Abstract
Photoreceptor guanylate cyclase (GC1) is a transmembrane protein and responsible for synthesis of cGMP, the secondary messenger of phototransduction. It consists of an extracellular domain, a single transmembrane domain, and an intracellular domain. It is unknown how GC1 targets to the outer segments where it resides. To identify a putative GC1 targeting signal, we generated a series of peripheral membrane and transmembrane constructs encoding extracellular and intracellular mouse GC1 fragments fused to eGFP. The constructs were expressed in X. laevis rod photoreceptors under the control of the rhodopsin promoter. We examined the localization of GFP-GC1 fusion proteins containing the complete GC1 sequence, or partial GC1 sequences, which were membrane-associated via either the GC1 transmembrane domain or the rhodopsin C-terminal palmitoyl chains. Full-length GFP-GC1 targeted to the rod outer segment disk rims. As a group, fusion proteins containing the entire cytoplasmic domain of GC1 targeted to the OS, whereas other fusion proteins containing portions of the cytoplasmic or the extracellular domains did not. We conclude that GC1 likely has no single linear peptide-based OS targeting signal. Our results suggest targeting is due to either multiple weak signals in the cytoplasmic domain of GC1, or co-transport to the OS with an accessory protein.
Keywords: Guanylate cyclase, transgenic Xenopus, targeting signals, membrane associated EGFP-fusion protein, membrane protein transport
1. Introduction
Mammalian rod and cone outer segment membranes are renewed continuously with replacement every ten days (Young, 1967), a process which requires rapid and efficient vectorial transport of membrane-associated proteins from the inner segment to outer segment (Sung and Tai, 2000; Deretic, 1998). Transmembrane (TM) proteins are synthesized by endoplasmic reticulum (ER)-associated ribosomes, incorporated into vesicles budding at the ER exit sites and delivered to the Golgi complex (Rodriguez-Boulan et al., 2005). The proteins are then sorted at the trans-Golgi network (TGN), a process that requires targeting signals (“zip codes”) that specify the final destination of a protein. Using transgenic frogs expressing GFP-rhodopsin fusion proteins, it was shown that the C-terminal eight amino acids of rhodopsin contain ROS targeting information as long as the fusion peptide is membrane-associated (Tam et al., 2000). However, the identification of targeting signals embedded in the primary sequence of membrane proteins has been successful only for a few outer segment proteins (Luo et al., 2004; Tam et al., 2004).
Sorting of newly synthesized membrane proteins at the TGN for delivery to their final destinations requires small GTPases like Arf4 (Deretic et al., 2005), Rab8 (Deretic et al., 1995; Moritz et al., 2001) and rab11 (Deretic, 1997; Satoh et al., 2005) as well as targeting signals (“zip codes”) that specify the final destination of a protein. C-terminal rhodopsin truncation mutants lacking QVA(S)PA could not rescue the phenotype of Rho−/− rods which form no outer segments (Concepcion et al., 2002; Humphries et al., 1997), whereas full-length rhodopsin and cone pigments (C-terminus KVGPH) could, suggesting a sorting signal consensus sequence XVXPX-COOH (Deretic et al., 2005). Photoreceptor retinol-dehydrogenase 8 (RDH8 or prRDH), membrane-associated by C-terminal acylation, also carries a closely related -V(I)XPX signal at its C-terminus (Luo et al., 2004). Peripherin/rds, a structural protein of rod outer segment discs, carries its targeting signal within a 20 residue segment (317–336) near its C-terminus while its closely related binding partner, ROM1, does not (Tam et al., 2004). In contrast, CNG channel subunits carried no targeting signals and required ankyrin-G as a binding partner, as shown by transgenic expression of CNGB1 in X. laevis rods (Kizhatil et al., 2009). R9AP, the anchoring protein of the GAP complex, relies exclusively on the TM region for targeting of GAP to the outer segments (Baker et al., 2008). Syntaxin-3, structurally related to R9AP, is part of the SNARE complex located at ribbon synapses in photoreceptors (Morgans et al., 1996) and is involved in trafficking from the TGN to the ribbon synapse in rods (Sharma et al., 2006). Syntaxin 3 contains a targeting signal FMDE in the N-terminal region necessary and essential for apical targeting (Sharma et al., 2006). Interestingly, simple removal of the synapse-specific targeting signal redirected this protein to the outer segment suggesting that some membrane proteins that lack specific targeting information may be delivered to the outer segment by default (Baker et al., 2008).
The gene Gucy2e encodes a single span transmembrane guanylate cyclase, termed GC1, which localizes to the rod and cone outer segments (Baehr et al., 2007) and is responsible for the synthesis of the internal messenger, cyclic GMP. Deletion of GC1 in mouse resulted in cone dystrophy (Yang et al., 1999) whereas ablation of both GC1 and GC2 resulted in rod/cone dystrophy mimicking human Leber congenital amaurosis (LCA) (Baehr et al., 2007). We observed that cone pigments mislocalized throughout the cell of Gucy2e−/− cones, transport of peripheral membrane proteins (i.e., transducin, PDE6, GRK1) to the mutant COS was impeded and proteins that failed to traffic were degraded (Baehr et al., 2007). In GC double knockout rods, trafficking defects were largely limited to the peripheral membrane proteins, PDE6 and guanylate cyclase activating proteins, GCAP1 and GCAP2. We further observed that heterotrimeric kinesin-II is involved in intraflagellar transport (IFT) of GC1 in cones, but is not required for IFT of GC1 and other transmembrane proteins in rods (Avasthi et al., 2009). The identity of cargo for inner segment or ciliary (intraflagellar) transport is unknown. However, GST-pull downs and co-immunoprecipitation with anti-IFT88 antibody identified GC1, the heat-shock protein HSC70, the chaperone MRJ and rhodopsin (Bhowmick et al., 2009).
