Abstract
Disconnected Interacting Protein 1 (DIP1), a member of the double-stranded RNA-binding protein family based on amino acid sequence, was shown previously to form complexes with multiple transcription factors in Drosophila melanogaster. To explore this protein further, we have undertaken sedimentation equilibrium experiments that demonstrate that DIP1-c (longest isoform of DIP1) is a dimer in solution, a characteristic common to other members of the dsRNA-binding protein family. The closest sequence identity for DIP1 is found within the dsRBD sequences of RNA editase enzymes. Consistent with this role, we demonstrate binding of DIP1-c to a potential physiological RNA target: pre-tRNA. In addition, DIP1-c was shown to interact with ADAT, a tRNA deaminase that presumably modifies pre-tRNAs. From these data, we hypothesize that DIP1 may serve an integrator role by binding its dsRNA ligand and recruiting protein partners for the appropriate metabolism of the bound RNA.
Keywords: dsRNA-binding protein, DIP1, ADAT, high affinity, tRNA, Gel retardation
Disconnected Interacting Protein 1 (DIP1)1 is a member of the double-stranded RNA (dsRNA) binding protein family and, based on sequence alignment, contains two dsRNA-binding domains (dsRBDs) [1,2]. DIP1-c, which is the longest isoform of DIP1, has been shown to bind a number of RNA targets in vitro [1,3]. Proteins with dsRBDs, motifs that are 65–68 amino acids in length [4], have been shown to function in a wide variety of RNA processes (reviewed in [5,6]).
Proteins with dsRBDs bind dsRNA with similar affinity (~nM range) (reviewed in [1,3]), and interaction is thought to occur mainly through the ribose-phosphate backbone and not the individual RNA bases [4,7]. How can these proteins discriminate among all RNA molecules in the cell? Currently, it is thought that specificity for dsRNA arises from the spatial arrangement of multiple dsRBDs [8], with the binding footprint for dsRBDs reported to be a minimum of 11 base pairs [9,10] or as large as 18–20 base pairs [11].
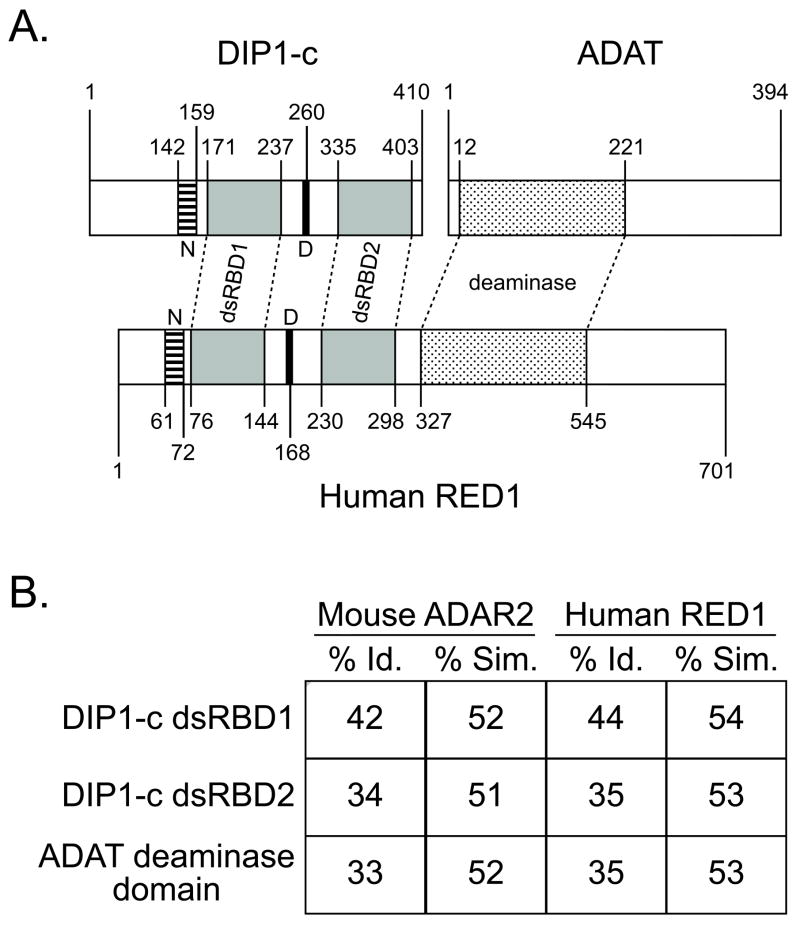
The function of DIP1 and the role of its dsRBDs are currently unknown, but its interactions with Ultrabithorax (Ubx) [1], Disconnected [2], and Su(var)3–9 [12], suggest this protein is involved in transcription regulation [1,2] and chromatin remodeling [12]. However, we suggest here that DIP1 may also contribute to tRNA maturation. Indeed, the closest sequence identity for DIP1 is found within the dsRBD sequences of RNA editase enzymes, such as RED1 [13]. Based on domain arrangements, DIP1-c appears to be comparable to the N-terminal half of human RED1 (Figure 1). Interestingly, the C-terminal half of RED1 appears to mimic the domain structure of ADAT, a tRNA deaminase [14].
