stimulated by proIGF-II, while no phosphorylation was observed in either ER−PR− cell lines. Interestingly, insulin stimulated IRS-1 phosphorylation in the AA CRL-2329 (ER+PR+) cells while no IRS-1 phosphorylation was observed when CA MCF-7 (ER+PR+) cells were treated with insulin. Similarly, breast tissues from AA women expressed significantly higher INSR-A and higher IRS-1 and FAK phosphorylation than comparable tissues from CA patients. Therefore, our results show that INSR-A, IRS-1 and FAK changes observed in breast cancer cells established from AA and CA patients correlated with results obtained from the breast tissues. These data suggests that changes in the IGF-Insulin signaling pathways are maintained in the cells established from the primary tumors. Cells that predominantly express INSR-B (CA patient cells) will activate metabolic pathways when stimulated by insulin or IGF-II while the AA cells with higher INSR-A will respond to the same treatment by activating the mitogenic pathways. If INSR isoforms are coexpressed, the formation of hybrid receptors occurs (IGF1R/INSR-A, IGF1R/INSR-B, INSR-A/INSR-B) and the hybrid receptors show different binding affinities for insulin and IGF-II. Levels of hybrid INSR/IGF1R have been shown to be increased in breast tumors (Pandini et.al, 1995), leading to increased affinity for IGF-II over insulin. Therefore, the coexpression of the INSR-A, INSR-B isoforms increases the hybrid receptor formation, reduces the metabolic effect of INSR-B and promotes mitogenic and survival signals by IGF-II.
Similarly, increased IGF-II and INSR-A expression in association with elevated INSR and/or IGF1R (hybrid receptors) activation induces increased IRS-1 and FAK phosphorylation, leading to inhibition of apoptosis, cell proliferation, metastasis and insulin resistance. FAK represents a point of convergence in the actions of the extracellular matrix (ECM) and growth factors. In fact, insulin (but not IGFs) stimulates c-Abl tyrosine phosphorylation, with consequent FAK dephosphorylation and mediation of insulin metabolic effects thru the INSR-B (Frasca et.al, 2007). In contrast, we observed that the FAK signaling cascade was highly activated in the ER−PR− AA cell line CRL-2335 by IGF-II/insulin. Thus, INSR-A and formation of hybrid INSRs will increase IGF-II and Insulin signaling to promote cell migration, proliferation and survival (Andersson et.al, 2009). In consequence, higher levels of INSR-A among AA women results in increased cellular IGF-II signaling and may contribute to higher breast density, increased risk of breast cancer, more aggressive and rapid progression, insulin resistance and type-2 diabetes.
The mechanisms of INSR-A over-expression in breast cancer are not well understood, however, p53 inactivation or gain of function mutations may play an important role in this regard since wild-type p53 acts as a repressor of the INSR promoter as well as of the IGF-II promoter (Webster et.al., 1996, Zhang et.al., 1996). This is significant since AA breast cancer patients have higher levels of p53 inactivation and mutations (Jones et.al, 2004, Olopade et.al., 2003) and mutated p53 “gain of function” results in stimulation of IGF-II (Zhang et.al., 1998) thus, a similar mechanism may increase INSR-A expression among AA.
Of great significance is the potential link between proIGF-II in diabetes and cancer observed among AA breast cancer patients. Epidemiological studies looking at the development of type 2 diabetes and the metabolic syndrome, which are diseases highly prevalent among AA women, have shown that both diseases are also strongly associated with increased breast cancer risk (Rose et.al., 2007, Ido et.al., 2005 review). Interestingly, overexpression of IGF-II leads to type 2 diabetes in a transgenic mouse model through a direct effect of IGF-II on β-cell proliferation via activation of the IGF-IR, INSR and/or hybrid receptors (Devedjian et.al., 2000). Transgenic mice overexpressing IGF-II in β-cell were hyperinsulinemic early in life and showed altered glucose and insulin tolerance tests, and developed insulin resistance. Thus, elevated IGF-II protein levels in normal AA women as compared to normal CA women may not only increase the risk for breast cancer, but may also contribute to the development of insulin resistance and diabetes observed in this ethnic group. Of note, the IGF-II E-peptide derived from the cleavage of proIGF-II regulates insulin secretion (Buchanan et.al., 2001). Since proIGF-II is detected at higher levels in cells and tissues from AA patients while little or no proIGF-II was detected in breast tissues from CA patients (Kalla Singh et.al, 2010a) we can speculate that perhaps there is an impairment in the processing of proIGF-II leading to increased proIGF-II levels and increased risk of breast cancer and diabetes among AA.
