Abstract
Serotonin modulates brain physiology and behavior and has major roles in brain diseases involving abnormal mood and cognition. Enhancing brain serotonin has been found to regulate glycogen synthase Kinase-3 (GSK3), but the signaling mechanism and functional significance of this regulation remain to be determined. In this study, we tested the signaling mechanism mediating 5-HT1A receptor-regulated GSK3 in the hippocampus. Using mutant GSK3 knock-in mice, we also tested the role of GSK3 in the behavioral effects of 5-HT1A receptors and the serotonin reuptake inhibitor fluoxetine. The results showed that activation of 5-HT1A receptors by 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT) increased phosphorylation of the N-terminal serine of both GSK3α and GSK3β in several areas of the hippocampus. The effect of 8-OH-DPAT was accompanied by an increase in the active phosphorylation of Akt, and was blocked by LY294002, an inhibitor of phosphoinositide 3-kinases (PI3K). Phosphorylation of GSK3β, but not GSK3α, was necessary for 5-HT1A receptors to suppress the hippocampus-associated contextual fear learning. Furthermore, acute fluoxetine treatment up-regulated both phospho-Ser21-GSK3α and phospho-Ser9-GSK3β in the hippocampus. Blocking phosphorylation of GSK3α and GSK3β diminished the anti-immobility effect of fluoxetine treatment in the forced swim test, wherein the effect of GSK3β was more prominent. These results together suggest that PI3K/Akt is a signaling mechanism mediating the GSK3-regulating effect of 5-HT1A receptors in the hippocampus, and regulation of GSK3 is an important intermediate signaling process in the behavioral functions of 5-HT1A receptors and fluoxetine.
Keywords: Serotonin, 5-HT1A, fluoxetine, GSK3, Akt, behavior
Introduction
Serotonin is synthesized in serotonin neurons that arise in the dorsal and median raphe nucleus of the brain stem. Serotonin neuron projections reach throughout the brain, where serotonin is released to affect functions of multiple neurons in the brain through a large family of serotonin receptor subtypes [1]. Serotonin is a crucial neurotransmitter involved in regulation of brain physiological and behavioral states, such as mood, anxiety, and cognition, and drugs that block serotonin reuptake have clinical implications in the treatment of depression and anxiety.
In brain, serotonin was found to regulate the activity of glycogen synthase kinase-3 (GSK3) [2, 3], a serine/threonine protein kinase that is highly expressed in brain, and plays important roles in regulating neurotransmitter receptor activity [4-6], gene expression [7], synaptic plasticity [8], neurogenesis [9], apoptosis [10], and behaviors [11]. GSK3 is also a major pharmacological target of neuropsychiatric treatments, including lithium, monoamine-regulating antidepressants, and antipsychotics [11-13]. Both isoforms of GSK3, GSK3α and GSK3β, are constitutively active in vivo [14], but they normally undergo inhibitory regulation by upstream protein kinases, such as Akt [15], protein kinase A (PKA) [16], and protein kinase C (PKC) [17], via phosphorylation of a N-terminal serine residue, the serine-21 of GSK3α and the serine-9 of GSK3β, respectively. This regulation prevents GSK3 from access to its substrates, therefore resulting in inhibition of its kinase activity [14]. In mice with serotonin synthesis deficiency, the level of phospho-Ser9-GSK3β was lower than in wild type mice [2]. Reversely, increasing synaptic serotonin by d-fenfluramine or blocking serotonin reuptake by fluoxetine results in increased level of phospho-Ser9-GSK3β, an effect that can be blocked by 5-HT1A receptor antagonist [3]. Among all subtypes of serotonin receptors, 5-HT1A receptors have been shown to increase phospho-Ser9-GSK3β in brain [3, 18].
This study aimed to identify the signaling mechanism mediating the GSK3-regulating effect of 5-HT1A receptors and to determine the behavioral significance of regulating GSK3 by 5-HT1A receptors and fluoxetine. Findings of this study demonstrate that phosphorylation of GSK3 in the hippocampus by activation of 5-HT1A receptors was mediated by the PI3K/Akt signaling pathway. Phosphorylation of brain GSK3β is a necessary process for 5-HT1A receptor-regulated contextual fear learning and for the anti-immobility effect of fluoxetine.