These results imply a role of GCs in transport of membrane-associated proteins from the inner segment to the outer segment (Karan et al., 2010; Karan et al., 2008). To gain insight into the transport pathway and sorting signals of GC1, we generated several deletions in the cytoplasmic and extracellular domains of GC1 and monitored the ability of each construct to traffic to the outer segments using EGFP-rhodopsin/GC chimeras expressed in transgenic Xenopus laevis. We further generated truncated GC1 transmembrane proteins fused to EGFP containing either the extracellular or the intracellular domains of GC1, or the entire GC1 protein fused to EGFP at the C-terminus. The results suggest the requirement of an entire cytoplasmic domain of GC1 for correct targeting to the OS, and that GC1 likely has no single signal motif embedded in its primary sequence.
2. Materials and Methods
2.1. Expression constructs
All X. laevis expression constructs were based on pEGFP-C1 (acc. no. U55763; BD Biosciences Clontech, Palo Alto, CA). This vector was modified by replacing the CMV promoter with a proximal 0.8 kb fragment of the X. laevis opsin promoter (Tam et al., 2004) to yield XOP-0.8EGFP-C1 vector. XOP-0.8EGFP-C1 was used for generation of transmembrane constructs GCtm3-GCtm4 (Fig. 1). Sequences encoding the C-terminal 44 amino acids of rhodopsin lacking the distal 5 amino acids (QVSPA) were fused to the C-terminus of EGFP in XOP-0.8EGFP-C1 (Tam et al., 2000). The resulting vector XOP-0.8EGFP-rhoCT44del5 was used to generate peripheral membrane (PM) associated fusion proteins GCct1-GCct8 (Fig. 4). DNA fragments encoding regions of mouse GC1 were PCR-amplified from mouse retina cDNA prepared from retina poly(A) mRNA by reverse transcription. Sense and antisense primers were flanked by two unique restriction sites suitable for cloning (Table 1). For PM constructs, the fragments were cloned into the XOP-0.8eGFP-rhoCT44del5 vector downstream of and in frame with the region encoding the rhodopsin C-terminus. For transmembrane constructs GCtm1 and GCtm2, the fragments were cloned into XOP-0.8EGFP-C1 in-frame with the N-terminus of EGFP. To generate constructs GCtm3 and GCtm4, the 60 amino acid leader sequence of GC1 was PCR-amplified using primers flanked by NheI and AgeI. This fragment was fused in-frame with the N-terminus of GFP in the XOP-0.8eGFP-C1 vector. Subsequently, a 2 kb fragment of cDNA encoding the TM-C-terminus of GC1 was amplified and cloned into the same vector in-frame with and following the region encoding the C-terminus of GFP. The final constructs were verified by sequencing. Expression vectors were linearized by digestion with NotI and purified using a GeneClean kit (Q-BIOgene, Carlsbad, CA).
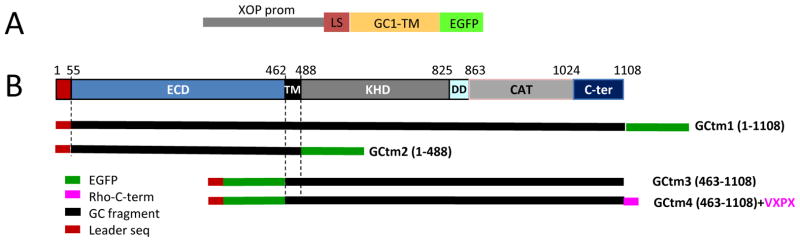
Fig. 1.
Schematic overview of transmembrane GC1-EGFP fusion constructs. A, targeting construct containing the Xenopus opsin promoter (XOP prom), mouse GC1 leader sequence (LS) and parts of the GC1 coding sequence to which EGFP is fused. B, overview of the GC1 gene structure depicting extracellular (ECD) and cytoplasmic (KHD, DD, CAT) domains GC1-EGFP fusion constructs. Below, the four transmembrane (TM) constructs (GCtm1-4) used to generate transgenic tadpoles. EGFP is fused either at the C-terminus or N-terminus. GCtm4 contains in addition a VXPX targeting signal.
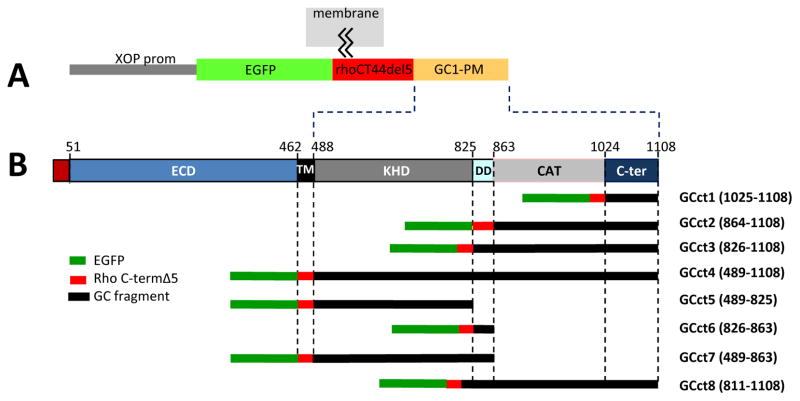
Fig. 4.