Figure 1. Evolutionary correlation between DIP1-c, ADAT, and vertebrate ADAR2/RED1.
A) Alignment of domains within Drosophila DIP1-c and ADAT to human RED1. ADARs in all higher organisms contain two important motifs: dsRBDs and deaminase domains. Interestingly, vertebrate ADAR2 [22] (human RED1 shown) appears to be a fusion between two protein sequences that highly resemble DIP1-c and ADAT. DIP1-c resembles the N-terminal half of ADAR2/RED1, in which there are two dsRBDs (gray), a nuclear localization sequence (N, striped), and a DEAD box (D, black); ADAT contains a deaminase domain (polka dot) similar to the C-terminal half of ADAR2/RED1. B) Sequence comparisons of DIP1-c and ADAT with mammalian ADARs. Shown are the percent identity (% Id.) and similarity (% Sim.) between each DIP1-c dsRBD and ADAT deaminase domain and the corresponding sequence within either mouse ADAR2 or human RED1.
In the present work, we examine the assembly structure of DIP1-c and its interaction with ADAT to show that these two proteins interact in vitro. We further examine the dsRNA-binding behavior of DIP1-c and identify a potential physiological target of DIP1, pre-tRNAs [15], which are presumed to be modified by ADAT.
MATERIALS AND METHODS
Expression and Purification of DIP1-c
Expression and purification of DIP1-c has been described [3]. Given that the histidine tag was shown not to alter DIP1-c affinity for dsRNA [1,3], His6-DIP1-c was used for these experiments.
Analytical Ultracentrifugation (AU)
Micromolar amounts of DIP1-c were dialyzed against 1 L of AU Buffer (20 mM NaH2PO4, 200 mM NaCl, 2% glucose, 0.1 mM dithiothreitol) at 4 °C with four exchanges with pH decreasing from pH 7.8, to pH 7.6, and two steps with pH 7.5. After each exchange, protein was dialyzed for at least 30 minutes. All sedimentation equilibrium experiments were performed at 20 °C according to the manufacturer’s specifications on an Optima XL-A Analytical Ultracentrifuge (Beckman Coulter, Brea, CA) using an An-60 Ti Rotor, charcoal-filled epon 6-channel centerpieces, and Beckman XL-A version p4.5 software. Experiments were performed at four speeds (9,000, 12,000, 15,000 and 18,000 RPM) with wavelengths scans of 235, 237, and 280 nm after 14 hours at each speed. To ensure that sedimentation equilibrium had been achieved, a second scan was taken at least one hour later at each speed.
Analysis of the AU data was performed using UltraScan 6.2 software [16]. Data were analyzed with a GlobalFit regime [17] using the “Ideal and Non-interacting species” model in the software. The parameters used were calculated based on DIP1-c sequence and buffer: Vbar (partial specific volume) was 0.72495 mL/g; density and viscosity of the buffer were 1.0169 g/mL and 1.0734 cp, respectively.
Cloning of GST-ADAT
The ADAT sequence (a gift from Mary O’Connell, Western General Hospital, Edinburgh, U.K.) was cloned between the EcoRI and XhoI sites of the pGEX-6P-2 vector (GE Healthcare, Piscataway, NJ). The resulting vector expresses ADAT fused with the glutathione S-transferase (GST) domain on its N-terminus.
Cloning of tRNA Constructs
The sequence of Bombyx mori (silkworm moth) tRNAAla (a gift from Walter Keller, University of Basel, Czech Republic) was placed under T7 RNA polymerase (RNAP) control in pUC118 (Takara Shuzo Company, Tokyo, Japan). Other constructs were cloned by synthesizing the entire sequence behind the T7 RNAP promoter (5′-TAATACGACTCACTATAG-3′) as two separate oligonucleotides (Biosources, Inc., Camarillo, CA). The sequences were flanked by BamHI and EcoRI sites for cloning into pUC18 (Novagen, Madison, WI). The clones designed were: B. mori pre-tRNAAla [18], Drosophila melanogaster pre-tRNAAla [19], and D. melanogaster tRNAAla [19]. To allow for proper polymerase “runoff” during transcription, all of the above constructs include a restriction site 3′ of the template sequence of either SwaI (for 3′ poly-U constructs) or BstNI (for 3′ CCA constructs).