Materials and Methods
Animals and treatment
The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved all experimental protocols of this study. C57BL/6 wild type (WT) mice (Frederick Cancer Research, MD) were accommodated in the university animal facility for one week before used for pharmacological treatments. GSK3 knock-in (KI) mice bearing the serine21 to alanine (S21A) mutant of GSK3α or S9A mutant of GSK3β were derived from the S21A/S9A-GSK3α/β KI mice [19], all were backcrossed 10 generations into C57BL/6 background. Homozygous GSK3 KI and littermate WT mice were continuously bred from heterozygous breeders.
Pharmacological treatment
Eight-12 week-old adult male mice were treated with intraperitroneal (i.p.) injections of 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT, Sigma, St. Louis, MO), N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]- N-(2-pyridyl)cyclohexanecarboxamide (WAY100635, Sigma, St. Louis, MO), 3-Methyl-N-[(1R)-1-methyl-3-(4-methyl-1-piperidinyl)propyl]-N-methylbenzenesulfonamide (SB258719, Tocris, Ellsville, MO), or fluoxetine (NIMH Chemical Synthesis and Drug Supply Program), all were dissolved in saline (vehicle). All drugs and vehicle (saline) for i.p. injections were administered at a volume of 5 μl/g body weight with the exception of fluoxetine, which was administered at a volume of 10 μl/g body weight for complete solubility.
For intrahippocampal (i.h.) injection, mice were anesthetized with a mixture of ketamine and xylazine (100 mg/kg:10 mg/kg) and placed in a stereotaxic frame. Bilateral burr holes were drilled 2.0 mm posterior to and 1.5 mm lateral to Bregma. A guide cannula (Plastic One, Roanoke, VA) with the projection length of 1.8 mm was inserted through the burr holes to give access to the surface of dorsal hippocampus. After post-surgery recovery for 5-7 days, mice were lightly anesthetized with isofluorane and placed in the stereotaxic frame. An injector projecting 0.5 mm past the guide was inserted into the guide cannula, and LY294002 or vehicle were infused at 0.5 μl volume into each hippocampus over 2 min.
Protein preparation and immunoblotting
At the end of treatment, mice were rapidly decapitated. The cerebral cortex, hippocampus, and striatum were rapidly dissected and homogenized in lysis buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% NP-40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin, 0.1 mM β-glycerophosphate, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium vanadate, and 100 nM okadaic acid. The lysate was collected after homogenates were centrifuged at 20,800 g for 10 min to remove insoluble debris [3]. Proteins were resolved in 10% SDS-polyacrylamide gels, and immunoblotted with antibodies to phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, phospho-Thr308-Akt, phospho-Ser473-Akt, total Akt (Cell Signaling Technologies, Danvers, MA), and total GSK3α/β (Upstate Biotech, Lake Placid, NY), followed by horseradish peroxidase-conjugated anti-mouse or goat anti-rabbit IgG. Immunoreactions were detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ), and protein bands were quantified with densitometry software.
Immunohistochemistry
The immunohistochemistry method was as described previously [20, 21]. After decapitation, brains were immediately immersed in Bouin’s solution and fixed overnight at 4°C. Fixed brains were processed in paraffin, and 7 μm brain sections were prepared on a microtome. Deparaffinized sections were incubated with antibodies to phospho-Ser9-GSK3β, total GSK3β, phospho-Thr308-Akt, and total Akt (Cell Signaling Technologies, Danvers, MA), labeled with horseradish peroxidase-conjugated anti-rabbit IgG, and developed with a TSA-Plus kit (Perkin-Elmer, Waltham, MA). Sections were counter-stained with Hoechst 33,258 (Sigma, St. Louis, MO). Immuno-fluorescence in brain sections was viewed with an Olympus BX-51 fluorescence microscope, and fluorescence intensity was measured using MicroBrightField Stereo Investigator Software (MBF Bioscience, Williston, VT).
