Abstract
Col1a2-deficient (oim) mice synthesize homotrimeric type I collagen due to nonfunctional proα2(I) collagen chains. Our previous studies revealed a postnatal, progressive type I collagen glomerulopathy in this mouse model, but the mechanism of the sclerotic collagen accumulation within the renal mesangium remains unclear. The recent demonstration of the resistance of homotrimeric type I collagen to cleavage by matrix metalloproteinases (MMPs), led us to investigate the role of MMP-resistance in the glomerulosclerosis of Col1a2-deficient mice. We measured the pre- and post-translational expression of type I collagen and MMPs in glomeruli from heterozygous and homozygous animals. Both the heterotrimeric and homotrimeric isotypes of type I collagen were equally present in whole kidneys of heterozygous mice by immunohistochemistry and biochemical analysis, but the sclerotic glomerular collagen was at least 95–98% homotrimeric, suggesting homotrimeric type I collagen is the pathogenic isotype of type I collagen in glomerular disease. Although steady-state MMP and Col1a1 mRNA levels increased with the disease progression, we found these changes to be a secondary response to the deficient clearance of MMP-resistant homotrimers. Increased renal MMP expression was not sufficient to prevent homotrimeric type I collagen accumulation.
Keywords: collagen, extracellular matrix, glomerular sclerosis, fibrosis, matrix metalloproteinase
1. Introduction
Type I collagen is predominantly found in tissues as a heterotrimeric isotype consisting of two α1(I) chains and one α2(I) chain, [α1(I)2α2(I)]. However the homotrimers consisting of three α1(I) chains, [α1(I)3] [1–4] have been shown to be present during embryogenesis [5], in tumors [6–8], fibrotic tissues [9–12] and in stressed mesangial cells [13].
Haralson et al demonstrated that cultured wildtype rat mesangial cells synthesized the homotrimeric type I collagen in what was postulated to be a wound response [13]. They hypothesized that the synthesis of the homotrimeric isotype may contribute to sclerotic accumulation of type I collagen within the renal mesangium. Historically, glomerulosclerosis has been attributed to an imbalance of collagen synthesis and degradation [14–17]. Mesangial cells are thought to be responsible for the excessive expression of collagen in the glomerular mesangium during disease progression [18–21]. Although the resulting matrix accumulation leads to glomerulosclerosis [22–25], the initiating mechanisms of the pathology and the specific role of homotrimeric type I collagen still remain unclear.
A clue to answering the latter question may be contained in the recent finding that type I collagen homotrimers are much more resistant to degradation by matrix metalloproteinases (MMPs) than the heterotrimers [8, 26]. MMPs are known regulators of the extracellular matrix (ECM) and are of keen interest in glomerulosclerotic disease [27–30]. To maintain ECM homeostasis, human kidneys express collagenases (MMP-1, -13 and -14), gelatinases (MMP-2 and -9) and stromelysin-1 (MMP-3) [31]. MMP-2 has been most widely studied across species and shown to be constitutively expressed by both mesangial and glomerular epithelial cells [31–33] and is postulated to act as a pro-inflammatory activator of mesangial cells [34, 35] while possibly contributing to epithelial-to-mesenchymal-transition (EMT) [36]. MMP-9 [31, 37, 38] is also synthesized by glomerular epithelial cells, but unlike MMP-2, has been shown to have restricted temporal expression during development and in adult rodent tissues [39, 40] and is differentially regulated in several animal models [41–44]. Though less well studied in kidneys, MMP-3 is an important activator of MMP-2, -9 and -13, degrading many of the same substrates including gelatin [45]. It has also been shown to be elevated in the serum of human renal transplant patients with chronic nephropathy [46]. MMP-13 is synthesized by mesangial cells, activated by MMP-2, -3 and -9, and plays an important role in cancer progression [8, 31]. The membrane collagenase MMP-14 (also known as MT1-MMP) has multiple functions, including but not limited to pericellular collagen cleavage and activation of MMP-2 [47]. It is known to be crucial for general collagen turnover [48] as well as wound healing and tumor invasion [49]. MMP-3, -13 and -14 have also been identified as primary players in fibrosis due to EMT [50].
Mice constitutively expressing the MMP-resistant homotrimers provide an unprecedented opportunity to examine the potential pathogenic role of this collagen isotype in glomerulosclerotic disease without confounding factors of artificially induced renal injury. The Col1a2-deficient mouse has a single nucleotide deletion that causes a frame-shift and disrupts the carboxyl terminus (C-propeptide) of the α2(I) chain, required for the association with α1(I) procollagen chains to form the heterotrimeric triple helix [51]. The resulting exclusion of the α2(I) chain from the type I collagen triple helix leads to synthesis and secretion of only the homotrimeric isotype in homozygous Col1a2-deficient (−/−) mice. The genetic defect itself, reduced bone mineral density and increased bone fragility in the Col1a2-deficient mouse [52–54] resemble a patient with an autosomal recessive form of osteogenesis imperfecta [55–57], which is why this mouse is also known as the osteogenesis imperfecta murine (oim) model. In addition to the bone phenotype, our previous studies of the Col1a2-deficient mouse revealed postnatal accumulation of homotrimeric type I collagen in the renal mesangium, resulting in progressive glomerulosclerosis, podocyte foot process effacement and proteinuria [12, 58]. These findings and the synthesis of the homotrimers by mesangial cells that have no genetic Col1a2 deficiency [13] beg the question whether this isotype is the primary pathogenic collagen in glomerulosclerotic lesions.
In the present study, we addressed the potential role of delayed degradation and clearance of this MMP-resistant collagen isotype from renal mesangium. We compared pre- and post- translational expression and distribution of type I collagen isotypes and MMPs in whole kidneys and isolated glomeruli from 1 month old and 3 month old heterozygous and Col1a2-deficient mice. Since mouse MMP-1 is only synthesized during embryogenesis [31, 59, 60], we limited the study to MMP-2, -3, -9, -13 and -14.
2. Materials and methods
2.1. Animals
Heterozygous B6C3Fe a/a- Col1a2oim/J (−/+) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred to produce wildtype (+/+), heterozygous (+/−) and homozygous (−/−) animals. Animals were housed and fed (Purina 5008 Formulab Diet; Purina Mills Inc., Richmond, IN) ad libitum in an AAALAC accredited animal facility in accordance with an approved University of Missouri Animal Care and Use protocol. Animal genotypes were determined as previously described [61] and aged to 1 (n=168 mice) or 3 months (n=151) of age. Animals were sacrificed and kidneys or glomeruli harvested as described below.
2.2. Glomerular isolation
Wildtype, heterozygous and Col1a2-deficient mice were aged to 1-month [−/−, n=20; +/+, n=21; and +/−, n=38] and 3-months [−/−, n=13; +/+, n=22; and +/−, n=23] of age and anesthetized prior to kidney perfusion [62]. Perfusion of 8×107 tosylactivated Dynabeads® magnetic beads (deactivated according to the manufacturers instructions) in 1M PBS were perfused through the body via the heart. Perfused kidneys were removed, weighed and minced followed by a 30 minute digestion in 1mg/ml collagenase A (Invitrogen Corporation, Carlsbad, CA), 100units/ml DNase (Invitrogen Corp., Carlsbad, CA) and Hanks Balanced Salt Solution (HBSS) (GibCo-Invitrogen Corporation, Carlsbad, CA) at 37°C. The digested slurry was sieved twice through 100µm cell strainers (BD Bioscience, San Jose, CA) with the addition of HBSS, followed by centrifugation at 1500rpm for 15 minutes. The pellet was resuspended in HBSS and placed onto a magnetic particle concentrator for 1 minute to separate glomeruli from extraneous tissue, and repeated 5 times. Remaining glomeruli were resuspended in HBSS and assessed for purity (greater than 98% purification) and yield using a hemocytometer, followed by snap-freezing and storage at −80°C.
2.3. Microscopy and glomerular counting
Longitudinal sections (5µm) of formalin-fixed kidneys from 1 and 3 month wildtype, heterozygous and Col1a2-deficient [n=8 of each genotype] were embedded in paraffin and stained with picrosirius red (PSR) fibrillar collagen stain. The PSR-stained sections were examined by conventional light microscopy and with polarized light. Glomeruli within individual sections were evaluated blindly to obtain a glomerular lesion score for each kidney as previously described [58], and to assess the average glomerular number within longitudinal sections. Mean glomerular number was calculated as the average of the number of glomeruli within 4 sections.