GST-ADAT Pulldown Assay
GST-ADAT was generated using transcription/translation (TNT) reactions supplemented with [35S]-methionine in the T7 PCR Quick Master Mix (Promega, Madison, WI) (see Supplemental Data in [1]). TNT reactions contained either template for DIP1-c, a plasmid carrying the sequence of D. melanogaster pre-tRNAAla downstream of the T7 RNA polymerase promoter, or both plasmids. TNT reactions were mixed with lysates containing GST-ADAT or “empty” cell lysates. A similar experiment was performed with only the plasmid containing the DIP1-c sequence for the TNT reaction, with addition of exogenous D. melanogaster pre-tRNAAla in amounts ranging from 50 pM to 50 nM. To ensure stability of the pre-tRNAAla in the reaction, an RNase inhibitor (SUPERaseIN, Ambion, Austin, TX) was added. The pre-tRNAAla was added to the wash buffer (20 mM Tris-HCl, pH 7.5, 100 mM KCl, 5 mM MgCl2) to maintain the potential ternary complex of pre-tRNAAla, DIP1-c, and GST-ADAT throughout the experiment.
RNA preparation and handling
Pre-tRNA and tRNA templates were grown in 50 mL LB overnight cultures of either XL1-Blue (Stratagene, La Jolla, CA) or DH5α (Invitrogen Corp., Carlsbad, CA) E. coli cells, and the plasmids were purified using a Qiagen miniprep kit (Valencia, CA). To allow for proper T7 RNAP polymerase “runoff,” each construct was digested several hours by either SwaI (for 3′ poly-U constructs) or BstNI (for 3′ CCA constructs), based on manufacturer’s instructions (New England Biolabs, Ipswich, MA). The digested plasmids were precipitated with ethanol and resuspended in water treated with diethylpyrocarbonate (Sigma, St. Louis, MO). RNA ligands were transcribed in vitro, gel-purified, dephosphorylated, folded, and radiolabeled as described [1,3].
RNA Gel Retardation
Gel retardation assays and analysis were performed as described [1,3].
RESULTS
Establishing DIP1-c Protein Character for Detailed RNA Binding Analysis
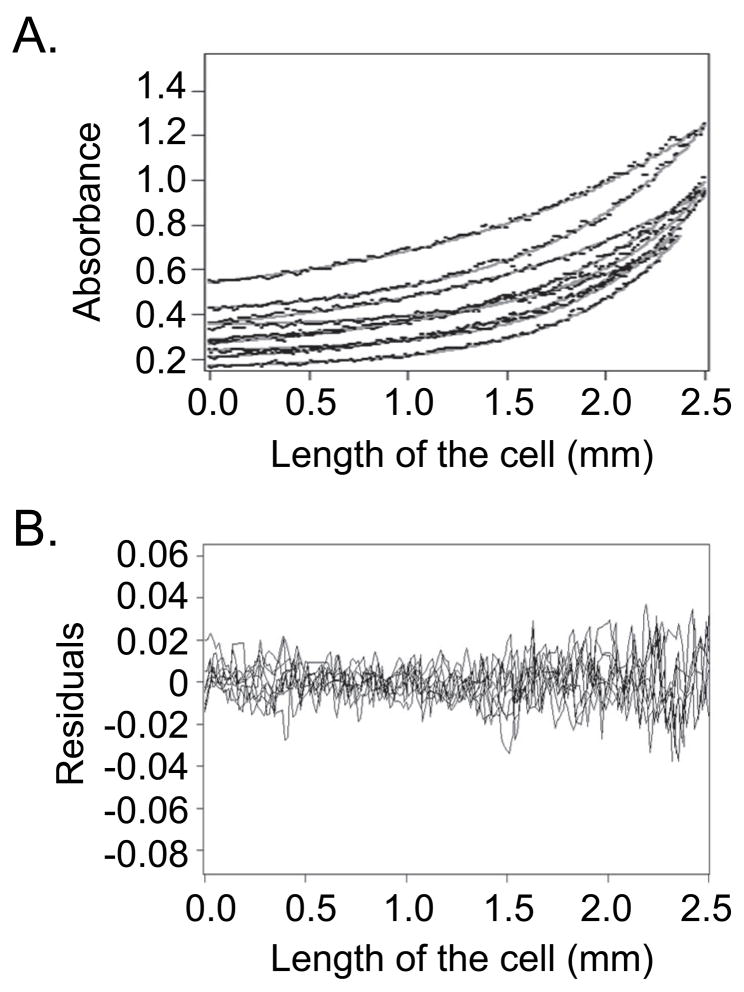
Precise binding parameters require determining the functional binding unit of DIP1-c. Other proteins with dsRBDs are reported to dimerize [6,20,21], and DIP1-c self- associates in a yeast two-hybrid assay (Peter Pelka, personal communication). To confirm this feature, analytical ultracentrifugation was used to determine the solution state of DIP1-c. Sedimentation equilibrium experiments yielded a molecular weight for DIP1-c of ~90 kDa (Figure 2), consistent with a dimer in solution. This oligomeric state agrees with observations for other dsRNA-binding proteins [6,20,21] and the fact that DIP1-c contains one Type B dsRBD, which promotes cooperative dsRNA binding and/or dimerization [20]. Under the conditions tested, a single dimeric species was observed. Given the DIP1-c concentration, the dimer dissociation constant can be estimated to be < 10−7 M.