Fear Conditioning
Mice were placed in an operant chamber inside an sound-attenuating cubicle to allow exploring the operant chamber for 2 min. After this acclimation, mice were administered three rounds of a 15-second, 75-db white noise tone each followed immediately by a 0.5 mA foot shock. Twenty-four hr after training, mice were tested for the contextual memory by placing them back into the same operant chamber for 5 min. Three hr after contextual testing, the cued memory was tested by placing mice in a novel context for 3 min (pre-tone), followed by a 75-db white noise tone. Freezing was monitored using Video Freeze software [22].
Forced Swim Test (FST)
Mice were placed in an automated apparatus consisting of clear Plexiglas cylinders containing distilled water (23-25°C) and outfitted with 2 rings of photo-beam detectors (Kinder Scientific, Poway, CA) [23]. Movements were continuously monitored by computer for 6 min. Data were recorded using Motor Monitor Software (Kinder Scientific, Poway, CA) and transferred into Microsoft Excel. The immobility time was represented by recording the resting time (seconds without beam breaks,) during the last 4 minu of testing [21, 22].
Statistics
Statistical analyses were completed using SigmaStat software. Statistical significance was determined by Student’s t-test for comparisons between two groups and one-way ANOVA followed by post-hoc testing for experiments with multiple treatments. Studies examining treatment effects in different genotypes were analyzed using two-way ANOVA followed by post-hoc testing. Values are expressed as mean ± SEM and are considered significant when p<0.05.
Results
We previously found that serotonin-induced increase in phospho-Ser9-GSK3β is primarily mediated by 5-HT1A receptors [3]. In this study, we further tested GSK3-regulating effect of 5-HT1A receptors in the hippocampus where 5-HT1A receptors are highly expressed [24] and regulate specific behaviors [25]. Systemic injection of the 5-HT1A receptor agonist 8-OH-DPAT (1 mg/kg, i.p., 30 min) caused a small but significant increase in the level of phospho-Ser21-GSK3α, and robustly increased the level of phospho-Ser9-GSK3β in the hippocampus (Figure 1A), but 8-OH-DPAT did not alter the levels of total GSK3α or GSK3β. The 8-OH-DPAT-induced increases in phospho-Ser21-GSK3α and phospho-Ser9-GSK3β were attenuated by the 5-HT1A receptor antagonist WAY100635 (42±10% and 40±6% responses of 8-OH-DPAT alone on GSK3α and GSK3β, respectively), but not by the 5-HT7 receptor antagonist SB258719 (107±41% and 169±13% response of 8-OH-DPAT alone on GSK3α and GSK3β, respectively). GSK3β was ubiquitously expressed in the hippocampus, with high levels of immunoreactivity in neuronal cell bodies and dendritic processes (Figure 1B). 8-OH-DPAT caused a prominent increase in phospho-Ser9-GSK3β in the dendrites and cell bodies of CA3, and an observable increase in the cell bodies and projections of dentate granule cells and in the dendrites of CA1. Luminescence quantification of phospho-Ser9-GSK3β as a ratio of nuclear stain in the subfields of the hippocampus revealed significant increases in the stratum pyramidale and the stratum radiatum/stratum lucidum of CA3 and in the Hilus of the dentate gyrus (Figure 1B). A trend of increase was also seen in the stratum pyramidale and the stratum radiatum of CA1, and in the granule cell layer of the dentate gyrus, but the change did not reach statistical significance.
Figure 1.