2.4. Immunohistochemistry (IHC) for α1(I) and α2(I) collagen
Longitudinal sections (10µm) of zinc fixed kidneys were embedded in paraffin and placed on slides. Heat-induced epitope retrieval in target retrieval solution (1X TRS) (Dako, Carpinterea, CA) was followed by quenching of endogenous biotin using a avidin/biotin block. Endogenous peroxidase was removed by treating slides with 3% hydrogen peroxide and non-specific antibody binding was blocked with a 5% bovine serum albumin (BSA) solution. Next, kidneys were incubated in either rabbit polyclonal anti-α1(I) collagen primary antibody (MD Biosciences, St Paul, MN) diluted 1:600 or rabbit polyclonal anti-α2(I) collagen primary antibody diluted 1:3000. The anti-α2(I) collagen primary antibody was produced by the antibody production core at UT-Southwestern Medical Center. In brief, two rabbits (U6410 and U6425) were immunized genetically with 1 mg each of cDNA encoding murine α2(I) collagen chain three times over 42 days. The rabbits were boosted every two weeks (five times total) with collagen purified from homozygous Col1a2-deficient mice. Pre-immune serum and sera collected after the final boost were evaluated by ELISA for reactivity with collagen isolated from wildtype and Col1a2-deficient mice animals. Following the addition of the primary antibodies, biotinylated swine anti-rabbit secondary antibody diluted 1:300 was added, followed by a strepavidin horseradish peroxidase conjugate, and 3,3’ diaminobenzidine tetrahydrochloride (DAB) substrate with hematoxylin counterstaining. Staining was performed on a Dako Autostainer Universal Staining System. Anti-α1(I) and anti-α2(I) collagen antibody specificity was confirmed by western blot analysis of wildtype and Col1a2-deficient mouse tail tendon collagen. The anti-α2(I) collagen also specifically binds acid-solublized and pepsin treated rat and human α2(I) collagen chains by western blot analyses.
2.5. Biochemical analysis of collagen composition in glomeruli and kidney
One half of harvested whole kidney was homogenized by ice-bath sonication in 0.5M acetic acid and 0.5 % Brij 35 for 1 minute and treated overnight with 0.5 mg/ml pepsin (EMD Biosciences, Darmstadt, Germany) at 4°C. Solublized collagen was precipitated with 1M NaCl. Glomerular preparations were thawed, precipitated by centrifugation, homogenized, pepsin treated, and precipitated with NaCl as described above. The beads with precipitated collagen were separated from pepsin solution by centrifugation and washed with 70% ethanol, to remove residual acetic acid.
For analysis, kidney or glomerular collagen were resuspended in 0.1M Na2CO3, 0.5M NaCl, pH 9.3 and labeled with mono-reactive Cy5 (GE Healthcare, Piscataway, NJ) as previously described [63]. Aliquots of 3–4µg of protein were denatured for 5 min at 90°C in 4X LDS sample buffer (Invitrogen) and analyzed on precast 3–8% Tris-acetate mini gels (Invitrogen) with α1(I) molecular standards. Selected bands were excised from the gel, treated with CNBr (Sigma, St. Louis, MO), and re-analyzed on precast 12% Tris-glycine gels (Invitrogen). All gels were scanned on a FLA5000 fluorescent scanner (FUJI Medical Systems, Stamford, CT) and analysized by ScienceLab software. The estimated detection limit of this assay is 0.2–0.5 pg collagen/glomerulus.
2.6. Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Snap frozen glomeruli from 1 month and 3 month old +/+, +/− and −/− mice were homogenized in TRIzol Reagent (Invitrogen Corporation) using a TissueLyser homogenizer (QIAGEN, Valencia, CA), or RNeasy Kit (QIAGEN) and total RNA was isolated according to manufacturer’s protocol. Total glomerular RNA was transcribed following the manufacturer’s protocol (Superscript First Strand Synthesis or VILO, Invitrogen Corporation). Real-time RT-PCR amplification was performed on individual mouse samples and standard curves generated as previously described [58]. One month [+/+ (lesion score G0), n=10; +/− (lesion score G1–4), n=16; −/− (lesion score G3–4), n=16] and 3 month [+/+ (lesion score G0), n=16; +/− (lesion score G1), n=16; −/− (lesion score G1–4), n=17] old mice were individually evaluated for COL1A1, COL1A2 MMP-2, -3, and -9 transcript levels, and 1 month [+/+ (lesion score G0), n=8; +/− (lesion score G1–4), n=9; −/− (lesion score G3–4), n=8] and 3 month [+/+ (lesion score G0), n=8; +/− (lesion score G1), n=9; −/− (lesion score G1–4), n=8] old mice were evaluated for MMP-13 and -14. PCR primer sequences for COL1A1, COL1A2 MMP-2, -3, and -9 are found in Table 3. MMP-13 and -14 transcripts were evaluated with purchased primer/probe sets (Applied Biosystems, Foster City, CA) and individually evaluated using TaqMan® gene expression assay. RNA copy number values were evaluated and normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) [64].
Table 3.
Quantitative real time PCR primers
| Primer | Sequence | Amplicon size |
|---|---|---|
| COL1A1 forward | 5' - TGG ATT CCC GTT CGA GTA CG - 3' | 202bp |
| COL1A1 reverse | 5’ – ATT AGG CGC AGG AAG GTC AG - 3’ | |
| COL1A2 forward | 5’ – TGA AGT GGG TCT TCC AGG TCT TTC – 3’ | 236bp |
| COL1A2 reverse | 5’ – CAC CCT TGT TAC CGG ATT CTC CTT – 3’ | |
| MMP-2 forward | 5’ – AAA GGA CTC GGG TTG TCT GA – 3’ | 150bp |
| MMP-2 reverse | 5’ – CAA GAA GGC TGA GCA GGA AG – 3’ | |
| MMP-3 forward | 5’ – TAA AGA CAG GCA CTT TTG GC – 3’ | 114bp |
| MMP-3 reverse | 5’ – GTA ACC TCA TAT GCA GCA TCC – 3’ | |
| MMP-9 forward | 5’ – TCC AGT ACC AAG ACA AAG CC – 3’ | 169bp |
| MMP-9 reverse | 5’ – TGA AGC AAA GAA GGA GCC C – 3’ | |
| MMP-13 | Mm00439491_m1 TaqMan® assay (Applied Biosciences) | 65 bp |
| MMP-14 | Mm01318966_m1 TaqMan® assay (Applied Biosciences) | 82 bp |
2.7. Quantitation of MMPs
Protein levels of MMP-2, MMP-3, and MMP-9 in 1 month [n=9 +/+; n=11 −/−] and 3 month [n=10 +/+; n=10 −/−] wildtype and Col1a2-deficient mouse glomeruli were measured commercially using the Thermo Scientific SearchLight multiplex assay (Pierce Scientific, Rockford, IL) [65, 66]. Briefly, snap frozen glomeruli were thawed on ice and protease activity inhibited by the addition of phenylmethyl sulfonyl fluoride (PMSF) to a final concentration of 2mM. Glomeruli were disrupted by a 10 minute ice-bath followed by a 1 hour incubation in 96-well format at room temperature with agitation (200rpm) for antigen-protein binding to MMP-2, -3, and -9 antigens. Wells were washed and incubated with biotinylated detection antibodies for 30min, followed by a 30 min incubation with strepdavidin horseradish peroxidase conjugate and imaged by SuperSignal ELISA Femto Chemiluminescence substrate.
2.8. Statistics
Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC) by complete randomization design in which genotype and age were arranged as a 3 × 2 factorial (3 genotypes, 2 ages). Stabilization of heterogeneous variations were made when necessary by using the log transformation of the mean values. Mean differences were determined using Fisher’s protected least significant differences (LSD). Means and standard errors presented are untransformed values, although all p values, with the exception of MMP-14 mRNA data, were determined from transformed data. All results are presented as mean ± standard error with statistical significance as noted.
3. Results
3.1. Kidney size and glomerular content
Col1a2-deficient mice had smaller total body weights [51, 53, 67] and kidney weights than their wildtype and heterozygous littermates, but larger kidney/body weight ratios (Table 1); although the increased kidney/body weight ratio was significant (p ≤ 0.005) only at one month of age. The yield of glomeruli isolated by perfusion from Col1a2-deficient kidneys (6510 ± 1350 glomeruli) was similar to the wildtype and heterozygous kidneys at 1 month of age, and much lower at 3 month of age (900 ±122 glomeruli, p ≤ 0.005) (Table 1). To investigate this further, 1 and 3 month wildtype, heterozygous and Col1a2-deficient deficient kidneys were evaluated histologically and the glomerular number determined (Table 2). This confirmed that there was not a decrease in the number of glomeruli (per field of view) in the 3 month old Col1a2-deficient animals, but rather suggested that the decreased glomerular yield was a result of inefficient perfusion of sclerotic glomeruli in the older Col1a2-deficient animals. The increase in glomerular number in Col1a2-deficient kidneys (Table 2) can likely be attributed to the reduction in size and area of Col1a2-deficient kidneys (Table 1) as compared to their littermates, and rather than a true increase in the total number of glomeruli.
Table 1.