In summary, we propose that higher levels of proIGF-II combined with higher INSR-A among AA women results in increased cellular IGF-II signaling which may contribute to higher breast density, more aggressive and rapid breast cancer progression, insulin resistance and possibly, type-2 diabetes. Furthermore, our recent study (Kalla Singh et.al, 2010b) demonstrated that there was a significantly lower IGF-IIR expression in cells and in breast tissues from AA breast cancer patients. This further increases the risk of developing breast cancer and diabetes because lower IGF-IIR contributes to higher levels of “bioavailable IGF-II” and impaired glucose uptake. In conclusion, IGF-II, INSR-A and INSR-B isoforms play a major role in the onset and progression of BC, making it clear that a better understanding of these receptors in different populations can lead to new and more effective therapeutic agents. To our knowledge, this is the first study that looks at the different insulin receptor isoforms expression in AA and CA breast cancer cell lines and in paired breast tissue samples and demonstrate a potential link of the IGF-II signaling pathway in breast cancer and diabetes. Finally, the differential activation of the INSRs between AA and CA coupled with differential activation of the IGF-II downstream signaling pathways may contribute to the more aggressive breast cancer phenotype observed among AA breast cancer patients and represent, along with IGF-II, potential therapeutic targets to reduce breast cancer mortality and its link to diabetes.
Footnotes
Declaration of Interest
This research was supported by 5P20 MD001632, and NIGMS 5R25GM060507. No conflicts of interest are declared by the authors.
References
- Abbas A, Samani, Yakar S, Leroith D, Brodt P. The role of the IGF system in cancer growth and Metastasis: overview and recent insights. Endocrine Reviews. 2007;28(1):20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, D’Arcy P, Larsson O, Sehat B. Focal adhesion kinase activates and stabilizes IGF1 receptor. Biochem Biophys Res Commun. 2009;387:36–41. doi: 10.1016/j.bbrc.2009.06.088. [DOI] [PubMed] [Google Scholar]
- Avruch A. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182:31–48. [PubMed] [Google Scholar]
- Bates P, Fisher R, Ward A, Richardson L, Hill DJ, Graham CF. Mammary cancer in transgenic mice expressing insulin-like growth factor II. British J of Cancer. 1995;72:1189–1193. doi: 10.1038/bjc.1995.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF1 receptors in human cancer. Current Pharmaceutical Design. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptors isoforms and insulin receptor/IGF receptor hybrids in physiology and disease. Endocrine Reviews. 2009;30 (6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Phillips AR, Cooper GJ. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet b-cells and enhances insulin secretion. Biochem J. 2001;360:431–439. doi: 10.1042/0264-6021:3600431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414 – 418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. Transforming potential of the insulin receptor substrate 1 signaling. Cell Growth Differ. 1995;6:557–562. [PubMed] [Google Scholar]
- Devedjian JC, George M, Casellas A, Pujol A, Visa J, Pelegrin M, Gros L, Bosch F. Transgenic mice overexpressing insulin-like growth factor II in b cells develop type 2 diabetes. J Clin Invest. 2000;105:731–740. doi: 10.1172/JCI5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Malaguarnera R, Mandarino A, Messina RL, Sciacca L, Belfiore A, Vigneri R. Role of c-Abl in directing metabolic versus mitogenic effects in insulin receptor signaling. J Biol Chem. 2007;282(36):26077–26088. doi: 10.1074/jbc.M705008200. [DOI] [PubMed] [Google Scholar]
