Abstract
Objective
To explore predictors of change in measures of carotid atherosclerosis among rheumatoid arthritis (RA) patients without known cardiovascular disease (CVD) at baseline
Methods
RA patients underwent carotid ultrasonography at two timepoints, separated by an average of 3.2 ± 0.3 years. The associations of baseline and average patient characteristics with the average yearly change in mean maximal intima-medial thickness (IMT) of the common (CCA) and internal carotid arteries (ICA), and with incident or progressive plaque in the ICA/carotid bulb, were explored.
Results
Among the 158 RA patients, maxCCA-IMT increased in 82% (median=16 μm/year; p<0.001) and maxICA-IMT increased in 70% (median=25 μm/year; p<0.001). Incident plaque was observed in 14% without baseline plaque [incidence rate=4.2/100 person-years (95% CI 1.61–6.82)]. Plaque progression was observed in 5% with baseline plaque. Among RA predictors, the adjusted average yearly change in maxCCA-IMT was significantly greater in patients with earlier RA vs. longer disease. Those prescribed TNF inhibitors at baseline had a 37% lower adjusted rate of maxCCA-IMT progression vs. non-users (14 vs. 22 μm/year; p=0.026). For maxICA-IMT, cumulative prednisone exposure was associated with progression [1.2 μm/year per gram (95% CI 0.1–2.4)] after adjustment, and was lower in patients prescribed statins concomitant with prednisone. Higher swollen joint count and higher average CRP were both associated with incident or progressive plaque, primarily in patients with elevated baseline CVD risk based on the Framingham score.
Conclusions
These prospective data provide evidence for inflammation as a contributor to subclinical atherosclerosis progression in RA, potentially modified favorably by TNF inhibitors and detrimentally by glucocorticoids.
Keywords: Atherosclerosis, Inflammation, prediction, carotid ultrasound
INTRODUCTION
Cardiovascular disease (CVD) morbidity and mortality rates are higher in RA patients compared to the general population(1), leading to reduced lifespan(2)and quality of life, and increased medical expenditures(3). Underlying higher CVD rates, cross-sectional studies have identified a greater burden of atherosclerosis in both the coronary(4, 5)and carotid(6, 7) vasculature of RA patients. Among RA patients without traditional CVD risk factors or events, a greater thickness of the intima-medial layer (IMT) of the common carotid artery (CCA) and evidence of focal plaques were each predictive of incident CVD events(8, 9).
RA may predispose to accelerated atherosclerosis through a number of mechanisms; most compelling being the atherogenic effect of chronically elevated inflammatory cytokines. Augmentation of TNF-α induces atherosclerosis in animals(10)and elevated TNF-α(11)and IL-6(12)predict CVD events in humans. However, other mechanisms, including reduced physical fitness, traditional CVD risk factors, and the impact of RA pharmacotherapies (both beneficial and detrimental), may also be significant contributors. Understanding the causal determinants of atherosclerosis in RA could facilitate targeted early intervention, thereby reducing incident events and mortality. However, as the majority of studies of carotid atherosclerosis in RA have been cross-sectional(13), identifying causal determinants is difficult. In particular, cross-sectional assessments of exposures that vary with time, such as laboratory markers of systemic inflammation, may not reflect cumulative levels. Few longitudinal studies of the natural history and determinants of subclinical atherosclerosis in RA have been performed(8, 14, 15). Those that are available are limited by small sample size and insufficient accounting for important confounders.
Carotid measures may not be equally predictive of subsequent events. While progression of CCA-IMT has been linked to CVD events(16), there is some indication that increased IMT in the internal carotid artery (ICA) may be a better predictor of events than the CCA (17). In addition, risk factors for progression may be artery-specific(18). Recently(6), we reported higher IMT and plaque in the ICA/bulb, but not CCA-IMT, in a cross-sectional investigation of RA patients compared to a matched non-RA control group.
Therefore, we investigated the associations of baseline and averaged patient characteristics with the rate of change in measures of carotid atherosclerosis in both the ICA and CCA among a group of RA patients without prior CVD. Additionally, we explored whether rates of change in IMT or plaque differed between groups stratified by CVD risk factors, RA characteristics, and RA pharmacotherapies. We hypothesized that both traditional CVD risk factors and RA disease characteristics (i.e. clinical disease activity measures and laboratory measures of systemic inflammation) would manifest with a more rapid increase in IMT and predict incident plaque or progression of lumenal narrowing of established plaques.
METHODS
Study Participants and Timing of Visits
Participants were enrolled in ESCAPE RA (Evaluation of Subclinical Cardiovascular disease And Predictors of Events in Rheumatoid Arthritis), a prospective cohort study investigating subclinical CVD in RA described in detail previously(4, 6). Participants met 1987 RA classification criteria(19), had RA ≥6 months from diagnosis, and were 45–84 years of age without known prior pre-specified cardiovascular events. Among the 197 RA patients completing the baseline visit, 186 (94%) returned for the second visit (occurring an average of 21 ± 3 months post-baseline) and 158 (80%) returned for the third visit, (occurring an average of 39 ± 4 months post-baseline). Carotid ultrasonography occurred at visits 1 and 3. Clinical data and biospecimens were collected at each visit. The study was approved by the Institutional Review Board of the Johns Hopkins Hospital. Enrollment occurred between October 2004 and May 2006. The final follow-up visit occurred in April 2009.
