Abstract
Background
The ApcMin/+ mouse, an animal model of colorectal cancer and cachexia, has a heterologous mutation in the Apc tumor suppressor gene, predisposing the mouse to intestinal and colon tumor development. This mouse develops intestinal polyps by ~4 weeks of age, and loses body weight gradually between ~14 and ~20 weeks of age. The strengths of this cachexia model derive from several features that mimic human cancer, including a gradual increase in tumor burden, chronic inflammation, and anemia. Little is known about the role of gut barrier dysfunction and endotoxemia in the development of cancer cachexia. We sought to determine how gut permeability and resultant endotoxemia change with the progression of cachexia.
Methods
Intestinal gut barrier integrity was assessed by permeability to FITC-dextran (MWav = 4,000 kDa; FD4). Plasma glucose and triglycerides were measured by enzymatic assays, IL-6 by ELISA, and endotoxin by the limulus amoebocyte assay. Body temperature was measured using a rectal probe.
Results
Progression of cachexia was accompanied by development of gut barrier dysfunction (permeability to FD4), hypertrophy of mesenteric lymph nodes, and an increase in plasma endotoxin concentration. Changes in blood glucose and glucose tolerance, plasma IL-6, triglycerides, and body temperature were characteristic of endotoxemia.
Conclusion
We propose a role for gut barrier dysfunction (GBD) and subsequent endotoxemia in the development of inflammation and progression of cachexia in the ApcMin/+ mouse.
Keywords: cachexia, colorectal cancer, endotoxin, gut barrier dysfunction, gut permeability, inflammation
1.1 INTRODUCTION
Cachexia, a condition characterized by severe wasting of muscle and adipose tissue, is a common complication of late-stage cancers, especially cancers of the gastrointestinal system. It contributes to at least 20% of deaths from cancer [1]. Cachexia is a metabolic syndrome associated with underlying illness causing a loss of muscle mass and fat mass [2]. It is commonly associated with increases in acute phase proteins and pro-inflammatory cytokines in plasma, particularly TNF-∝ and IL-6 [3–5]. The ApcMin/+ mouse is an animal model for colon cancer research [6, 7], which has a mutation in the Apc tumor suppressor gene. This mouse develops intestinal polyps, beginning at ~4 weeks of age and begins to develop a progressive cachexia between 12 and ~20 weeks of age, that culminates in a 20–25% decrease in body weight, The progression of cachexia is associated with an increased concentration of plasma IL-6, and cachexia is inhibited in an IL-6 knockout (ApcMin/+ × IL-6−/−) mouse [8] despite the presence of intestinal and colon tumors. The cachectic response can be restored by systemic IL-6 over-expression in the ApcMin/+ × IL-6−/− mouse, while IL-6 over-expression in C57BL/6 mice does not induce cachexia. Thus, IL-6 is necessary, but not sufficient, for induction of cachexia in mice.
Gut barrier dysfunction (GBD), characterized by breakdown and leakage of the gut epithelial barrier, leads to systemic inflammation because of entry of bacterial cell wall components (endotoxin), also known as lipopolysaccharide (LPS), or intact bacteria into the circulation [9]. The resulting inflammation is a common problem in critical care medicine and can lead to multiple organ dysfunction syndrome [10]. Endotoxemia may be caused by a variety of stresses [11], burn injury [12], traumatic brain injury [13] or stroke [14], chronic heart failure [15], pancreatitis [16, 17], and even strenuous exercise [18, 19]. The gut receives a substantial fraction of cardiac output and impairment of the blood supply leads to hypoxia, which may be a common mediator of the development of gut barrier dysfunction and endotoxemia. Indeed, hemorrhagic shock [20] followed by resuscitation (HS/R) induces GBD in mice [21], as measured by increased intestinal permeability to FITC-dextran and translocation of bacteria to mesenteric lymph nodes. Like the ApcMin/+ model, development of cachexia in the HS/R model, GBD is dependent on increased circulating IL-6 concentration; IL-6 knock-out mice do not develop GBD after HS/R [21]. The observation that IL-6 is required, but not sufficient, for development of cachexia in both the ApcMin/+ [8] and HS/R models [21], and that GBD is implicated in the pathogenesis of cachexia in the HS/R model led us to investigate the possible role of GBD and endotoxemia in development and progression of cachexia in the ApcMin/+mouse.
1.2 MATERIALS AND METHODS
1.2.1 Animals
C57BL/6 and ApcMin/+ mice were originally purchased from Jackson Laboratories (Bar Harbor, ME), and breeding was continued at the University of South Carolina’s Animal Resource Facility, as previously described [22]. The room was maintained on a 12:12 light: dark cycle with the light period starting at 0700. Mice were provided standard rodent chow (Harlan Teklad Rodent Diet, #8604, Madison, WI) and water ad libitum. Body weights were measured weekly. Male mice (n = 5–10 animals) were used in each group for all experiments. All animal experimentation was approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
1.2.2 Tissue sampling and physiological measurements
Mice were anesthetized with a ketamine/xylazine/acepromazine cocktail (1.4 ml/kg body weight), and tissues were removed, weighed and frozen at −80 °C until further analysis. Blood samples were collected in heparinized capillary tubes from the retroorbital sinus under brief isoflurane anesthesia.
Rectal temperature was measured using a rectal probe designed for mice (Thermalert TH-5, Physitemp, Clifton, NJ). Temperature was measured at the same time of day bi-weekly for the duration of the study.
