Abstract
A series of tri- and bimetallic titanium-gold, titanium-palladium and titanium-platinum derivatives of general formulas [Ti{η5-C5H4(CH2)nPPh2(AuCl)}2].2THF n = 0 (1); n = 2 (2); n = 3 (3) and [TiCl2{η5-C5H4κ-(CH2)nPPh2}2(PtCl2)].2THF (M = Pd, n = 0 (4); n = 2 (5); n = 3 (6); M = Pt, n = 0 (7); n = 2 (8); n = 3 (9)) have been synthesized and characterized by different spectroscopic techniques and mass spectrometry. The molecular structures of compounds 1–9 have been investigated by means of density-functional calculations. The calculated IR spectra of the optimized structures fit well with the experimental IR data obtained for 1–9. The stability of the heterometallic compounds in deuterated solvents (CDCl3, d6-dmso, mixtures 50:50 d6-dmso/D2O, 1:99 d6-dmso/D2O at acidic pH and at neutral pH) has been evaluated by 31P and 1H NMR spectroscopy showing a higher stability for these compounds than for Cp2TiCl2 or precursors [Ti{η5-C5H4(CH2)nPPh2}2]. The new compounds display a lower acidity (1 to 2 units) than Cp2TiCl2. The decomposition products have been identified over time. Complexes 1–9 have been tested as potential anticancer agents and their cytotoxicity properties were evaluated in vitro against HeLa human cervical carcinoma and DU-145 human prostate cancer cells. TiAu2 and TiPd compounds were highly cytotoxic for these two cell lines. The interactions of the compounds with Calf Thymus DNA have been evaluated by Thermal Denaturation (1–9) and by Circular Dichroism (1, 3, 4, 7) spectroscopic methods. All these complexes show a stronger interaction with DNA than that displayed by Cp2TiCl2 at neutral pH. The data is consistent with electrostatic interactions with DNA for TiAu2 compounds and for a covalent binding mode for TiM (M = Pd, Pt) complexes.
Keywords: cytotoxic, prostate cancer, titanocene, phosphines, heterometallic, gold, palladium, platinum
Introduction
Metallocene dihalides (Cp2MCl2, Cp = cyclopentadienyl, M = Ti, V, Nb, Mo, Re) were the first organometallic compounds with antitumor properties to be identified.1,2 Titanocene dichloride (Cp2TiCl2, Chart 1) was the first non-platinum metal complex to enter clinical trials in 1993.3 Cp2TiCl2 antitumor activity exhibited considerable in vitro and in vivo experimental models with significant cytotoxicity even in cisplatin resistant cells and tumors generally difficult to treat.4,5 However the efficacy of Cp2TiCl2 in Phase II clinical trials in patients with metastatic renal cell carcinoma6 or metastatic breast cancer was too low to be pursued.7 Nevertheless, the absence of any effect on proliferative activity on the bone marrow, which is the usual dose-limiting side-effect of organic drugs, was a promising result that suggested Cp2TiCl2 may have significant potential for possible use in combination therapy. The hydrolysis chemistry and mode of action of Cp2TiCl2 has been investigated.4,5,8 Cp2TiCl2 hydrolizes at pH above 4 and there is protonation and loss of Cp ligands and formation of insoluble Ti-oxo (Ti-O-Ti)n species. This instability in solution and lack of standard formulation were contributing factors to discontinue Cp2TiCl2 from clinical trials (although promising results have been recently obtained with different mixtures of solvents and aged solutions9 or by a controlled release system by electrospun fiber10). accumulates in Ti derived from administered Cp2TiCl2 the nucleic acid rich regions of tumor cells and exhibits pronounced inhibition of nucleic acid synthesis. An interaction with biomolecules may occur to facilitate the transport, uptake and delivery of Ti to the nucleus. It was demonstrated that Cp2TiCl2 and other Ti compounds interact with transferrin at blood plasma pH values taking the vacant Fe(III) binding sites in transferrin. The bound Ti(IV) is released to ATP at cellular endosomal pH values.11 It was proposed that ATP would facilitate the transport of Ti(IV) to the nucleus and interaction with DNA.12 Recent studies on the interaction of Ti compounds with transferrin13 point out to a non-redox release of Ti from transferrin into the cell (different from that of iron).13a Computational and some experimental studies have provided information about titanocene binding sites for Human Serum Albumin14 and DNA.15 The pH dependent hydrolysis of the Cp ligands is a critical property required for activity. Slow hydrolysis permits the Ti(IV) species to be maintained in a lipophilic environment on a time scale that allows uptake of Ti by transferrin. Cytotoxic Ti derivatives which have more stable Cp-Ti bonds may have a different mechanism of action.4 During the last 10 years, new cytotoxic Cp-derived and other coordination Ti complexes have been reported (selected examples in Chart 1).1,4,5,16–20
Chart 1.
Selected Cytotoxic Titanocene Derivatives.
The majority of derivatives have incorporated electron donating substituents. One of the main advances in the field came from the introduction of a basic group (alkylammonium substituents) in the Cp ring through a general route patented by McGowan and co-workers in 2004.21 Substitution of the Cp groups with new protonated cyclic and aryl amines19 and some other aryl and alkyl side chains (extensive work by Tacke et al.16 and some others22) increased the solubility in water of titanocene dichloride or improved the cytotoxicity. From these Cp-substituted titanocenes those named titanocene C and titanocene Y (Chart 1) were the more successful. Titanocene Y was tested against a range of freshly explanted human tumors and its sensitivity was highly remarkable in the case of renal cell, ovarian, non-small cell lung, colon and prostate cancer,19,23 as well as in MCF-7 xenograft models24 and A431 xenografts in vivo.25 More impressive were the studies in vivo on human renal cancer cells (Caki-1) in mice26 which may lead to clinical tests against metastatic renal cancer.26,27 Titanocene Y was highly cytotoxic also in prostate cancer cell lines and induced more apoptosis (specifically DNA-damage-induced apoptosis)19 than cisplatin for these cell lines by over expression of Bcl-2.28 Modifications on the substituents of titanocenes based on Y29–31 or substitution of chloride ligands by fluoride,32 oxalate33 or other anions34 may be another approach to increase the cytotoxicity of titanocenes. More recently cytotoxic steroid-functionalized titanocenes have been described.35 The mode of action of Cp2TiCl2 and titanocene C has been investigated but only limited information has been obtained dealing with the transport of Ti inside the cells, accumulation in the nucleus and DNA damage.5
The number of polymetallic complexes (homo or heterometallic) that have been evaluated as anticancer agents is more limited,36 especially for titanium.37 The hypothesis is that the incorporation of two different cytotoxic metals in the same molecule may improve their activity as anti-tumor agents due to interaction of the different metals with multiple biological targets. We have chosen gold, palladium and platinum as second cytotoxic metals. While the cytotoxicity of platinum derivatives has long been known,38 the number of reports with phosphine ligands is much more limited.39 Gold compounds are currently considered as a class of metallodrugs with great potential for cancer treatment40 (including some examples from our laboratories41) and cytotoxic palladium derivatives have also been reported.42 As far as we are aware, trimetallic TiAu2 complexes have not been investigated before.43
We have used previously reported titanocene-phosphine derivatives (Scheme 1 a–c)44 as backbones to obtain heterometallic titanocene compounds. While this work was in progress, cytotoxic bimetallic Ti-Ru compounds incorporating a similar skeleton with only one phosphine-containing Cp were reported.37b
Scheme 1.
Synthesis of Titanocene-Phosphine Trimetallic TiAu2 and Bimetallic TiM (M = Pd, Pt) Complexes (1–9).
We describe here the preparation, characterization, stability in solution and study of the cytotoxic properties of trinuclear TiAu2 and dinuclear TiM (M = Pd, Pt) heterometallic titanocene compounds. (Scheme 1). The study of their interactions with Calf Thymus DNA by spectroscopic techniques is also reported.
Results and Discussion
1. Chemistry
The new heterometallic compounds can be obtained in moderate to high yields following the procedure depicted in Scheme 1. The titanocene-phosphine derivatives a–c had been described before.44 All the heterometallic compounds obtained are stabilized with 2 molecules of THF (see experimental section). Gold(I) compounds (1–3) have a linear configuration for the AuI center whereas PdII (4–6) and PtII (7–9) centers have a square-planar arrangement with a cis disposition of the phosphine and chloro ligands as demonstrated by their 31P chemical shifts δ and 195Pt-31P coupling constants (J).45 When starting materials MCl2(NCPh)2 (M = Pd, Pt) were reacted with titanocene-phosphine a instead of MCl2(cod), a mixture of cis and trans isomers in a 50:50 ratio were obtained: δ (cis) = 30.8 (s) ppm (4), 8.0 ppm (s) JP-Pt=3642 Hz (7); δ (trans) = 16.9 (s) ppm (Pd), 12.2 ppm (s) JP-Pt=2396 Hz (Pt). Thus titanocene-phosphine derivatives b and c were reacted with MCl2(cod) (M =Pd, Pt) for the preparation of pure cis compounds 5, 6, 8, 9. Related complexes [TiCl2{η5-C5H4κPPh2}2M(C6F5)2)] (M = Pd, Pt) were obtained as the trans isomers although no specification was made and they were not characterized by crystallographic methods.46 In bimetallic [Cp2Ti(OSO2(CH2)2PPh2}2MCl2] M= Pd, Pt (where the Ti is bonded to two phosphines through the oxygen of the sulfonato group) the Pd and Pt are also in a cis environment.37a All the new compounds (1–9) have been fully characterized (see experimental section) and they are air-stable solids for short periods of time (2 days) but can be stored under nitrogen for months. Unfortunately we could not get single crystals of enough quality to perform an X-ray diffraction study of these compounds.