Outcomes
Carotid Imaging
Carotid imaging was performed as previously described(6). Videotaped scans were analyzed at the Multi-Ethnic Study of Atherosclerosis (MESA)Ultrasound Reading Center. Baseline and follow-up scans were re-analyzed concurrently by a single MESA reader aware of the temporal ordering but unaware of clinical characteristics. Maximal IMT was measured in end-diastole at each of the near and far walls of the right and left CCA, and the anterior oblique, lateral and posterior oblique views of the carotid bulb and ICA. The mean maximal IMTs of the CCA (maxCCA-IMT) and carotid bulb+ICA (maxICA-IMT) were obtained by averaging the bilateral maximal measurements from the near and far walls at each projection. When an atherosclerotic plaque was present at the measurement site, it was included in the IMT measurement. The average yearly rate of change in IMT was calculated by subtracting baseline from follow-up IMT divided by the number of years between visits. Plaque was investigated in the ICA/carotid bulb only. Plaque extent was categorized into groups based on the percentage of lumenal stenosis: 0; 1–24%; 25–49%; 50–74%; 75–99%; and 100%. Participants had incident plaque if there was no lesion at baseline and a lesion of any degree of stenosis at follow-up. Participants had “progression” of plaque on transition to a higher stenosis group at follow-up, and “regression” on transition to a lower group. Intra-observer reliability, assessed periodically at the reading center, was consistent with prior reports(6).
Covariates
Data on exposure variables were collected concurrent with carotid imaging by trained study personnel using standardized forms and procedures. Although carotid imaging occurred only at visits 1 and 3, data on sociodemographics, CVD risk factors, RA disease characteristics, and laboratory covariates were collected at all three visits.
Sociodemographic and Lifestyle Covariates
Demographics and smoking history were assessed by self-report. Physical activity was assessed with the 7-Day Physical Activity Recall Questionnaire(20). Current use and dosage of medications were ascertained from prescription bottles. Body mass index (BMI) was calculated as body weight (kg) divided by height (meters2).
CVD Risk Factors
Hypertension was defined as systolic BP≥140 mmHg, diastolic BP≥90, or antihypertensive medication use. Diabetes was defined as a fasting serum glucose≥126 mg/dL or use of anti-diabetic medications. Metabolic syndrome was defined according to the National Cholesterol Education Program-Adult Treatment Panel III criteria(21). Framingham Hard-10 year CVD risk was calculated according to published criteria(22).
RA Disease Characteristics
Forty-four joints were examined by a single trained assessor. RA disease duration was assessed from the self-reported date of diagnosis. RA disease activity was calculated using the Disease Activity Score for 28 joints with CRP (DAS28-CRP)(23). Current and past use of glucocorticoids and disease-modifying antirheumatic drugs (DMARDs) was queried by detailed examiner-administered questionnaires. _The Stanford Health Assessment Questionnaire (HAQ)(24)was used to assess disability related to common activities. Single view, anterior-posterior radiographs of the hands and feet were scored using the Sharp-van der Heijde (SvH) method(25)by a single trained radiologist.
Laboratory Covariates
High sensitivity C-reactive protein (CRP), IL-6, fibrinogen, s-ICAM, e-Selectin, and homocysteine were measured as previously described(26). Plasma lipids and glucose were measured by standard assays; LDL-cholesterol was estimated using the Friedewald equation. Rheumatoid factor (RF) was assessed by ELISA, with seropositivity ≥40 units. Anti-CCP antibody was assessed by ELISA, with seropositivity ≥60 units. HLA alleles bearing the “shared epitope” were investigated by DRB1 gene sequencing as previously described(6).
Statistical Analysis
The distributions of all baseline variables were examined. Cumulative averages of repeated measures of continuous variables were calculated from the area under the curve divided by the total number of interim days. Robust regression was used to explore the changes in maxCCA and maxICA IMT, first in null models testing the mean annual change in the outcome against the null hypothesis of no change. Univariate robust regression models were constructed with participant characteristics included as covariates, with beta coefficients and 95% confidence intervals (CIs) calculated. Where required, variables were transformed to normality.
Multivariable models were constructed, first in extended models including all covariates with associations from univariate models with p≥0.20 (to allow for residual confounding). Simpler models were constructed by excluding the covariates with the weakest associations, with the impact of excluding the covariate tested using Akaike’s Information Criterion (AIC) for nested models. Variance inflation factors were calculated to ensure that variables with excessive collinearity were not modeled simultaneously. Potential heterogeneities in the associations of covariates with the change in maxIMT across strata of characteristics were modeled in interaction models and tested using Analysis of Covariance (ANCOVA) for a preselected set of stratification variables: age (dichotomized at the median); gender; Caucasian vs. other ethnicity; RA duration (stratified into tertiles); baseline DAS28 (dichotomized at the median); biologic DMARD use; Framingham score (stratified at <5% vs. ≥ 5% or diabetes); and lipid lowering medication use.