Gut barrier integrity was assessed by permeability to FITC-dextran (MWav = 4,000; FD4). The FD4 was administered by gavage (600 mg/kg BW; 125 mg/ml of phosphate-buffered saline) to fasted mice. Plasma was sampled prior to the gavage and 1 hr after the procedure and measured for fluorescence, as described by Yang et al.[21]
1.2.3 Biochemical assays
Plasma endotoxin was measured by a chromogenic Limulus Amoebocyte Lysate (LAL) assay (product no. HIT302; Hycult Biotech, Plymouth Meeting, PA). Plasma triglycerides were measured using a colorimetric assay (Thermo Scientific), and IL-6 using a mouse IL-6 ELISA (Invitrogen), performed according to manufacturer’s instructions. An intraperitoneal glucose tolerance test was performed after an overnight fast by injecting a 20% glucose solution (2 g glucose/kg body weight). Glucose was measured at 0, 15, 30, 60, and 90 minutes after injection using a Bayer (Mishawaka, IN) Ascencia Contour glucometer.
1.2.4 IL-6 over-expression
At 12wk of age mice were electroporated with either an empty vector (vector) or an IL-6 plasmid (IL-6), as previously described [8]. The IL-6 plasmid, driven by the cytomegalovirus (CMV) promoter, was used to increase endogenous IL-6 production in the mice. The mice were anesthetized with a mixture of isoflurane and oxygen during the procedure. While unconscious the right leg was shaved and cleaned with alcohol. A small incision was made over the quadriceps muscle and 50µ of either control vector or IL-6 plasmid was injected into the muscle. To promote uptake of the plasmid into the myofibers a series of eight 100 volt pulses lasting 50 ms each were used on the quadriceps muscle. Each pulse consisted of a 1 second train of square bipolar pulses delivered every other second. Each train consists of 1000 pulses of 200 microseconds in length. The skin was then closed with a wound clip. Mice were then sacrificed at 14wk of age.
1.2.5 Statistical analysis
Repeated measures ANOVA was used to examine changes in body weight and rectal temperature over time in mice categorized by stage of cachexia. One-way ANOVAs or independent t-tests were used to determine significance for all other variables. Post-hoc analyses were performed with Student-Newman-Keuls methods. Significance was set at p<0.05.
1.3 RESULTS
1.3.1 Progression of cachexia ApcMin/+ mice
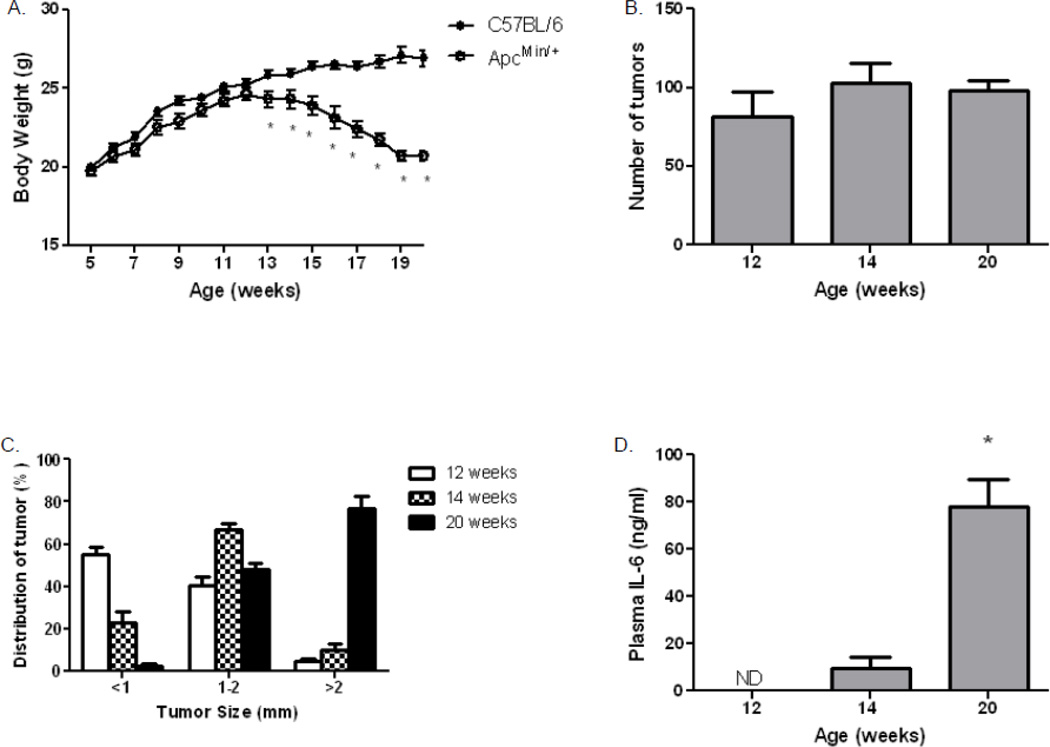
ApcMin/+ mice develop tumors by ~4 weeks of age, but continue to grow in parallel with C57BL/6 mice until ~12 weeks, when they begin to lose body weight (Fig. 1A). By 20 weeks of age, ApcMin/+ mice typically lose 20–25% of body mass, compared to either their maximal weight or the C57BL/6 control mice (Fig. 1A). Polyp number reaches a plateau at ~12 weeks (Fig. 1B), Tumor size continues to increase during the 12th–20th week, as the ApcMin/+ mice develop cachexia; eventually ~80% of the polyps grow to 2 mm or greater in diameter (Fig. 1C). The weight loss and increase in tumor size is accompanied by a significant increase in plasma IL-6 concentration (Fig. 1D). As previously reported, IL-6, a marker of inflammation, increases with severity of cachexia and age in the ApcMin/+ mice [23], consistent with a strong inflammatory response during cachexia [8, 24].