Regulation of GSK3 by 5-HT1A receptors in the hippocampus. C57BL/6 wild type mice were treated with the 5-HT1A receptor agonist 8-OH-DPAT (DPAT, 1 mg/kg, i.p.) or saline (Sal) for 30 min. (A) Representative immunoblots and quantification of phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, total GSK3α, and total GSK3β in the hippocampus. Data is calculated as % Control (saline-treated). Mean ± SEM, n=12-16, *p<0.05 in Student’s t-test when 8-OH-DPAT treatment is compared to saline treatment. (B) Immunohistochemical images of total GSK3β and phospho-Ser9-GSK3β in the hippocampus. Red, total GSK3β or phospho-Ser9-GSK3β; Blue, Hoescht 33342 nuclear marker. Immunofluorescence intensity of phospho-Ser9-GSK3β in the dentate gyrus (DG), CA3, and CA1 is quantified, and data is expressed as ratio of phospho-Ser9-GSK3β to Hoescht 33342. Mean ± SEM, n=3-4. *p<0.05 in Student’s t-test when 8-OH-DPAT treatment is compared to saline treatment. GL, Granule cell layer; SP, stratum pyramidale; SR, stratum radiatum; SL, stratum lacunosum.
The PI3K/Akt is a major signaling pathway that regulates GSK3 [15]. To test if 5-HT1A receptors also regulate Akt in mouse brain hippocampus, protein lysates from the hippocampus were immunoblotted for phosphorylated Akt (representing active Akt) and total Akt. Treatment with 8-OH-DPAT significantly increased the level of phospho-Thr308-Akt, caused a small but significant increase in phospho-Ser473-Akt, but did not change the level of total Akt (Figure 2A). To further test if the PI3K/Akt signaling pathway mediates 5-HT1A receptor-regulated GSK3 phosphorylation, mice received bilateral intrahippocampal infusion of the PI3K inhibitor LY294002 (2.5 nmoles/hippocampus, 90 min prior to 8-OH-DPAT). Although LY294002 itself had little effect, it completely blocked 8-OH-DPAT-induced increases in phospho-Thr308-Akt and phospho-Ser9-GSK3β in the hippocampus (Figure 2B).
Figure 2.
The effect of PI3K/Akt signaling in 5-HT1A receptor-regulated GSK3 in the hippocampus. (A) Mice were treated as described in Figure 1. Representative immunoblots and quantification of phospho-Thr308-Akt, phospho-Ser473-Akt and total Akt (left panel). Data is expressed as % Control (saline-treated). Mean ± SEM, n=12-16, *p<0.05 in Student’s t-test when 8-OH-DPAT treatment is compared to saline treatment. Immunohistochemical images of total Akt and phospho-Thr308-Akt (right panel). Red, Akt or phospho-Thr308-Akt; Blue, Hoescht 33342 nuclear marker. (B) Intrahippocampal (ih) infusion of LY294002 (LY, 2.5 nmole/side, 90 min) followed by 8-OH-DPAT (DPAT, 1 mg/kg, i.p., 30 min). Representative immunoblots and quantified data of phospho-Thr308-Akt, total Akt, phospho-Ser9-GSK3β, and total GSK3β. Data is expressed as % Control (saline-treated). Mean ± SEM, n=4-6, *p<0.05 in one-way ANOVA followed by Holm-Sidak comparison.
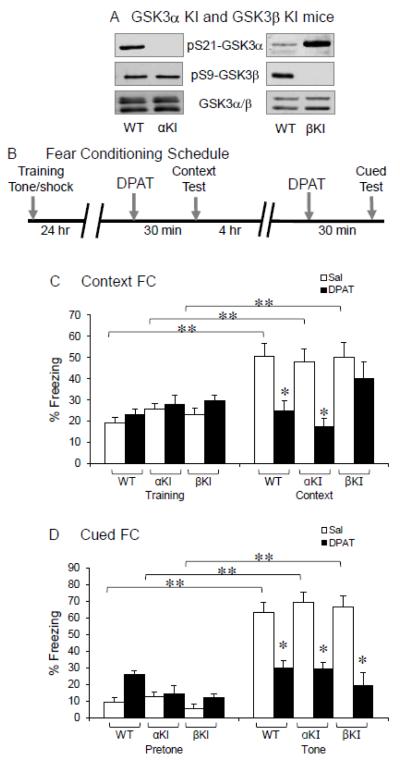
To determine if regulation of GSK3 by 5-HT1A receptors in the hippocampus has functional significance, we used the S21A-GSK3α KI and S9A-GSK3β KI mice that express normal levels of GSK3α and GSK3β, but the serine-21 of GSK3α or the serine-9 of GSK3β was resistant to phosphorylation by Akt [19, 22] (Figure 3A).