Incidence, progression, and regression rates for carotid plaque were calculated as the total count divided by the pertinent follow-up person-years. Poisson regression was used to model the association of participant characteristics with the rate of incident or progressive plaque, as described above, with the exception of the likelihood ratio test used for testing nested models. Probability functions were plotted to inspect for cutpoints in the associations of continuous covariates with the probability of incident or progressive plaque. All statistical calculations were performed using Intercooled Stata 10 (StataCorp, College Station, TX). A two-tailed α of 0.05 was used throughout.
RESULTS
A total of 158 RA patients underwent carotid ultrasonography at both visits, separated by an average of 3.2 ± 0.3 years. Baseline characteristics and cumulative averages for continuous characteristics are summarized in Table 1. In general, cumulative average values did not substantially differ from baseline values, with the exception of CRP and IL-6, in which cumulative average values were 40% and 25% higher, respectively, than baseline values.
Table 1.
Baseline and Follow-up Characteristics of Study Participants
| Characteristic | Baseline (n = 158) | Cumulative Average during Study Period (n = 158) |
|---|---|---|
| Follow-up time, years | -- | 3.2 ± 0.3 |
| Age, years | 59 ± 8 | -- |
| Male, n (%) | 57 (36) | -- |
| Caucasian, n (%) | 138 (87) | -- |
| Any college, n (%) | 123 (78) | -- |
| Reported exercise, minutes/day | 33 (9 – 84) | 40 (16 – 76) |
| Walking for exercise, min/day | 13 (0 – 43) | 17 (5 – 43) |
| Sports and activities, min/day | 0 (0 – 9) | 0 (0 – 13) |
| Conditioning exercise, min/day | 0 (0 – 17) | 2 (0 – 18) |
| Hormone replacement(women only), n (%) | 15 (15) | -- |
| Body mass index, kg/m2 | 28.1 ± 5.1 | 28.4 ± 5.1 |
| Ever smoking, n (%) | 88 (56) | -- |
| Current smoking, n (%) | 15 (9) | -- |
| Diabetes, n (%) | 9 (6) | -- |
| Hypertension, n (%) | 82 (52) | -- |
| Systolic blood pressure, mm Hg | 126 ± 17 | 129 ± 16 |
| Diastolic blood pressure, mm Hg | 75 ± 9 | 76 ± 8 |
| LDL-C, mg/dL | 117 ± 31 | 114 ± 28 |
| HDL-C, mg/dL | 56 ± 20 | 57 ± 19 |
| Triglycerides, mg/dL | 104 (74 – 144) | 104 (73 – 138) |
| Use of lipid lowering medication, n (%) | 26 (16) | -- |
| Metabolic syndrome, n (%) | 34 (22) | -- |
| Framingham 10-year risk score, (%) | 4 (2 – 10) | -- |
| Fibrinogen | 334 (279 – 416) | 373 (310 – 430) |
| s-ICAM | 293 (228 – 340) | 290 (240 – 345) |
| e-Selectin | 46.7 (28.3 – 69.8) | 45.5 (33.3 – 64.0) |
| Homocysteine | 9.1 (7.6 – 10.4) | 9.9 (8.5 – 11.9) |
| Baseline RA duration, years | 8.5 (4 – 17) | -- |
| RF or anti-CCP seropositive at baseline, n (%) | 118 (75) | -- |
| Any SE alleles at baseline, n (%) | 110 (71) | -- |
| DAS28-CRP | 3.57 (2.88 – 4.31) | 3.31 (2.63 – 4.08) |
| Swollen joint count(0 – 42) | 7 (3 – 10) | 5 (3 – 8) |
| Tender joint count(0 – 44) | 6 (2 – 13) | 6 (2 – 14) |
| CRP, mg/L | 2.2 (1.1 – 6.7) | 3.1 (1.2 – 6.5) |
| IL-6, pg/mL | 3.6 (1.7 – 7.5) | 4.5 (2.3 – 11.8) |
| HAQ | 0.63 (0.13 – 1.25) | 0.70 (0.16 – 1.34) |
| Total Sharp-van der Heijde score | 44 (13 – 116) | -- |
| Cumulative prednisone, grams | 3.1 (0 – 9.1) | 0 (0 – 3.8) |
| Baseline non-biologic DMARDs, n (%) | 135 (86) | -- |
| Baseline methotrexate, n (%) | 101 (64) | -- |
| Baseline hydroxychloroquine, n (%) | 39 (25) | -- |
| Baseline biologics, n (%) | 70 (45) | -- |
| Baseline TNF-inhibitors, n (%) | 66 (42) | -- |
values are presented as mean ± SD or median (IQR) unless otherwise noted
LDL-C=large density lipoprotein cholesterol; HDL-C=high density lipoprotein cholesterol; RF=rheumatoid factor; CCP=cyclic citrullinated peptide; SE=shared epitope; DAS=disease activity score; CRP=C-reactive protein; IL=interleukin; HAQ=Health Assessment Questionnaire; DMARD=disease modifying anti-rheumatic drug; TNF=tumor necrosis factor
Changes in maxCCA-IMT, maxICA-IMT, and Plaque Stenosis During Follow-up
All 158 participants had interpretable maxCCA-IMT measurements for both time-points, while 155 and 154 had interpretable maxICA and plaque measurements, respectively. For maxCCA-IMT, the median (IQR) yearly rate of change was 16 (6–31) μm (p<0.001 for the comparison against the null hypothesis of a yearly rate of change of 0 μm). An increase in maxCCA-IMT (i.e. a change in CCA-IMT > 0 μm) was observed in 129 patients (82%). For maxICA-IMT, the median (IQR) yearly rate of change was 25 (−8 – 91) μm (p<0.001 for the comparison against the null hypothesis of a yearly rate of change of 0 μm). Any increase in maxICA-IMT was observed in 109 (82%).