Figure 2. Analytical ultracentrifugation demonstrates DIP1-c is a homodimer in solution.
A) Representative curves of the global fit [17] of the raw data of the sedimentation equilibrium experiments with DIP1-c protein. The raw data (black dots) of the exponentials overlay well with the fitted curves (gray lines) using the Ultrascan 6.2 program [16]. B) The residuals comparing the raw data versus the fitted data. The residuals are largely within 0.02 units of agreement, indicating a good fit. These data predict DIP1-c to be a homodimer of ~90 kDa in solution (monomer molecular weight is 46 kDa).
DIP1-c Physically Interacts with ADAT, a tRNA Deaminase
Although the DIP1-c sequence contains two dsRBDs with closest similarity to RNA editases [13] (Figure 1), the catalytic domain for editing is missing in DIP1-c [1,2]. In contrast, ADAT, a tRNA deaminase in Drosophila, contains an adenosine deaminase domain but does not possess any recognizable RNA-binding motifs [14]. How this protein (or its orthologues) binds to tRNA is unknown, although ADAT is capable of deaminating tRNAAla in vitro [14,22]. ADAT and DIP1 may be evolutionarily linked (Figure 1) given that all metazoans possess ADARs with both dsRBDs and adenosine deaminase domains [6,23]. Eisenberg’s Rosetta Stone theory [24] predicts protein•protein associations based on the existence of multidomain proteins containing domains homologous to individual proteins.
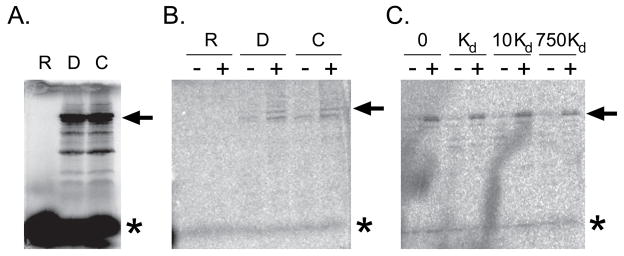
To explore such an interaction, association between DIP1-c and ADAT was determined by a glutathione S-transferase (GST) pulldown assay. [35S]-Methionine-labeled DIP1-c was incubated with glutathione resin and bacterial cell lysate, with or without overexpressed GST-ADAT. [35S]-DIP1-c was precipitated only in the presence GST-ADAT (Figure 3B), although the interaction appeared weak, consistent with observations by yeast two-hybrid assay. Thus, DIP1-c can preferentially interact with GST-ADAT in presence of competing bacterial proteins. We further added D. melanogaster pre-tRNAAla to the GST pulldown assay in two separate ways: expression and exogenous addition. In the first experiment, the expression of both pre-tRNAAla and DIP1-c were under T7 RNA polymerase control. In the second experiment, pre-tRNAAla that had been transcribed in vitro and purified was added exogenously to the GST pulldown reaction (Figure 3C). In both cases, the addition of pre-tRNAAla, even at very high concentrations (e.g., 750 times the Kd) did not alter the association between [35S]-DIP1-c and GST-ADAT, indicating that there is not an RNA dependence for the interaction between the two proteins.
Figure 3. DIP1 interacts physically with ADAT.
A) 12% SDS-PAGE gel depicting in vitro expression of full-length DIP1-c (arrow) labeled with [35S]-methionine. The TNT reactions included the D. melanogaster pre-tRNAala alone (R), DIP1-c alone (D), or both constructs (C). Unincorporated [35S]-methionine is depicted by an asterisk (*). B) Coexpression glutathione S-transferase (GST) pulldown assay gel. Lysates of untransformed bacteria (−) or lysates of bacteria expressing GST-ADAT (+) were mixed with TNT reactions containing either pre-tRNAAla alone (R), DIP1-c alone (D), or both constructs (C). C) Addition of exogenous pre-tRNAAla GST pulldown assay gel. A similar experiment as panel B was performed by adding exogenous D. melanogaster pre-tRNAAla to the GST pulldown reaction. The amounts of pre-tRNAAla added are indicated at the top of the gel; “Kd” denotes the affinity of DIP1-c for the pre-tRNAAla (Figure 4).
DIP1-c Binds with High Affinity to Insect Pre-tRNAs
Given the association between DIP1-c and ADAT, we examined DIP1-c binding to the presumed physiological target of ADAT, pre-tRNAAla [14]. Pre-tRNAs and tRNAs fold into distinct secondary structures, although the duplex stretches are generally no more than seven base pairs in length [25]. Key differences between pre-tRNAs and mature tRNAs include: 1) 5′ and 3′ extensions in pre-tRNAs that are removed [25]; 2) intervening sequences or micro-introns in some tRNAs that are removed [26]; and 3) multiple chemical modifications (i.e., methylation, deamination, isomerization, etc.) that occur in mature tRNA [15].