Figure 3.
5-HT1A receptor-induced inhibition of fear conditioning. (A) Immunoblots of phospho-Ser21-GSK3α, phospho-Ser9-GSK3β and total GSK3α and GSK3β in WT, S21A-GSK3α KI (αKI), and S9A-GSK3β KI (βKI) mice. (B) Fear conditioning and drug treatment schedule. (C) Contextual fear conditioning (FC) 30 min after saline (Sal) or 8-OH-DPAT (DPAT, 1 mg/kg) treatment in WT, S21A-GSK3α KI (αKI), and S9A-GSK3β KI (βKI) mice. (D) Cued fear conditioning 30 min after drug treatment. Mean ± SEM, n=8-11, *p<0.05 in Student’s t-test, **p<0.05 in two-way ANOVA followed by Holm-Sidak comparison.
In S21A-GSK3α KI, S9A-GSK3β KI, and littermate WT mice, we tested the expression of contextual and cued fear learnings (Figure 3B) that are hippocampus-associated behaviors and are known to be modulated by 5-HT1A receptors [26, 27]. In WT mice, a standard contextual fear test significantly increased the freezing behavior (Figure 3C). The freezing effect was lost when mice were treated with 8-OH-DPAT (1 mg/kg) 30 min prior to the contextual test. Similarly to WT mice, S21A-GSK3α KI mice had a significant increase in freezing during contextual fear test, and 8-OH-DPAT treatment significantly reduced freezing. However, in contrast to its effect in either WT or S21A-GSK3α KI mice, 8-OH-DPAT did not cause significant reduction of contextual freezing in S9A-GSK3β KI mice. In cued fear conditioning, a conditioned tone significantly increased freezing in WT mice. Systemic administration of 8-OH-DPAT 30 min prior to testing prevented the increase in freezing induced by the conditioned tone (Fig. 3D). Similarly to WT mice, both S21A-GSK3α KI and S9A-GSK3β KI mice had a significant increase in freezing during cued fear test. Unlike that seen in contextual fear test, 8-OH-DPAT treatment significantly reduced cued freezing in both S21A-GSK3α KI and S9A-GSK3β KI mice. Thus, inhibition of GSK3β, but not GSK3α, by 5-HT1A receptors is an intermediate process for 5-HT1A receptor-regulated contextual fear learning. This function of GSK3β is specific to the contextual fear, but not cued fear learning.
Several studies have found that both acute and chronic fluoxetine treatment up-regulates phospho-Ser9-GSK3β in mouse brain [2, 3, 20, 28]. In WT mice, a 30-min fluoxetine treatment (20 mg/kg, i.p.) caused moderate increases in both phospho-Ser21-GSK3α and phospho-Ser9-GSK3β in the hippocampus, but fluoxetine did not change the total level of GSK3α or GSK3β (Figure 4A).
Figure 4.
The effect of fluoxetine in regulation of GSK3 and in FST. (A) WT mice were treated with saline (S or Sal) or fluoxetine (F or FLX, 20 mg/kg, i.p.) for 30 min, Representative immunoblots and quantification of phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, total GSK3β and total GSK3β of serine-phosphorylated GSK3α and GSK3β in the hippocampus. Data is expressed as % Control (saline-treated). Mean ± SEM, n=11-14, *p<0.05 in Student’s t-test when fluoxetine is compared to saline treatment. (B) WT, S21A-GSK3α KI (αKI), S9A-GSK3β KI (βKI), and S21/9A-GSK3α/β double-KI (α/βKI) mice were treated with saline or fluoxetine (20 mg/kg, i.p.) for 30 min, followed by testing immobility in the FST. Mean ± SEM, n=6-10, *p<0.05 between saline and fluoxetine in the same genotype by Holm-Sidak comparison in two-way ANOVA. This test found no interaction between the four genotypes and the two treatments (saline and fluoxetine) (DF=3, F-1.954, p=0.131).