Figure 1. Changes in body weight and tumor distribution in ApcMin/+ mice.
A) Body weight was measured weekly, C57BL/6 (●, N = 10) and ApcMin/+ (○, N = 9) mice. (B and C) Intestines were evaluated in mice that were sacrificed at 12, 14 and 20 weeks of age. Polyps were counted and categorized by diameter. D) Changes in IL-6 concentration during progression of cachexia. Data are expressed as means ± SE. *p<0.05 indicates significant difference from controls.
1.3.2 Gut Barrier Permeability increases during cachexia
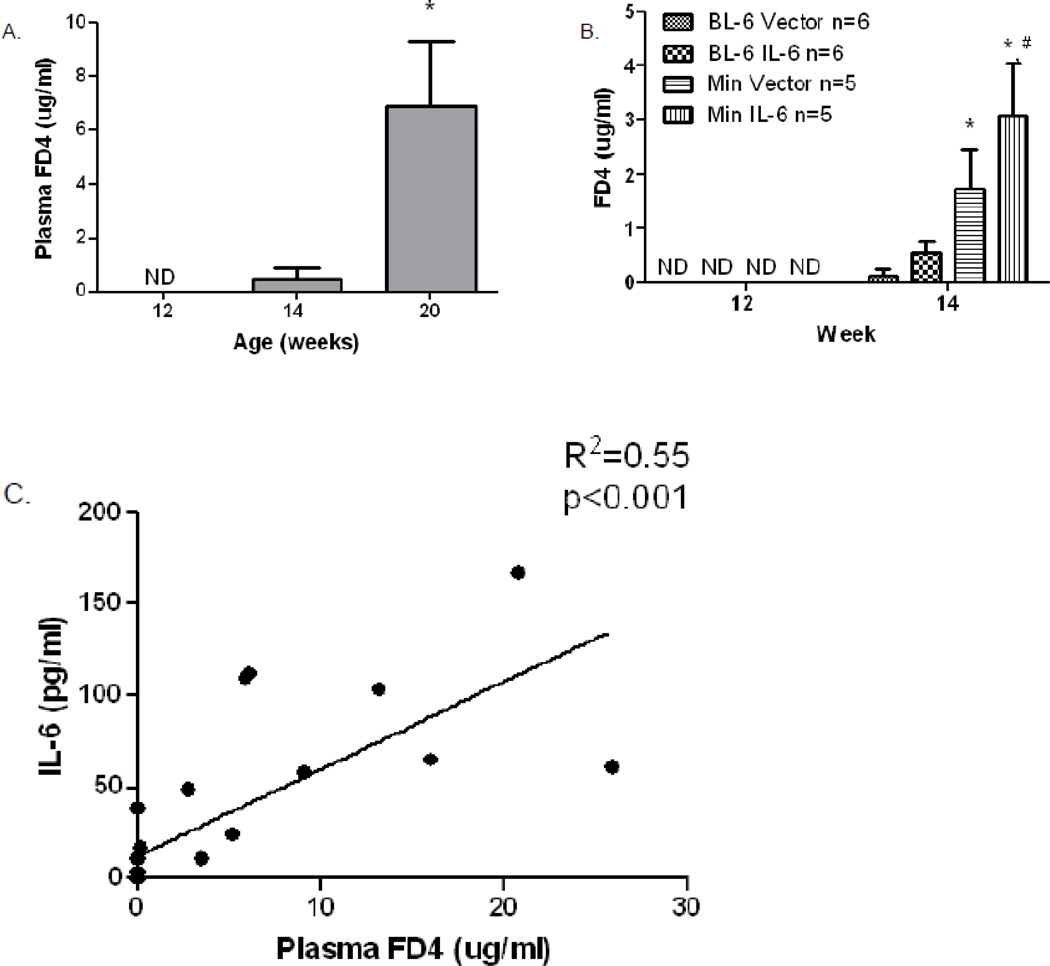
The integrity of the gut barrier can be assessed by measuring its permeability to neutral hydrophilic polymers, such as FD4. Gut barrier permeability was negligible in C57BL/6 controls throughout the 20 weeks of the study, while permeability to FD4 begins to increase in ApcMin/+ mouse, starting at 12–14 weeks of age (Fig. 2A), corresponding to the time of onset of cachexia (Fig. 1A). By 20 weeks of age, which corresponds with the severely catechetic condition, there was a significant increase in gut permeability in ApcMin/+ mice. There was a strong correlation between plasma IL-6 and intestinal permeability (r2 = 0.55, p <0.001) in untreated ApcMin/+ mice at 20 weeks (Fig. 2C) and a significant correlation between the number of large polyps (> 2 mm) and intestinal permeability (r2 = 0.38; p = 0.003). Intestinal permeability also increased 3-fold, compared to vector-injected controls, when IL-6 was over-expressed in ApcMin/+ mice for 2 weeks at the onset of cachexia (12 weeks of age) (Fig. 2B), consistent with a role for IL-6 in development of GBD. There was also a measurable increase in FD4 permeability in the vector-injected mice, compared to C57Bl/6 controls, at 14 weeks, which is attributable to the normal increase in intestinal permeability in ApcMin/+ at this time.