At baseline, 58 RA patients (38%) showed no evidence of plaque in the ICA or carotid bulb, 85 (55%) showed mild plaque with stenosis of 1–24% of the luminal diameter, and 11 (7%) showed plaque stenosis of 25–50%. No patients showed high-grade plaque stenosis (i.e. > 50% of the luminal diameter). Among those without plaque at baseline, 8 (14%) showed evidence for new plaque at follow-up, resulting in an incidence rate of 4.2 per 100 person-years (95% CI 1.6–6.8). Among those with plaque at baseline, 5 (5%) showed evidence of greater plaque stenosis at follow-up, resulting in a progression rate of 1.6 per 100 person-years (95% CI 0.2–5.6). Of the remaining 91 patients with plaque at baseline, the majority (89%) showed the same degree of plaque stenosis at follow-up; however, a lower degree of plaque stenosis was observed in 6 patients (6%). Any carotid plaque at either visit was observed in 104 patients (68%).
Predictors of Change in maxCCA-IMT
We modeled the associations of baseline and cumulative average patient characteristics with the yearly progression rate of maxCCA-IMT (Supplemental Table 1). In the final multivariable prediction model, higher amounts of reported daily intentional exercise and baseline cigarette smoking were significantly associated with a higher adjusted average yearly maxCCA-IMT progression rate. Those reporting higher amounts of exercise had a more favorable CVD risk profile compared to those reporting lower amounts, including significantly lower BMI and fat mass, a lower prevalence of hypertension and metabolic syndrome, and a lower average e-Selectin and CRP levels (data not shown). Walking for exercise was more strongly associated with max-CCA IMT progression rate than sporting activities or conditioning exercise (data not shown). Trends to significance were noted in the associations of higher body surface area and baseline maxCCA-IMT predicting a higher average rate of change in maxCCA-IMT. No other traditional CVD risk factors were significantly associated with the average yearly maxCCA-IMT progression rate.
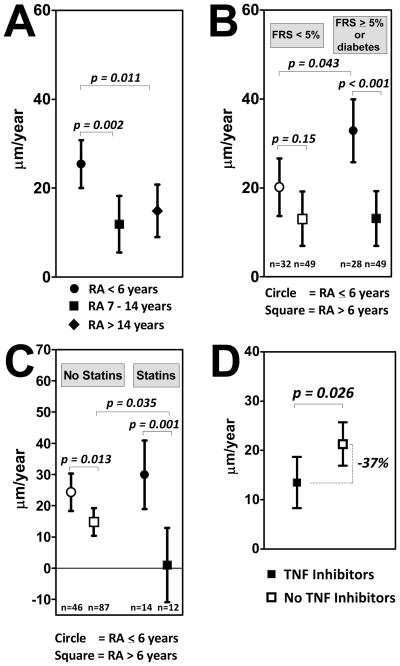
Two RA characteristics were inversely associated with the maxCCA-IMT progression rate after adjusting for the associated non-RA factors described above: RA duration and the use of TNF inhibitors. For RA duration, the association with the average yearly change in maxCCA-IMT was not linear, but was more rapid in patients in the first tertile of baseline RA duration (≤6 years; Figure 1.a.). Specifically, the adjusted rate of change in average maxCCA-IMT was approximately double for the patients in the first tertile of RA duration compared to those with longer disease, differences that were statistically significant. There was no significant difference in the adjusted average yearly change in maxCCA-IMT for those in the 2nd vs. the 3rd tertiles of RA duration. Although demographic and CVD risk factors did not differ significantly according to RA duration (data not shown), the adjusted rate of yearly change in maxCCA-IMT was significantly higher for the subgroup in the 1st tertile of disease duration with higher Framingham risk scores or diabetes compared to earlier RA patients with lower Framingham risk scores (33 vs. 20 μm per year, respectively; p=0.043: Figure 1.b.), suggesting an effect modification among this subgroup. In contrast, IMT progression rates were identical for RA patients with longer standing disease regardless of Framingham score.
Figure 1. Adjusted associations of baseline RA duration and TNF inhibitor use with the yearly change in maximum intima-medial thickness of the common carotid artery (μm per year).