Wildtype (+/+), heterozygous (+/−), and Col1a2-deficient (−/−) perfusion data (mean ± S.E.M.)
| Genotype (n) | Age (mo) |
Animal Wt (g) |
Kidney Wt (g) |
Kidney/ Animal Wt |
Total Glomerular Yield |
Glomerular Yield/Kidney Wt |
|---|---|---|---|---|---|---|
| Wildtype (21) | 1 | 18.15 ± 0.54 | 0.279 ± 0.010 | 0.0155 ± 0.0004 | 6942 ± 818 | 25826 ± 3204 |
| Heterozygous (38) | 1 | 17.38 ± 0.45 | 0.281 ± 0.008 | 0.0163 ± 0.0004 | 6750 ± 659 | 25160 ± 2797 |
| Col1a2-deficient (20) | 1 | 12.79 ± 0.62a | 0.225 ± 0.011a | 0.0178 ± 0.0006a | 6510 ± 1350 | 30568 ± 6905 |
| Wildtype (22) | 3 | 29.40 ± 0.66 | 0.437 ± 0.013 | 0.0149 ± 0.0004 | 4679 ± 805 | 10880 ± 2041b |
| Heterozygous (23) | 3 | 27.91 ± 0.95 | 0.441 ± 0.022 | 0.0157 ± 0.0004 | 5398 ± 1080 | 12011 ± 2322b |
| Col1a2-deficient (13) | 3 | 21.05 ± 1.13a | 0.343 ± 0.032a | 0.0162 ± 0.0012 | 900 ± 122a,b | 3087 ± 584a,b |
p≤0.005 compared to age-matched wildtype;
p≤0.005 compared to same genotype at 1 month of age
Table 2.
Average number of glomeruli per field in kidney cortex and juxtamedullary regions (mean ± S.E.M.)
| Genotype (n) | Age (mo) | Cortical Glomeruli | Juxtamedullary Glomeruli | Total Glomeruli |
|---|---|---|---|---|
| Wildtype (8) | 1 | 5.09 ± 0.43 | 2.06 ± 0.32 | 3.58 ± 0.46 |
| Heterozygous (11) | 1 | 4.16 ± 0.50 | 2.28 ± 0.21 | 3.22 ± 0.34 |
| Col1a2-deficient (8) | 1 | 7.00 ± 0.92b | 3.63 ± 0.65a,b | 5.31 ± 0.70b |
| Wildtype (8) | 3 | 4.28 ± 0.47 | 2.38 ± 0.31 | 3.33 ± 0.37 |
| Heterozygous (8) | 3 | 4.34 ± 0.43 | 2.22 ± 0.26 | 3.28 ± 0.37 |
| Col1a2-deficient (8) | 3 | 5.34 ± 0.76 | 3.71 ± 0.58b | 4.53 ± 0.51a |
p≤0.05 compared to age-matched heterozygous;
p≤0.05 compared to age-matched wildtype
3.2. Glomerular accumulation of homotrimeric collagen
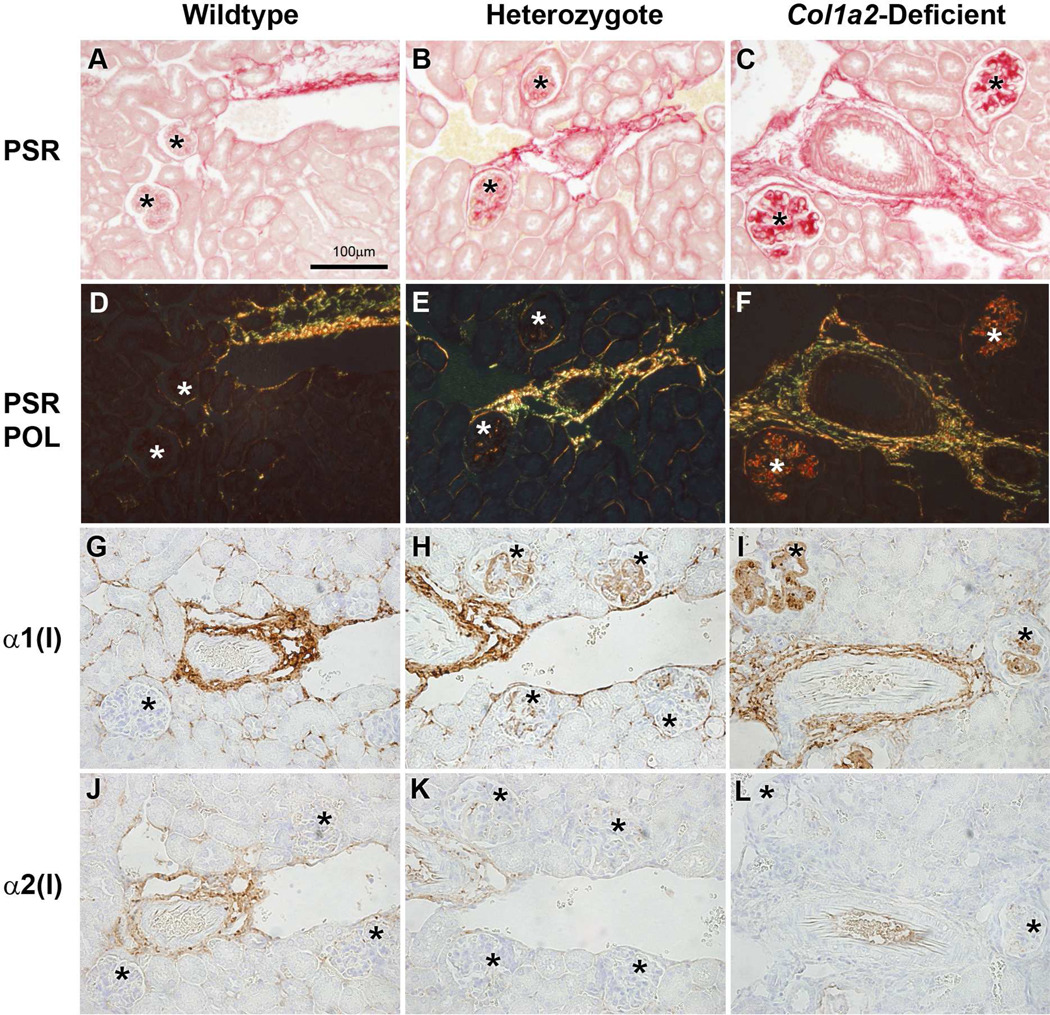
Immunohistological examination of kidney sections demonstrated a divergence between the α1(I) and α2(I) chain localizations, suggesting that only homotrimeric type I collagen is depositing in the glomeruli (Figure 1). The adventitia of the renal vasculature of all genotypes, where type I collagen is normally expressed, showed picrosirius red (PSR) collagen staining and anti-α1(I) antibody staining. As expected, the same region also showed anti-α2(I) staining in wildtype and heterozygous kidneys, but not in Col1a2-deficient kidneys. Sclerotic glomeruli in heterozygous and Col1a2-deficient kidneys were positive for both anti-α1(I) and PSR, but negative for anti-α2(I).
Figure 1.
Identification of type I collagen homotrimer in wildtype (+/+) (A,D,G,J), heterozygous (+/−)(B,E,H,K) and homozygous (−/−)(C,F,I,L) glomeruli. Picrosirius red (PSR) staining under normal (A,B,C)and polarized light (D,E,F) of wildtype glomeruli (A,D) show no deposition of type I collagen, heterozygous glomeruli (B,E) show mild deposition of type I collagen, and homozygous glomeruli (C,F) show severe deposition of collagen within glomeruli. Anti-α1(I) collagen immunohistochemistry (IHC)(G–I) of wildtype kidneys (G) demonstrate no localization of α1(I) chains within glomeruli, only in the vasculature. Heterozygous kidneys (H) show localization of type I collagen α1(I) chains within the glomeruli and vasculature. Homozygous kidneys (I) mice also show evidence of type I collagen α1(I) in the glomeruli and vasculature. Anti-α2(I) collagen IHC (J–L) of wildtype (J), heterozygous (K), and homozygous (L) kidneys demonstrate the presence of α2(I) chains in the vasculature of wildtype and heterozygous kidneys, and the absence of anti-α2(I) positive staining within glomeruli of wildtype, heterozygous, and homozygous kidneys. Asterisks indicate glomeruli.
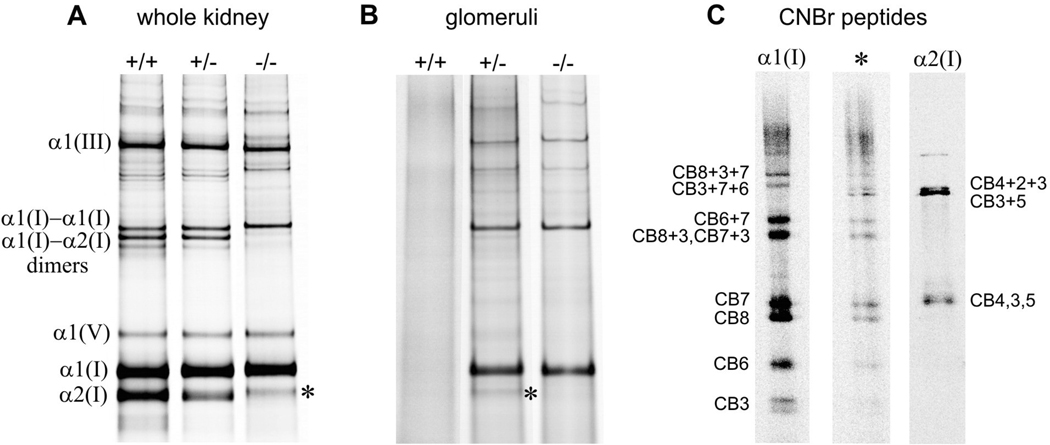
Further biochemical examination of extracted collagen showed that type I collagen comprised ~80% of total fibrillar collagen in whole kidneys, while type III and V collagens comprised ~12–16% and ~5%, respectively (Figure 2A). These ratios are consistent with the composition of collagen isolated from cultured fibroblasts. Additionally, type I collagen was present in wildtype kidneys only as the heterotrimeric isotype, in Col1a2-deficient kidneys only as the homotrimeric isotype, and approximately equal amounts of both homotrimeric and heterotrimeric isotypes were present in heterozygous kidneys (Figure 2A).