Figure 2. Gut permeability (barrier dysfunction) increases during development of cachexia in ApcMin/+ mice.
A) Measurements were taken at 12 (n = 8), 14 (n = 7) and 20 (n = 10) weeks of age. B) Measurements were taken before and after two weeks of IL-6 over-expression (n=5–6 per group). C) IL-6 correlates with increases in gut permeability in mice measured at 12, 14, and 20 weeks of age. Serum fluorescein dextran concentrations (λex = 485; λem = 535 nm) were measured at one hour after gavage. Data are expressed as means ± SE. **p<0.05, indicates significant difference from other groups; *p<0.05 compared with 14 week BL-6 Vector; # p<0.05 compared with 14 week BL-6 IL-6; ND = not detected.
1.3.3 Endotoxemia develops during cachexia
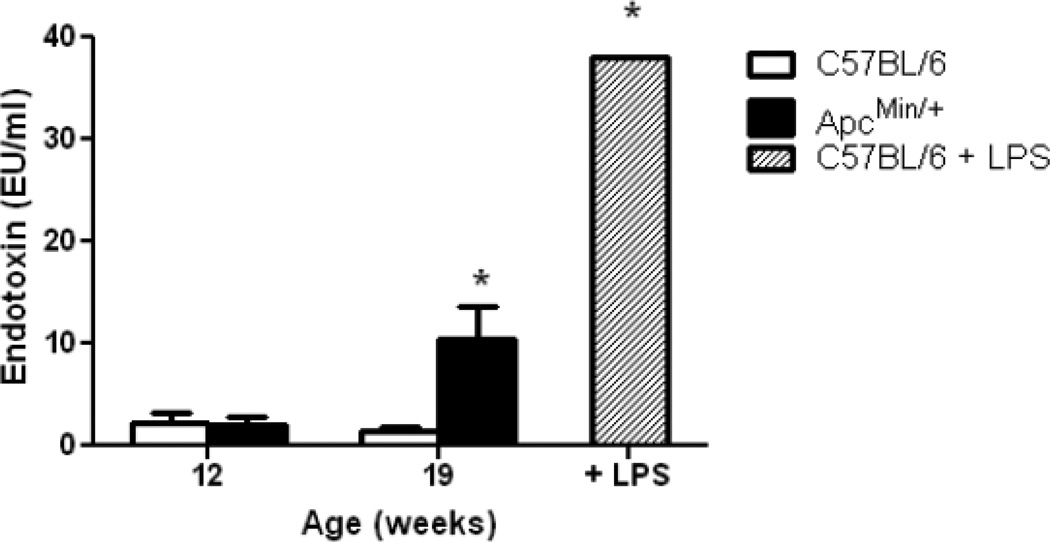
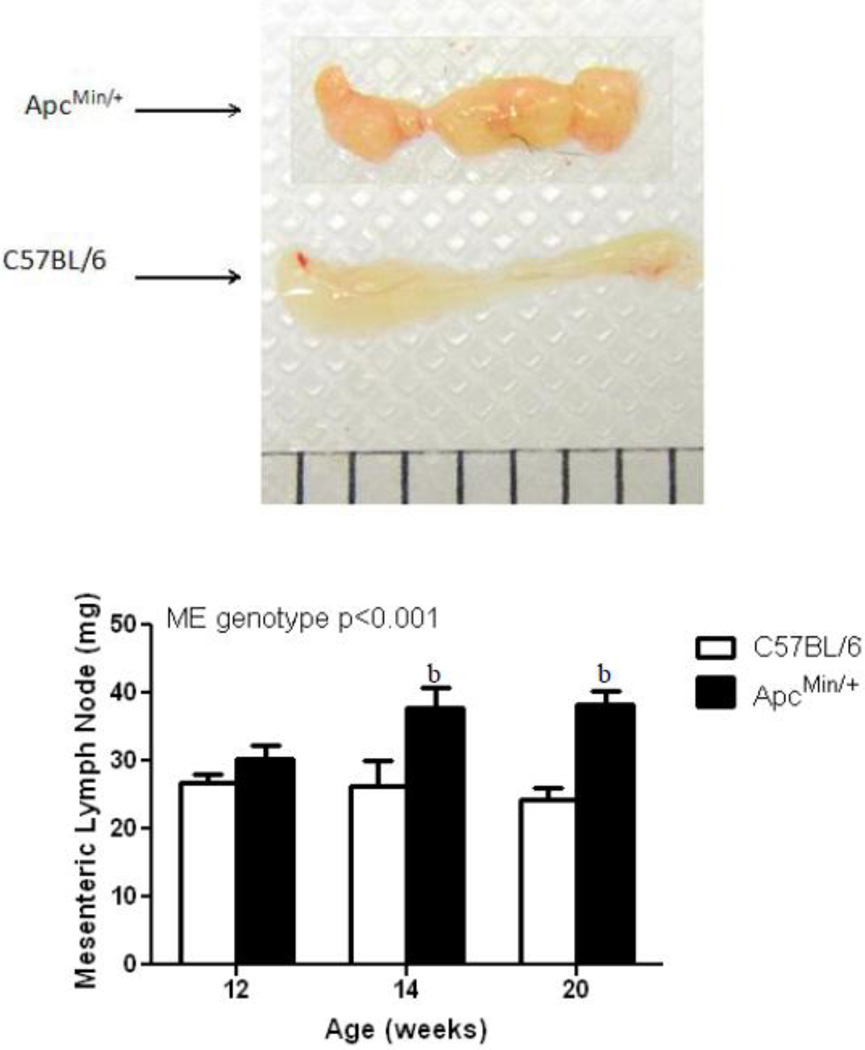
Trace levels of endotoxin are detectable in plasma of control mice, and similar levels were detected in pre-cachectic ApcMin/+ mice at 12 weeks of age. However, endotoxin increased by ~5-fold in severely cachectic animals at 19 weeks of age (Fig. 3), corresponding to the increase in gut permeability to FD4. The bar on the right in this figure represents plasma endotoxin concentration at 12 hours after intraperitoneal injection of 250 µg LPS into a C57BL/6 mouse at 20 weeks of age. Intestinal lymph nodes were also significantly enlarged between 12 and 14 weeks of age and remained enlarged through 20 weeks (Fig. 4), possibly as a consequence of LPS or bacterial penetration into the mesenteric lymphatic system.