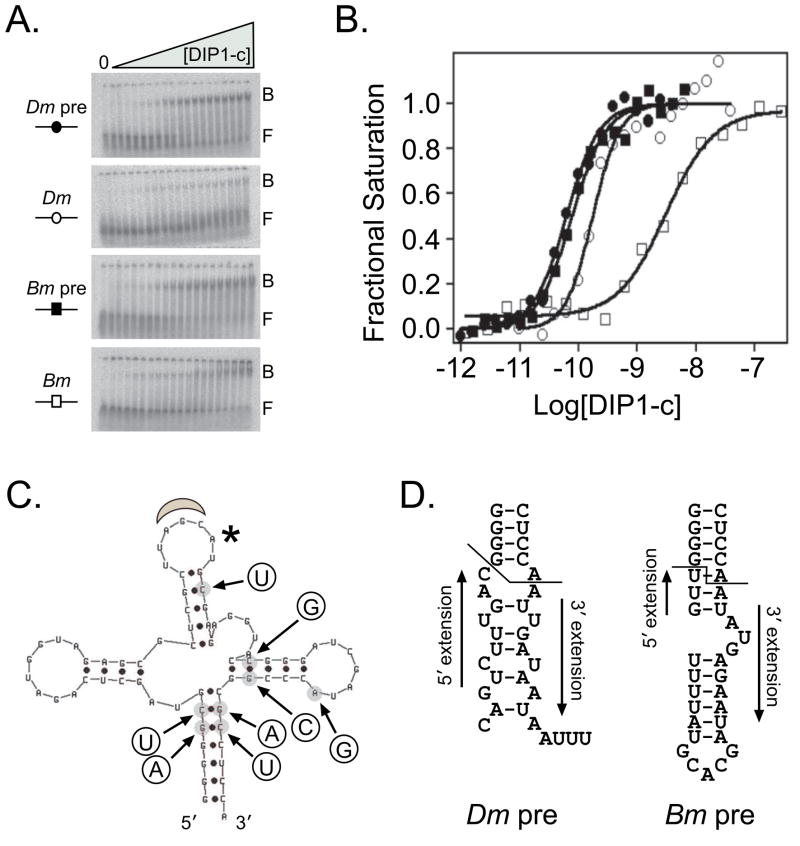
In the present study, we investigated the binding affinity of DIP1-c for two insect pre-tRNAAla and tRNAAla using gel retardation. The only differences between the pre-tRNAsAla and tRNAsAla that were transcribed in vitro were the presence of the 5′ and 3′ extensions on the pre-tRNAs and the addition of CCA, the position of aminoacylation [27], on the 3′ end of the mature tRNA sequence. Since Bombyx mori (silkworm moth) tRNAAla was the only reported ligand that Drosophila ADAT was shown to deaminate in vitro [14], we tested DIP1-c binding to this species and its pre-tRNAAla counterpart. DIP1-c demonstrated an almost 30-fold preference for and cooperativity in binding to pre-tRNAAla versus tRNAAla (Kd = 70 ± 26 pM, n = 1.5 vs. 2000 ± 1100 pM, n= 1.1, respectively) (Figure 4B). Since the difference between the silkworm moth ligands was significant, we investigated whether the same pattern existed for the fruit fly ligands. DIP1-c bound preferentially, but with lower differential and similar cooperativity, to D. melanogaster pre-tRNAAla versus tRNAAla (Kd = 63 ± 15 pM, n = 1.5 vs. 160 ± 59 pM, n = 1.9) (Figure 4B). The sequences and predicted secondary structures of the 5′ and 3′ extensions of the pre-tRNAs for the two insect species are divergent (Figure 4D), but whether and how DIP1-c may be recognizing these differences is unknown. Interestingly, DIP1-c demonstrates more than ten-fold preference for Drosophila tRNAAla versus Bombyx tRNAAla, although none of the eight base changes between the two species is predicted to alter the secondary structure (Figure 4C).
Figure 4. DIP1-c binds preferentially to insect pre-tRNAAla.