Figure 2.
Analysis of collagen composition in whole kidney (A) and glomeruli (B) from wildtype (+/+), heterozygous (+/−) and homozygous Col1a2-deficient (−/−) mice. The fraction of homotrimeric type I collagen was determined from the intensity ratios of α1(I) : α2(I) and α1(I)-α1(I) : α1(I)-α2(I) as previously described [90]. A faint band labeled with *, migrating close to the expected position of the α2(I) chain, was observed in kidneys from homozygous mice and glomeruli from some heterozygous mice. CNBr peptide patterns (C) from the * band of the homozygous kidney and heterozygous glomeruli were similar and consistent with the α1(I) chain cleaved within CB6 or CB5. Such proteolytic degradation of α1(I) homotrimers is often observed in Col1a2-deficient tissues [68]. However, we could not completely exclude the presence of the α2(I) chain in the * band from heterozygous glomeruli. Indeed, the most intense bands of α2(I) CNBr peptides are co-migrating CB3, CB4, and CB5 as well as co-migrating partial CNBr cleavage products CB3+5 and CB4+2+3 (C). The first group migrates close to and may overlap with α1(I)-CB7. The second group migrates close to and may overlap with α1(I)-CB3+7+6, in which CB6 truncated by the proteolytic cleavage discussed above. Based on the observed */α1(I) intensity ratio, absence of the well defined α1(I)-α2(I) dimer band, and relative intensities of different bands in the CNBr cleavage pattern of the * band, we estimated that type I collagen from heterozygous glomeruli consisted of at least 95–98 % α1(I) homotrimers. Note that fluorescent labeling with Cy5 allows evaluation of the relative content of α1(I) and α2(I) chains with approximately 5% accuracy.
Of key interest, no glomerular type I collagen was detected in wildtype mouse glomeruli at 0.2–0.5 pg/glomerulus detection limit, but over 100 pg of homotrimeric type I collagen per glomerulus (evidenced by the lack of α2(I) chains) was found in an age-matched Col1a2-deficient mouse. Analysis of heterozygous mice revealed <0.5 pg/glomerulus in one of six animals, 0.8–7 pg/glomerulus in four animals and 34 pg/glomerulus in a single animal with the most severe lesions of the six heterozygous mice evaluated (Figure 2B). In Figures 2A & B, a faint pepsin-resistant protein species (indicated by the asterick) was seen migrating slightly slower than the pepsin-treated α2(I) chains in the extracts from kidneys from homozygous mice and glomeruli from some heterozygous mice. To determine if this protein was of α1(I) or α2(I) chain origin CNBr pepetide mapping was done (Figure 2C). The CNBr peptide patterns from of this protein species (*) was similar and consistent with the α1(I) chain cleaved within CB6 or CB5. Such proteolytic degradation of α1(I) homotrimers is often observed in Col1a2-deficient tissues [68]. Based on the observed protein species (*)/α1(I) intensity ratio, absence of the well defined α1(I)-α2(I) dimer band, and the relative intensities of different fragments in the CNBr cleavage pattern of the protein species (*), we estimated that at least 95–98% of type I collagen molecules in heterozygous glomeruli were α1(I) homotrimers, confirming selective accumulation of the homotrimeric isotype.
3.3. Col1a1 and col1a2 transcription
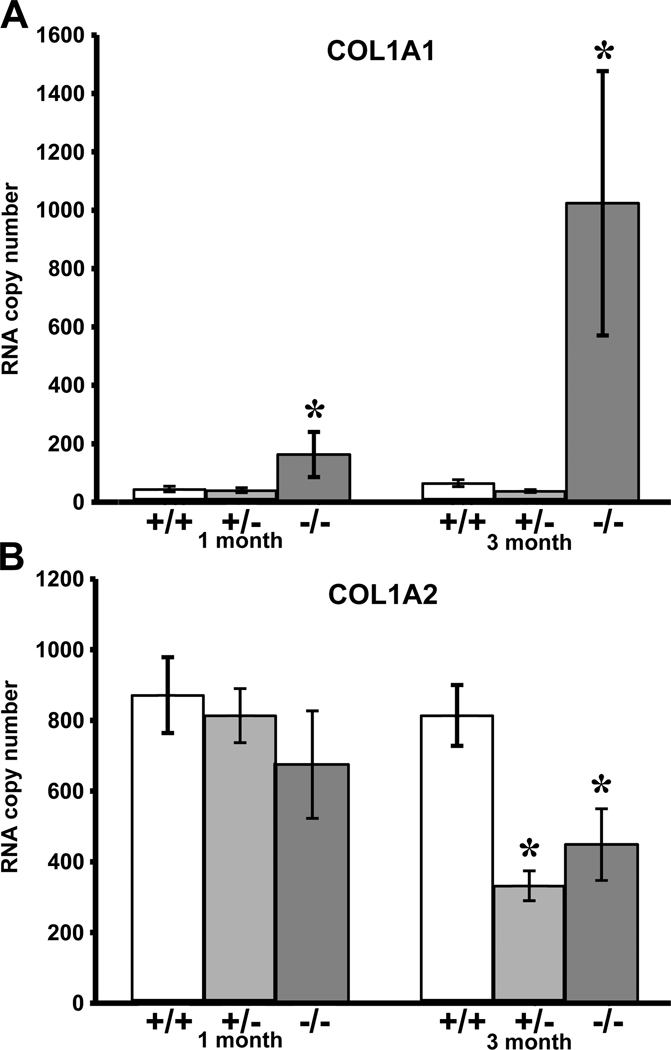
Analysis of steady-state mRNA levels by quantitative RT-PCR (Figure 3A) revealed similar col1a1 expression in wildtype and heterozygous glomeruli (both at one and at three month of age). Col1a1 expression in Col1a2-deficient glomeruli was elevated 3-fold at one month (p ≤ 0.003) and 15-fold at three month of age (p ≤ 0.0001), consistent with previously observed 2-fold increase in steady-state col1a1 mRNA transcripts in the whole kidney at one month [58].
Figure 3.
Quantitative RT-PCR steady-state mRNA expression of COL1A1 (top) and COL1A2 (bottom) transcripts in wildtype (+/+), heterozygous (+/−) and homozygous (−/−) Col1a2-deficient glomeruli. 1-month and 3-month old homozygous glomeruli demonstrate increases in proα1(I) collagen mRNA copy as compared to age-matched wildtype and heterozygous glomeruli (*p <0.003 and *p <0.0001 respectively). Proα2(I)collagen mRNA copy number decreases in 3-month old heterozygous (*p <0.0001) and homozygous (*p <0.003) glomeruli as compared to age-matched wildtype glomeruli.
Although Col1a2-deficient mice do not incorporate α2(I) chains in their type I collagen triple helix, they continue to synthesize col1a2 transcripts which are hypothesized to be translated and the aberrant protein product degraded shortly thereafter [4, 69]. Both in heterozygous and Col1a2-deficient glomeruli, we observed progressive reduction in col1a2 steady-state mRNA with age, compared to matched wildtype glomeruli (Figure 3B).