All graphs depict means and 95% confidence intervals. Panel A: Tertiles of RA duration are depicted. Associations are adjusted for habitual exercise, current smoking, baseline CCA-IMT, body surface area, and TNF inhibitor use. Panel B: The adjusted association of RA duration (dichotomized at the first tertile of 6 year) with the outcome in groups stratified by baseline cardiovascular disease risk is depicted. Open markers indicate participants with a Framingham Risk Score < 5%. Closed markers indicate those with a Framingham Risk Score ≥ 5% or with diabetes. Analyses were adjusted for the same variables as described above. Panel C: The adjusted association of RA duration (dichotomized at the first tertile of 6 year) with the outcome in groups stratified by statin use is depicted. Open markers indicate participants not using statins at baseline. Closed markers indicate those using statins at baseline. Analyses were adjusted for the same variables as described above. Panel D: The adjusted yearly change in the outcome for participants receiving TNF inhibitors at baseline (closed marker) and those not receiving TNF inhibitors at baseline (open marker). Analyses were adjusted for habitual exercise, current smoking, baseline CCA-IMT, body surface area, and RA duration. RA=rheumatoid arthritis; FRS=Framingham Risk Score; TNF=tumor necrosis factor
RA patients in the 1st tertile of RA duration who were receiving statin therapy at baseline did not significantly differ in their adjusted yearly change in maxCCA-IMT compared to those with early RA who were not receiving statins (Figure 1.c.). However, RA patients with longer duration disease who were receiving statins at baseline had, on average, no change in the adjusted average rate of change in maxCCA-IMT (Figure 1.c.), a rate that was significantly lower compared to RA patients with longer duration disease who were not receiving statin therapy at baseline (1 vs. 15 μm per year, respectively; p=0.035). Levels of other characteristics (age, gender, ethnicity, RF or anti-CCP antibody status, RA disease activity, or average CRP level during follow-up) did not significantly modify the association of RA duration with the adjusted average yearly rate of change in maxCCA-IMT (data not shown).
RA patients receiving TNF inhibitors at baseline had a 37% lower rate of change in the adjusted average maxCCA-IMT compared to patients not receiving TNF inhibitors (14 vs. 22 μm per year, respectively; p=0.026: Figure 1.d.). The association of TNF inhibitor use with the rate of change in maxCCA-IMT was not modified by levels of other demographic or lifestyle characteristics, Framingham score, or other RA characteristics (data not shown).
Predictors of Change in maxICA-IMT
Among the non-RA disease related characteristics modeled as predictors of the yearly rate of change in maxICA-IMT, only higher reported exercise was significantly associated with a higher average yearly rate of change in maxICA-IMT (Supplemental Table 2). Strong trends to significance were observed indicating higher rates of change in maxICA-IMT for ever smokers and those with higher baseline body surface area. Other demographic, lifestyle, and CVD risk factors were not associated with change in maxICA-IMT for the total RA group.
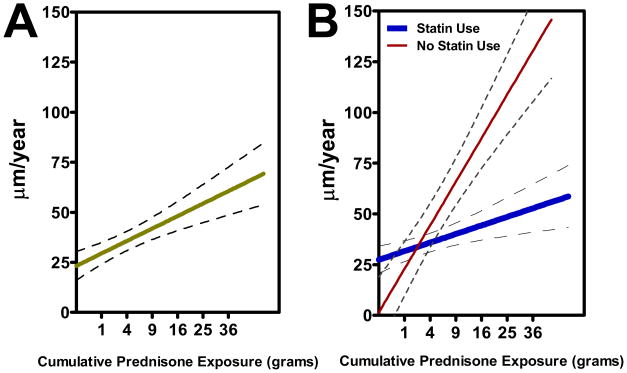
Among RA characteristics, higher prior exposure to glucocorticoids was significantly associated with a higher adjusted yearly change in maxICA-IMT compared to lower exposure (Figure 2.a.). This association was modified by statin use, as the association of cumulative prednisone exposure with the adjusted average yearly change in maxICA-IMT was attenuated, yet remained significant, in participants receiving statins at baseline (Figure 2.b.). The association of cumulative prednisone with change in maxICA-IMT was not significantly modified by the levels of any other characteristics (data not shown). Neither current prednisone use nor other RA disease or treatment characteristics were significantly associated with the adjusted yearly change in maxICA-IMT.
Figure 2. Adjusted associations of cumulative prednisone exposure (grams)with the yearly change in maximum intima-medial thickness of the internal carotid artery (μm per year).
Graphs depict the least squares estimator for the linear association and the 95% confidence interval around the point estimate. Cumulative prednisoneis depicted on the quadratic scale. Panel A depicts the unstratified association, adjusted for exercise, ever smoking, and body surface area [β = 1.26 (95% CI 0.07, 2.44)]. Panel B depicts the association stratified on statin use at baseline, adjusted for the same covariates as above [β = 0.85 (95% CI −0.53, 2.23) for statin use and β = 3.93 (95% CI 1.41, 6.45) for no statin use; p-value for test of interaction = 0.036]
Predictors of Change in ICA-bulb Plaque
After considering all Table 1 covariates as predictors of incident or progressive plaque, four covariates (baseline age, hormone replacement therapy (HRT) use, cumulative average swollen joint count, and cumulative average CRP) were significantly associated with the outcome at or below the p<0.20 level of significance in univariate models (Table 2, Model 1). All were significantly associated with incident or progressive plaque (at the p<0.05 level) when modeled in the same multivariate model (Table 2, Model 2). Including Framingham score and diabetes into the model only reduced the magnitude and significance of the association with baseline age (Table 2, Model 3). Neither biologic DMARD use nor glucocorticoid use (current or cumulative) were associated, either positively or negatively, with incident or progressive carotid plaque (data not shown).
Table 2.