We therefore tested if this effect of fluoxetine on GSK3 has an impact in its acute behavior effect in the FST, a commonly applied animal behavior to test acute antidepressant effect [29]. Among WT, S21A-GSK3α KI, S9A-GSK3β KI, and S21/9A-GSK3α/β double-KI mice, the baseline immobility (saline treatment) was not significantly different (Figure 4B). WT mice responded to fluoxetine (20 mg/kg, i.p., 30 min) with a significant 56% reduction in immobility when compared to saline-treated mice (t=3.935, p<0.001). In S21A-GSK3α KI mice, fluoxetine caused a 36% reduction in immobility, but it was not significantly different from saline treatment. The anti-immobility effect of fluoxetine in S9A-GSK3β KI mice, however, was markedly diminished, with only 17% non-significant reduction in immobility. To confirm that the significant lack of effect by fluoxetine in S9A-GSK3β KI mice was not due to compensatory increase in the baseline phospho-Ser21-GSK3α, the anti-immobility effect of fluoxetine was further tested in GSK3α/β double-KI mice, wherein fluoxetine only caused a non-significant 20% reduction in immobility. Therefore, both GSK3α and GSK3β may be involved in the anti-immobility effect of fluoxetine, but the effect of GSK3β is more prominent.
Discussion
Serotonin has been found to regulate brain GSK3β by phsophorylation at serine-9 residue [2, 3], and this effect of serotonin is mediated by activated 5-HT1A receptors [3]. 5-HT1A receptors classically couple to the inhibitory Gi protein which inhibits adenylyl cyclase (AC) activity and reduces cAMP production. Activation of this signaling pathway by 5-HT1A receptors therefore results in inactivation of PKA. Since PKA is one of the protein kinases to upregulate phosphorylation of GSK3 [16], it is unlikely that the 5-HT1A receptor-induced Gi-mediated inactivation of PKA is responsible for the increase in GSK3β phosphorylation. Alternatively, activation of PI3K and Akt by 5-HT1A receptors has been reported in 5-HT1A receptor-expressing cells and primary hippocampal neurons [30-32]. Results of this study further suggest that the GSK3-regulating effect of 5-HT1A receptors is mediated by the PI3K/Akt signaling pathway, since 8-OH-DPAT activates Akt (by increasing its phosphorylation) in the hippocampus, and direct hippocampal inhibition of PI3K abolished 5-HT1A receptor agonist-induced increases in phospho-Thr308-Akt and phospho-Ser9-GSK3β.
In this study we chose systemic administration of 5-HT1A receptor agonist because this is the most likely route of drug delivery in clinical settings. However, this treatment involves activation of 5-HT1A autoreceptors and heteroreceptors located in the raphe nucleus and hippocampus [24], thus the result of this study cannot rule out that the PI3K/Akt-mediated phosphorylation of GSK3β in the hippocampus is an indirect post-synaptic response to serotonin [33], which remain to be determined in future studies.
The immunohistochemical findings in the hippocampus show that 5-HT1A receptor activation leads to the greatest increase in phospho-Ser9-GSK3β in the CA3 pyramidal glutamatergic neurons and their dendrites, suggesting that regulation of GSK3β by 5-HT1A receptors may have an effect on the glutamatergic circuits of the hippocampus. GSK3β has been shown to be a mediator of glutamate receptor activity and synaptic plasticity in the hippocampus [5, 34, 35], thus regulation of GSK3β by 5-HT1A receptors in the hippocampus may further link this signaling mechanism to an important learning and memory task of glutamate neurotransmission in the hippocampus.