3.4. MMP transcription and translation
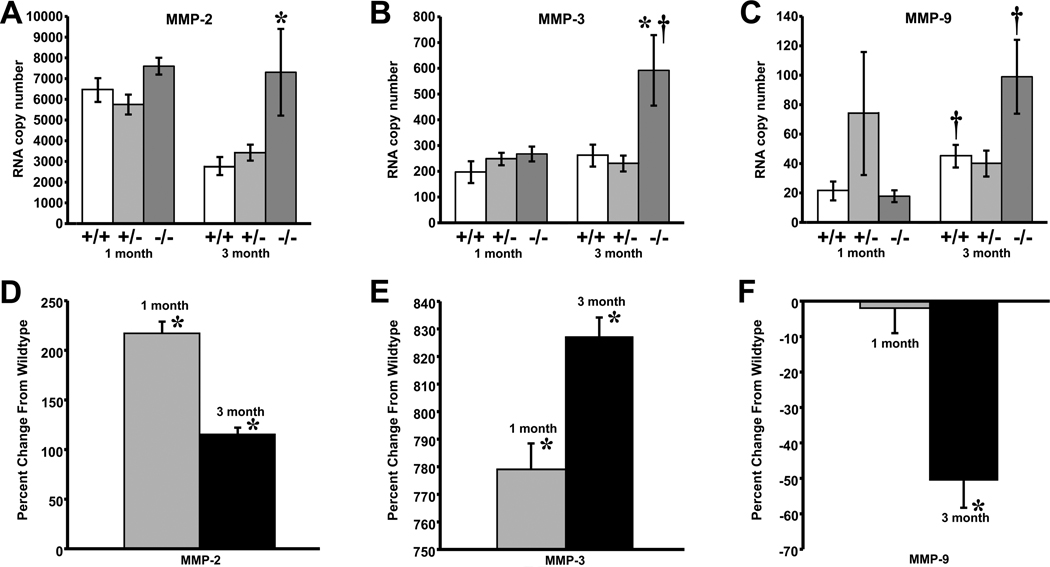
Analysis of mRNA showed similar MMP-2 expression in all genotypes at one month of age (Figure 4A). MMP-2 mRNA levels decreased 2-fold in wildtype and heterozygous glomeruli at three months of age, but remained elevated in Col1a2-deficient glomeruli (p ≤ 0.0004). Analysis of the protein showed a 3-fold and 2-fold elevation in MMP-2 in Col1a2-deficient glomeruli at one month (p ≤ 0.001) and three months (p ≤ 0.008), respectively (Figure 4D).
Figure 4.
Quantitative RT-PCR demonstrates an increase in MMP-2 (A), and MMP-3 (B) mRNA expression in 3-month Col1a2-deficient (−/−) glomeruli compared to age-matched wildtype (+/+) [MMP-2, *p <0.0004; MMP-3,*p <0.04]. MMP-3 (B) and MMP-9 (C) demonstrate significant increases between 1-month and 3-month wildtype and Col1a2-deficient glomeruli [MMP-3, †p <0.04 Col1a2-deficient glomeruli; MMP-9, †p <0.01 wildtype and MMP-9, †p <0.0007 Col1a2-deficient glomeruli]. 1-month and 3-month Col1a2-deficient glomeruli show an increase in MMP-2 (D) (*p ≤0.001 and *p ≤0.008 respectively), and MMP-3 (E) (*p ≤0.0005 and *p ≤0.0003 respectively) protein expression as compared to age-matched wildtype mice, while MMP-9 (F) shows a decrease in protein expression at three months of age (*p ≤0.05). Data expressed as mean ±SEM.
MMP-3 steady-state transcript levels (Figure 4B) appeared similar in all genotypes at one month of age. At three months of age, they increased more than 2-fold in Col1a2-deficient (p ≤ 0.002) and remained unchanged in wildtype and heterozygous glomeruli. At the protein level, however, Col1a2-deficient mice had 9-fold higher MMP-3 content compared to age-matched wildtype mice (p ≤ 0.0005), both at one and at three months (Figure 4E). Furthermore, the MMP-3 protein content increased 2.6 fold at three months compared to one month in both genotypes.
MMP-9 steady-state mRNA levels (Figure 4C) were similar in one month old wildtype and Col1a2-deficient glomeruli and 70% greater, though not significantly, in heterozygous glomeruli. At three months of age, MMP-9 mRNA levels were similar in wildtype and heterozygous glomeruli, and 2-fold higher in Col1a2-deficient glomeruli, although not significant. MMP-9 protein was similar in one month old Col1a2-deficient and wildtype glomeruli, but MMP-9 protein level was 2-fold lower in Col1a2-deficient glomeruli than in the wildtype (p≤0.05) at three months of age, opposite to the change in mRNA (Figure 4F).
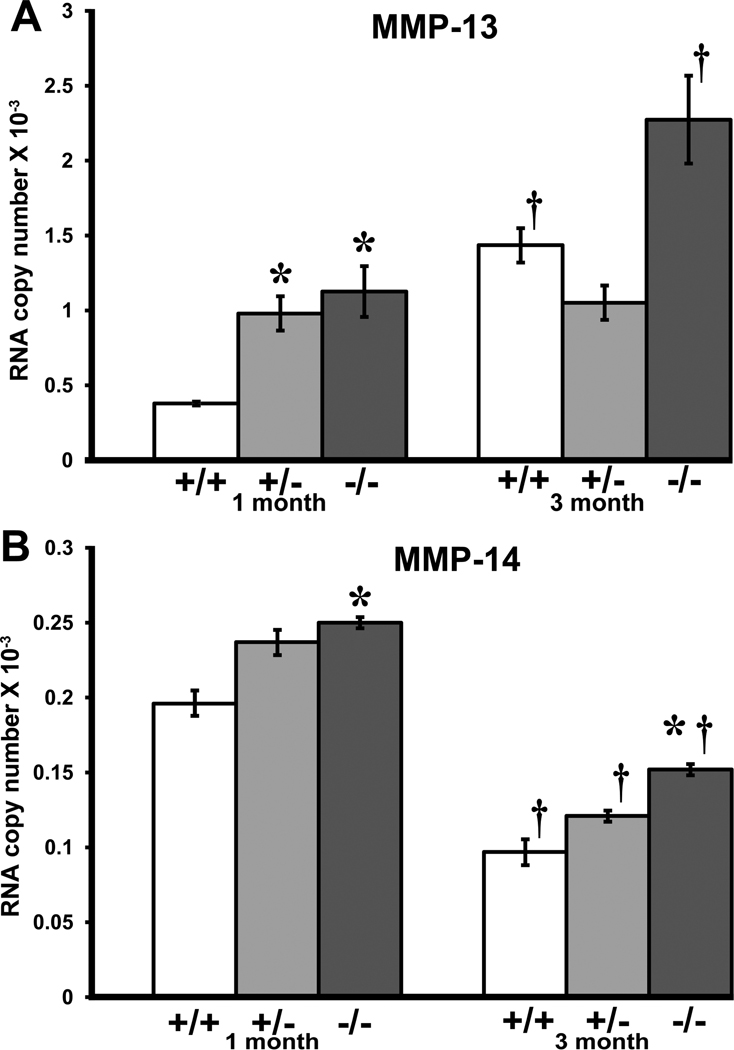
MMP-13 steady-state mRNA levels (Figure 5A) were higher in Col1a2-deficient than wildtype glomeruli (p ≤ 0.02) and increased with age (p ≤ 0.02). MMP-14 steady state mRNA levels (Figure 5B) were also higher in Col1a2-deficient glomeruli than in wildtype glomeruli (p ≤ 0.02), but decreased in all genotypes with age (p≤0.0001). Due to very small amounts of tissue and very low expression levels, protein data was not analyzed for MMP-13 and MT1-MMP.
Figure 5.
Quantitative RT-PCR steady-state expression of MMP-13 and MMP-14 transcripts in wildtype (+/+), heterozygous (+/−) and homozygous (−/−) Col1a2-deficient glomeruli. 1-month homozygous (*p <0.02) and heterozygous Col1a2-deficient glomeruli (*p <0.03) show an increase in MMP-13 (A) mRNA expression compared to age-matched wildtype glomeruli and increases in wildtype (†p<0.02) and Col1a2-deficient glomeruli (†p<0.02) between 1 and 3-months of age. MMP-14 (B) mRNA transcript levels are elevated in 1-month homozygous glomeruli compared to age-matched wildtype glomeruli (*p<0.02), and in 3-month homozygous glomeruli (*p ≤0.02). Wildtype glomeruli show a decrease in MMP-14 mRNA transcripts at 3 months of age compared to 1-month wildtype glomeruli and this trend is seen across all genotypes (†p ≤0.0001). Data expressed as mean ±SEM.
4. Discussion
4.1. Collagen accumulation results in increased number and diminished filtration capacity of glomeruli in Col1a2-deficient mice
In utero, fetal glomeruli function to generate the placental fluid, however glomerular functionality and filtration is dramatically changed postnatally. In the postnatal mouse kidney, functionality of the glomeruli appears to start with the innermost glomeruli, increasing circumferentially outward with age until all glomeruli are functioning [58, 70]. Col1a2 deficiency in oim mice results in a progressive glomerulopathy caused by accumulation of type I collagen [58], and the reduced glomerular yields seen in three month Col1a2-deficient kidneys are potentially due to narrowing of glomerular capillaries as a result of collagen deposition and mesangial expansion suggesting that the glomerular capillaries may become restricted to the flow of blood as sclerosis increases, thus diminishing their filtering capacity as well as potentially altering their mechanical properties similar to that observed in glomeruli of the Col4a3−/− and HIV-associated nephropathy mice [71].