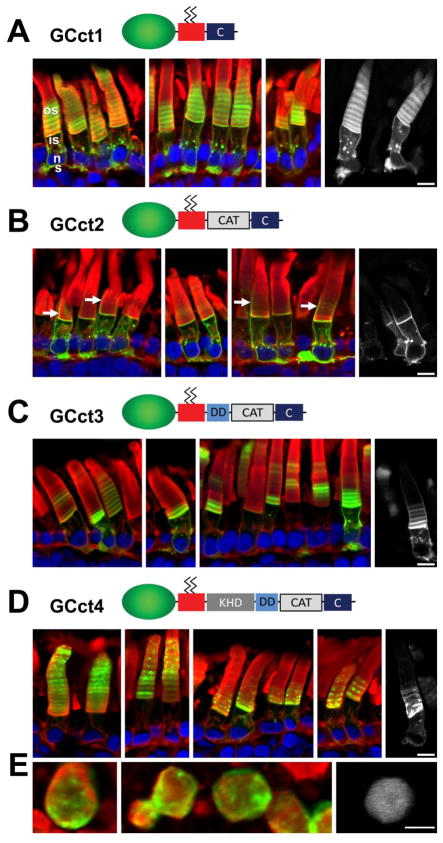
Peripheral membrane (PM) GC1 fusion protein constructs. A, all EGFP-GC1 fusion proteins are expressed under the control of the Xenopus opsin promoter (XOP). EGFP is fused upstream of the C-terminal 44 amino acids of rhodopsin lacking the VXPX signal. The VXPX domain is replaced by various GC1 cytoplasmic fragments. The fusion proteins are membrane-attached via two palmitoyl anchors. B, schematic representation of the fusion proteins containing cytoplasmic domains of GC1 (GCct1 - GCct8) depicting the extent of the cytoplasmic domains present.
Table 1.
Primer sequences used for generation of GC1 subdomains. Sense and antisense primers were flanked by two unique restriction sites (third column) suitable for cloning into the XOP-0.8EGFP-C1 vector.
| Construct | Sense primer | Antisense primer | Sites |
|---|---|---|---|
| GCct1 | 5′-ATGAGCACTGTTCGGATTC | 5′-CCAGTTTACTGGGAAGTGA | BamHI;HpaI |
| GCct2 | 5′-GTGGCTGAGGCCCTGAAGA | 5′-CCAGTTTACTGGGAAGTGA | EcoRI;AatII |
| GCct3 | 5′-ATGCTGGAGCAGTACTCT | 5′-CCAGTTTACTGGGAAGTGA | EcoRI;AatII |
| GCct4 | 5′-TACTTGAGGCACAGGCTGC | 5′-CCAGTTTACTGGGAAGTGA | EcoRI;AatII |
| GCct5 | 5′-TACTTGAGGCACAGGCTGC | 5′-ATCGACTCCATGCTTCGGTAA | EcoRI;AatII |
| GCc6 | 5′-ATGCTGGAGCAGTACTCTC | 5′-CAGATGCTGCCTCCATCTTAA | EcoRI;AatII |
| GCct7 | 5′-TACTTGAGGCACAGGCTGC | 5′-CAGATGCTGCCTCCATCTTAA | EcoRI;AatII |
| GCct8 | 5′-ATGGACCTCACCTTTGACCT | 5′-CCAGTTTACTGGGAAGTGA | EcoRI;AatII |
| GCtm1 | 5′-ATGAGCGCTTGGCTCCTGCC | 5′-CAGGCCAGTTTACTGGGAAG | NheI |
| GCtm2 | 5′-ATGAGCGCTTGGCTCCTGCC | 5′-GGCACAGGCTGCTACACATG | NheI |
| GCtm3 | 5′-GTGATCTGCAACG | 5′-TGGGAAGTGA | BglII; EcoRI |
| GCtm4 | 5′-ATGAGCGCTTGGCTCCTGCCAG | 5′-GGCCAGTTTACTGGGAAGACGGA-GACCAGCCAGGTGGCTCCAGCCTGA | BglII; EcoRI |
2.2. Transgenesis, GFP screening and tadpole rearing
To generate transgenic tadpoles, X. laevis sperm nuclei were incubated for 10 min with 0.3X high speed egg extract, 0.05 U of restriction enzyme and 100–200 ng of linearized vector DNA in 18μl of total volume. Following incubation, the reaction mixture was diluted to 0.3 nuclei/nl and 10 nl was injected per egg. Resulting embryos were reared in 0.1 X Marc’s-modified Ringer containing 6% Ficoll and 50 μg/ml gentamicin for 24 hours. The embryos were then transferred to 0.1 X Gerhart’s Ringer solution (Wu and Gerhart, 1991). At 21 dpf, the tadpoles were screened for GFP expression by using a Zeiss Axioplan2 microscope equipped with epifluorescence optics and a GFP filter set. GFP-expressing tadpoles were identified by the green fluorescence emitted from their eyes. All animals were raised at 18°C on a 12h:12h light cycle (Moritz et al., 1999; Ishibashi et al., 2008).
2.3. Confocal microscopy
At P21, transgenic tadpoles were sacrificed and the eyes were fixed using 4% paraformaldehyde in 0.1M sodium phosphate buffer, pH 7.5. Fixed eyes were embedded (OCT medium, Ted Pella Inc., Redding, CA) and cryosectioned (14μm sections). Frozen sections were stained with Alexa Fluor 594-conjugated wheat germ agglutinin (Invitrogen-Molecular Probes) and Hoechst 33342 (Sigma-Aldrich) to identify Golgi/post-Golgi membranes and nuclei, respectively (Tam et al., 2000). Sections were mounted in Mowiol (Calbiochem, San Diego, CA). Images of fluorescently labeled sections were acquired using a Zeiss LSM 510 confocal microscope, equipped with a C-Apochromat 40X W Korr, numerical aperture 1.2 objective. At least five independently derived transgenic animals per construct were examined by confocal microscopy.