Among 5-HT1A receptor-regulated behaviors, inhibition of fear conditioning is a hippocampus-associated learning process [36]. The 5-HT1A receptor-induced inhibition of this behavior was compared between WT mice and either S21A-GSK3α KI or S9A-GSK3β KI mice because result of this study revealed that 5-HT1A receptor activation not only increased phospho-Ser9-GSK3β, but also had a small effect in increasing phospho-Ser21-GSK3α. We present evidence here that phosphorylation of GSK3β, but not GSK3α, is necessary for 5-HT1A receptor-induced inhibition of contextual fear learning. This is another example showing that despite both GSK3α and GSK3β are expressed in brain, the two isoforms of GSK3 have different substrates and mediate different physiological functions [4, 37, 38]. Importantly, regulation of GSK3β phosphorylation only mediates 5-HT1A receptor-regulated contextual fear, but not the cued fear conditioning. The result suggests that inhibition of GSK3β may have important pharmacological effect in selectively eliminate 5-HT1A receptor-regulated behaviors.
Several studies have found up-regulation of phospho-Ser9-GSK3β by fluoxetine in mouse brain [2, 3, 20, 28]. In this current study, acute fluoxetine treatment caused equivalent moderate increases in phospho-Ser21-GSK3α and phospho-Ser9-GSK3β in the hippocampus. This differs from the uneven responses of GSK3α and GSK3β to the 5-HT1A receptor agonist 8-OH-DPAT wherein the response of GSK3β is robust. Since increasing synaptic serotonin by fluoxetine may activate serotonin receptor subtypes other than 5-HT1A receptors, the effect of fluoxetine in the hippocampus could be mediated by several serotonin receptor subtypes that remain to be identified in future studies.
Phosphorylation of either GSK3α or GSK3β at their N-terminal serine results in inhibition [14], and inhibition of GSK3 by small molecule inhibitors or in GSK3-deficient mice elicit anti-immobility effect in the FST [39-43], which is a similar effect seen with antidepressants, including fluoxetine [44, 45]. We therefore hypothesized that phosphorylation of GSK3 by fluoxetine could be an intermediate signaling process for its anti-immobility effect. We tested the acute effect of fluoxetine on GSK3 and behavior because a signaling protein kinase GSK3 is likely an early mediator of the behavioral effect of serotonin and its receptors. The effect of fluoxetine on GSK3 and on behavior is tested after an identical fluoxetine treatment to further demonstrate that the acute regulation of GSK3 and behavior occurs within the same time period. Indeed, results from GSK3 KI mice demonstrate that phosphorylation of GSK3β is necessary for the acute anti-immobility effect of fluoxetine. Although phosphorylation of GSK3α may also contribute to the anti-immobility effect of fluoxetine, the effect is likely minimal because the reduced anti-immobility effect of fluoxetine in S21/9A-GSK3α/β double-KI mice was not superior to that in S9A-GSK3β KI mice. Additionally, taking into account that increasing synaptic serotonin by fluoxetine may activate several serotonin receptor subtypes and the FST is not a hippocampus-defined behavior, it is likely that GSK3α and GSK3β mediate the behavioral effect of fluoxetine through several serotonin receptor subtypes in different brain regions.
Taken together, regulation of primarily GSK3β by 5-HT1A receptors and fluoxetine is an important signaling mechanism for serotonin-regulated behaviors. These findings provide crucial information on the signaling pathogenesis of brain disorders that involve impairment of serotonin function and on new treatment development targeting GSK3 and its signaling mechanisms.
Highlights.
-
➢
5-HT1A receptors regulate phosphorylation of GSK3 in the hippocampus.
-
➢
PI3K/Akt signaling pathway mediates regulation of GSK3 by 5-HT1A receptors.
-
➢
5-HT1A receptor-induced inhibition of contextual fear learning is GSK3 -dependent.
-
➢
Fluoxetine regulates phosphorylation of GSK3 in the hippocampus.
-
➢
Regulation of GSK3 is an intermediate of fluoxetine’s anti-immobility effect.