4.2. Mice that synthesize heterotrimeric and homotrimeric type I collagen accumulate the homotrimeric isotype in glomeruli
Type I collagen is normally found in the kidney vasculature, to a lesser extent interstitium, but not within the glomerular mesangium [19, 72]. However, during glomerulosclerosis, increases in type I collagen mRNA and accumulation of the protein has been shown within the mesangium [73–75], yet it is not known which isotype of type I collagen is present, or why it accumulates. Although we have previously shown homotrimeric type I collagen accumulation in sclerotic glomeruli of Col1a2-deficient kidneys [12, 58], we did not examine sclerotic glomeruli in heterozygous mice, which synthesize both collagen isotypes, to determine whether homotrimer was indeed the pathogenic accumulating collagen in the glomeruli. As shown in Figures 1&2, homotrimer is the predominant type I collagen isotype (95–98%) accumulating in sclerotic heterozygous Col1a2-deficient glomeruli, while both heterotrimeric and homotrimeric type I collagen appear equally present in tissues outside the glomeruli, suggesting a major role of homotrimeric type I collagen in the pathogenesis of the disease.
4.3. The homotrimer accumulation in heterozygous glomeruli is caused by deficient degradation of the homotrimers rather than increased synthesis of α1(I) chains
Stressed mesangial cells were shown to produce the homotrimeric collagen isotype [13]. However, our examination of steady-state mRNA revealed no increases in COL1A1 expression in heterozygous glomeruli at 1 and 3 months (Figure 3). COL1A1 expression was increased only in homozygous animals as a response to more severe overall collagen deficiency, consistent with previous observations in whole kidneys [58]. Apparently, increased COL1A1 expression was not the primary cause but a contributing factor to glomerulosclerosis severity in these animals.
Our data suggest that type I collagen is weakly expressed in wildtype, heterozygous, and Col1a2-deficient glomeruli. The protein seems to be effectively degraded in wildtype glomeruli, so that it is usually not detected and it does not accumulate. Collagen accumulation in heterozygous glomeruli with normal or even reduced expression of COL1A1 and COL1A2 mRNA (Figure 3) indicates that normal heterotrimers might be recognized and degraded more efficiently, leaving homotrimers lingering within the mesangial matrix.
The inefficient degradation appears to be associated with the absence of the α2(I) chain in homotrimer molecules. Although the 97% homology between amino acid compositions of the α1(I) and α2(I) chains has been evolutionarily conserved for the past 500 million years [1, 2], absence of the α2(I) chain leads to significant changes in type I collagen properties. The α2(I) chain alters crosslinking and tensile strength of collagen fibers [76] as well as interactions between the triple helices in fibers [77]. It reduces the overall triple helix stability [78, 79] and changes the local stability of differing regions along the helix [79]. The α2(I) chain has been proposed to play an important role in collagen recognition and subsequent cleavage by MMPs [80–82]. Our recent study has revealed that α1(I)3 homotrimers are 5–10 times more resistant to cleavage by all collagenolytic MMPs than normal type I heterotrimers [8] due to increased triple helix stability at the cleavage site [26].
4.4. MMP upregulation follows the homotrimer build up but is not sufficient to degrade the accumulating collagen
Collagenases (MMP-13, -14), gelatinases (MMP-2, -9), and stromelysin (MMP-3) are all crucial enzymes for type I collagen degradation in soft mouse tissues [83]. Although MMP-3 does not cleave collagen by itself, it is essential for activating collagenolytic MMPs. In addition to collagen degradation, the same MMPs also have other functions and substrates that may be important in EMT induction and fibrosis [84]. MMPs not only activate themselves as previously mentioned, but have been shown to be transcriptionally regulated by TGF-α and other growth factors that take part in the process of fibrosis[42, 85, 86]. Further, MMP collagenolytic activity has also been shown to be influenced by the presence of TIMPs." Nonetheless, we have observed significant changes in MMP mRNA expression only in homozygous animals and only at three month of age (Figures 4 and 5). In these mice, homotrimer accumulation begins as early as 1 week after birth, suggesting that the MMP upregulation is not a primary pathogenic event [58].
The simplest interpretation of our observations is that the upregulation of the collagen degradation pathway occurs in response to collagen accumulation, although it is not sufficient for degradation of the MMP-resistant homotrimers. On a cautionary note, however, increased levels of MMP-2 and MMP-3 proteins seem to occur before an increase in their mRNA. These discrepancies suggest potential changes in protein translation, circulation, or life span of the MMPs in the tissue. Alterations in circulating levels of MMPs have been associated with inflammation and tumor progression [46, 50, 87–89]. However, decreases in MMP-9 protein seem to suggest that it has little or no role in this type of glomerulopathy. Additional investigation is necessary to elucidate the regulation and functional mechanisms of MMPs in Col1a2-deficient glomeruli.
4.5. ER stress may exacerbate Col1a2-deficient glomerulopathy
The aberrant proα2(I) chains, which are not incorporated into the collagen triple helix, appear to be translated and retained in the endoplasmic reticulum (ER), triggering an unfolded protein response (UPR) and degradation [2, 55, 90]. The resulting ER stress may explain why syntheses on nonfunctional proα2(I) chains leads to severe skeletal deformities [55] while complete lack of proα2(I) chain translation does not [91]. ER stress due to UPR has recently been found to play a role in abnormal skeletal formation and renal disease [92–96]. ER stress and UPR may result not only in cell malfunction but also in increased synthesis of type I collagen homotrimers, which is a known response of mesangial cells to stress [13]. ER stress and UPR may underlie the increased COL1A1 expression and decreased COL1A2 expression in homozygous animals at three month of age. The increased homotrimer synthesis may worsen the disease. Further studies are needed to define the potential role of ER stress in the glomerulopathy in Col1a2-deficient mice.
In summary, our findings demonstrate that homotrimeric type I collagen is the pathogenic type I collagen isotype accumulating in sclerotic glomeruli of heterozygous and homozygous mice with Col1a2 deficiency. This accumulation appears to be associated with the type I collagen homotrimer resistance to MMPs and inefficient homotrimer “clean-up” within the mesangium. It may be exacerbated by UPR to nonfunctional proα2(I) chains, resulting in ER stress, and accompanying upregulation of the COL1A1 gene and down-regulation of the COL1A2 gene. Alterations in MMP-2, -3, -9, -13 and -14 expression appear to be a secondary response rather than primary events in the disease process. Increased expression of collagenolytic MMPs is insufficient to prevent accumulation of homotrimeric type I collagen in the glomeruli of the Col1a2-deficient mouse.
Acknowledgements
The authors are grateful to Dr. Rolf Brekken (University of Texas-Southwestern) for assistance with antibody development, and to Stephanie Carleton, PhD, and Bettina Gentry, DVM, Kristin Dietiker, and Lauren Hibler for technical assistance.
Role of the funding source
This work was supported in part by the Leda J. Sears Trust Foundation Grant, NIH/NIDDK Grant, DK069522, NIH/Clinical Biodetectives Training Grant, R90-DK071510-03 and the Intramural Research Program of NICHD. The funding sources had no involvement in the study design, collection, analysis or interpretation of data, writing of the report or in the decision to submit the paper for publication.
Abbreviations
- +/+
wildtype mouse
- +/−
heterozygous oim mouse
- −/−
homozygous oim mouse
- BSA
bovine serum albumin
- DAB
3,3’ diaminobenzidine tetrahydrochloride
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal-transition
- ER
endoplasmic reticulum
- HBSS
Hanks Balanced Salt Solution
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- IHC
immunohistochemistry
- MMP
matrix metalloproteinase
- OI
osteogenesis imperfecta
- oim
osteogenesis imperfecta murine
- PBS
phosphate buffered saline
- PMSF
phenylmethyl sulfonyl fluoride
- PSR
picrosirius red
- TRS
target retrieval solution
- UPR
unfolded protein response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the American Society for Matrix Biology Meeting (San Diego, CA December 9, 2008).