We were able to investigate the molecular structure of 1–9 by means of density functional calculations (see experimental). The optimized structures show a distorted tetrahedral arrangement of the Cp rings and chloro ligands around the Ti centers in all cases (Table 1). The calculated distances Ti-Cl and Ti-C are within the range for those in a related heterometallic Ti-Rh sandwich complex whose crystal structure is known [Ti{η5-C5H4(CH2)2PPh2RhCl(CO)}2] (Ti-Cl 2.382(2), 2.375(2) Å; Ti-C 2.321(6)-2.435(5) Å).47 The Au centers are in a nearly linear arrangement (See structure of 3 in Figure 1) whereas the Pd and Pt centers are in a distorted square-planar arrangement with the ligands in a cis disposition (See structure of 4 in Figure 2). The two parts in the molecule of TiAu2 containing the Cp rings and phosphine-gold-chloride fragments are symmetrical (Cs symmetry for the molecule) in the case of the compounds with the alkyl spacer (2, 3).
Table 1.
Structural Parameters and Frequencies of Selected Normal Modes (IR Spectroscopy) of complexes 1–9 obtained from DFT calculations.a
| Ti-Au | Ti-Pd | Ti-Pt | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Average bond length Ti-Cp (Å) | 2.451 | 2.420 | 2.420 | 2.425 | 2.436 | 2.435 | 2.425 | 2.437 | 2.435 |
| Average bond length Ti-Cl (Å) | 2.319 | 2.361 | 2.363 | 2.345 | 2.344 | 2.347 | 2.343 | 2.344 | 2.353 |
| Angle Cl-Ti-Cl (°) | 98.15 | 94.91 | 94.88 | 95.40 | 96.78 | 94.66 | 95.40 | 96.75 | 93.36 |
| Average bond length M-Cl (Å) | 2.309 | 2.309 | 2.310 | 2.398 | 2.365 | 2.370 | 2.367 | 2.379 | 2.382 |
| Angle P1-M-Cl1 (°) | 177.31 | 179.28 | 179.28 | 88.13 | 87.09 | 86.74 | 89.28 | 87.85 | 87.28 |
| Angle P2-M-Cl2 (°)c | 179.28 | 179.28 | 179.28 | 84.72 | 81.70 | 82.58 | 85.38 | 82.38 | 82.69 |
| Average angle Cl1-M-Cl2 (°) | ---- | ---- | ---- | 88.31 | 90.15 | 90.05 | 86.63 | 87.94 | 87.30 |
| Average bond length M-P (Å) | 2.294 | 2.289 | 2.290 | 2.338 | 2.356 | 2.349 | 2.302 | 2.319 | 2.317 |
| Average angle P1-M-P2 (°) | ---- | ---- | ---- | 98.77 | 103.89 | 103.45 | 98.65 | 103.57 | 104.15 |
| IR M-Cl (cm−1) Calculated |
339 | 337 | 337 | Ab 304 | Ab 296 | Ab 296 | Ab 299 | Ab 294 | Ab 291 |
| Sb 327 | Sb 317 | Sb 317 | Sb 324 | Sb 316 | Sb 315 | ||||
| IR M-Cl (cm−1) Experimental | 303 | 308 | 307 | 281 | 283 | 285 | 270 | 281 | 273 |
| IR M-P (cm−1) Calculated |
526 | 538 | 529 | Ab 448 | Ab 479 | Ab 431 | Ab 524 | Ab 456 | Ab 530 |
| Sb 455 | Sb 495 | Sb 437 | Sb 559 | Sb 487 | Sb 540 | ||||
| IR M-P (cm−1) Experimental | 477 | 477 | 472 | 420 | 450 | 423 | 448 | 480 | 477 |
Gaussian 09 program package (see experimental).
A = asymmetric vibration, S = symmetric vibration.
In the case of TiAu2 derivatives: P1-Au1-Cl1 and P2-Au2-Cl2. Compounds 2 and 3 are symmetric and values are identical; in the case of TiM (M = Pd, Pt) derivatives: P1-M-Cl1 and P2-M-Cl2.
Figure 1.
Optimized structure of [Ti{η5-C5H4(CH2)3PPh2(AuCl)}2] (3) obtained from DFT calculations.
Figure 2.

Optimized structure of [TiCl2{η5-C5H4κPPh2}2(PdCl2)] (4) obtained from DFT calculations.
When there is no alkyl spacer between the phosphorous and the Cp ring the molecule has a degree of distortion and there is no symmetry in this case (1).
The distortion in the square-planar arrangement of the Pd and Pt compounds is weaker for the compounds with a phosphine ligand incorporating an alkyl spacer (5, 6, 8 and 9). The angles P1-M-P2 are wider than the expected 90° (table 1) in all cases (4–9) indicating a wide ‘bite angle’ of the Ti-containing bidentate phosphine ligands. The distances M-Cl and M-P are within the expected range for AuI, PdII and PtII complexes and don’t merit further explanation.
Frequencies of selected normal modes (in IR spectroscopy) were also determined by DFT methods (Table 1) for the optimized structures of compounds 1–9. The calculated and the experimental frequencies of M-Cl and M-P stretching modes are in reasonable agreement to validate the calculated structures for these complexes. It is worth mentioning that reported DFT frequencies are not refined by any empirical scaling procedures, therefore are expected to be slightly overestimated (by about 10–15%).48 This is due partly to computational limitation, such as basis set truncation or electron correlation effects, and, to a lesser extent, to anharmonicity of a given vibration.
All the complexes are completely soluble in DMSO and in mixtures 1% DMSO: 99% H2O. The DMSO is necessary to exchange the coordinated THF and favor their solubility in H2O. The pH values of the new heterometallic complexes have been measured in 1% DMSO: 99% H2O and they are less acidic than titanocene dichloride under the same conditions (pH = 3.12). The more acidic gold derivatives are those without a spacer (n = 0: 1 pH = 4.16) whereas the introduction of a spacer or chain between the phosphine atom and the Cp ring increases the basicity of the resulting TiAu compound (n = 2: 2 pH = 5.05; n = 3: 3 pH = 5.09). In the case of Pd and Pt different effects were observed (n = 0: 4 pH = 4.40; 7 pH 4.25; n = 2: 5 pH = 3.90; 8 pH = 4.55; n = 3: 6 pH = 4.06; 9 pH 4.15) although the pH values are very similar in these cases. The fact that they are less acidic than titanocene dichloride is of importance in their future application as anticancer agents. As commented before, Cp2TiCl2 hydrolyzes at pH above 4 and there is protonation and loss of Cp ligands and formation of insoluble Ti-oxo (Ti-O-Ti)n species. This instability in solution and lack of standard formulation were contributing factors to discontinue Cp2TiCl2 from clinical trials.
By 31P NMR spectroscopy we have been able to quickly evaluate their stability in solution. Compounds (1–3) are stable for months in CDCl3 at 0 °C and Pd and Pt complexes (4–9) are also stable at room temperature (RT). Importantly for biological testing, the compounds are stable in d6-DMSO solutions at RT for at least 2 days. For titanocene dichloride, DMSO resulted in a faster Cp loss than water or other organic solvents.49 Studies in mixtures 50:50 d6-DMSO:D2O have shown that all derivatives are stable for at least 24 hours (most of them for even 2–3 weeks). Detailed studies in mixtures of d6-DMSO:D2O (1:99) have shown that the compounds are stable for 24 hours and after that time decomposition takes place (see detailed table and selected spectra in supplementary material). The heterometallic compounds are in all cases more stable than the parent phosphine-containing titanocenes (a–b) which are totally decomposed after 1 week (a) or just 36 h (b and c). Compounds of TiPd and TiPt containing Ti fragments a–c (4–9) are still present (60%) after one week, while the TiAu2 derivatives are less stable (1–3 present at 30–40% after one week). The main products of decomposition are phosphine-oxides indicating a cleavage of the phosphine-second metal (Au, Pd or Pt) and a cleavage of the Ti-Cp bonds. The first cleavage seems to be for the Ti-Cp bond as species that can be assigned to cis-Pt(PPh2Cp)2Cl2 are identified as the first decomposition products by 31P{1H} NMR spectroscopy for the TiPt derivatives (see table and selected spectra in supporting information). The second cleavage M-P occurs faster for TiAu2 and TiPd compounds. For all these complexes the decoordination of the Cp ring from the Ti center can be observed in the 1H NMR when the products start to decompose. The stability of [Ti{η5-C5H4PPh2}2] a and its heterometallic complexes [Ti{η5-C5H4(CH2)nPPh2(AuCl)}2] 1, [TiCl2{η5-C5H4κ-(CH2)nPPh2}2(MCl2)] (M = Pd 4 and Pt 7) was studied in a mixture of d6-DMSO:D2O at physiological pH 7.39 (5mM tris/NaClO4 buffer (50 mM NaClO4)) and compared with the stability of Cp2TiCl2 in D2O at pH 7.39. While the titanocene-phosphine derivative a decomposed to phosphine oxide almost completely upon addition of the deuterated buffer, the decomposition of the heterometallic complexes was slower and after 24 h a 60%-54% of the TiAu2 and TiM species was still present at neutral pH (see supporting information). Titanocene dichloride was totally decomposed after 16 hours. Thus the stability of the new heterometallic compounds is higher than that of titanocene dichloride or the starting titanocene-phosphine derivatives at neutral pH.