- Jones BA, Kasl SV, Howe CL, et al. African American/white differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- Kalla-Singh S, Tan QT, Brito C, De León M, De León D. Differential insulin like growth factor-II (IGF-II) expression: A potential role for breast cancer survival disparity. Growth Hormone & IGF Research. 2010a;20:162–70. doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla-Singh S, Tan QT, Brito C, De León M, De León D. Insulin-like growth factors I and II receptors in the breast cancer survival disparity among African-American women. Growth Hormone and IGF Res. 2010b;20:245–54. doi: 10.1016/j.ghir.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla-Singh S, Moretta D, Almaguel F, Wall NR, DeLeon M, DeLeon D. Differential effect of proIGF-II and IGF-II on resveratrol induced cell death by regulating survivin cellular localization and mitochondrial depolarization in breast cancer cells. Growth Factors. 2008;25(6):363–372. doi: 10.1080/08977190801886905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, Waters SB, Okada S, Pessin JE. Insulin stimulates the phosphorylation of the 66- and 52- kilodalton Shc isoforms by distinct pathways. Endocrinology. 1997;138:2474–2480. doi: 10.1210/endo.138.6.5203. [DOI] [PubMed] [Google Scholar]
- Louvi A, Accili D, Efstratiadis A. Growth promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- Moller DE, Yokota A, Caro JF, Flier JS. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989;3:1263–1269. doi: 10.1210/mend-3-8-1263. [DOI] [PubMed] [Google Scholar]
- Moorehead RA, Hojilla CV, De Belle I, Wood GA, Fata JE, Adamson ED, Watson KL, Edwards DR, Khokha R. Insulin-like growth factor-II regulates PTEN expression in the mammary gland. J Biol Chem. 2003;278(50):50422–7. doi: 10.1074/jbc.M306894200. [DOI] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R. IGF-II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci. 1997;94:3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, White MF. Insulin signal transduction and the IRS proteins. Annual Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- Olopade OI, Fackenthal JD, Dunston G, Tainsky MA, Collins F, Whitfield-Broome C. Breast cancer genetics in African Americans. Cancer. 2003;97:236–45. doi: 10.1002/cncr.11019. [DOI] [PubMed] [Google Scholar]
- Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. IGF1R overexpression in breast cancers leads to insulin/IGF1 hybrid receptor overexpression: evidence for a second mechanism of IGF- signaling. Clin Cancer Res. 1995:1935–1944. [PubMed] [Google Scholar]
- Pravtcheva DD, Wise TL. Metastasizing mammary carcinomas in H19 enhancers-Igf2 transgenic mice. The Journal of Exp Zool. 1998;281(1):43–57. [PubMed] [Google Scholar]
- Pravtcheva DD, Wise TL. Transgene instability in mice injected with an in vitro methylated Igf2 gene. Mutation Res. 2003;529:35–50. doi: 10.1016/s0027-5107(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and White American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
- Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng GMD, Pegram KC, Podratz J, Crown DJ, Slamon, Duffy MJ. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Annals of Oncology. 2006;17:567–604. doi: 10.1093/annonc/mdj121. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Greer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Constantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology. 2003;144:2650–2658. doi: 10.1210/en.2002-0136. [DOI] [PubMed] [Google Scholar]
- Singer CF, Mogg M, Koestler W, Pacher M, Marton E, Kubista E, Schreiber M. Insulin-Like Growth Factor (IGF)-I and IGF-II Serum Concentrations in Patients with Benign and Malignant Breast Lesions: Free IGF-II Is Correlated with Breast Cancer Size. Clin Cancer Res. 2004;10:4003–4009. doi: 10.1158/1078-0432.CCR-03-0093. [DOI] [PubMed] [Google Scholar]
- Webster NJ, Resnik JL, Reichart DB, Strauss B, Haas M, Seely BL. Repression of the insulin receptor promoter by the tumor suppressor gene product p53: a possible mechanism for receptor overexpression in breast cancer. Cancer Res. 1996;56:2781–2788. [PubMed] [Google Scholar]
- Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncology. 2005;6:103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kashanchi F, Zhan Q, Brady JN, Fornace AJ, Seth P, Helman LJ. Regulation of IGF-II P3 promoter by p53: a potential mechanism for tumorigenesis. Cancer Res. 1996;65 (6):1367–1373. [PubMed] [Google Scholar]
- Zhang LJ, Zhan QM, Zhan SL, Kashanchi F, Fornace AJ, Seth P, Helman LJ. p53 regulates human insulin-like growth factor II gene expression through active P4 promoter in rhabdomyosarcoma cells. DNA and Cell Biology. 1998;17:125. doi: 10.1089/dna.1998.17.125. [DOI] [PubMed] [Google Scholar]