Crude and Adjusted Predictors of Incident or Progressive Plaque
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| IRR | (95%CI) | IRR | (95%CI) | IRR | (95%CI) | |
| Baseline age, per year | 1.04 | (0.99, 1.07)** | 1.05 | (1.00, 1.11)* | 1.04 | (0.79, 1.86) |
| Baseline HRT use | 3.60 | (1.23, 10.55)* | 2.68 | (1.11, 6.51)* | 2.46 | (1.02, 5.95)* |
| Average swollen joint count, per joint | 1.08 | (1.01, 1.16)* | 1.10 | (1.01, 1.20)* | 1.12 | (1.02, 1.22)* |
| Average CRP > 12.0 mg/L*** | 5.06 | (1.84, 13.89)* | 3.81 | (1.34, 10.85)* | 3.48 | (1.25, 9.66)* |
Model 1: Covariates listed were modeled individually with no adjustment
Model 2: Covariates listed were modeled simultaneously
Model 3: Covariates listed were modeled simultaneously with additional adjustment for Framingham Risk Score and Diabetes
IRR=incidence rate ratio; CI=confidence interval; HRT=hormone replacement therapy; CRP=C-reactive protein
p <0.05
0.10 > p > 0.05
A total of 20 of 154 participants with scans adequate to assess plaque had a CRP > 12.0 mg/L
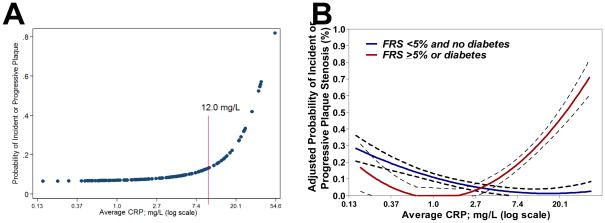
The association of cumulative average CRP with the probability of incident or progressive plaque was not linear across the extent of the exposure (Figure 3.a.), as the probability of the outcome was minimal until a threshold of approximately 12 mg/L, after which the probability began to sharply increase. For this reason, average CRP was modeled as a dichotomous, rather than a continuous, variable. Interestingly, the threshold for cumulative average CRP at which the adjusted risk of incident or progressive plaque began to increase was lower for RA patients with a higher Framingham scores or diabetes at baseline, with a cutpoint at approximately 5 mg/L (Figure 3.b.). Average CRP was not associated with incident or progressive plaque in RA patients with lower Framingham scores or who did not have diabetes (p-value for the interaction of Framingham level on the association of average CRP with the outcome of incident or progressive plaque=0.008)
Figure 3. Associations of average CRP and swollen joint count with the probability of incident or progressive carotid plaque.
Panel A depicts the unadjusted average probability of incident or progressive carotid plaque according to the average serum CRP over the study interval. A cutoff of approximately 12 mg/dl is a level at which the probability of plaque appears to increase over that observed at lower average CRP levels. Panel B depicts the same relationship as Panel A, but stratified by Framingham risk score < 5% (blue line) vs. Framingham risk score ≥ 5% or diabetes (red line) adjusted for age, current hormone replacement, and average swollen joint count [IRR=0.86 per log unit increase in average CRP for the low risk group (p=0.17) vs. IRR=2.16 per log unit increase in average CRP for the high risk group (p=0.042; p-value for interaction on Framingham risk level = 0.009)]. CRP=C-reactive protein; FRS=Framingham Risk Score
DISCUSSION
This investigation, to our knowledge, is the largest published to date exploring longitudinal predictors of carotid atherosclerosis in RA. RA characteristics were associated with site-specific changes in subclinical atherosclerosis measures. Specifically, after accounting for pertinent traditional CVD risk factors, progression of maxCCA-IMT was more rapid in RA patients of shorter disease duration vs. those with longer disease, an association modified by baseline CVD risk and statin use. RA patients treated with TNF inhibitors had a significantly lower rate of progression of maxCCA-IMT compared to those not receiving treatment, an association not observed with other DMARDs. Increasing cumulative prednisone exposure was the only RA characteristic associated with maxICA-IMT progression, an association modified favorably in patients prescribed statins at baseline. Lumenal stenosis due to plaque located in the ICA/bulb was generally stable over the study interval in most patients; however, higher levels of systemic inflammation and swollen joint counts were both associated with a higher probability of developing plaque or increasing stenosis by prevalent plaque.
The mechanism underlying the more rapid progression of maxCCA-IMT in patients with earlier RA is not readily apparent, although the association was stronger in patients with higher overall CVD risk. Differing rates of carotid IMT progression according to RA duration were suggested in a previous cross-sectional study(27). It remains possible that this represents a lagged effect of the early inflammatory milieu of RA in synergy with CVD risk factors; however, we did not detect a difference in baseline or average CRP levels among those with earlier RA compared to longer-standing disease. Another possible explanation is a survival effect, as those RA patients with longer disease who were rapid progressors would likely have CVD events earlier in their disease course, and were thus ineligible for our study of subclinical disease. There is debate as to what threshold of yearly progression of CCA-IMT is associated with increased events. In one study of men with coronary atherosclerosis (16), a CCA-IMT change ≥0.34 mm/year was associated with a 3.7-fold higher risk of events vs. those with ≤0.11 mm/year. Although this 0.34 mm threshold was met for many of our study participants with RA<6 years, it is unclear whether this cutpoint is a relevant predictor of clinical CVD events for our cohort. Follow-up is ongoing to track the relationship between progression of subclinical measures and CVD events.