3. Results
3.1. X. laevis rod photoreceptors properly traffic mouse GC1 via targeting information contained within its cytoplasmic region
Mouse and X. laevis GC1 (Gucy2e) amino acid sequences display 57% overall sequence similarity, which increases to 71% in the cytoplasmic domain, but decreases to 37% in the extracellular domain (ECD) of the peptide. The less conserved extracellular domain, located in the lumen of discs, consists of over 400 amino acids and has no known function. We generated a full-length GC1-EGFP fusion construct (GCtm1) to determine whether mouse GC1 traffics correctly to X. laevis rod outer segments (Fig. 1). We also prepared transgenes in which either the entire N-terminus of GC1 (GCtm3, GCtm4), or the entire C-terminus of GC1 (GCtm2) was replaced by EGFP. All transmembrane fusion proteins contained the mouse GC1 leader sequence, which is essential for proper translocation of the polypeptide chain through the ER membrane. After cleavage of the leader sequence and correct folding, it was expected that the mature fusion proteins would exit the ER by incorporating into ER-Golgi transport carriers and traffic to the Golgi powered by microtubule-based molecular motors, following the classical secretory pathway of transmembrane proteins. Upon exiting the TGN, membrane proteins sort based on an integral ‘zip code,’ or by association with a complex possessing a zip code, and travel to their final intracellular destination. Prior studies suggest intracellular transport carriers with cargo destined for the outer segment fuse with the plasma membrane near the periciliary ridge complex and their cargo is subsequently delivered to the outer segment by IFT.
The full-length mouse GC1-GFP fusion protein (GCtm1) localized predominantly to the outer segment, indicating that X. laevis rods are capable of recognizing and properly trafficking mouse GC1 (Fig. 2A). However, GFP fluorescence was occasionally observed in inner segments either as puncta associated with Golgi, or as faint fluorescence throughout the inner segment suggestive of retention in ER membranes. A fusion protein in which the entire cytoplasmic region of GC1 was replaced by EGFP (GCtm2) distributed throughout the inner segment, the synapse and the outer segment suggesting absence of targeting information in the ECD (Fig. 2C). In contrast, the GCtm3 GFP fusion protein containing only the cytoplasmic portions of GC1 distributed predominantly to the outer segments (Fig. 3A). As observed for the full-length construct GCtm1, EGFP fluorescence was present in the inner segment membranes of some cells, but not the plasma membrane or synapse (Fig. 2A). Accumulation of the GCtm1 and GCtm3 fusion proteins in the ER may be indicative of either a small propensity to misfold or saturation of some component of the GC1 trafficking pathway. To test these possible scenarios, we added the rhodopsin C-terminal sequence TETSQVSPA (GCtm4) in order to provide an alternate trafficking pathway which is unlikely to be saturated. However, even with VXPX at the far C-terminal we still observed relatively minor inner segment EGFP fluorescence (Fig. 3C) suggesting that fusion with EGFP causes a minor folding defect.
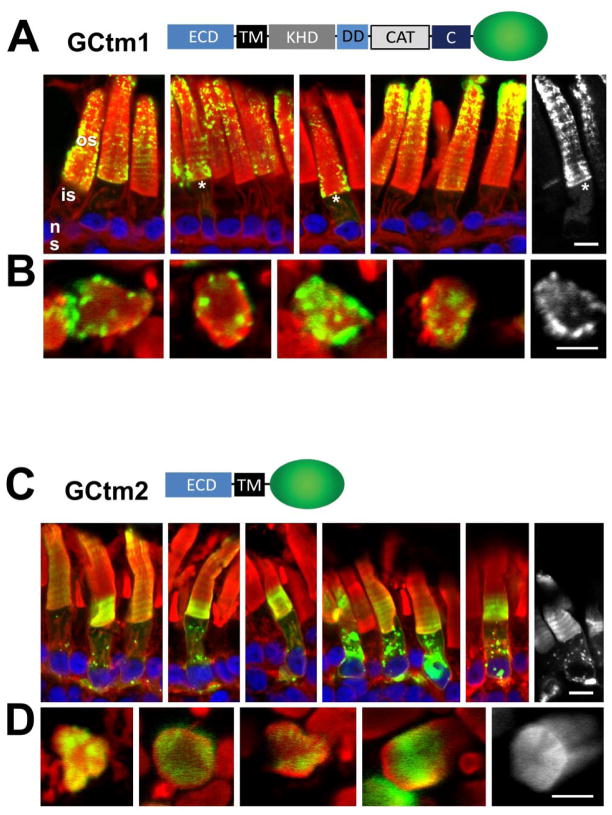
Fig. 2.
Correct targeting of GC1 requires its cytoplasmic domain. A, GCtm1 containing the entire GC1 coding sequence. GCtm1 predominantly targets to the outer segments although faint EGFP fluorescence is present in the inner segments of some rods (*). GC1-EGFP fluorescence has a particulate appearance in the outer segments, but appears uniformly distributed in inner segment endomembranes. B, Cross-sections of GCtm1 show localization to the edges of disc membranes. C, GCtm2 containing the entire extracellular domain of GC1 including TM. The ECD-EGFP fusion protein distributes among membranes of the outer segment, inner segment and synaptic terminals. D, Cross-sections of GCtm2. While not targeting correctly, the GCtm2 fusions protein locates diffusely to the disc edges. The distribution of GCtm1 suggests correct targeting although overexpression or fusion to EGFP may cause the fusion protein to misfold and accumulate in the ER. In contrast, the diffuse localization of GCtm2 suggests that targeting information is absent. Green, EGFP fluorescence; red, Alexa Fluor 594-conjugated wheat germ agglutinin; blue, Hoechst 33342. Scale bars: 10uM.