Figure 3. Plasma endotoxin concentration increases during development of cachexia in ApcMin/+ mice.
N = 7–9 per group. Right hand bar represents plasma endotoxin concentration in C57BL/6 mice at 12 hr after intraperitoneal injection of 250µg of endotoxin (N = 2). Data are means ± SE. * p<0.05. vs. all other groups.
Figure 4. Mesenteric lymph nodes are enlarged in ApcMin/+ compared to C57BL/6 mice at 12, 14, and 20 weeks of age.
Mesenteric lymph nodes were taken in C57BL/6 and ApcMin/+ mice at 12 (n = 8/8), 14 (n = 8/8) and 20 (n = 9/6) weeks of age. Data are expressed as means ± SE. bp<.05 compared to 12 week ApcMin/+.
1.3.4 Hypothermia develops during cachexia
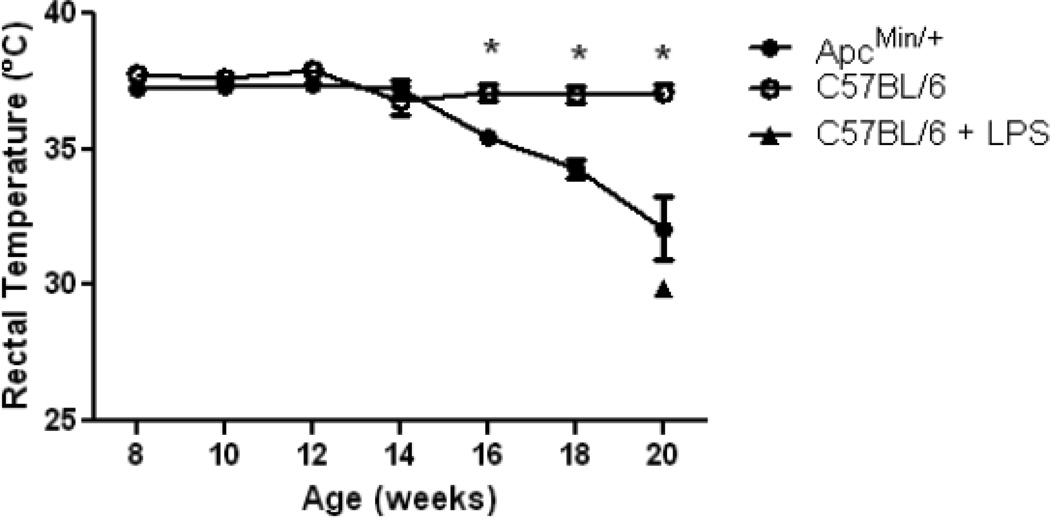
Acute injection of LPS into normal mice induces hypothermia [25], possibly as a protective mechanism to limit tissue damage from the inflammatory response [26]. As shown in Fig. 5, ApcMin/+ mice also experience a gradual decline in body temperature during development of cachexia. Intraperitoneal injection of a bolus of 250 µg endotoxin into control C57BL/6 mice led to a similar decrease in body temperature to 29.9 ± 0.2 °C at 12 hours (n = 2). Although their temperatures were comparable, the LPS-injected control animals were more lethargic than ApcMin/+ mice at 16–20 weeks of age, consistent with lower-level, chronic exposure to endotoxin in the cachectic animals (Fig. 3).
Figure 5. Decrease in body temperature with development of cachexia in ApcMin/+ mice.
Mean rectal temperature is shown for C57BL/6 (●, N = 10) and ApcMin/+ (○, N = 9) mice, C57BL/6 mice at 12 hr after intraperitoneal injection of 250µg of endotoxin (▲, N = 2). Data are expressed as means ± SE. *p<.05 indicates significant difference from controls.