The ability of DIP1-c to bind the silkworm moth (Bm) and fruit fly (Dm) pre-tRNAAla and tRNAAla was investigated using gel retardation. A) Representative native gels of the four RNAs tested. The bound (B) and free (F) species in each gel are denoted. RNA concentration was ≤ 1 pM in each reaction. The first lane on the left side of each gel contained no DIP1-c protein, and protein concentrations ranged left to right in lanes 2–20 from 1 pM to 6.3 nM (Dm pre, Bm pre), 10 pM to 30 nM (Dm), and 1 pM to 180 nM (Bm). B) Representative binding isotherms for the four RNAs tested. Data were fitted and analyzed as described [1,3]. DIP1-c binds with high affinity to both pre-tRNAsAla; Kd ~60–70 pM, n = 1.5 (Dm pre, filled circles), n = 1.5 (Bm pre, filled squares). The protein loses about 2- to 3-fold affinity for Dm tRNAAla (Kd ~160 pM, n = 1.9, open circles); however, there is a larger decrease in affinity (~28-fold) for Bm tRNAAla (Kd ~2000 pM, n = 1.1, open squares). C) The predicted secondary structure of the B. mori pre-tRNAAla (5′ on the left moving clockwise to 3′). The anticodon is identified by the gray moon at the top and an asterisk denotes the adenosine at position 37 that is modified by ADAT [14]. There are eight base differences (circles) between B. mori and D. melanogaster tRNAAla; none of these substitutions are predicted to change the secondary structure. D) The predicted secondary structures of the D. melanogaster and B. mori 5′ and 3′ extensions. The line denotes where the extensions begin in each pre-tRNAAla.
DISCUSSION
Growing evidence indicates that dsRNA-binding domains recognize and bind their ligands through interactions beyond the ribose-phosphate backbone [4,7]. Previous structural work has shown contacts between dsRBDs and ssRNA regions in multiple NMR structures [23,28]; however, the field is limited by the available number of physiological dsRNA targets. Staufen is known to bind with high specificity to bicoid mRNA [29], but the affinity of this interaction is unknown. A chimeric ADAR1 protein substituted with Protein Kinase R dsRBDs was less effective in deaminating synthetic and physiological substrates, presumably because of distinct selectivity and specificity [30]. The yeast RNase III protein demonstrates specific nuclease activity on hairpins that contain a tetraloop with the sequence 5′-AGNN-3′ [31], where “N” is any of the four ribonucleotides. This protein binds hairpins with random tetraloop sequences with comparable affinity in the nanomolar range, but cleavage does not occur unless the 5′-AGNN-3′ tetraloop is present [31]. Each of these proteins with dsRBDs, which have been shown in many cases to bind dsRNA with comparable affinity in vitro, must recognize their specific RNA target or subset of targets among all RNA species in vivo [6].
We sought to define further the capacity and binding preference for DIP1-c, a member of the dsRBD family. DIP1-c has multiple protein partners, including the Hox transcription factor, Ubx [1], Disconnected, a zinc-finger transcription factor [2], and Su(var)3–9, a histone methyltransferase [12]. DIP1-c interacts with itself in a yeast two-hybrid assay, and we show here that DIP1-c is a dimer in solution, a trait shared with Protein Kinase R [6,20] and ADAR2 [21]. Dimerization has been presumed to occur through type B dsRBDs [20], consistent with the DIP1-c sequence containing one type B dsRBD in its C-terminus, although each dsRBD within DIP1-c exhibits some dsRNA binding capability [2]. We consistently observe cooperative behavior (n values > 1) with higher affinity ligands (here and [3]), suggesting a thermodynamic linkage between assembly of the DIP1-c dimer and high affinity binding to dsRNA.
In some respects, DIP1-c behaves like other members of the dsRNA-binding protein family, with preferential binding for dsRNA over ssRNA or DNA [1,3]. However, DIP1-c is also unique with its ability to discriminate between different RNA ligands [1,3]. Despite the presumed lack of physiological relevance, DIP1-c binds very tightly (Kd ~50 pM) to adenovirus VA1 RNA, and this tight binding is not solely due to a simple dsRNA duplex within VA1 [3]. Indeed, DIP1-c also binds a microRNA (miRNA) precursor stemloop with the highest affinity to dsRNA currently reported in the literature (Kd ~45 pM) [3]. Binding to this miRNA precursor may have physiological significance, as this stemloop gives rise to a miRNA that inhibits Ubx activity in vivo [32]. DIP1-c must recognize elements (sequence or structural) to bind with higher affinity to a subset of RNAs, since DIP1-c binds with an affinity in the nanomolar to submicromolar range to multiple RNAs of similar size that contain duplexed regions [1,3]. We expanded our investigation of DIP1-c to a potential physiological target selected based on its ability to associate with ADAT, a tRNA deaminase [14].
Given the high affinity of DIP1-c for the pre-tRNAAla and the physical interaction with ADAT demonstrated by yeast two-hybrid and GST pulldown experiments, a role for DIP1-c in tRNA maturation appears likely. DIP1-c and ADAT are homologous to sequence segments that are fused in vertebrate proteins [22]. The association between DIP1-c and ADAT would create a complex capable of deaminating the pre-tRNAAla adenosine at position 37 [14]. To our knowledge, Drosophila ADAT activity has not been examined on any Drosophila pre-tRNA or tRNA ligands; however, B. mori tRNAAla is deaminated faithfully only at position 37 by ADAT [14]. An attractive model is that DIP1-c binds pre-tRNAAla and recruits ADAT to deaminate this ligand. This possibility is consistent with the lack of RNA inhibition observed in the GST pulldown assay between these two proteins.