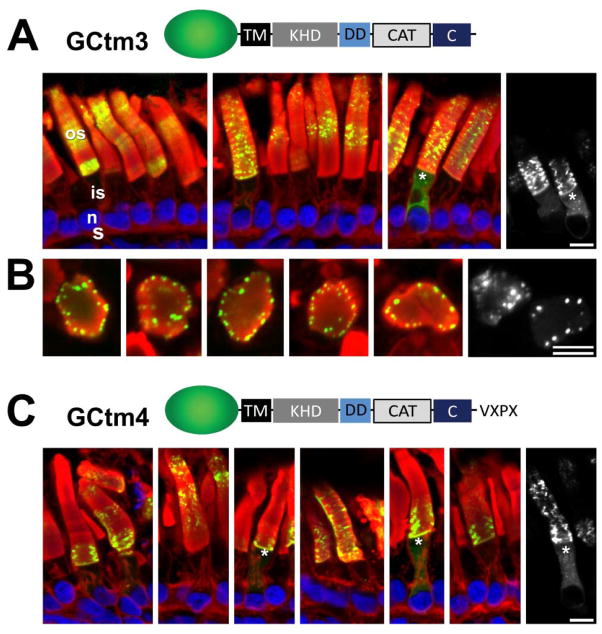
Fig. 3.
Correct targeting of GC1 does not require its extracellular domain. A, GCtm3 containing the TM and entire cytoplasmic domain of GC1. The distribution pattern of GCtm3 is similar to that of the full-length GCtm1, i.e. predominantly outer segment (Fig. 2A). B, Transgenic rod outer segments in cross-section, showing that GCtm3 tends to segregate to the rim regions of rod discs. C, GCtm4 containing the TM and entire cytoplasmic domain of GC1 and a C-terminal sequence containing the rhodopsin VXPX sorting signal (TETSQVSPA). Both GCtm3 and GCtm4 show nearly identical expression patterns. This indicates that the small amount of inner segment fluorescence is probably due to misfolding rather than mistargeting. Green, EGFP fluorescence; red, Alexa Fluor 594-conjugated wheat germ agglutinin; blue, Hoechst 33342. Scale bars: 10uM.
3.2. Transmembrane fusion proteins containing the cytoplasmic region of GC1 localize primarily to the OS disc periphery
Distribution of the transmembrane fusion proteins, with the exception of ECD-TM-GFP (GCtm2), appeared punctate and peripheral in the outer segment discs (Fig. 2,3). When we examined outer segments in cross-section, we observed that GCtm1, GCtm2, and GCtm3 localized to the edge of disc membranes as if very much restricted in lateral diffusion (Figs. 2B, 2D, 3B). This localization pattern does not represent calycal processes (finger-like extensions of the inner segment plasma membrane that form a basket around the proximal outer segment) since in longitudinal confocal sections the GFP signal is seen in the interior of outer segments and not confined to the surface, as would be the case for labeling of calycal processes. Therefore, the regularly spaced labeling is likely related to the regularly spaced lobes and incisures of the disks. Ultrastructural localization of GC1 is not unequivocally known, but peripheral labeling of intact discs has been shown in monkey rods by immuno-EM (Liu et al., 1994). Others, however, observed GC1 localization throughout disc membranes (Yang and Garbers, 1997), but also in association with actin filaments and the axoneme (Fleischman et al., 1980; Hallett et al., 1996). Our result confirms that GC1 is localized to the disc periphery (Fig. 2B, 2D, 3B), a location that is optimal for effecting rapid localized changes in the concentration of cGMP in the immediate vicinity of the cGMP-gated cation channels of the outer segment plasma membrane.
3.3. Generation of peripheral membrane-associated EGFP-GC1 fusion proteins
The results from the transmembrane GC1 fusion proteins indicated that the ECD of GC1 is devoid of targeting information, but that the transmembrane domain or one of the cytoplasmic domains may contain a contiguous outer segment targeting signal. Previously, short peptide based outer segment targeting signals have been identified in the C-termini of rhodopsin, peripherin/Rds and retinol hydrogenase (Tam et al., 2000; Tam et al., 2004; Luo et al., 2004). We therefore generated fusion proteins in which GFP was fused to the C-terminal 44 amino acids of rhodopsin lacking the last five amino acids. The EGFP-rhoCT44del5-GC1 fusion proteins (short: EGFP-GC1 fusion proteins) are palmitoylated at two adjacent cysteines and are expected to be attached peripherally to membranes and transit through the TGN as previously demonstrated (Tam et al., 2000; Tam et al., 2004). Unlike transmembrane proteins, the EGFP-GC1 fusion proteins are synthesized in the cytosol, and become stably membrane associated only after S-palmitoylation by membrane-associated palmitoyl transferases in the rough ER (St Jules et al., 1990). Therefore, it is likely that our peripheral membrane fusion proteins are present in post-TGN transport carriers, and participate in IFT through the connecting cilium (Karan et al., 2008).
Because EGFP-rhoCT44del5 contains no specific targeting information and is localized to multiple post-Golgi membranes including OS discs, OS plasma membrane, IS plasma membrane and synaptic membranes (Tam et al., 2000), we fused various length of cytoplasmic GC1 to EGFP-rhoCT44del5 in order to identify potential OS targeting sequences embedded in the GC1 primary sequence. The cytoplasmic region of GC1 consists of a kinase homology domain (KHD), a dimerization domain (DD), and a catalytic (CAT) domain. We selected a strategy in which an 83 residue distal C-terminal fragment of mouse GC1 (construct GCct1, Fig. 4B) was gradually extended to contain CAT, DD, and KHD (constructs GCct2, GCct3, and GCct4, Fig. 4B). We also tested individual domains (KHD, DD, constructs GCct5 and GCct6, respectively) and combinations of domains (constructs GCct7, GCct8) for the presence of targeting signals.