1.3.5 Changes in lipemia and insulin resistance during development of cachexia
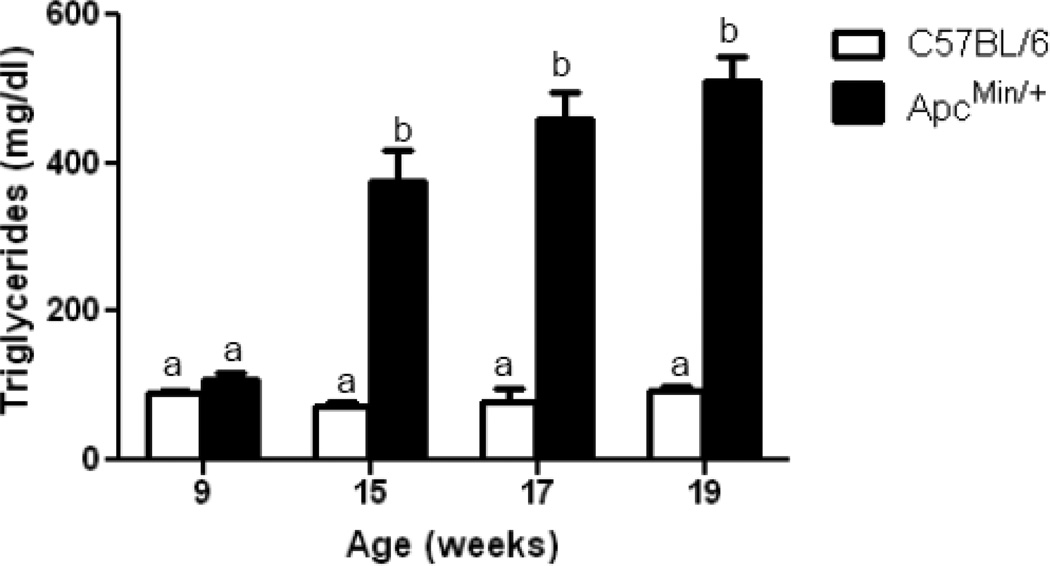
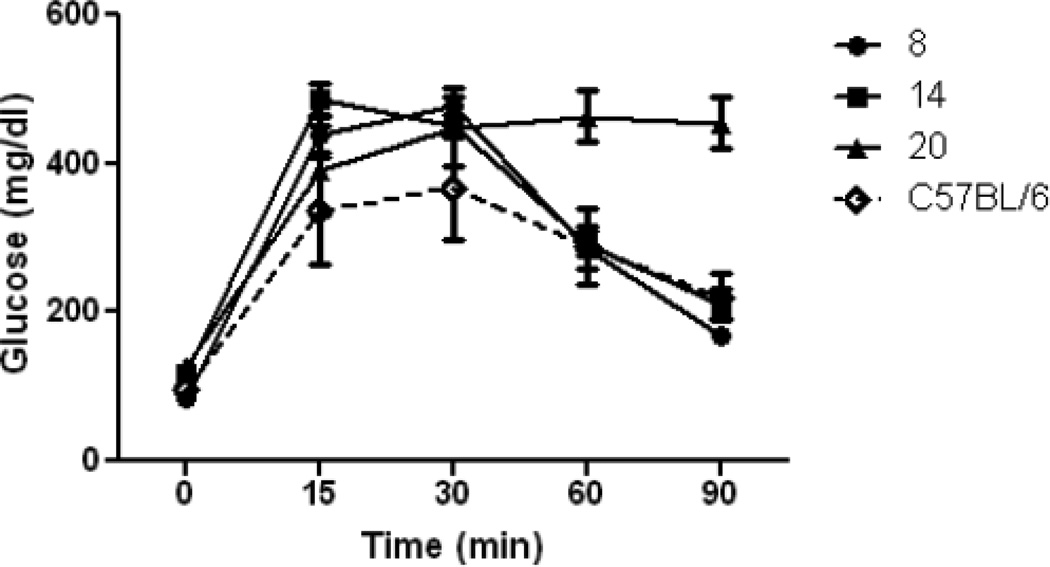
ApcMin/+ mice developed severe hypertriglyceridemia, which is maintained during the progression of cachexia (Fig. 6). Although fasting blood glucose was not significantly altered, glucose tolerance tests were abnormal in cachectic animals, based on delayed clearance of glucose at 90 minutes and the 29% increase (p = 0.05) in Area-Under-the Curve (AUC) for the glucose tolerance test (Fig. 7). Alterations in lipemia and insulin resistance are commonly seen in cachexia [27]. These changes are indicative of major shifts in energy metabolism in response to inflammation in cachectic animals and, as discussed below, are also common sequellae of endotoxemia.
Figure 6. Plasma triglyceride concentration increases during development of cachexia in the ApcMin/+ mouse.
Plasma triglyceride concentration was measured in the same animals over time, N = 7–9. Data are expressed as means ± SE. bp<.05 indicates significant difference from controls and ApcMin/+ at 9 weeks.
Figure 7. Insulin resistance increases during development of cachexia in ApcMin/+ mice.
Glucose (2.0 g / kg of body weight) was injected intraperitoneally. Glucose was measured at 0, 15, 30, 60 and 90 min. Results are means ± SE, N = 6.
1.4 DISCUSSION
1.4.1 Role of endotoxemia in cachexia
Cachexia is a challenging complication of end-stage cancer, affecting a patient’s overall health and vitality, ability to withstand infection, and to respond to chemotherapy or other interventions. Dealing aggressively with cachexia is critical for increasing patient survival. While there has been considerable focus on the distal consequences of cachexia, i.e. the mechanisms involved in adipose and muscle wasting, it is equally, if not more, important from a clinical perspective to elucidate the primary, proximal mechanisms initiating cachexia. The ApcMin/+ mouse is an accepted model of colorectal cancer cachexia. In this model the mice grow at a normal rate until approximately 12 weeks when intestinal polyps reach a plateau in numbers. After this the polyps grow in size without increasing in number and the animals begin to lose weight gradually until 16 weeks of age when there is a more severe decrease in body weight. These data suggest that it is not just the presence of the polyps that leads to cachexia, but the size of the polyps contributes to the degree of weight loss.