2. Cytotoxicity Studies
Cytotoxicity results for the heteronuclear compounds are collected in Table 2 and in tables in the supporting information section. The cytotoxicity (by a live-cell imaging method, see experimental) was evaluated against two selected cell lines: HeLa human cervical carcinoma and DU-145 human prostate cancer cell line. The new compounds are much more cytotoxic than titanocene dichloride (Cp2TiCl2) for the HeLa cell line with the best values obtained in the case of TiAu2 and TiPd derivatives (especially for ligands with the spacer (CH2)3 n=3). For HeLa cells, the gold compounds 1, 3 and the Pd derivative 6 are also more citotoxic than cisplatin (IC50 = 14.9 μM).41a More importantly, some of the compounds (like TiAu (1, 3) and TiPd with no spacer 4) result quite cytotoxic for the DU-145 human prostate cell line. The more cytotoxic titanocene compounds against this cell line described to date (titanocene Y and C) had values of IC50 of 30–50 μM19 (cisplatin for this cell line was less cytotoxic19 and we have obtained a value of 70 μM50). TiPd 4, and especially TiAu2 compounds 1 and 3 are more cytotoxic for this cell line than the titanocene derivatives and cisplatin. The TiPt compound with a propylic spacer (9) displays a cytotoxicity similar to that of cisplatin. The compounds which do not show values had a much lower cytotoxicity. They did not kill half of the cells at the highest soluble concentrations tested and at lower concentrations their IC50 values were too low. Unfortunately, at high concentrations the compounds precipitated in the media and thus their IC50 values could not be calculated. However, it seems reasonable to assume that their cytotoxicity is quite low (values in tables in the supporting information).
Table 2.
IC50 of titanium complexes obtained after exposing them to human cancer cells. All values in the table are in μM. All compounds were dissolved in 1% of DMSO and diluted with water. Titanocene dichloride, Cp2TiCl2, was diluted in water.
| Compound | HeLa | DU-145 | |
|---|---|---|---|
| [Ti{η5-C5H5}2Cl2] titanocene dichloride C1 | † | † | |
| [Ti{η5-C5H4PPh2}2Cl2] a C2 | 16.87 | † | |
| [{AuCl}2(μ-dppe)] C3 | 13.17 | 3.87 | |
| [PdCl2(dppe)] C4 | † | † | |
| [PtCl2(dppe)] C5 | † | † | |
| TiAu2 derivatives | 1 | 2.44 | 27.26 |
| 2 | 35.58 | † | |
| 3 | 1.12 | 14.16 | |
| TiPd derivatives | 4 | 37.53 | 49.84 |
| 5 | 19.16 | † | |
| 6 | 4.25 | † | |
| TiPt derivatives | 7 | 33.58 | † |
| 8 | † | † | |
| 9 | † | 73.51 | |
Complexes showing low toxicity against human cancer cells. When the maximum toxicity observed experimentally was less than 30% it was considered risky to calculate the IC50, therefore the titanium compound was categorized as low toxic. For these cases see tables with values in supporting information.
We studied the cytotoxicity of the titanocene-phosphine starting material a (C2 in Table 2) as well as the cytotoxicity of complexes of Au(I), Pd(II) and Pt(II) with a bidentate phosphine (diphenylphosphinoethane: dppe) in order to make comparisons with the new heterometallic complexes. However these highly lipophilic dppe compounds result only soluble in DMSO and precipitate easily in concentrations above 4–15 μM (depending on the metal, see SI) while added to the culture media. Compound a (C2) and the gold derivative [{AuCl}2(μ-dppe)] (C3) display cytotoxicities in HeLa cells which are similar to that of cisplatin (Table 2). The new compounds 1, 3 and 6 are more cytotoxic than C2 and C3. The Pd and Pt controls (C4 and C5) have a very low cytotoxicity (Table 2 and supporting information). Besides, we studied the cytotoxicity of combinations of titanocene dichloride Cp2TiCl2 and the bidentate phosphine metal complexes to asses a possible additive or synergistic effect (see supporting information). Combinations of Cp2TiCl2 (C1) and [{AuCl}2(μ-dppe)] C3, C1 and [PdCl2(dppe)] C4, C1 and [PtCl2(dppe)] C5 (see details in supporting information) showed in all cases a low cytotoxicity (non additive or synergistic effect) in HeLa cells.
In DU145 cells, the cytotoxicity was low for the combinations of Ti and Pd or Ti and Pt compounds (C1 and C4, C1 and C5) and a little higher for the combination of C1 and the gold compound (C3). In these cases, an additive effect was observed for the gold and palladium combinations and no effect was observed for the platinum compounds. The gold compound itself C3 was surprisingly quite cytotoxic for this cell line (more cytotoxic than the more cytotoxic TiAu2 and TiPd compounds reported here). Some gold (III) compounds seem to hold excellent potential as drugs for prostate cancer40b but we are not aware so far on reports on gold(I) derivatives.
These results are consistent with what was found for bimetallic titanocene-Pt derivatives37b and for the recently reported bimetallic titanocene-Ru complexes.37a In both cases, the cytotoxicity of the bimetallic species is higher than that of monometallic Ti, Pt and Ru species.37 It could be argued that the cytotoxicity could come from degraded species (after 24 h in physiological media at neutral pH there is 55%–60% of heterometallic species present in solution). The main decomposition product (phosphine oxide) is not cytotoxic. NMR data indicate that the heterometallic complexes decompose to species containing the second metal (Au, Pd or Pt) prior to total decomposition. However, from experiments done with equivalent second metal-containing species (C3–C5) we have demonstrated that the heterometallic species are in general more cytotoxic.
It seems clear that the new trinuclear and dinuclear compounds possess peculiar chemicophysical properties with respect to their precursors responsible for the observed biological effects (especially in HeLa cell lines). These results also strongly support the main hypothesis of our proposal: that the incorporation of a second different cytotoxic metal in titanocenes can improve their activity as anti-tumor agents.
3. Interactions with Calf Thymus DNA
Since the DNA biopolymer is involved in cellular replication, it becomes an interesting target in cancer chemotherapy. It is well known that most cytotoxic platinum drugs present strong covalent bonds with the DNA bases51 although a variety of platinum compounds act as DNA intercalators while coordinated to the appropriate ancillary ligands.52 There are also reports on palladium derivatives interacting with DNA in covalent42a,53 and noncovalent ways.54 While most gold(III) and gold(I) compounds display reduced affinity for DNA,40,41a gold(I)-phosphine derivatives with weakly bound ligands (such as halides) bind in a nondenaturing fashion to DNA.55 Although initial studies suggested that the effect of titanocene derivatives might be related to DNA interaction,56 later investigations indicate that metallocene dihalides neither bind strongly to DNA at neutral pH nor suppress DNA-processing enzymes.57 More recent reports provide experimental evidence of titanium being accumulated in the cellular nucleic acid-rich regions, particularly in the chromatin.58 Moreover it has been suggested that Cp2TiCl2 or, more likely, Ti(IV) ions can interact weakly with the phosphate groups of nucleotides at neutral pH.15b,59,60 The DNA binding ability of the 9 heterometallic complexes was investigated by use of two different techniques and directly compared to that of cisplatin and titanocene dichloride (Cp2TiCl2).
3.1. Thermal Denaturation Experiments
The melting point technique is a sensitive and easy tool to detect even slight DNA conformational changes. It is known that a destabilizing interaction with the double helix (typically, covalent) is observed as a decrease in the melting point (Tm), while a stabilizing interaction (usually by intercalation or by electrostatic attraction) induces an increase of the Tm. Bearing that in mind, Calf Thymus DNA (CT DNA) was incubated for different periods of time (1 h or 24 h) with each drug at a ratio DNA:drug 2:1 (see experimental for details). The results are summarized in Table 3. These experiments indicate that all the compounds tested (including TiCp2Cl2 and cisplatin) have interactions with DNA, as demonstrated by a change in Tm.
Table 3.
Changes in the Tm of CT DNA after incubation with complexes cisplatin,a Cp2TiCl2,a 1 – 9b for 1h in 5mM tris/NaClO4 buffer (50 mM NaClO4) at pH 7.39 and r = 0.5.
| Compound | ΔTm (°C)/1h | ΔTm (°C)/24h |
|---|---|---|
| DNA-cisplatin | −7.08 | −6.35 |
| DNA-titanocene dichloride | 0.65 | 0.20 |
| DNA-1 (TiAu2, n=0) | 8.65 | 5.67 |
| DNA-2 (TiAu2, n=2) | 6.80 | 3.04 |
| DNA-3 (TiAu2, n=3) | 4.25 | 2.05 |
| DNA-4 (Ti-Pd, n=0) | 5.91 | −1.10 |
| DNA-5 (Ti-Pd, n=2) | 4.15 | −0.60 |
| DNA-6 (Ti-Pd, n=3) | 2.15 | −0.35 |
| DNA-7 (Ti-Pt, n=0) | 6.51 | −1.97 |
| DNA-8 (Ti-Pt, n=2) | 5.05 | −1.43 |
| DNA-9 (Ti-Pt, n=3) | 4.25 | −1.01 |
Compounds dissolved in buffer;
Compounds dissolved in a 1:99 DMSO:buffer solution.