3.4. The C-terminal region of GC1 does not contain a contiguous targeting signal
Apart from the last seven amino acids, the mouse and X. laevis C-terminal regions of GC1 show high sequence similarity. In particular, the DD and CAT domain are highly conserved. Targeting signals characteristic for rhodopsin or RDH8 are not discernible. To determine whether this region contains targeting information, we generated construct GCct1 in which 83 C-terminal amino acid residues of mouse GC1 was fused to EGFP-rhoCT44del5. As shown by confocal microscopy of retinal sections, the fusion protein distributed throughout membranes of the synapse, inner segment, and the outer segment discs (Fig. 5A), a pattern resembling that of EGFP-rhoCT44del5. Presence of the GCct1 fusion protein prominently in rod outer segments is consistent with co-transport of GCct1 with other OS bound transmembrane proteins participating in intraflagellar transport through the connecting cilium (Tam et al., 2000; Baker et al., 2008). In contrast, the GCct2 fusion protein containing mouse GC C-terminal and CAT domains (amino acids 864–1108) primarily associated with membranes in the inner segment (Fig. 5B). In some cells, calycal processes reveal EGFP fluorescence (arrows in Fig. 5B). Calycal processes arise from the apex of the inner segment and surround the base of the outer segment, and are contiguous with the inner segment plasma membrane. Although faint bands of EGFP fluorescence are present in the discs, the bulk of the fusion proteins are largely excluded from the outer segment. Inner segments show strongly fluorescent particles, often at or adjacent to the nucleus and Golgi membranes, suggesting inability to exit the ER or TGN. These results suggest that the extreme C-terminus and the CAT of mouse GC1 do not harbor a contiguous outer segment targeting signal. The inefficient trafficking of GCct2 to the OS suggests that the fusion protein is rejected as IFT cargo. The cause for rejection is unknown, but misfolding can be excluded, as the GFP fusion protein is prominently present in the plasma membrane and therefore not subject to ER quality control mechanisms.
Fig. 5.
The cytoplasmic region of GC1 can direct outer segment targeting of a membrane-associated fusion protein. A, GCct1 containing the distal C-terminal 83 amino acids of GC1. GCct1 is present in outer segment, inner segment and synaptic membranes. B, GCct2 containing the distal C-terminus and the catalytic domain. GCct2 is found primarily in the inner segment endomembranes, plasma membrane, the calycal processes (white arrows), and synaptic terminals. Interestingly, the GCct2 fusion protein appears significantly excluded from outer segments. C, GCct3 containing DD, CAT and C-term. GCct3 is present in outer segment, inner segment and synaptic membranes. In contrast to GCct2, GCct3 is not excluded from the OS. D, GCct4 containing the entire GC1 cytoplasmic domain. As seen for the full-length GC1 fusion protein (GCtm1, Fig. 2A), GCct4 traffics almost exclusively to the OS. Inner segments reveal only faint EGFP fluorescence in an ER-like distribution. E, cross-sections of GCct4 (left and middle) showing tendence of GCct4 to segregate to disc edges. Green, EGFP fluorescence; red, Alexa Fluor 594-conjugated wheat germ agglutinin; blue, Hoechst 33342. Scale bars: 10uM.
In construct GCct3, the GC1 portion was extended to include the dimerization domain, and in construct GCct4, to include the entire cytoplasmic domain of GC1. Confocal microscopy revealed that GCct3 distributed to the outer segment, the inner segment (including the plasma membrane and calycal processes) and synapse. Thus, addition of the DD to the CAT and C-terminal domains results in the fusion protein no longer being excluded from the outer segment (Fig. 5C). The subcellular distribution of GCct4 (Fig. 5D) strongly resembled that of GCtm3 (i.e. primarily outer segment but with some minor localization to the inner segment membranes) and thus supports the presence of outer segment targeting information residing within the C-terminus of GC1. Cross-sections of GCct4 (Fig. 5D, lower panel) showed predominant labeling of disc edges, very similar to that seen with GCtm1-3 (Fig. 2,3).
3.5. KHD, DD, KHD-CAT domains cannot individually target fusion proteins to the OS
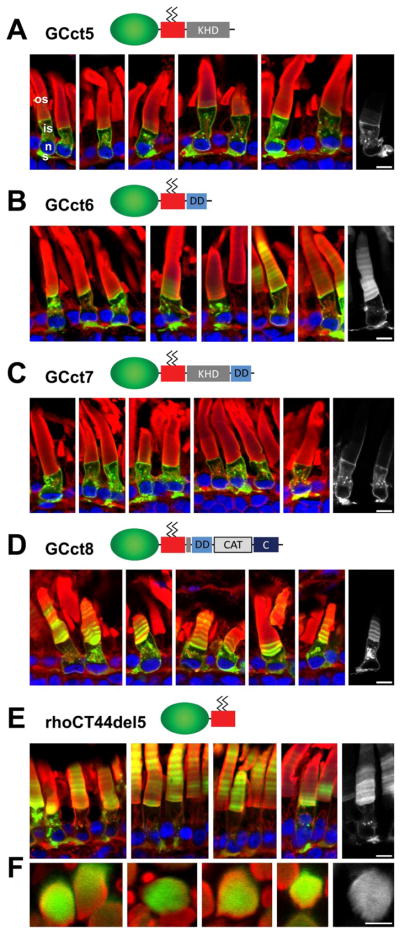
The KHD, DD and CAT domains of mouse and X. laevis show high sequence similarity. Although the function of the KHD is unclear, it was shown to be essential for interaction with guanylate cyclase activating proteins (GCAPs) (Duda et al., 1996; Laura and Hurley, 1998). Further, it was shown that photoreceptor GC1 auto-phosphorylates serine residues of the 529-GSRSSLGARS motif (Bereta et al., 2010). The predominantly outer segment localization of GCct4 suggested the possibility that the KHD might contain targeting information. However, analysis by confocal microscopy (Fig. 6) showed that GCct5 (containing KHD) was unable to enter the outer segment, and appeared to be excluded from IFT (Fig. 6A), observations similar to those obtained with GCct2 (containing the catalytic domain) (Fig. 5B).
Fig. 6.