Notably, statin use was associated, on average, with almost no progression of CCA-IMT in RA patients with longer disease duration, an observation supporting the use of statins in RA. Interestingly, however, statin use was not associated with lower maxCCA-IMT progression among participants with earlier disease. This may suggest differing mechanisms for CCA-IMT progression in early vs. late disease. Alternately, as statin exposure prior to enrollment was not quantified, cumulative exposure to stains could be systematically lower in early RA patients compared to those with longer disease. Otherwise, lipid levels and other traditional CVD risk factors (such as hypertension) were not strong independent predictors of atherosclerosis progression. Despite this lack of association of individual risk factors, higher composite CVD risk (i.e. higher Framingham score) was necessary for higher levels of cumulative inflammation predict CCA-IMT and plaque progression. In contrast, higher reported exercise was associated with more rapid progression of IMT in our study. The mechanism behind this observation is unclear, as CVD risk factors, systemic inflammatory markers, and circulating markers of endothelial activation were not higher in the group reporting more exercise. Since fitness was not measured directly, but approximated by self-reported duration of exercise activities, it is possible that the association could be confounded by unmeasured factors related to exercise self-report. Self-reported physical activity is frequently overestimated compared to objective measurement with accelerometers(28), with accuracy increased for more vigorous activities. Importantly, the associations of RA characteristics with IMT progression rates were not meaningfully affected by the exclusion of exercise from the models.
In animal studies, TNF-α gene knockout or anti-TNF treatment were associated with a reduction in atherosclerosis progression(10, 29). Our observation that TNF inhibitor treated patients had a 37% reduction in the rate of change in maxCCA-IMT provides some human confirmation of a link between cytokines and atherosclerosis. However, it is unclear whether this effect is due to TNF inhibition per se, and thus a unique effect of TNF inhibitors, or a general anti-inflammatory effect. We did not observe the same reduction among users of other RA treatments, such as methotrexate or hydroxychloroquine, despite similar levels of disease activity and systemic inflammation. Our findings corroborate those of a smaller study(30)that also identified a reduction in CCA-IMT progression in RA patients treated with TNF inhibitors.
Most carotid ultrasound studies, including those of RA patients, have focused on the CCA and have not studied the ICA separately. However, the associations of CVD risk factors with the rate of change in ICA compared to CCA-IMT may differ(18, 31), emphasizing the importance of studying the ICA separately. However, whether higher rates of ICA-IMT progression are more or less predictive of subsequent CVD events than CCA-IMT progression is unclear. In our study, higher prior exposure to glucocorticoids was strongly associated with a more rapid progression of maxICA-IMT. Glucocorticoids are associated with an increase in CVD events in the general population(31)and in RA(32), and have been cross-sectionally linked to carotid atherosclerosis (33). Interestingly, we observed that the association of glucocorticoid exposure with maxICA-IMT progression was attenuated in statin treated patients, independent of lipid levels. This finding deserves additional study and, short of a confirmatory trial, suggests that statins could be considered in RA patients receiving glucocorticoids.
Carotid plaque may be more synonymous with atherosclerosis than elevated IMT, as IMT may be reflective off actors such as arterial wall aging, body size, and muscularity. Accordingly, plaque was shown to be more predictive of CVD events than IMT (34). In contrast to IMT progression, new or progressive ICA/bulb plaque was strongly associated with clinical measures of inflammation and RA disease activity in our study, with CRP and swollen joints contributing independently to risk. Participants with higher levels of systemic inflammation were at higher risk of new or progressive plaque, even in those with a low number of swollen joints. Further, average levels of CRP and IL-6 were more predictive of plaque progression than baseline levels. This could suggest that CVD risk assessment in RA patients should include serial assessment of systemic inflammation, with consideration of modification of therapy in the setting of persistently elevated inflammatory markers, even in those with apparently controlled articular symptoms. Our study also supports the concept of interaction between inflammation and traditional CVD risk factors on atherogenesis, since elevated CRP was only predictive of incident or progressive plaque in patients with higher Framingham scores or diabetes. Based on this, one could propose that CVD risk prevention in RA should focus as much on traditional risk factor control as on inflammation reduction.
Among limitations, our cohort was not followed from RA inception. Thus, although we were able to ascertain cumulative inflammatory and disease activity measures over time, we may have missed relevant exposure time prior to enrollment. Similarly, we used clinical and laboratory measures from three time points to cumulate average levels, potentially missing relevant fluctuations between visits. Pharmacotherapies were not randomly allocated, so confounding by indication must be considered when interpreting any beneficial or detrimental treatment effects. Finally, a limitation of repeat carotid ultrasound is measurement variability, particularly for ICA-IMT, and the subjectivity of interpreting plaques. We sought to reduce these issues by following a standard protocol used in other large epidemiologic studies, with rigorous training and quality control measures in place. Most importantly, baseline scans were reread concurrently with follow-up scans by a single interpreter, a necessity for tracking plaque.
In summary, we identified several RA characteristics as predictors of site-specific changes in subclinical carotid atherosclerosis suggesting that progression may be more rapid in RA patients with earlier disease, and accelerated by systemic/articular inflammation and glucocorticoids. Since statins and TNF antagonists may function to reduce the impact of these factors, interventional studies are warranted to determine effects on future CVD event risk.