Complexes Cp2TiCl2 and 1–9 produce a stabilizing effect (increase in Tm) on DNA. This increase is especially pronounced for the gold compounds and for the compounds which don’t contain the alkylic spacer between the Cp ring and the phosphine (1, 4 and 7). The increase is significantly higher than that of titanocene dichloride (Table 3).
Since the studies of cytotoxicity are performed at 20 h and subsequent more detailed DNA conformational studies (circular dichroism) are also performed after 20 h of incubation with DNA we studied the behavior of DNA after 24 h of incubation with the complexes and controls. We have found that compounds 1–9 are stable in a solution DMSO:H2O (1:99) for 24 h as studied by NMR spectroscopy (results and supporting information section). At physiological neutral pH we have found that the compounds are less stable but they are more stable than Cp2TiCl2 or starting titanocene-phosphine and after 24 h there is still 60% of TiAu2 or 55% of Ti-M (M = Pd, Pt) heterometallic compounds. However when DNA is present in the neutral solutions it interacts with the drugs (Table 3) but we cannot anticipate if the decomposition of the complexes or the Cp cleavage from Ti may be accelerated or slowed down.
After 24 h of incubation with DNA the stabilizing effect is observed only for the three Ti-Au2 derivatives. A very small effect is observed for titanocene dichloride indicating a small interaction with DNA. The complexes Ti-M (M = Pd, Pt) show a decrease in Tm indicating a covalent bonding to DNA similar to that produced by cisplatin (Table 3). The increase or decrease of Tm is quite similar at 24 h for the complexes with an ethyl or propyl spacer between the Cp and the phosphine ligand for all the heterometallic complexes. It seems that the stabilizing effect observed for all the heteronuclear compounds after 1h incubation with DNA is due to an initial electrostatic interaction with the titanium center (which hydrolyses very quickly exchanging Cl− ligands by OH− groups).49 When the Cp-Ti bonds start to be cleaved (in less than 24 h), titanium ions may be released and the interaction of the DNA with the metal may be due at least in part to the second metal- (Au, Pd or Pt) containing degraded species.
In order to asses if the TiAu2 complexes are stabilized by electrostatic interaction (a most plausible interaction for these complexes since its structure does not seem suitable for an intercalation effect) a different ionic strength of 5 mMTris/HCl buffer was employed (25 mM NaClO4, Table 4). At low Na+ concentrations the increase of Tm is higher, most likely due to the complexes stabilizing DNA through electrostatic binding. On the other hand at high salt concentration the stabilizing effects are reduced since the electrostatic component is lowered by the increase of concentrations of Na+ counterions. The Tm is lower in all cases after the incubation for 24h which resembles better the conditions for the cytotoxicity experiments. In the case of the compounds with an alkyl spacer (2 and 3) at high salt concentration (double amount of Na+) the Tm is reduced to half after 24 h. This effect is smaller for compound 1 but still noticeable.
Table 4.
Changes in the Tm of CT DNA after incubation with complexes 1–3 for 1h and 24 h with different ionic strengths of 5mM tris/NaClO4 buffer at pH 7.39 and r = 0.5.
| Compound | ΔTm (°C)/1h Incubation/25 mM NaClO4 |
ΔTm (°C)/1h Incubation/50 mM NaClO4 |
ΔTm (°C)/24h Incubation/25 mM NaClO4 |
ΔTm (°C)/24h Incubation/50mM NaClO4 |
|---|---|---|---|---|
| DNA-1 (TiAu2, n=0) | 11.39 | 8.65 | 7.35 | 5.67 |
| DNA-2 (TiAu2, n=2) | 9.75 | 6.80 | 6.31 | 3.04 |
| DNA-3 (TiAu2, n=3) | 7.75 | 4.25 | 5.73 | 2.05 |
From these experiments it seems clear that all new compounds show an interaction with DNA. This interaction (which is stronger for the heterometallic compounds than for titanocene dichloride) seems to be covalent in nature for TiM (Pd and Pt) complexes and mostly of electrostatic nature for the TiAu2 derivatives.
3.2. Circular Dichroism Spectroscopy Studies
More detailed DNA conformational alterations can be detected by means of circular dichroism (CD) spectroscopy. We selected the TiAu2 derivatives (1) and the TiM complexes (Pd 4 and Pt 7) which display the stronger interaction with DNA by Tm experiments (Table 3) to carry out these studies. Additionally we studied the interaction of the most cytotoxic compound TiAu2 (3). Figure 4 shows a CD spectrum of DNA in its B conformation treated with increasing amounts of titanocene dichloride Cp2TiCl2. The band of the CD spectrum of DNA at 275 nm is due to stacking between base pairs, while the band at 248 nm is originated because of the right-handed helicity.61 In the CD technique the interaction of the drug with DNA in the region between 275 nm and 248 nm and these changes in the curve can be attributed to conformational modifications in the double helix.
Figure 4.
CD spectra of CT incubated for 24 h at 37 °C with Cp2TiCl2 at 0, 0.1, 0.25, 0.5 and 1 ratios.
When CT DNA is incubated with increasing amounts of Cp2TiCl2 there are not noticeable changes at small DNA-drug ratios (up to r = 0.5) and only at ratios of 1 there is an small increase of the positive band indicating an stabilizing effect most plausibly by intercalation or electrostatic effects. We have not found publications describing CD spectroscopic studies for DNA-titanocene dichloride interactions. The results are in agreement with what we found for the melting point experiments with titanocene dichloride. It seems that the interaction of titanocene dichloride with DNA is not very strong and does not seem to be covalent in nature. This is in agreement with recent investigations of titanocene dichlorides which indicate they do not interact strongly with DNA at neutral pH.57
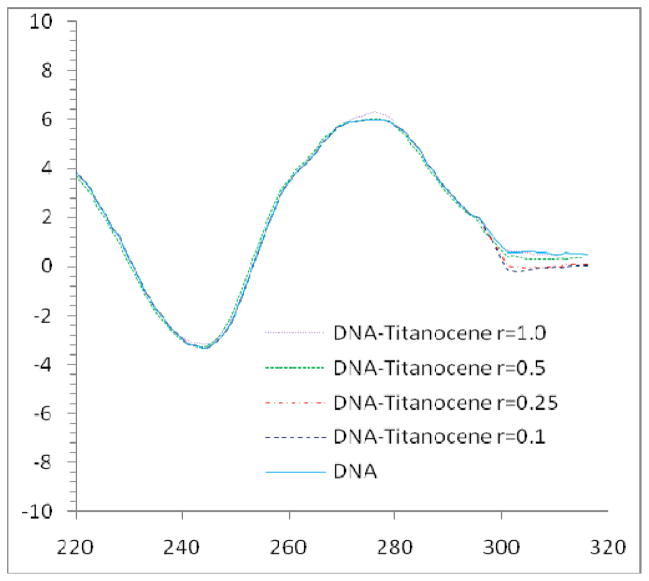
When CT DNA is incubated with increasing amounts of TiAu2 derivatives (1 and 3), a slight increase of the intensities of the positive and especially the negative bands are observed (Figure 5). These changes in the wavelength and the ellipcity of free DNA indicate modifications on the secondary structure of DNA as consequence of the interaction of the complexes with DNA.62 This type of change has been previously observed in similar experiments with gold(I)-phosphine-halide derivatives such as AuCl(PEt3).55 For the positive bands there is also a shift to lower energy when increasing the amount of drug added (from 278 to 274 cm−1 1 and to 271 cm−1 for 3). The negative bands show a shift to higher energy when increasing the amount of drug added (from 238 cm−1 to 242 cm−1 for 1 and to 244 cm−1 for 3). This interaction has been reported before as the binding of the AuPR3+ cations to the heterocyclic bases of DNA in a nondenaturating and reversible fashion. This only happens for compounds with a weakly bound ligand such as Cl− or Br−.55 In these cases increasing the ionic strength of the medium served to decrease the extent of the interaction between the gold(I) complex and DNA.55 This is totally in agreement with what we observed in the Tm studies and indicates an interaction of the TiAu2 compounds with DNA of electrostatic nature. When CT DNA is incubated with increasing amounts of TiM derivatives (TiPd 4 and TiPt 7), a slight decrease of the intensities of the negative and especially the positive bands are observed. Those changes in the stacking and the helicity of CT DNA are consistent with a covalent binding mode to DNA in a cis-bidentate fashion.63 These changes are more noticeable for the TiPt derivative 7 (as indicated by the Tm experiment, see Table 3). In both cases there is a bathochromic shift for the positive (from 278 to 270 cm−1 4 and to 274 cm−1 for 7) and for the negative (from 238 cm−1 to 243 cm−1 for 4 and to 243 cm−1 for 7) bands.
Figure 5.
CD spectra of CT incubated for 24 h at 37 °C with TiAu2 derivatives 1 (A) and 3 (B) at 0, 0.1, 0.25, 0.5 and 1 ratios.