1.4.2
In the present study, we have developed both direct and indirect evidence supporting a role for endotoxemia in development of cachexia in the ApcMin/+ mouse model of colon cancer. The direct evidence is the detection of GBD (increased FD4 permeability) and the appearance of endotoxin in the circulation during the progression of cachexia. The plasma endotoxin concentration, while only modestly (~5-fold) increased, represents a steady state balance between its rate of entry into the circulation through the mesenteric lymph ducts and its rate of clearance from plasma in reticuloendothelial and other organs. The enlargement of mesenteric lymph nodes may be consistent with entry of endotoxin (or intact bacteria) through the intestinal lymphatic system [28, 29]. Acute intravenous administration of endotoxin also increases intestinal permeability in rodents [30, 31] and humans [32], suggesting a possible amplification loop in which gastrointestinal damage promotes endotoxin leakage, which then promotes an escalating cycle of endotoxin exposure and intestinal leakage. Indirect evidence for endotoxemia in cachectic mice includes a number of pathological changes that are characteristic of endotoxemia, including hypothermia [25, 26, 33], hypertriglyceridemia [34, 35], and insulin resistance [36, 37], as well as the chronic inflammatory state characterized by increased plasma concentrations of IL-6 [8]. However, the increase in the inflammatory biomarker IL-6 alone is not sufficient to cause these changes since IL-6 injection does not induce cachexia in control C57Bl/6 mice [8] or GBD (increased FD4 permeability) in ApcMin/+ mice (Fig. 2B).
1.4.3 Source of GBD
While we have documented increases in GBD and endotoxemia in the ApcMin/+ mouse, we do not have sufficient information at this stage to identify the primary cause of GBD. Tumor growth or macrophage infiltration and inflammation in the intestinal wall may affect gastrointestinal permeability, either locally or throughout the intestine through alterations in epithelial tight junctions. Soler et al. showed that in humans and rats tight junction permeability is increased in the region surrounding intestinal tumors [38]. Tight junction protein such as ZO-1 and occludin are also decreased in tumor rich regions of the intestines and colon in humans [39]. Decreases in tight junction proteins would increase permeability and allow passage of large molecules such as LPS into the lymphatic circulation. Changes in mucin secretion and mucin profiles in gastrointestinal carcinomas [40, 41] may also contribute to increased gut permeability. It is also possible that, as observed in HS/R [21] and acute pancreatitis [42], decreased splanchnic blood flow and resultant hypoxia might contribute to GBD. Hypoxia may also develop as a result of severe anemia, which may also cause hypoxia. We have reported previously [43] that blood hemoglobin concentration and red cell hemoglobin content fall by ~50% during the rapid phase of weight loss (16–20 weeks) in the ApcMin/+ mouse, suggesting that decreases in oxygen transport to the intestine may underlie the development of hypoxia. All of these mechanisms may be linked through hypoxia-induced mucosal damage and subsequent lifting of the intestinal epithelia. This lifting of the epithelia disrupts junctional proteins and leads to increased gut permeability [44].
1.4.4 Role of inflammation in GBD
Increases in circulating IL-6 are necessary for development of cachexia in the ApcMin/+ mouse [8]. We show here that plasma IL-6 concentration also increases in concert with changes in intestinal permeability, and that tumor size also correlates with increased intestinal permeability. The strong correlations between permeability to FD4, tumor size, and plasma IL-6 in the ApcMin/+ mouse, as well as the increase in permeability following IL-6 overexpression, suggest that GBD and endotoxemia are associated with, and perhaps the immediate cause of, both inflammation and cachexia. While more work is required to define the mechanisms underlying the increase in gut permeability, the general observations regarding compromised barrier function in this model of colon cancer may also apply to other cancers with a high frequency of cachexia, such as pancreatic or lung cancer.
1.5 CONCLUSION
Effective treatment of cachexia would have a significant impact on survival of patients with colorectal and other cancers. We show data to support that gut barrier dysfunction and endotoxemia develops concurrently with a surge in IL-6 and tumor growth present during cachexia. While our studies on cachexia are limited thus far to the ApcMin/+ mouse model, our observations suggest that endotoxemia should be evaluated in clinical studies of cachectic patients, especially those with gastrointestinal cancers. If endotoxemia or GBD is a common feature of cachexia, then efforts to limit gastrointestinal toxicity and/or preserve the integrity of the gut epithelial barrier during therapy may have a significant impact on cancer morbidity and mortality.
Highlights.