Supplementary Material
Acknowledgments
We would like to thank the Johns Hopkins Bayview Medical Center General Clinical Research Center and staff, the field center of the Baltimore MESA cohort, and the MESA Coordinating Center at the University of Washington, Seattle.
We are indebted to the dedication and hard work of the ESCAPE RA Staff: Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, Shawn Franckowiak, and Brandy Miles and to the participants in the ESCAPE RA study who graciously agreed to take part in this research.
Drs. Uzma Haque, Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Andrea Marx, Howard Hauptman, Achini Perera, Peter Holt, Alan Matsumoto, Megan Clowse, Gordon Lam and others generously recommended their patients for this study.
We would like to thank Dr William Scott Jr. for interpreting all baseline radiographs.
FUNDING
This work is supported by Grant Numbers AR050026-01 (JMB) and 1K23AR054112-01 (JTG) from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases; a Clinical Investigator Fellowship Award from the Research and Education Foundation of the American College of Rheumatology (JTG); and the Johns Hopkins Bayview Medical Center General Clinical Research Center (Grant Number M01RR02719). Funding for this research was also made possible by the American College of Rheumatology Research and Education Foundation’s Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign(JMB). MESA is funded by contracts N01-HC-95159 through N01-HC-95166 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
All authors attest that they have no financial conflicts of interest pertaining to this investigation
Author Contributions
Study Design: Bathon, Giles, Post, Blumenthal, Szklo
Data Acquisition: Bathon, Giles, Post
Statistical Analyses: Giles
Interpretation of Data and Manuscript Writing: All authors
References
- 1.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.Joyce AT, Smith P, Khandker R, Melin JM, Singh A. Hidden cost of rheumatoid arthritis (RA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol. 2009;36(4):743–52. doi: 10.3899/jrheum.080670. [DOI] [PubMed] [Google Scholar]
- 4.Giles JT, Szklo M, Post W, Petri M, Blumenthal RS, Lam G, et al. Coronary arterial calcification in rheumatoid arthritis: comparison to the multi-ethnic study of atherosclerosis. Arthritis Res Ther. 2009;11(2):R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Giles JT, Polak JF, Blumenthal RS, Leffell MS, Szklo M, et al. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol. 2010;37(4):730–9. doi: 10.3899/jrheum.090670. [DOI] [PubMed] [Google Scholar]
- 7.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144(4):249–56. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Juanatey C, Llorca J, Martin J, Gonzalez-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38(5):366–71. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Evans MR, Escalante A, Battafarano DF, Freeman GL, O’Leary DH, Del Rincon I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011 Jan 28; doi: 10.1002/art.30265. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, et al. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180(1):11–7. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of Tumor Necrosis Factor-{alpha} and Increased Risk of Recurrent Coronary Events After Myocardial Infarction. Circulation. 2000;101(18):2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 13.Tyrrell PN, Beyene J, Feldman BM, McCrindle BW, Silverman ED, Bradley TJ. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30(5):1014–26. doi: 10.1161/ATVBAHA.109.198424. [DOI] [PubMed] [Google Scholar]
- 14.Nagata-Sakurai M, Inaba M, Goto H, Kumeda Y, Furumitsu Y, Inui K, et al. Inflammation and bone resorption as independent factors of accelerated arterial wall thickening in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(11):3061–7. doi: 10.1002/art.11327. [DOI] [PubMed] [Google Scholar]
- 15.Sodergren A, Karp K, Boman K, Eriksson C, Lundstrom E, Smedby T, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. 2010;12(4):R158. doi: 10.1186/ar3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 18.Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: the carotid atherosclerosis progression study. Stroke. 2004;35(9):2150–4. doi: 10.1161/01.STR.0000136720.21095.f3. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C for the Conference Participants. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire Functional Disability Index in patients with rheumatoid arthritis. J Rheumatol. 1988;15(10):1480–8. [PubMed] [Google Scholar]
- 25.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–3. [PubMed] [Google Scholar]
- 26.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83(6):1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Rincon I, O’Leary DH, Freeman GL, Escalante A. Acceleration of atherosclerosis during the course of rheumatoid arthritis. Atherosclerosis. 2007;195(2):354–60. doi: 10.1016/j.atherosclerosis.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Richardson MT, Ainsworth BE, Jacobs DR, Leon AS. Validation of the Stanford 7-day recall to assess habitual physical activity. Ann Epidemiol. 2001;11(2):145–53. doi: 10.1016/s1047-2797(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 29.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 30.Del Porto F, Lagana B, Lai S, Nofroni I, Tinti F, Vitale M, et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2007;46(7):1111–5. doi: 10.1093/rheumatology/kem089. [DOI] [PubMed] [Google Scholar]
- 31.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141(10):764–70. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 32.Davis JM, 3rd, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56(3):820–30. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]
- 33.del Rincon I, O’Leary DH, Haas RW, Escalante A. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum. 2004;50(12):3813–22. doi: 10.1002/art.20661. [DOI] [PubMed] [Google Scholar]
- 34.Stein JH, Korcarz CE, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: summary and discussion of the American Society of Echocardiography consensus statement. Prev Cardiol. 2009;12(1):34–8. doi: 10.1111/j.1751-7141.2008.00021.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.