In conclusion, the experiments of DNA-drug interactions have shown that Cp2TiCl2 seems not to interact strongly with DNA at physiological pH in vitro and that this interaction is electrostatic in nature. The new heterometallic compounds interact more strongly with DNA. In the same period of time that cytotoxicity studies are carried out, this type of drug-DNA interaction depends partly on the second metal-containing degraded species. Thus the interaction of TiAu2 derivatives with DNA is dominated by electrostatic effects while the interaction of DNA with d8 metals (PdII and PtII) seems to be covalent in nature. The length of the alkyl chain (n = 0, 2, 3) between the phosphorous and the Cp ring is also an important factor and the strength of the interaction decreases while increasing n (more flexibility of the “bidentate titanocene phosphine” in compounds with n = 2 and 3). However the cytotoxicity of the compounds may not be related in all cases to these metal-DNA interactions. In the case of TiAu2, interactions of the Au cations with thiol or selenol groups of transport and mitochondrial proteins may play a major role.40, 51b
Conclusions
Organometallic compounds are currently being studied as potential anticancer agents since they exhibit chemicophysical properties intermediate between organic drugs and metallic coordination compounds. We have prepared trinuclear TiAu2 and binuclear TiM (M = Pd, Pt) complexes based on a titanocene-phosphine backbone. Their stability in different deuterated solvents (including buffer solutions) can be quickly evaluated by 31P {1H} NMR spectroscopy. These complexes are less acidic and more stable in DMSO:D2O (1:99) solutions at acidic and neutral pH than titanocene dichloride. TiPd and, especially TiAu2 complexes are highly cytotoxic in vitro against HeLa human cervical carcinoma and DU-145 human prostate cancer cells. In general the most cytotoxic compounds incorporate the propyl spacer between the phosphorous atom and the Cp ring. They are more active than their parent titanocene dichloride and titanocene-phosphine derivatives and second metal-related precursors (with the exception of Au for the DU-145 cell line).
The interactions of the compounds with Calf Thymus DNA have been evaluated by Thermal Denaturation and by Circular Dichroism spectroscopic methods. All these complexes show a stronger interaction with DNA than that displayed by Cp2TiCl2 at neutral pH. The data is consistent with electrostatic interactions with DNA for TiAu2 compounds and for a covalent binding mode for TiM (M = Pd, Pt) complexes. However with this data we cannot propose that DNA is the ultimate biomolecular target for these compounds. These preliminary results strongly support that the incorporation of a second different cytotoxic metal in titanocenes can improve their activity as anti-tumor agents as it has been recently reported for a related family of Ti-Ru bimetallic complexes.37b We are currently working on ligand/anion modifications for the most cytotoxic compounds to increase their stability as well as in the study of reactions of the compounds with transport and mitochondrial proteins to gain an insight into the plausible mode of action of this type of heteronuclear complexes.
Experimental Section
1. Synthesis and Characterization of the Heterometallic Complexes
All manipulations involving syntheses of titanium complexes (a–c) and heterometallic complexes were performed at an argon/vacuum manifold using standard Schlenk-line techniques under an argon atmosphere or in a glove-box MBraun MOD System. Solvents were purified by use of a PureSolv purification unit from Innovative Technology, Inc. The phosphine substrates were purchased from Aldrich and used without further purification. Titanocene-diphenylphosphine complexes (a–c),44 [AuCl(tht)],64 [PdCl2(cod)]65 and [PtCl2(cod)]66 were prepared as previously reported. NMR spectra were recorded in a Bruker AV400 (1H NMR at 400MHz, 13C NMR at 100.6 MHz, 31P NMR at 161.9 MHz, 195Pt NMR at 86.1 MHz). Chemical shifts (δ) are given in ppm using CDCl3 as solvent, unless otherwise stated. 1H and 13C resonances were measured relative to solvent peaks considering TMS = 0 ppm, 31P{1H} was externally referenced to H3PO4 (85%). Coupling constants J are given in Hz. Infrared spectra (4000–250 cm−1) were recorded on a Nicolet 6700 FT-IR spectrophotometer on KBr pellets. Elemental analyses were performed on a Perkin Elmer 2400 CHNS/O Analyzer, Series II. Mass spectra (ESI) were performed on Agilent Analyzer and on a Bruker Analyzer. DNA thermal denaturation experiments were performed with a Cary 100 Bio UV-Visible Spectrophotometer. Circular Dichroism spectra were taken in a Chirascan CD Spectrometer equipped with a thermostated cuvette holder. pH was measured in an OAKTON pH/conductivity meter in 1% DMSO:99% H2O solutions.
[Ti{η5-C5H4(CH2)nPPh2(AuCl)}2].2THF n = 0 (1); n = 2 (2); n = 3 (3)
[AuCl(tht)] (0.06 g, 0.20 mmol) was added to a dichloromethane solution (15 mL) of a (0.07 g, 0.10 mmol, for the preparation of 1), b (0.07 g, 0.10 mmol, 2) or c (0.08 g, 0.10 mmol, 3) at 0°C (ice bath). The reaction mixture was stirred for 20 min. afterwards, the ice bath was removed and it was stirred for 10 min at room temperature. The yellow oil formed was collected after evaporation and the volatiles were removed under vacuum. The residue was washed with n-hexane (10 mL) and diethyl ether (10 mL) and dried under reduced pressure to afford 1 as a white solid, or 2 and 3 as pale yellow solids. 1: Yield: 0.070 g, 60%. Anal. Calc. For C42H44Au2Cl4O2P2Ti (1226.38): C, 41.13; H, 3.62; found: C, 41.02; H, 3.64;. MS(ESI+) [M/Z, (%)]:1225 [M]+. 31P{1H} NMR (CDCl3) δ = 30.8 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 2.73 (m, 16H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.71 (−, Cipso-C6H4). Value of pH ([8.15 × 10−4M] in 1% DMSO:99% H2O) = 4.16. 2: Yield: 0.064 g, 50%. Anal. Calc. For C46H52Au2 Cl4O2P2Ti (1282.49): C, 43.08; H, 4.09; found: C, 43.28; H, 4.01;. MS(ESI+) [M/Z, (%)]: 1281 M]+. 31P{1H} NMR (CDCl3) δ = 29 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 1.66 (m, 8H, THF), 2.13 (t 2H, P-CH2, 3JHH = 8), 2.45 (t 2H, C5H4-CH2, 3JHH = 8), 3.66 (m, 8H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6 (−, PCH2), 34.2 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([7.79 × 10−4M] in 1% DMSO:99% H2O) = 5.05. 3: Yield: 0.08 g, 59%. Anal. Calc. For C48H56 Au2Cl4O2P2Ti (1310.54): C, 43.99; H, 4.31; found: C,44.01; H,4.27;. MS(ESI+) [M/Z, (%)]:1309 [M]+. 31P{1H} NMR (CDCl3) δ = 28 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = (m, 8H, THF), 3.15 (dd 2H, CH2), 4.62 (dd, 1H, 3JHH = 10, 3JHH = 5,), 5.47 (m, 1H, ,) 6.68 (m, 1H, C5H4), 6.90 (m, 2H, C5H4), 7.18 (m, 1H, C6H4), 7.33 (m, 1H, C6H4), 7.42 (m, 1H, C6H4), 8.09 (m, 1H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6 (−, PCH2), 34.2 (−, CH2), 46.8 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([7.67 × 10−4M] in 1% DMSO:99% H2O) = 5.09.
[TiCl2{η5-C5H4κ-(CH2)nPPh2}2(PdCl2)].2THF n = 0 (4); n = 2 (5); n = 3 (6)
[PdCl2(cod)] (0.03 g, 0.10 mmol) was added to a dichloromethane solution (15 mL) of a (0.07 g, 0.10 mmol, for the preparation of 4), b (0.07 g, 0.10 mmol, 5) or c (0.08 g, 0.10 mmol, 6) at room temperature. The reaction mixture was stirred for 30 min. The red (4), yellow (5) or orange-yellow (6) oil formed was collected by evaporation and the volatiles were removed under vacuum. The residue was washed with cold n-hexane (10 mL) at 0 degrees and dried under reduced pressure to afford 4 as a red solid, 5 as a pale yellow solid or 6 as a pale orange solid. 4: Yield: 0.058 g, 62%. Anal. Calc. For C42H44PdCl4O2P2Ti (938.86): C 53.73; H 4.72; found: C, 53.99; H, 4.80;. MS(ESI+) [M/Z, (%)]:[M]−. 937. 31P{1H} NMR (CDCl3) δ = 30.8 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 2.73 (m, 16H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.71(−, Cipso-C6H4). Value of pH ([9.77 × 10 −4M] in 1% DMSO:99% H2O) = 4.40. 5: Yield: 0.051 g, 51%. Anal. Calc. For C46H50PdCl4O2P2Ti (994.97): C 55.53; H 5.25; found: C, 55.22; H, 5.09;. MS(ESI+) [M/Z, (%)]: 993 [M]+. 31P{1H} NMR (CDCl3) δ = 30.8 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 1.66 (m, 8H, THF), 2.13 (t 2H, P-CH2, 3JHH = 8), 2.45 (t 2H, C5H4-CH2, 3JHH = 8), 3.66 (m, 8H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6 (−, PCH2), 34.2 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([9.72 × 10 −4M] in 1% DMSO:99% H2O) = 3.90. 6: Yield: 0.073 g, 72%. Anal. Calc. For C48H54PdCl4O2P2Ti (1023.03): C 56.35; H 5.52; found: C, 56.21; H, 5.68;. MS(ESI+) [M/Z, (%)]: 1022 [M]+. 31P{1H} NMR (CDCl3) δ = 30.8 (s). 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 2.73 (m, 16H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6 (−, PCH2), 34.2 (−, CH2), 46.8 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([9.75 × 10 −4M] in 1% DMSO:99% H2O) = 4.06.