Isolated domains of the GC1 cytoplasmic region do not direct outer segment targeting. A, GCct5 containing only the KHD domain. GCct5 remains in the inner segment and is excluded from the outer segment. B, GCct6 containing the dimerization domain. GCct6 is present in outer segment, inner segments and synaptic membranes. C, GCct7, containing KHD and DD. GCct7 is mislocalized to the plasma membrane, ER/Golgi and synaptic membranes of the inner segment, and fails to enter the OS. D, GCct8, containing DD (extended by 15 amino acids of KHD), CAT and C-terminus. GCct8 is present in the outer segment, inner segment and synaptic membranes. E, Localization of the fusion protein rhoCT44del5 (the base construct for GCct1-8). RhoCT44del5 is present in outer and inner segment membranes, including synaptic membranes. F. cross-sectioned rods expressing rhoCT44del5 – the fusion protein has no affinity for disc rims. Green, EGFP fluorescence; red, Alexa Fluor 594-conjugated wheat germ agglutinin; blue, Hoechst 33342. Scale bars: 10uM.
Contribution of the DD to outer segment targeting (GCct6) was also tested. The DD is a small, highly conserved domain (Laura et al., 1996) with a predicted coiled-coil structure. A specific role for the DD in the dimerization of the natriuretic peptide receptor A (NPR-A) intracellular domain has long been established (Wilson and Chinkers, 1995). The DD is an attractive candidate to enable targeting, as GCs are known to preferentially form homodimers (Yang and Garbers, 1997) and dimerization may facilitate trafficking and enzymatic activity. DD missense mutations of GC1 have been linked to cone-rod dystrophy (Downes et al., 2001; Wilkie et al., 2000; Tucker et al., 1999). We reasoned that interaction of intrinsic GC1 with transgenic fusion proteins expressing the DD may enable targeted trafficking. However, a fusion protein containing only the DD (GCct6) mislocalized to the inner segment and synapse besides being trafficked to the outer segment (Fig. 6B). The lack of faithful targeting suggests that the fusion protein did not dimerize with endogenous GC1. Similar results were observed when the DD (extended by 15 amino acids of KHD, as the border between the KHD and DD is poorly defined) was linked to the CAT and C-terminal domains of GC1 (GCct8, Fig. 6D). Furthermore, when the DD and KHD were combined (GCct7, Fig. 6C), the resulting fusion protein was almost entirely excluded from the outer segment, similar to the distribution of GCct5. Localization of GCct1-8 to specific photoreceptor membrane compartments in these studies can be attributed to GC sequences, as the rhoCT44del5 base construct distributes throughout inner and outer segment membrane compartments as previously described (Tam et al., 2000) (Figure 6E) and is localized diffusely in outer segment disks (Figure 6F).
4. Conclusion
The results described in this paper indicate that the cytoplasmic domain of GC1 contains targeting information that directs GC1 transport to the disc membranes of the rod outer segment, and more specifically, to the outer segment disc rims. However, in contrast to rhodopsin, peripherin/rds and retinol dehydrogenase, GC1 does not possess a short sorting motif (analogous to QVAPA) within this domain that can independently direct outer segment targeting of an otherwise unrelated membrane protein. It is possible that an accessory GC1-interacting protein may be necessary for correct GC1 transport, just as Ankyrin-G is required for CNGB1 transport (Kizhatil et al., 2009). One promising candidate is the RD3 protein identified by EST data mining, originally termed C1orf36 (Lavorgna et al., 2003), and later by positional cloning of the rd3 locus (Friedman et al., 2006). In the naturally occurring rd3 mouse, the RD3 protein is truncated by a stop codon at position 106, causing a rapid LCA-like photoreceptor degeneration (Friedman et al., 2006). Inspection of the RD3 primary sequence does not reveal an obvious targeting signal. However, the RD3 polypeptide was found to colocalize and interact with GC1 and GC2 in rod and cone photoreceptor outer segments (Azadi et al., 2010). Both GC1 and GC2 are undetectable in rd3 photoreceptors and the GC-activating proteins, GCAP1 and GCAP2, are mislocalized. It remains to be seen whether the RD3 polypeptide alone is sufficient to target GC1 correctly in vivo, or whether additional proteins are also required.
Highlights.
GFP fused to the entire cytoplasmic region of GC1 targets to rod outer segments
This fusion localizes specifically to outer segment disk rims
Smaller fragments of the GC1 cytoplasmic region do not target GFP to outer segments
Outer segment localization information is contained within the cytoplasmic region of GC1
In contrast to rhodopsin, GC1 has no single linear peptide-based targeting signal
Acknowledgments
This work was supported by National Institute of Health grants EY08123, EY019298 (WB), EY014800-039003 (NEI core grant), by a Center Grant of the Foundation Fighting Blindness, Inc., to the University of Utah, by an individual grant BR-CMM-0810-0603-UUT (WB), by an operating grant from the Canadian Institutes of Health Research (OLM), and unrestricted grants to the Departments of Ophthalmology at the University of Utah from Research to Prevent Blindness (RPB; New York). OLM is a recipient of a CIHR New Investigator Award. WB is a recipient of a Research to Prevent Blindness Senior Investigator Award.