References
- 1.Bornstein P, Sage H. Structurally distinct collagen types. Annual review of biochemistry. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- 2.Deak SB, Nicholls A, Pope FM, Prockop DJ. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. The Journal of biological chemistry. 1983;258:15192–15197. [PubMed] [Google Scholar]
- 3.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. Journal of cell science. 2007;120:1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 4.Kielty CH, I, Grant M. Connective Tissue and its Heritable Disorders - Molecular, Genetic, and Medical Aspects. New York: Wiley-Liss; 1993. [Google Scholar]
- 5.Jimenez SA, Bashey RI, Benditt M, Yankowski R. Identification of collagen alpha1(I) trimer in embryonic chick tendons and calvaria. Biochem Biophys Res Commun. 1977;78:1354–1361. doi: 10.1016/0006-291x(77)91441-3. [DOI] [PubMed] [Google Scholar]
- 6.Moro L, Smith BD. Identification of collagen alpha1(I) trimer and normal type I collagen in a polyoma virus-induced mouse tumor. Archives of biochemistry and biophysics. 1977;182:33–41. doi: 10.1016/0003-9861(77)90280-6. [DOI] [PubMed] [Google Scholar]
- 7.Rupard JH, Dimari SJ, Damjanov I, Haralson MA. Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. The American journal of pathology. 1988;133:316–326. [PMC free article] [PubMed] [Google Scholar]
- 8.Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, Leikin S. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 2010;70:4366–4374. doi: 10.1158/0008-5472.CAN-09-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–719. [PubMed] [Google Scholar]
- 10.Narayanan AS, Page RC, Meyers DF. Characterization of collagens of diseased human gingiva. Biochemistry. 1980;19:5037–5043. doi: 10.1021/bi00563a016. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich HP, Brown H, White BS. Evidence for type V and I trimer collagens in Dupuytren's Contracture palmar fascia. Biochem Med. 1982;28:273–284. doi: 10.1016/0006-2944(82)90080-1. [DOI] [PubMed] [Google Scholar]
- 12.Phillips CL, Pfeiffer BJ, Luger AM, Franklin CL. Novel collagen glomerulopathy in a homotrimeric type I collagen mouse (oim) Kidney Int. 2002;62:383–391. doi: 10.1046/j.1523-1755.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- 13.Haralson MA, Jacobson HR, Hoover RL. Collagen polymorphism in cultured rat kidney mesangial cells. Laboratory investigation; a journal of technical methods and pathology. 1987;57:513–523. [PubMed] [Google Scholar]
- 14.Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71:755–763. doi: 10.1038/sj.ki.5002108. [DOI] [PubMed] [Google Scholar]
- 15.Eikmans M, Baelde JJ, de Heer E, Bruijn JA. ECM homeostasis in renal diseases: a genomic approach. J Pathol. 2003;200:526–536. doi: 10.1002/path.1417. [DOI] [PubMed] [Google Scholar]
- 16.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- 17.Schnaper HW. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr Nephrol. 1995;9:104–111. doi: 10.1007/BF00858986. [DOI] [PubMed] [Google Scholar]
- 18.Gibson IW, More IA. Glomerular pathology: recent advances. J Pathol. 1998;184:123–129. doi: 10.1002/(SICI)1096-9896(199802)184:2<123::AID-PATH16>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Glick AD, Jacobson HR, Haralson MA. Mesangial deposition of type I collagen in human glomerulosclerosis. Human pathology. 1992;23:1373–1379. doi: 10.1016/0046-8177(92)90057-a. [DOI] [PubMed] [Google Scholar]
- 20.Sakatsume M, Saito A, Takeda T, Yamazaki H, Narita I, Nishi S, Nakagawa Y, Arakawa M. Mesangial cell-matrix interactions: modulation of matrix expression in mesangial cells by extracellular matrices. Experimental nephrology. 1995;3:362–368. [PubMed] [Google Scholar]
- 21.Striker LJ, Doi T, Elliot S, Striker GE. The contribution of glomerular mesangial cells to progressive glomerulosclerosis. Seminars in nephrology. 1989;9:318–328. [PubMed] [Google Scholar]
- 22.Akhtar M, Al Mana H. Molecular basis of proteinuria. Advances in anatomic pathology. 2004;11:304–309. doi: 10.1097/01.pap.0000146219.03058.ea. [DOI] [PubMed] [Google Scholar]
- 23.Kwoh C, Shannon MB, Miner JH, Shaw A. Pathogenesis of nonimmune glomerulopathies. Annual review of pathology. 2006;1:349–374. doi: 10.1146/annurev.pathol.1.110304.100119. [DOI] [PubMed] [Google Scholar]
- 24.Latta H. An approach to the structure and function of the glomerular mesangium. J Am Soc Nephrol. 1992;2:S65–S73. doi: 10.1681/ASN.V210s65. [DOI] [PubMed] [Google Scholar]
- 25.Miner JH. A molecular look at the glomerular barrier. Nephron. 2003;94:e119–e122. doi: 10.1159/000072495. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Makareeva E, Kuznetsova NV, DeRidder AM, Sutter MB, Losert W, Phillips CL, Visse R, Nagase H, Leikin S. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J Biol Chem. 2010;285:22276–22281. doi: 10.1074/jbc.M110.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelongt B, Ronco P. Role of matrix metalloproteinases in kidney development and glomerulopathy: lessons from transgenic mice. Nephrol Dial Transplant. 2002;17 Suppl 9:28–31. doi: 10.1093/ndt/17.suppl_9.28. [DOI] [PubMed] [Google Scholar]
- 28.Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- 29.Norman JT, Lewis MP. Matrix metalloproteinases (MMPs) in renal fibrosis. Kidney international. 1996;54:S61–S63. [PubMed] [Google Scholar]
- 30.Shiozawa S. Participation of macrophages in glomerular sclerosis through the expression and activation of matrix metalloproteinases. Pathology international. 2000;50:441–457. doi: 10.1046/j.1440-1827.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- 31.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol. 2007;292:F905–F911. doi: 10.1152/ajprenal.00421.2006. [DOI] [PubMed] [Google Scholar]
- 32.Davies M, Thomas GJ, Martin J, Lovett DH. The purification and characterization of a glomerular-basement-membrane-degrading neutral proteinase from rat mesangial cells. The Biochemical journal. 1988;251:419–425. doi: 10.1042/bj2510419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH. Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. The Journal of biological chemistry. 1996;271:15074–15083. doi: 10.1074/jbc.271.25.15074. [DOI] [PubMed] [Google Scholar]
- 34.Mertens PR, Harendza S, Pollock AS, Lovett DH. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. The Journal of biological chemistry. 1997;272:22905–22912. doi: 10.1074/jbc.272.36.22905. [DOI] [PubMed] [Google Scholar]
- 35.Turck J, Pollock AS, Lovett DH. Gelatinase A is a glomerular mesangial cell growth and differentiation factor. Kidney Int. 1997;51:1397–1400. doi: 10.1038/ki.1997.191. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. The American journal of pathology. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda T, Yoshida Y, Kamiie J, Kovalenko P, Nameta M, Fujinaka H, Yaoita E, Endo T, Ishizuka S, Nakabayashi K, Yamada A, Nagasawa T, Yamamoto T. Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis in WKY rats. Clinical and experimental nephrology. 2004;8:206–215. doi: 10.1007/s10157-004-0289-8. [DOI] [PubMed] [Google Scholar]
- 38.Lelongt B, Legallicier B, Piedagnel R, Ronco PM. Do matrix metalloproteinases MMP-2 and MMP-9 (gelatinases) play a role in renal development, physiology and glomerular diseases? Current opinion in nephrology and hypertension. 2001;10:7–12. doi: 10.1097/00041552-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 39.McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH. Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. The Journal of clinical investigation. 1996;97:1094–1101. doi: 10.1172/JCI118502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang M, Huang H, Li J, Huang W, Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen. 2007;15:817–824. doi: 10.1111/j.1524-475X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 41.Portik-Dobos V, Harris AK, Song W, Hutchinson J, Johnson MH, Imig JD, Pollock DM, Ergul A. Endothelin antagonism prevents early EGFR transactivation but not increased matrix metalloproteinase activity in diabetes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R435–R441. doi: 10.1152/ajpregu.00300.2005. [DOI] [PubMed] [Google Scholar]
- 42.Uchio K, Manabe N, Tamura K, Miyamoto M, Yamaguchi M, Ogura A, Yamamoto Y, Miyamoto H. Decreased matrix metalloproteinase activity in the kidneys of hereditary nephrotic mice (ICGN strain) Nephron. 2000;86:145–151. doi: 10.1159/000045733. [DOI] [PubMed] [Google Scholar]
- 43.Wu K, Setty S, Mauer SM, Killen P, Nagase H, Michael AF, Tsilibary EC. Altered kidney matrix gene expression in early stages of experimental diabetes. Acta anatomica. 1997;158:155–165. doi: 10.1159/000147926. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer L, Han X, August C, Matzkies F, Lorenz T, Schaefer RM. Differential regulation of glomerular gelatinase B (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in obese. Zucker rats Diabetologia. 1997;40:1035–1043. doi: 10.1007/s001250050785. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Molecular and cellular biochemistry. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigo E, Lopez-Hoyos M, Escallada R, Fernandez-Fresnedo G, Ruiz JC, Pinera C, Cotorruelo JG, Zubimendi JA, de Francisco AL, Arias M. Circulating levels of matrix metalloproteinases MMP-3 and MMP-2 in renal transplant recipients with chronic transplant nephropathy. Nephrol Dial Transplant. 2000;15:2041–2045. doi: 10.1093/ndt/15.12.2041. [DOI] [PubMed] [Google Scholar]