Acknowledgment
The authors thank Dario Alessi for the original strain of GSK3α/β knock-in mice, and the Evelyn F. McKnight Brain Research Foundation to provide animal behavioral facility at UAB. This work was supported by NIH grants MH64555, MH73723, and MH86622 (XL), MH38752 (RSJ), NS61788 (Training Program in the Neurobiology of Cognition and Cognitive Disorders, awardee AP), and NS57098 (Alabama Neuroscience Blueprint Core).
Abbreviations
- FST
forced swim test
- GSK3
glycogen synthase kinase-3
- 5-HT1A
serotonin type 1A receptors
- i.p.
intraperitoneal
- KI
knock-in
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
These authors claim no conflict of interest.
References
- [1].Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- [2].Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen L, Salinas GD, Li X. Mol Pharmacol. 2009;76:1150–1161. doi: 10.1124/mol.109.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, Tragon T, Hunsberger JG, Machado-Vieira R, Drevets W, Wang YT, Manji HK. Proc Natl Acad Sci U S A. 2010;107:11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wei J, Liu W, Yan Z. J Biol Chem. 2010;285:26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jope RS, Johnson GV. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [8].Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. Br J Pharmacol. 2008;153(Suppl 1):S428–437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hur EM, Zhou FQ. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beurel E, Jope RS. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li X, Jope RS. Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beaulieu JM, Gainetdinov RR, Caron MG. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- [13].Jope RS, Roh MS. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doble BW, Woodgett JR. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- [16].Fang X, Yu SX, Lu Y, Bast RC, Jr., Woodgett JR, Mills GB. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goode N, Hughes K, Woodgett JR, Parker PJ. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- [18].Chen S, Owens GC, Crossin KL, Edelman DB. Mol Cell Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [19].McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- [21].Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, Peng SL, Jope RS, Li X. Biol Psychiatry. 2009;65:150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Polter AM, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon LL, Bartolucci AA, Li X, Jope RS. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kurtuncu M, Luka LJ, Dimitrijevic N, Uz T, Manev H. J Neurosci Methods. 2005;149:26–30. doi: 10.1016/j.jneumeth.2005.04.010. [DOI] [PubMed] [Google Scholar]
- [24].Hoyer D, Hannon JP, Martin GR. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- [25].Polter AM, Li X. Cell Signal. 2010;22:1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stiedl O, Misane I, Spiess J, Ogren SO. J Neurosci. 2000;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Porsolt RD, Le Pichon M, Jalfre M. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- [30].Cowen DS, Johnson-Farley NN, Travkina T. J Neurochem. 2005;93:910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hsiung SC, Tamir H, Franke TF, Liu KP. J Neurochem. 2005;95:1653–1666. doi: 10.1111/j.1471-4159.2005.03496.x. [DOI] [PubMed] [Google Scholar]
- [32].Hsiung SC, Tin A, Tamir H, Franke TF, Liu KP. J Neurosci Res. 2008;86:2326–2338. doi: 10.1002/jnr.21676. [DOI] [PubMed] [Google Scholar]
- [33].Manahan-Vaughan D, Anwyl R, Rowan MJ. Br J Pharmacol. 1994;112:1083–1088. doi: 10.1111/j.1476-5381.1994.tb13194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- [35].Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- [36].Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- [37].Wang QM, Park IK, Fiol CJ, Roach PJ, DePaoli-Roach AA. Biochemistry. 1994;33:143–147. doi: 10.1021/bi00167a018. [DOI] [PubMed] [Google Scholar]
- [38].Liang MH, Chuang DM. J Biol Chem. 2006;281:30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- [39].Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [40].Gould TD, Einat H, Bhat R, Manji HK. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- [41].Rosa AO, Kaster MP, Binfare RW, Morales S, Martin-Aparicio E, Navarro-Rico ML, Martinez A, Medina M, Garcia AG, Lopez MG, Rodrigues AL. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1549–1556. doi: 10.1016/j.pnpbp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- [42].O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- [44].Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F. Exp Brain Res. 2002;143:191–197. doi: 10.1007/s00221-001-0979-3. [DOI] [PubMed] [Google Scholar]
- [45].Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]