[TiCl2{η5-C5H4κ-(CH2)nPPh2}2(PtCl2)].2THF n = 0 (7); n = 2 (8); n = 3 (9)
[PtCl2(cod)] (0.03 g, 0.10 mmol) was added to a dichloromethane solution (15 mL) of a (0.07 g, 0.10 mmol for the preparation of 7), b (0.07 g, 0.10 mmol, 8) or c (0.08 g, 0.10 mmol, 9) at room temperature. The reaction mixture was stirred for 30 min. The yellow oil formed was collected by evaporation and the volatiles were removed under vacuum. The residue was washed with cold n-hexane (10 mL) at 0 degrees and dried under reduced pressure to afford 7 as a white solid, 8 as a pale yellow solid or 9 as a beige solid. 7: Yield: 0.080 g, 80%. Anal. Calc. For C42H44PtCl4O2P2Ti (1027.53): C 49.09; H 4.32; found: C, 48.89; H, 4.28;. MS(ESI+) [M/Z, (%)]: 1026 [M]. 31P{1H} NMR (CDCl3) δ = 8.0 (s) JP-Pt = 3642 Hz. 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 2.73 (m, 16H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.71 (−, Cipso-C6H4). Value of pH ([9.72 × 10−4M] in 1% DMSO:99% H2O) = 4.25. 8: Yield: 0.087 g, 80%. Anal. Calc. For C46H50PtCl4O2P2Ti (1081.62): C 50.99; H 4.84; found: C, 50.20; H, 4.78;. MS(ESI+) [M/Z, (%)]:1080 [M]. 31 P{1H} NMR (CDCl3) δ = 8.3 (s) JP-Pt = 3648 Hz. 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 1.66 (m, 8H, THF), 2.13 (t 2H, P-CH2, 3JHH = 8), 2.45 (t 2H, C5H4-CH2, 3JHH = 8), 3.66 (m, 8H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6 (−, PCH2), 34.2 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([9.72 × 10 − 4M] in 1% DMSO:99% H2O) = 4.55. 9: Yield: 0.090 g, 82%. Anal. Calc. For C48H54PtCl4O2P2Ti (1109.67): C 51.86; H 5.08; found: C, 51.00; H,5.06;. MS(ESI+) [M/Z, (%)]: 1108 [M]−. 31P{1H} NMR (CDCl3) δ = 8.1 (s) JP-Pt = 3640 Hz. 1H NMR (plus COSY, plus NOESY, CDCl3) δ = 2.73 (m, 16H, THF), 7.54 (m, 8H, C5H4), 7.56 (m, 8H, C6H4), 7.75 (m, 12H, C6H4). 13C{1H} NMR (plus APT, plus HSQC, CDCl3) 22.6(−, PCH2), 34.2(−, CH2), 46.8 (−, C5H4CH2), 122.4, 123.1, 124.9 (all +, C5H4), 125.5 (+, C6H4), 127.6 (+, C5H4), 128.9, 129.3, 129.5 (all +, C6H4), 130.0 (−, Cipso-C5H4), 139.7 (−, Cipso-C6H4). Value of pH ([9.73 × 10 − 4M] in 1% DMSO:99% H2O) = 4.15.
2. Computational Methods
All calculations reported here were carried out by the Gaussian 09 program package.67 All structures are found by full geometry optimization at the B3LYP/6–31G* level of density functional theory (DFT) in combination with relativistic effective-core potential cc-pVDZ-PP for heavy metals; palladium, platinum and gold in appropriate compounds to describe their core electrons and valence orbitals with the [4s, 4p, 3d, 1f], contracted Gaussians composition,68 that were treated explicitly in electronic structure calculations. Computed frequencies of all structures are positive, indicating that the optimized structures are at real minima of their ground-state potential-energy surfaces. In addition, a simple visual comparison of computed IR spectra with experimental spectra was performed to further validate reliability of computed structures.
3. Determination of Compound Cytotoxicity
Cells were seeded on 96-well microplate at 10,000 cells/well density in 200 μl culture media consisting of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with antibiotics (100 units/ml of penicillin, 100 μg/ml of streptomycin; Invitrogen, Carlsbad, CA) and 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT). Plates were incubated overnight at 37°C in a 5% CO2 humidified atmosphere to promote cell attachment, followed by cell exposure to experimental chemical compounds for 20 h essentially as described by Lema et al.69 One hour previously to capture the images in live-cell mode, each well was added with a mixture of two fluorescent dyes, Propidium iodide (PI; MP Biomedicals, Solon, OH) and Hoechst 33342 (Invitrogen, Eugene, OR) reaching a final concentration of 1 μg/ml.69 The fluorescence signal emitted from each individual fluorophore was captured in two separate channels, according with the dye emission properties. Images were acquired directly from the tissue culture microplate utilizing a BD Pathway 855 Bioimager system (BD Biosciences Rockville, MD). To obtain sufficient numbers of ROIs (Regions of Interest), for statistical analysis purposes, images from nine contiguous image fields (3×3 montages) were acquired per well utilizing a 20× objective.67 Data analysis determining the percentage of dead cells from each individual well was achieved by using BD AttoVision™ v1.6.2 software.69 Several controls were included in each experiment. DMSO was used as a solvent control at the same final concentration of 0.5 v/v used with the test compounds. Untreated cells were also used as negative controls, as an indicator of cell viability during the incubation period. Cell treated with 600 μM hydrogen peroxide were included as positive controls for cytotoxicity activity. The IC50 values were determined as previously described.70 Briefly, the average expressed as a percentage from triplicates of the two compound concentrations closest to the 50% cytotoxicity value was plotted against chemical compound concentration in a xy (scatter) chart function (Microsoft Excel). The best-fit regression line and its equation was used to calculate the concentration of chemical compound required to damage the plasma membrane of 50% of the cell population, as compared to solvent treated cells (DMSO). On the basis of this approach there is not standard deviation, because the IC50 values were calculated by linear extrapolation. Potentially the two molecules of THF released by dissolution of the new compounds in DMSO:Buffer could have a cytotoxic effect.71 However the amount of THF released is within the low micromolar range or lower. LD50 values for THF have been reported for concentrations in the millimolar ranges.71
4. Interaction of metal complexes with CT DNA by Thermal Denaturation (Melting Point) Experiments
Melting curves were recorded in media containing 50 mM NaClO4 or 25 mM NaClO4 (Tables 3 and 4) and 5 mM Tris/HCl buffer (pH=7.39). The absorbance at 260 nm was monitored for solutions of Calf Thymus DNA (70 μM) before and after incubation with a solution of the drug under study (35 μM in Tris/HCl buffer) for 1 h or 24 hours (Tables 3 and 4) at room temperature. The temperature was increased by 2 °C/min between 25 and 60 °C, by 1 °C/min between 60 and 70 °C, by 0.5° C/min between 70 and 85 °C and by 1 °C/min between 85 and 98 °C.
5. Interaction of metal complexes with CT DNA by Circular Dichroism Spectroscopy
Stock solutions (5 mM) of each complex were freshly prepared in DMSO-H2O prior to use. The right volume of those solutions was added to 3 mL samples of an also freshly prepared solution of CT DNA (195 μM) in Tris/HCl buffer (5 mM Tris/HCl, 50 mM NaClO4, pH = 7.39) to achieve molar ratios of 0.1, 0.25, 0.5, 1.0 drug/DNA. The samples were incubated at 37 °C for a period of 20 h. All CD spectra of DNA and of the DNA-drug adducts were recorded at 25 °C over a range 220–420 nm and finally corrected with a blank and noise reduction. The final data is expressed in molar ellipticity (millidegrees).
Supplementary Material
Figure 6.
CD spectra of CT incubated for 24 h at 37 °C with Ti-Pd 4 (A) and Ti-Pt 7 (B) derivatives at 0, 0.1, 0.25, 0.5 and 1 ratios.
Acknowledgments
We thank the financial support of a grant from the National Institute of General Medical Sciences (NIGMS), SC2GM082307 (M.C.) and a travel scholarship (J.F.G.-P.) from Becas Internacionales Bancaja-Universidad de Alcala 2009 (Spain). We also thank the staff of the Cell Culture and High Throughput Screening (HTS) Core Facility for services and facilities provided. This core facility is supported by grant 5G12RR008124 to the Border Biomedical Research Center (BBRC), granted to the University of Texas at El Paso from the National Center for Research Resources (NCRR) of the NIH. E.R.E was supported by NIGMS RISE grant 2R25GM069621-09.
Footnotes
This paper is dedicated to Prof. Roberto Sánchez-Delgado on the occasion of his 61st birthday
SUPPORTING INFORMATION PARAGRAPH. Tables of the stability of compounds 1–9 by 31P{1H} NMR spectroscopy (1:99 d6-DMSO:D2O) and for Cp2TiCl2 at different pH as well as selected spectra and complete cytotoxicity data for the compounds and controls are available on line at…..