The mechanism of recognition by DIP1-c for pre-tRNAs and tRNAs may differ from the miRNA and VA1 RNA model: None of the pre-tRNAs or tRNAs tested contain the minimal binding footprint of 11 contiguous base pairs [9,10], although B. mori pre-tRNAAla is close with 10 base pairs. We hypothesize the higher affinity of DIP1-c for pre-tRNAs may derive from the 5′ and 3′ extensions, the main distinction from the tRNAs. Despite lack of information on the specific mode of binding, the affinity of DIP1-c for all of the RNAs discussed here and elsewhere [1,3] demonstrates the tuning capacity of this protein. In general, high affinity originates with secondary structure; higher affinity derives from features outside of double-stranded regions of ligands tested and the spatial arrangement of multiple duplexed regions.
DIP1 is a remarkably multi-functional protein with the potential for binding a specific dsRNA and then recruiting one of its partner proteins with that RNA ligand in tow. DIP1 is expressed in all cells, but more highly expressed in the Drosophila nervous system [2], an arrangement consistent with a basal level activity of tRNA modification in all cells and a more specific transcriptional regulation in the nervous system. This multi-ligand binding allows DIP1 to be a “bridge” to bring specific proteins and RNAs together.
Research Highlights.
DIP1-c is a dimer in solution using analytical ultracentrifugation
DIP1-c interacts physically with ADAT, a tRNA deaminase
DIP1-c interaction with ADAT is not altered by the presence of pre-tRNAAla
DIP1-c binds with high affinity to insect pre-tRNAAla
Acknowledgments
The authors would like to thank: Mary O’Connell for the ADAT vector, Walter Keller for the B. mori tRNAAla vector, Yousif Shamoo for the T7 RNAP construct and for technical assistance, Susan Cates and Borries Demeler for assistance with analytical ultracentrifugation and the Ultrascan analysis program. We also would like to thank Sarah Bondos, Liam Keegan, Ana Campos, Dorothy DeSousa, and Peter Pelka for their scientific discussions.
Footnotes
This work was supported by grants from the Robert A. Welch Foundation (C-576) and the National Institutes of Health (GM22441) to KSM. DJC was trained on the National Institutes of Health Houston Area Molecular Biophysics Predoctoral Training Grant 5T32-GM08208.
ABBREVIATIONS:
ADAR, Adenosine Deaminase that acts on RNA, ADAT, Adenosine Deaminase that acts on tRNA, AU, analytical ultracentrifugation, DIP1, Disconnected Interacting Protein 1, DIP1-c, longest DIP1 isoform, dsRBD, double-stranded RNA-binding domain, dsRNA, double-stranded RNA, GST, Glutathione S-transferase, Kd, equilibrium dissociation constant, miRNA, microRNA, RED1, human RNA Editase 1, RNAP, RNA Polymerase, ssRNA, single-stranded RNA, TNT, transcription and translation, tRNAAla, tRNA Alanine, Ubx, Ultrabithorax
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bondos SE, Catanese DJ, Jr, Tan XX, et al. Hox transcription factor ultrabithorax Ib physically and genetically interacts with disconnected interacting protein 1, a double-stranded RNA-binding protein. J Biol Chem. 2004;279:26433–26444. doi: 10.1074/jbc.M312842200. [DOI] [PubMed] [Google Scholar]
- 2.DeSousa D, Mukhopadhyay M, Pelka P, et al. A novel double-stranded RNA- binding protein, disco interacting protein 1 (DIP1), contributes to cell fate decisions during Drosophila development. J Biol Chem. 2003;278:38040–38050. doi: 10.1074/jbc.M303512200. [DOI] [PubMed] [Google Scholar]
- 3.Catanese DJ, Jr, Matthews KS. High affinity, dsRNA binding by disconnected interacting protein 1. Biochem Biophys Res Commun. 2010;399:186–191. doi: 10.1016/j.bbrc.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St Johnston D, Brown NH, Gall JG, et al. A conserved double-stranded RNA- binding domain. Proc Natl Acad Sci USA. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall KB. RNA-protein interactions. Curr Opin Struct Biol. 2002;12:283–288. doi: 10.1016/s0959-440x(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 6.Saunders LR, Barber GN. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- 7.Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krovat BC, Jantsch MF. Comparative mutational analysis of the double- stranded RNA binding domains of Xenopus laevis RNA-binding protein A. J Biol Chem. 1996;271:28112–28119. doi: 10.1074/jbc.271.45.28112. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 10.Bycroft M, Grunert S, Murzin AG, et al. NMR solution structure of a dsRNA binding domain from Drosophila Staufen protein reveals homology to the N- terminal domain of the ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass BL, Hurst SR, Singer JD. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994;4:301–314. doi: 10.1016/s0960-9822(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 12.Krauss V, O’Grady M, Hoffmann J, et al. KLETT, a Drosophila chromatin protein with dsRNA binding motifs interacts with the heterochromatin-associated SU(VAR)3–9 protein. 17th European Drosophila Research Conference; Edinburgh (UK). 2001. p. F8. [Google Scholar]