Abbreviations
- GC
guanylate cyclase
- ER
endoplasmic reticulum
- TGN
trans-Golgi network
- EGFP
enhanced fluorescent protein
- IFT
intraflagellar transport
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Marc RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–14298. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi S, Molday LL, Molday RS. RD3, the protein associated with Leber congenital amaurosis type 12, is required for guanylate cyclase trafficking in photoreceptor cells. Proc Natl Acad Sci U S A. 2010;107:21158–21163. doi: 10.1073/pnas.1010460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Haeri M, Yoo P, Gospe SM, III, Skiba NP, Knox BE, Arshavsky VY. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–498. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereta G, Wang B, Kiser PD, Baehr W, Jang GF, Palczewski K. A functional kinase homology domain is essential for the activity of photoreceptor guanylate cyclase 1. J Biol Chem. 2010;285:1899–1908. doi: 10.1074/jbc.M109.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick R, Li M, Sun J, Baker SA, Insinna C, Besharse JC. Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic. 2009;10:648–663. doi: 10.1111/j.1600-0854.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision REs. 2002;42:417–426. doi: 10.1016/s0042-6989(01)00195-x. [DOI] [PubMed] [Google Scholar]
- Deretic D. Rab proteins and post-Golgi trafficking of rhodopsin in photoreceptor cells. Electrophoresis. 1997;18:2537–2541. doi: 10.1002/elps.1150181408. [DOI] [PubMed] [Google Scholar]
- Deretic D. Post-Golgi trafficking of rhodopsin in retinal photoreceptors. Eye. 1998;12 (Pt 3b):526–530. doi: 10.1038/eye.1998.141. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, HArgrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci U S A. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes SM, Payne AM, Kelsell RE, Fitzke FW, Holder GE, Hunt DM, Moore AT, Bird AC. Autosomal dominant cone-rod dystrophy with mutations in the guanylate cyclase 2D gene encoding retinal guanylate cyclase-1. Arch Ophthalmol. 2001;119:1667–1673. doi: 10.1001/archopht.119.11.1667. [DOI] [PubMed] [Google Scholar]
- Duda T, Goraczniak RM, Surgucheva I, Rudnicka-Nawrot M, Gorczyca WA, Palczewski K, Sitaramayya A, Baehr W, Sharma RK. Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:8478–8482. doi: 10.1021/bi960752z. [DOI] [PubMed] [Google Scholar]
- Fleischman D, Denisevich M, Raveed D, Pannbacker RG. Association of guanylate cyclase with the axoneme of retinal rods. Biochim Biophys Acta. 1980;630:176–186. doi: 10.1016/0304-4165(80)90419-5. [DOI] [PubMed] [Google Scholar]
- Friedman JS, Chang B, Kannabiran C, Chakarova C, Singh HP, Jalali S, Hawes NL, Branham K, Othman M, Filippova E, Thompson DA, Webster AR, Andreasson S, Jacobson SG, Bhattacharya SS, Heckenlively JR, Swaroop A. Premature truncation of a novel protein, RD3, exhibiting subnuclear localization is associated with retinal degeneration. Am J Hum Genet. 2006;79:1059–1070. doi: 10.1086/510021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett MA, Delaat JL, Arikawa K, Schlamp CL, Kong F, Williams DR. Distribution of guanylate cyclase within photoreceptor outer segments. J Cell Sci. 1996;109:1803–1812. doi: 10.1242/jcs.109.7.1803. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nature Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kroll KL, Amaya E. A method for generating transgenic frog embryos. Methods Mol Biol. 2008;461:447–466. doi: 10.1007/978-1-60327-483-8_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S, Frederick JM, Baehr W. Novel functions of photoreceptor guanylate cyclases revealed by targeted deletion. Mol Cell Biochem. 2010;334:141–155. doi: 10.1007/s11010-009-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48:442–452. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Baker SA, Arshavsky VY, Bennett V. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science. 2009;323:1614–1617. doi: 10.1126/science.1169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura RP, Dizhoor AM, Hurley JB. The membrane guanylyl cyclase, retinal guanylyl cyclase-1, is activated through its intracellular domain. J Biol Chem. 1996;271:11646–11651. doi: 10.1074/jbc.271.20.11646. [DOI] [PubMed] [Google Scholar]
- Laura RP, Hurley JB. The kinase homology domain of retinal guanylyl cyclases 1 and 2 specifies the affinity and cooperativity of interaction with guanylyl cyclase activating protein-2. Biochemistry. 1998;37:11264–11271. doi: 10.1021/bi9809674. [DOI] [PubMed] [Google Scholar]
- Liu X, Seno K, Nishizawa Y, Hayashi F, Yamazaki A, Matsumoto H, Wakabayashi T, Usukura J. Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp Eye Res. 1994;59:761–768. doi: 10.1006/exer.1994.1162. [DOI] [PubMed] [Google Scholar]
- Luo W, Marsh-Armstrong N, Rattner A, Nathans J. An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase. J Neurosci. 2004;24:2623–2632. doi: 10.1523/JNEUROSCI.5302-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Brandstatter JH, Kellerman J, Betz H, Wassle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J Neurosci. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Knox BE, Papermaster DS. Fluorescent photoreceptors of transgenic Xenopus laevis imaged in vivo by two microscopy techniques. Invest Ophthalmol Vis Sci. 1999;40:3276–3280. [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Sharma N, Low SH, Misra S, Pallavi B, Weimbs T. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Cell Biol. 2006;173:937–948. doi: 10.1083/jcb.200603132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jules RS, Smith SB, O’Brien PJ. The localization and timing of post-translational modifications of rat rhodopsin. Exp Eye Res. 1990;51:427–434. doi: 10.1016/0014-4835(90)90155-n. [DOI] [PubMed] [Google Scholar]
- Sung CH, Tai AW. Rhodopsin trafficking and its role in retinal dystrophies. Int Rev Cytol. 2000;195:215–267. doi: 10.1016/s0074-7696(08)62706-0. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15:2027–2037. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CL, Woodcock SC, Kelsell RE, Ramamurthy V, Hunt DM, Hurley JB. Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci U S A. 1999;96:9039–9044. doi: 10.1073/pnas.96.16.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, Hurley JB, Bhattacharya SS, Warren MJ, Hunt DM. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–3073. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Chinkers M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry. 1995;34:4696–4701. doi: 10.1021/bi00014a025. [DOI] [PubMed] [Google Scholar]
- Wu M, Gerhart J. Raising Xenopus in the laboratory. Methods Cell Biol. 1991;36:3–18. doi: 10.1016/s0091-679x(08)60269-1. [DOI] [PubMed] [Google Scholar]
- Yang RB, Garbers DL. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]
- Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci. 1999;19:5889–5897. doi: 10.1523/JNEUROSCI.19-14-05889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]