- 47.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis Semin. Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMPdeficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Tsao MS, Pagura M, Shalinsky DR, Khoka R, Fata J, Johnston MR. Early combined treatment with carboplatin and the MMP inhibitor, prinomastat, prolongs survival and reduces systemic metastasis in an aggressive orthotopic lung cancer model. Lung cancer (Amsterdam, Netherlands) 2003;42:335–344. doi: 10.1016/s0169-5002(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 50.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clinical & experimental metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 51.Chipman SD, Sweet HO, McBride DJ, Jr, Davisson MT, Marks SC, Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camacho NP, Hou L, Toledano TR, Ilg WA, Brayton CF, Raggio CL, Root L, Boskey AL. The material basis for reduced mechanical properties in oim mice bones. J Bone Miner Res. 1999;14:264–272. doi: 10.1359/jbmr.1999.14.2.264. [DOI] [PubMed] [Google Scholar]
- 53.Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL. Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim) Bone. 2008;42:681–694. doi: 10.1016/j.bone.2007.12.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McBride DJ, Jr, Shapiro JR, Dunn MG. Bone geometry and strength measurements in aging mice with the oim mutation. Calcified tissue international. 1998;62:172–176. doi: 10.1007/s002239900412. [DOI] [PubMed] [Google Scholar]
- 55.Nicholls AC, Osse G, Schloon HG, Lenard HG, Deak S, Myers JC, Prockop DJ, Weigel WR, Fryer P, Pope FM. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984;21:257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholls AC, Pope FM, Schloon H. Biochemical heterogeneity of osteogenesis imperfecta: New variant. Lancet. 1979;1:1193. doi: 10.1016/s0140-6736(79)91872-5. [DOI] [PubMed] [Google Scholar]
- 57.Pihlajaniemi T, Dickson LA, Pope FM, Korhonen VR, Nicholls A, Prockop DJ, Myers JC. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984;259:12941–12944. [PubMed] [Google Scholar]
- 58.Brodeur AC, Wirth DA, Franklin CL, Reneker LW, Miner JH, Phillips CL. Type I collagen glomerulopathy: postnatal collagen deposition follows glomerular maturation. Kidney Int. 2007;71:985–993. doi: 10.1038/sj.ki.5002173. [DOI] [PubMed] [Google Scholar]
- 59.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. The Journal of biological chemistry. 2001;276:10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 60.VanBuren V, Piao Y, Dudekula DB, Qian Y, Carter MG, Martin PR, Stagg CA, Bassey UC, Aiba K, Hamatani T, Kargul GJ, Luo AG, Kelso J, Hide W, Ko MS. Assembly, verification, and initial annotation of the NIA mouse 7.4K cDNA clone set. Genome research. 2002;12:1999–2003. doi: 10.1101/gr.633802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips CL, Bradley DA, Schlotzhauer CL, Bergfeld M, Libreros-Minotta C, Gawenis LR, Morris JS, Clarke LL, Hillman LS. Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone. 2000;27:219–226. doi: 10.1016/s8756-3282(00)00311-2. [DOI] [PubMed] [Google Scholar]
- 62.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. The American journal of pathology. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makareeva E, Cabral WA, Marini JC, Leikin S. Molecular mechanism of alpha 1(I)-osteogenesis imperfecta/Ehlers-Danlos syndrome: unfolding of an N-anchor domain at the N-terminal end of the type I collagen triple helix. The Journal of biological chemistry. 2006;281:6463–6470. doi: 10.1074/jbc.M511830200. [DOI] [PubMed] [Google Scholar]
- 64.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10 European journal of immunology. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 65.Austin BA, Liu B, Li Z, Nussenblatt RB. Biologically active fibronectin fragments stimulate release of MCP-1 and catabolic cytokines from murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:2896–2902. doi: 10.1167/iovs.08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem Res Toxicol. 2007;20:208–216. doi: 10.1021/tx060228l. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro JR, McBride DJ, Jr, Fedarko NS. OIM and related animal models of osteogenesis imperfecta. Connective tissue research. 1995;31:265–268. doi: 10.3109/03008209509010820. [DOI] [PubMed] [Google Scholar]
- 68.Deak SB, van der Rest M, Prockop DJ. Altered helical structure of a homotrimer of alpha 1(I)chains synthesized by fibroblasts from a variant of osteogenesis imperfecta. Collagen and related research. 1985;5:305–313. doi: 10.1016/s0174-173x(85)80020-0. [DOI] [PubMed] [Google Scholar]
- 69.Chu ML, Rowe D, Nicholls AC, Pope FM, Prockop DJ. Presence of translatable mRNA for pro alpha 2(I) chains in fibroblasts from a patient with osteogenesis imperfecta whose type I collagen does not contain alpha 2(I) chains. Collagen and related research. 1984;4:389–394. doi: 10.1016/s0174-173x(84)80006-0. [DOI] [PubMed] [Google Scholar]
- 70.Kleinman LI, Reuter JH. Maturation of glomerular blood flow distribution in the new-born dog. The Journal of physiology. 1973;228:91–103. doi: 10.1113/jphysiol.1973.sp010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyss HM, Henderson JM, Byfield FJ, Bruggeman LA, Ding Y, Huang C, Suh JH, Franke T, Mele E, Pollak MR, Miner JH, Janmey PA, Weitz DA, Miller RT. Biophysical properties of normal and diseased renal glomeruli. Am J Physiol Cell Physiol. 300:C397–C405. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexakis C, Maxwell P, Bou-Gharios G. Organ-specific collagen expression: implications for renal disease. Nephron. 2006;102:e71–e75. doi: 10.1159/000089684. [DOI] [PubMed] [Google Scholar]
- 73.Floege J, Alpers CE, Burns MW, Pritzl P, Gordon K, Couser WG, Johnson RJ. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model Laboratory investigation. a journal of technical methods and pathology. 1992;666:485–497. [PubMed] [Google Scholar]
- 74.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol. 1999;10:271–280. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- 75.Peten EP, Striker LJ, Garcia-Perez A, Striker GE. Studies by competitive PCR of glomerulosclerosis in growth hormone transgenic mice. Kidney international. 1993;39:S55–S58. [PubMed] [Google Scholar]
- 76.Misof K, Landis WJ, Klaushofer K, Fratzl P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. The Journal of clinical investigation. 1997;100:40–45. doi: 10.1172/JCI119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuznetsova N, McBride DJ, Jr, Leikin S. Osteogenesis imperfecta murine: interaction between type I collagen homotrimers. Journal of molecular biology. 2001;309:807–815. doi: 10.1006/jmbi.2001.4682. [DOI] [PubMed] [Google Scholar]
- 78.Miles CA, Sims TJ, Camacho NP, Bailey AJ. The role of the alpha2 chain in the stabilization of the collagen type I heterotrimer: a study of the type I homotrimer in oim mouse tissues. Journal of molecular biology. 2002;321:797–805. doi: 10.1016/s0022-2836(02)00703-9. [DOI] [PubMed] [Google Scholar]
- 79.Kuznetsova NV, McBride DJ, Leikin S. Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. Journal of molecular biology. 2003;331:191–200. doi: 10.1016/s0022-2836(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 80.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nerenberg PS, Stultz CM. Differential unfolding of alpha1 and alpha2 chains in type I collagen and collagenolysis. J Mol Biol. 2008;382:246–256. doi: 10.1016/j.jmb.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci U S A. 2008;105:2824–2829. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? Journal of cellular biochemistry. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 86.Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999;10:790–795. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]
- 87.Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu YH, Sung JS, Cha TL, Sun GH. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer science. 2008;99:905–913. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. The Journal of investigative dermatology. 2002;119:91–98. doi: 10.1046/j.1523-1747.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- 89.Wen D, Zhou XL, Li JJ, Hui RT. Biomarkers in aortic dissection. Clin Chim Acta. 412:688–695. doi: 10.1016/j.cca.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 90.Pace JM, Wiese M, Drenguis AS, Kuznetsova N, Leikin S, Schwarze U, Chen D, Mooney SH, Unger S, Byers PH. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. The Journal of biological chemistry. 2008;283:16061–16067. doi: 10.1074/jbc.M801982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwarze U, Hata R, McKusick VA, Shinkai H, Hoyme HE, Pyeritz RE, Byers PH. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. American journal of human genetics. 2004;74:917–930. doi: 10.1086/420794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bin-Abbas B, Al-Mulhim A, Al-Ashwal A. Wolcott-Rallison syndrome in two siblings with isolated central hypothyroidism. American journal of medical genetics. 2002;111:187–190. doi: 10.1002/ajmg.10495. [DOI] [PubMed] [Google Scholar]
- 93.Bonthron DT, Dunlop N, Barr DG, El Sanousi AA, Al-Gazali LI. Organisation of the human PAX4 gene and its exclusion as a candidate for the Wolcott-Rallison syndrome. Journal of medical genetics. 1998;35:288–292. doi: 10.1136/jmg.35.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamamura K, Yokota H. Stress to endoplasmic reticulum of mouse osteoblasts induces apoptosis and transcriptional activation for bone remodeling. FEBS letters. 2007;581:1769–1774. doi: 10.1016/j.febslet.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. American journal of physiology. 2008;295:F323–F334. doi: 10.1152/ajprenal.00050.2008. [DOI] [PubMed] [Google Scholar]
- 96.Lisse TS, Thiele F, Fuchs H, Hans W, Przemeck GK, Abe K, Rathkolb B, Quintanilla-Martinez L, Hoelzlwimmer G, Helfrich M, Wolf E, Ralston SH, Hrabe de Angelis M. ER stress-mediated apoptosis in a new mouse model of osteogenesis imperfecta. PLoS genetics. 2008;4:e7. doi: 10.1371/journal.pgen.0040007. [DOI] [PMC free article] [PubMed] [Google Scholar]