The ApcMin/+ model of colon cancer cachexia develops endotoxemia and gut barrier dysfunction
IL-6 over-expression increases intestinal permeability in ApcMin/+ mice
Intestinal permeability correlates with increases in tumor size and Il-6 levels
ACKNOWLEDGEMENTS
The authors thank Ms. Tia Davis for technical assistance with small animal experiments. The research described in this report was supported by a Seed Grant from the University of South Carolina Colorectal Cancer Program to JWB and JAC, funded by COBRE grant P20 RR-017698 from the National Center for Research Resources; and by research grant R01 CA121249 to JWB and JAC from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inagaki J, Rodriguez V, Bodey GP. Proceedings: Causes of death in cancer patients. Cancer. 1974;33:568–573. doi: 10.1002/1097-0142(197402)33:2<568::aid-cncr2820330236>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Fanelli F Rossi, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 5.Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003;21:198–213. doi: 10.1159/000073337. [DOI] [PubMed] [Google Scholar]
- 6.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479–490. doi: 10.1016/j.prp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 9.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 11.Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- 12.Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V, Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hang CH SJ, Li JS, Wu W, Yin HX. Alteration of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 2003;9:2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caso JR, Hurtado O, Pereira MP, Garcia-Bueno B, Menchen L, Alou L, Gomez-Lus ML, Moro MA, Lizasoain I, Leza JC. Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regul Integr Comp Physiol. 2009;296:R979–R985. doi: 10.1152/ajpregu.90825.2008. [DOI] [PubMed] [Google Scholar]
- 15.Sandek A, Anker SD, von Haehling S. The gut and intestinal bacteria in chronic heart failure. Curr Drug Metab. 2009;10:22–28. doi: 10.2174/138920009787048374. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas. 2008;36:192–196. doi: 10.1097/MPA.0b013e31815a399f. [DOI] [PubMed] [Google Scholar]
- 17.Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 18.Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the 'canary in the coal mine' during exercise-heat stress? Med Sport Sci. 2008;53:61–73. doi: 10.1159/000151550. [DOI] [PubMed] [Google Scholar]
- 19.Marshall JC. The gut as a potential trigger of exercise-induced inflammatory responses. Can J Physiol Pharmacol. 1998;76:479–484. doi: 10.1139/cjpp-76-5-479. [DOI] [PubMed] [Google Scholar]
- 20.Luyer MD, Buurman WA, Hadfoune M, Jacobs JA, Konstantinov SR, Dejong CH, Greve JW. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumor necrosis factor-alpha and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65–71. doi: 10.1097/01.shk.0000101671.49265.cf. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 22.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–1143. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 23.White JP. The regulation of skeletal muscle protein synthesis during the progression of cancer cachexia in the ApcMin/+ mouse. PLoS ONE. 2011 doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin's effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J Interferon Cytokine Res. 31:219–226. doi: 10.1089/jir.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habicht GS. Body temperature in normal and endotoxin-treated mice of different ages. Mech Ageing Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 26.Huet O, Kinirons B, Dupic L, Lajeunie E, Mazoit JX, Benhamou D, Vicaut E, Duranteau J. Induced mild hypothermia reduces mortality during acute inflammation in rats. Acta Anaesthesiol Scand. 2007;51:1211–1216. doi: 10.1111/j.1399-6576.2007.01419.x. [DOI] [PubMed] [Google Scholar]
- 27.Tisdale MJ. Cancer cachexia. Langenbecks Arch Surg. 2004;389:299–305. doi: 10.1007/s00423-004-0486-7. [DOI] [PubMed] [Google Scholar]
- 28.Cook MG. The size and histological appearances of mesenteric lymph nodes in Crohn's disease. Gut. 1972;13:970–972. doi: 10.1136/gut.13.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric lymph nodes seen at imaging: causes and significance. Radiographics. 2005;25:351–365. doi: 10.1148/rg.252045108. [DOI] [PubMed] [Google Scholar]
- 30.Deitch EA, Specian RD, Berg RD. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med. 1991;19:785–791. doi: 10.1097/00003246-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Go LL, Healey PJ, Watkins SC, Simmons RL, Rowe MI. The effect of endotoxin on intestinal mucosal permeability to bacteria in vitro. Arch Surg. 1995;130:53–58. doi: 10.1001/archsurg.1995.01430010055011. [DOI] [PubMed] [Google Scholar]
- 32.O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 33.Nautiyal KM, McKellar H, Silverman AJ, Silver R. Mast cells are necessary for the hypothermic response to LPS-induced sepsis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R595–R602. doi: 10.1152/ajpregu.90888.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouni I, Oka K, Etienne J, Chan L. Endotoxin-induced hypertriglyceridemia is mediated by suppression of lipoprotein lipase at a post-transcriptional level. J Lipid Res. 1993;34:139–146. [PubMed] [Google Scholar]
- 35.Uchiumi D, Kobayashi M, Tachikawa T, Hasegawa K. Subcutaneous and continuous administration of lipopolysaccharide increases serum levels of triglyceride and monocyte chemoattractant protein-1 in rats. J Periodontal Res. 2004;39:120–128. doi: 10.1111/j.1600-0765.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 36.McCowen KC, Ling PR, Ciccarone A, Mao Y, Chow JC, Bistrian BR, Smith RJ. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Crit Care Med. 2001;29:839–846. doi: 10.1097/00003246-200104000-00032. [DOI] [PubMed] [Google Scholar]
- 37.Siebler J, Galle PR, Weber MM. The gut-liver-axis: endotoxemia, inflammation, insulin resistance and NASH. J Hepatol. 2008;48:1032–1034. doi: 10.1016/j.jhep.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 39.Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45–54. [PMC free article] [PubMed] [Google Scholar]
- 40.Allen DC, Connolly NS, Biggart JD. Mucin profiles in ulcerative colitis with dysplasia and carcinoma. Histopathology. 1988;13:413–424. doi: 10.1111/j.1365-2559.1988.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 41.Gupta SC, Misra V, Singh PA, Misra SP, Srivastava M, Agrawal R. Mucin histochemistry--a simple and effective method for diagnosing premalignant and early malignant lesions of lower gastrointestinal tract. Indian J Pathol Microbiol. 1997;40:327–333. [PubMed] [Google Scholar]
- 42.Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7:26–35. doi: 10.1016/S1091-255X(02)00090-2. discussion 35-26. [DOI] [PubMed] [Google Scholar]
- 43.Baltgalvis KA, Berger FG, Pena MM, Davis J Mark, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol. 109:1155–1161. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg. 1970;101:484–488. doi: 10.1001/archsurg.1970.01340280036010. [DOI] [PubMed] [Google Scholar]