- 13.Melcher T, Maas S, Herb A, et al. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 14.Keegan LP, Gerber AP, Brindle J, et al. The properties of a tRNA-specific adenosine deaminase from Drosophila melanogaster support an evolutionary link between pre-mRNA editing and tRNA modification. Mol Cell Biol. 2000;20:825–833. doi: 10.1128/mcb.20.3.825-833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolin SL, Matera AG. The trials and travels of tRNA. Genes Dev. 1999;13:1–10. doi: 10.1101/gad.13.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Demeler B. UltraScan - A comprehensive data analysis software package for analytical ultracentrifugation experiments. In: Scott DJ, Harding SE, Rowe AJ, editors. Modern Analytical Ultracentrifugation: Techniques and Methods. Royal Society of Chemistry; Cambridge, U.K: 2005. pp. 210–229. [Google Scholar]
- 17.Ucci JW, Cole JL. Global analysis of non-specific protein-nucleic interactions by sedimentation equilibrium. Biophys Chem. 2004;108:127–140. doi: 10.1016/j.bpc.2003.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagenbuchle O, Larson D, Hall GI, et al. The primary transcription product of a silkworm alanine tRNA gene: identification of in vitro sites of initiation, termination and processing. Cell. 1979;18:1217–1229. doi: 10.1016/0092-8674(79)90234-4. [DOI] [PubMed] [Google Scholar]
- 19.Hori Y, Baba H, Ueda R, et al. In vitro hyperprocessing of Drosophila tRNAs by the catalytic RNA of RNase P the cloverleaf structure of tRNA is not always stable. Eur J Biochem. 2000;267:4781–4788. doi: 10.1046/j.1432-1327.2000.01534.x. [DOI] [PubMed] [Google Scholar]
- 20.Hitti EG, Sallacz NB, Schoft VK, et al. Oligomerization activity of a double- stranded RNA-binding domain. FEBS Lett. 2004;574:25–30. doi: 10.1016/j.febslet.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 21.Poulsen H, Jorgensen R, Heding A, et al. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA. 2006;12:1350–1360. doi: 10.1261/rna.2314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber A, Grosjean H, Melcher T, et al. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. EMBO J. 1998;17:4780–4789. doi: 10.1093/emboj/17.16.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefl R, Xu M, Skrisovska L, et al. Structure and specific RNA binding of ADAR2 double-stranded RNA binding motifs. Structure. 2006;14:345–355. doi: 10.1016/j.str.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Marcotte EM, Pellegrini M, Ng HL, et al. Detecting protein function and protein-protein interactions from genome sequences. Science. 1999;285:751–753. doi: 10.1126/science.285.5428.751. [DOI] [PubMed] [Google Scholar]
- 25.Deutscher MP. Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem. 1984;17:45–71. doi: 10.3109/10409238409110269. [DOI] [PubMed] [Google Scholar]
- 26.Abelson J, Trotta CR, Li H. tRNA splicing. J Biol Chem. 1998;273:12685–12688. doi: 10.1074/jbc.273.21.12685. [DOI] [PubMed] [Google Scholar]
- 27.Schurer H, Schiffer S, Marchfelder A, et al. This is the end: processing, editing and repair at the tRNA 3′-terminus. Biol Chem. 2001;382:1147–1156. doi: 10.1515/BC.2001.144. [DOI] [PubMed] [Google Scholar]
- 28.Ramos A, Grunert S, Adams J, et al. RNA recognition by a Staufen double- stranded RNA-binding domain. EMBO J. 2000;19:997–1009. doi: 10.1093/emboj/19.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrandon D, Elphick L, Nusslein-Volhard C, et al. Staufen protein associates with the 3′ UTR of bicoid mRNA to form particles that move in a microtubule- dependent manner. Cell. 1994;79:1221–1232. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Lei M, Samuel CE. Chimeric double-stranded RNA-specific adenosine deaminase ADAR1 proteins reveal functional selectivity of double- stranded RNA-binding domains from ADAR1 and protein kinase PKR. Proc Natl Acad Sci USA. 2000;97:12541–12546. doi: 10.1073/pnas.97.23.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Yang PK, Butcher SE, et al. A novel family of RNA tetraloop structure forms the recognition site for Saccharomyces cerevisiae RNase III. EMBO J. 2001;20:7240–7249. doi: 10.1093/emboj/20.24.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronshaugen M, Biemar F, Piel J, et al. The Drosophila microRNA iab-4 causes a dominant homeotic transformation of halteres to wings. Genes Dev. 2005;19:2947–2952. doi: 10.1101/gad.1372505. [DOI] [PMC free article] [PubMed] [Google Scholar]