References
- 1.Harding MM, Mokdsi G. Current Med Chem. 2000;7:1289. doi: 10.2174/0929867003374066.and refs. therein.
- 2.Köpf-Maier P, Köpf H. Angew Chem, Int Ed Engl. 1979;18:477. doi: 10.1002/anie.197904771. [DOI] [PubMed] [Google Scholar]
- 3.Berdel WE, Schmoll, Scheulen ME, Korfel A, Knoche MF, Harstrick A, Bach F, Saβ G. J Cancer Res Clin Oncol. 1994;120(Suppl):R172. [Google Scholar]
- 4.Abeysinghe PM, Harding MM. Dalton Trans. 2007:3474. doi: 10.1039/b707440a.and refs. therein.
- 5.Olszewski U, Hamilton G. Anti-Cancer Agents in Med Chem. 2010;10:320. doi: 10.2174/187152010791162306.and refs. therein.
- 6.Lummen G, Sperling H, Lubolt H, Otto T, Rubben H. Cancer Chemother Pharmacol. 1998;42:415. doi: 10.1007/s002800050838. [DOI] [PubMed] [Google Scholar]
- 7.Kroger N, Kleeberg UR, Mross K, Edler L, Sab G, Hossfeld D. Onkologie. 2000;23:288. [Google Scholar]
- 8.Chen X, Zhou L. J Molec Struct. 2010;940:45. [Google Scholar]
- 9.Ravera M, Cassino C, Monti E, Gariboldi M, Osella D. J Inorg Biochem. 2005;99:2264. doi: 10.1016/j.jinorgbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Wu QS, Ding YP, Chu M, Huang ZM, Hu W. Eur J Pharma Biopharma. 2010;76:413. doi: 10.1016/j.ejpb.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Guo M, Sun H, McArdle HJ, Gambling L, Sadler PJ. Biochem. 2000;39:10023. doi: 10.1021/bi000798z. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Sadler P. Dalton Trans. 2000:7. [Google Scholar]
- 13.a) Parker Siburt CJP, Lin EM, Brandt SJ, Tinoco AD, Valentine AM, Crumbliss AL. J Inorg Biochem. 2010;104:1006. doi: 10.1016/j.jinorgbio.2010.04.004. [DOI] [PubMed] [Google Scholar]; B) Tinoco AD, Incarvito CD, Valentine AM. J Am Chem Soc. 2007;129:3444. doi: 10.1021/ja068149j. [DOI] [PubMed] [Google Scholar]
- 14.Sarsam SW, Nutt DR, Strohfeldt K, Kimberly A. Metallomics: Int Biomet Sci. 2011;3:152. doi: 10.1039/c0mt00041h. [DOI] [PubMed] [Google Scholar]
- 15.a) Deng C, Zhou L. Struct Chem. 2010;21:735. [Google Scholar]; b) Erxleben A, Claffey J, Tacke M. J Inorg Biochem. 2010;104:390. doi: 10.1016/j.jinorgbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Strohfeldt K, Tacke M. Chem Soc Rev. 2008;37:1174. doi: 10.1039/b707310k. [DOI] [PubMed] [Google Scholar]
- 17.Caruso F, Rossi M. Mini-Rev Med Chem. 2004;4:49. doi: 10.2174/1389557043487565. refs. therein. [DOI] [PubMed] [Google Scholar]
- 18.Meléndez E. Crit Rev Oncol Hematol. 2002;42:309. doi: 10.1016/s1040-8428(01)00224-4. and refs. therein. [DOI] [PubMed] [Google Scholar]
- 19.Cuffe S, Dowling CM, Claffey J, Pampillon C, Hogan M, Fitzpatrick JM, Carty MP, Tacke M, Watson RWG. The Prostate. 2010 doi: 10.1002/pros.21227. [DOI] [PubMed] [Google Scholar]
- 20.Pizarro AM, Habtemariam A, Sadler PJ. Topics in Organomet Chem. 2010;32:21. [Google Scholar]
- 21.Allen OR, Croll L, Gott AL, Knox RJ, McGowan PC. Organometallics. 2004;23:60. [Google Scholar]
- 22.a) Kaluderovic GN, Tayurska V, Paschke R, Prashar S, Fajardo M, Gómez-Ruíz S. Appl Organomet Chem. 2010;24:656. [Google Scholar]; b) Kaluderovic GN, Pérez-Quintanilla D, Sierra I, Prashar S, del Hierro I, Zizak Z, Juranic ZD, Fajardo M, Gómez-Ruíz S. J Mat Chem. 2010;20:806. doi: 10.1002/chem.200900151. [DOI] [PubMed] [Google Scholar]
- 23.Oberschmidt O, Hanauske AR, Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M. Anti-Cancer Drugs. 2007;18:317. doi: 10.1097/CAD.0b013e3280115f86. [DOI] [PubMed] [Google Scholar]
- 24.Beckhove P, Oberschmidt O, Hanauske AR, Pampillón C, Schirrmacher V, Sweeney NJ, Strohfeldt K, Tacke M. Anti-Cancer Drugs. 2007;18:311. doi: 10.1097/CAD.0b013e328010a6f7. [DOI] [PubMed] [Google Scholar]
- 25.Bannon JH, Fitchner I, O’Neill A, Pampillón C, Sweeney NJ, Strohfeldt K, Watson RW, Tacke M, Mc Gee MM. British J Cancer. 2007;97:1234. doi: 10.1038/sj.bjc.6604021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichtner I, Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M. Anti-Cancer Drugs. 2006;17:333. doi: 10.1097/00001813-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hogan M, Gleeson B, Tacke M. Lett Drug Design & Discov. 2010;7:310. [Google Scholar]
- 28.O’Connor K, Gill C, Tacke M, Rehmann FJK, Strohfeldt K, Sweeney N, Fitzpatrick JM, Watson RWG. Apoptosis. 2006;11:1205. doi: 10.1007/s10495-006-6796-1. [DOI] [PubMed] [Google Scholar]
- 29.Hogan M, Gleeson B, Tacke M. Organometallics. 2010;29:1032. [Google Scholar]
- 30.Claffey J, Mueller-Bunz H, Tacke M. J Organomet Chem. 2010;695:2105. [Google Scholar]
- 31.Immel TA, Martin JT, Duerr CJ, Groth U, Huhn T. J Inorg Biochem. 2010;104:863. doi: 10.1016/j.jinorgbio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Eger S, Immel TA, Claffey J, Muller-Bunz H, Tacke M, Groth U, Hunh T. Inorg Chem. 2010;49:1292. doi: 10.1021/ic9022163. [DOI] [PubMed] [Google Scholar]
- 33.Claffey J, Hogan M, Muller-Bunz H, Pampillon C, Tacke M. ChemMedChem. 2008;3:729. doi: 10.1002/cmdc.200700302. [DOI] [PubMed] [Google Scholar]
- 34.Claffey J, Deally A, Gleeson B, Patil S, Tacke M. Appl Organomet Chem. 2010;24:675. [Google Scholar]
- 35.Gao LM, Vera JL, Matta J, Meléndez E. J Biolog Inorg Chem. 2010;15:851. doi: 10.1007/s00775-010-0649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selected recent examples: Zhang J, Wang L, Xing Z, Liu D, Sun J, Li X, Zhang Y. Anti-Cancer Agents Med Chem. 2010;10:272. doi: 10.2174/187152010791162270.Mattsson J, Govindaswamy P, Renfrew AK, Dyson PJ, Stepnicka P, Suss-Fink G, Therrien B. Organometallics. 2009;28:4350.Therrien B, Suss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew Chem, Int Ed. 2008;47:3773. doi: 10.1002/anie.200800186.Schmitt F, Govindaswamy P, Suss-Fink G, Ang WH, Dyson PJ, Juillerat-Jeanneret L, Therrien B. J Med Chem. 2008;51:1811. doi: 10.1021/jm701382p.Mendoza-Ferri MG, Hartinger CG, Eichinger RE, Stolyarova N, Severin K, Jakupec MA, Nazarov AA, Keppler BK. Organometallics. 2008;27:2405.Gabbiani C, Casini A, Messori L, Guerri A, Cinellu MA, Minghetti G, Corsini M, Rosani C, Zanello P, Arca M. Inorg Chem. 2008;47:2368. doi: 10.1021/ic701254s.de Hoog P, Boldron C, Gamez P, Sliedregt-Bol K, Roland I, Pitie M, Kiss R, Meunier B, Reedijk J. J Med Chem. 2007;50:3148. doi: 10.1021/jm0614331.
- 37.a) Pelletier F, Comte V, Massard A, Wenzel M, Toulot S, Richard P, Picquet M, Le Gendre P, Zava O, Edafe F, Casini A, Dyson PJ. J Med Chem. 2010;53:6923. doi: 10.1021/jm1004804. [DOI] [PubMed] [Google Scholar]; b) Wedgwood JL, Kresinski RA, Merry S, Platt AWG. J Inorg Biochem. 2003;95:149. doi: 10.1016/s0162-0134(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 38.Recent reviews and new trends in the preparation/mode of action of Pt cytotoxic compounds: Berners-Price S. Angew Chem Int Ed. 2011;50:804. doi: 10.1002/anie.201004552.Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113. doi: 10.1039/c0dt00292e.Mangrum JB, Farrell NP. Chem Commun. 2010;46:6640. doi: 10.1039/c0cc01254h.Olszewski U, Hamilton G. Anti-Cancer Agents Med Chem. 2010;10:293. doi: 10.2174/187152010791162306.Gibson D. Dalton Trans. 2009:10681. doi: 10.1039/b918871c.
- 39.For example: Kozelka JI, Segal E, Bois C. J Inorg Biochem. 1992;47:67. doi: 10.1016/0162-0134(92)84043-m.and refs. therein.
- 40.Selected recent reviews: Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L. Med Res Revs. 2010;30:550. doi: 10.1002/med.20168.Ronconi L, Aldinucci D, Dou QP, Fregona D. Anticancer Agents Med Chem. 2010;10:283. doi: 10.2174/187152010791162298.Pacheco E, Tiekink E, Whitehouse M. In: Gold Chemistry. Mohr F, editor. Ch 6. Wiley-VCH; 2009. p. 283.Bindoli A, Rigobello MP, Scutari G, Gabbiani C, Casini A, Messori L. Coord Chem Rev. 2009;253:1692.Sun RWY, Che CM. Coord Chem Revs. 2009;253:1682.Ott I. Coord Chem Rev. 2009;253:1670.
- 41.a) Shaik N, Martínez A, Augustin I, Giovinazzo H, Varela-Ramírez A, Aguilera RJ, Sanaú M, Contel M. Inorg Chem. 2009;48:1577. doi: 10.1021/ic801925k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vela L, Contel M, Palomera L, Azaceta G, Marzo I. J Inorg Biochem. doi: 10.1016/j.jinorgbio.2011.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Elie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramírez A, Aguilera RJ, Ovalle R, Contel M. Eur J Inorg Chem. 2009:3421. doi: 10.1002/ejic.200900279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.For example: Gao E, Zhu M, Liu L, Huang Y, Wang L, Shi S, Chuyue ZW, Sun Y. Inorg Chem. 2010;49:3261. doi: 10.1021/ic902176e.Prast-Nielsen S, Celbula M, Pader I, Arner ESJ. Free Rad Biol Med. 2010;49:1765. doi: 10.1016/j.freeradbiomed.2010.09.008.Vrzal R, Starha P, Dvorak Z, Travniceck Z. J Inorg Biochem. 2010:1130. doi: 10.1016/j.jinorgbio.2010.07.002.Spencer J, Casini A, Zava O, Rathnam RP, Velhanda SK, Pfeffer M, Callear SK, Hursthouse MB, Dyson PJ. Dalton Trans. 2009:10731. doi: 10.1039/b912096c.and refs. therein.
- 43.During revision of this manuscript a paper on related cytotoxic titanocene TiAu and Ti2Au derivatives was published on line. Wenzel M, Bertrand B, Eymin M-J, Comte V, Harvey JA, Richard P, Groessl M, Zava O, Amrouche H, Harvey PD, Le Gendre P, Picquet M, Casini A. Inorg Chem. doi: 10.1021/ic201155y. In press (dx.doi.org/10.1021/ic201155y)
- 44.a) LeBlanc C, Moise C, Maisonnat A, Poilblanc R, Charrier C, Mathey F. J Organomet Chem. 1982;231:C43. [Google Scholar]; b) Graham TW, Llamazares A, McDonald R, Cowie M. Organometallics. 1999;18:3490. [Google Scholar]; c) Kettenbach RT, Bonrath W, Butenschoen H. Chem Ber. 1993;126:1657. [Google Scholar]
- 45.Cobley Christopher J, Pringle Paul G. Inorg Chim Acta. 1997;265:107. and refs. therein. [Google Scholar]
- 46.Delgado E, Forniés J, Hernández E, Lalinde E, Mansilla N, Moreno MT. J Organomet Chem. 1995;494:261. [Google Scholar]
- 47.Graham TW, Llamazares A, McDonald R, Cowie M. Organometallics. 1999;18:3502. [Google Scholar]
- 48.Baker J, Jarzecki AA, Pulay P. J Phys Chem A. 1998:1412. [Google Scholar]
- 49.Modkdsi G, Harding MM. Metal Based Drugs. 1998;5:247. doi: 10.1155/MBD.1998.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IC50 obtained by Mosmann Tests (MTT reduction). Carreira M, Sanaú M, Calvo Sanjuán R, Marzo I, Contel M. Unpublished results.
- 51.a) Cardonna JP, Kippard SJ, Gait MJ, Singh M. J Am Chem Soc. 1982;104:5793. [Google Scholar]; b) Dabrowiak JC. Metals in medicine. John Wiley and Sons; 2009. [Google Scholar]
- 52.Liu HK, Sadler P. Acc Chem Res. 2011;44:349. doi: 10.1021/ar100140e. [DOI] [PubMed] [Google Scholar]
- 53.For example: Ruíz J, Cutillas N, Vicente C, Villa MD, López G, Lorenzo J, Avilés FX, Moreno V, Bautista D. Inorg Chem. 2005;44:7365. doi: 10.1021/ic0502372.
- 54.a) Mansori-Torshizi H, I-Moghaddam M, Divsalar A, Saboury AA. Biorg Med Chem. 2008;16:9616. doi: 10.1016/j.bmc.2008.08.021. [DOI] [PubMed] [Google Scholar]; b) Quiroga AG, Pérez JM, López-Solera I, Masaguer JR, Luque A, Roman P, Edwards A, Alonso C, Navarro-Ranninger C. J Med Chem. 1998;41:1399. doi: 10.1021/jm970520d. [DOI] [PubMed] [Google Scholar]; c) Paul AK, Mansuri-Torshizi H, Srivastava TS, Chavan SJ, Chitnis MP. J Inorg Biochem. 1993;50:9. doi: 10.1016/0162-0134(93)80010-7. [DOI] [PubMed] [Google Scholar]
- 55.Blank CE, Dabrowaik JC. J Inorg Biochem. 1984;21:21. doi: 10.1016/0162-0134(84)85036-9. [DOI] [PubMed] [Google Scholar]
- 56.Harding MM, Murray JH. J Med Chem. 1994;37:1936. doi: 10.1021/jm00039a005.and refs thereinChristodoulou CV, Eliopoulos AG, Young LS, Hodgkins L, Ferry DR, Kerr DJ. British J Cancer. 1998;77:2088. doi: 10.1038/bjc.1998.352.
- 57.a) Mascini M, Bagni G, Di Pietro ML, Ravera M, Baracco S, Osella D. BioMetals. 2006;19:409. doi: 10.1007/s10534-005-4340-3. [DOI] [PubMed] [Google Scholar]; b) Ravera M, Gabano E, Baracco S, Osella D. Inorg Chim Acta. 2009;362:1303. [Google Scholar]
- 58.Kopf Maier P. J Struct Biol. 1990;105:35. doi: 10.1016/1047-8477(90)90096-u. [DOI] [PubMed] [Google Scholar]
- 59.a) Mokdsi G, Harding MM. J Organomet Chem. 1998;565:29. [Google Scholar]; b) Yang P, Guo M. Coord Chem Rev. 1999;185–186:189. [Google Scholar]
- 60.Guo ML, Guo ZJ, Sadler PJ. J Biol Inorg Chem. 2001;6:698. doi: 10.1007/s007750100248. [DOI] [PubMed] [Google Scholar]
- 61.Fox K. Drug-DNA Interact Protocols. 1997;90:95. [Google Scholar]
- 62.a) Moreno V, Lorenzo J, Aviles FX, García MH, Ribeiro JP, Morais TS, Florindo P, Robalo MP. Bioinorg Chem Appl. 2010 doi: 10.1155/2010/936834. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martínez A, Rajapakse CSK, Sanchez-Delgado RA, Varela-Ramírez A, Lema C, Aguilera RJ. J Inorg Biochem. 2010;104:967. doi: 10.1016/j.jinorgbio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macquet JP, Butour JL. Eur J Biochem. 1978;83:375. doi: 10.1111/j.1432-1033.1978.tb12103.x. [DOI] [PubMed] [Google Scholar]
- 64.Usón R, Laguna A, Laguna M. Inorg Synth. 1989;26:85. [Google Scholar]
- 65.Drew D, Doyle JR, Shaver AG. Inorg Synth. 2007;13:52. [Google Scholar]
- 66.Baker MV, Brown DH, Simpson PV, Skelton BW, White AH, Williams CC. J Organomet Chem. 2006;691:5845. [Google Scholar]
- 67.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa R, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JN, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.1. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- 68.a) Peterson KA, Puzzarini C. Theor Chem Acc. 2005;114:283. [Google Scholar]; b) Peterson KA, Figgen D, Dolg M, Stoll H. J Chem Phys. 2007;126:124101. doi: 10.1063/1.2647019. [DOI] [PubMed] [Google Scholar]; c) Schuchardt KL, Didier BT, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL. J Chem Inf Model. 2007;47:1045. doi: 10.1021/ci600510j. [DOI] [PubMed] [Google Scholar]
- 69.Lema C, Varela-Ramírez A, Aguilera RJ. Submitted for publication. [Google Scholar]
- 70.Varela-Ramírez A, Costanzo M, Carrasco YP, Pannell KH, Aguilera RJ. Cell Biol Toxicol. 2011;3:159. doi: 10.1007/s10565-010-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moody DE. Drug Chem Toxicol. 1991;14:319. doi: 10.3109/01480549109011637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.