Abstract
Endocannabinoids (eCBs) modulate neurotransmission by inhibiting the release of a variety of neurotransmitters. The cannabinoid receptor agonist WIN 55,212-2 (WIN) can modulate organophosphorus (OP) anticholinesterase toxicity in rats, presumably by inhibiting acetylcholine (ACh) release. Some OP anticholinesterases also inhibit eCB-degrading enzymes. We studied the effects of the OP insecticide chlorpyrifos (CPF) on cholinergic signs of toxicity, cholinesterase activity and ACh release in tissues from wild type (+/+) and cannabinoid CB1 receptor knockout (−/−) mice. Mice of both genotypes (n=5–6/treatment group) were challenged with CPF (300 mg/kg, 2 ml/kg in peanut oil, sc) and evaluated for functional and neurochemical changes. Both genotypes exhibited similar cholinergic signs and cholinesterase inhibition (82–95% at 48 h after dosing) in cortex, cerebellum and heart. WIN reduced depolarization-induced ACh release in vitro in hippocampal slices from wild type mice, but had no effect in hippocampal slices from knockouts or in striatal slices from either genotype. Chlorpyrifos oxon (CPO, 100 μM) reduced release in hippocampal slices from both genotypes in vitro, but with a greater reduction in tissues from wild types (21% vs 12%). CPO had no significant in vitro effect on ACh release in striatum. CPF reduced ACh release in hippocampus from both genotypes ex vivo, but reduction was again significantly greater in tissues from wild types (52% vs 36%). In striatum, CPF led to a similar reduction (20–23%) in tissues from both genotypes. Thus, while CB1 deletion in mice had little influence on the expression of acute toxicity following CPF, CPF- or CPO-induced changes in ACh release appeared sensitive to modulation by CB1-mediated eCB signaling in a brain-regional manner.
Keywords: organophosphorus, insecticide, endocannabinoids, acetylcholinesterase, acute toxicity
Introduction
Organophosphorus (OP) insecticides remain the most widely used insecticides in the United States accounting for about 35% of all insecticides used in the latest year of analysis, 2007 (Kiely et al., 2004; Grube et al., 2011). While restrictions on household uses for CPF have been in place for a number of years, it remains the most the most commonly used OP insecticide, with 8–11 million pounds (roughly 10% of total amount of insecticides) used in the US in 2007 (Grube et al., 2011). OP insecticides elicit acute toxicity by complexing with acetylcholinesterase (AChE) leading to the formation of a stable, phosphylated enzyme (Eto, 1974; Casida and Quistad, 2005). This covalent modification blocks binding of ACh and its subsequent metabolic degradation, leading to ACh accumulation, persistent activation of post-synaptic cholinergic receptors and resulting signs of cholinergic toxicity (Mileson et al., 1998).
Endocannabinoids (eCBs, e.g., arachidonoylethanolamide [anandamide, AEA], and 2-arachidonylglycerol [2-AG]) are neuromodulators that can influence a variety of neurological processes throughout both the peripheral and central nervous systems (Hashimotodani et al., 2007; Pope et al., 2010). The eCB signaling system consists of the eCBs themselves, enzymes responsible for their synthesis and degradation, and specific cannabinoid receptors (CB1 and CB2). Anandamide is synthesized from N-arachidonoyl phosphatidyl ethanolamine by the enzyme, N-acyltransferase phosphatidyl ethanolamine-phospholipase D (Freund et al, 2003; Di Marzo et al, 2004; Pertwee, 2005). 2-Arachidonoyl glycerol is synthesized from membrane phospholipids by the action of diacylglycerol lipase. Endocannabinoid action is thought to be terminated by first reuptake into either the pre-synaptic terminal or the post-synaptic cell and then enzymatic degradation. Anandamide is primarily degraded by the enzyme fatty acid amide hydrolase while 2-AG is primarily inactivated by the enzyme monoacylglycerol lipase (Dinh et al, 2002; Hashimotodani et al, 2007; Chanda et al., 2010).
Neuron depolarization leads to the synthesis and release of eCBs, which diffuse across the synapse to activate cannabinoid receptors on the presynaptic terminal, thereby modulating neurotransmitter release. Endocannabinoids regulate the release of a variety of neurotransmitters in a brain regional and pathway-dependent manner. A number of studies reported inhibition of hippocampal ACh release by eCBs (Gessa et al, 1998; Gifford and Ashby 1996; Gifford et al, 1997, 2000; Tzavara et al, 2003; Degroot et al, 2006). In contrast, ACh release in striatum does not appear to be regulated by eCB signaling (Gifford et al., 1997; Kathmann et al., 2001). Thus, activation of eCB signaling could modulate the degree of ACh accumulation in a brain regional manner and potentially influence the expression of cholinergic toxicity following anticholinesterase exposure. Indeed, we previously reported that chemicals that can enhance eCB signaling (direct and indirect cannabinoid receptor agonists) reduced functional and neurobehavioral signs of toxicity in rats following OP anticholinesterase exposure (Nallapaneni et al., 2006, 2008; Wright et al., 2010). While effects on ACh release could mediate the modulation of OP anticholinesterase toxicity by eCBs, eCB-mediated changes in release of non-cholinergic transmitters, e.g., glutamate, could also play a role.
We have repeatedly observed relatively minimal classical signs of cholinergic toxicity in adult male Sprague Dawley rats following subcutaneous exposure to high dosages of CPF, even in the presence of extensive cholinesterase inhibition (Pope et al., 1992; Pope et al, 1995; Liu and Pope 1996, 1998; Karanth and Pope, 2000; Karanth et al., 2006). The active metabolite of CPF, i.e., chlorpyrifos oxon, is a potent inhibitor of eCB-degrading enzymes (Quistad et al., 2002, 2006; Pope et al., 2010). Thus, indirect activation of eCB signaling could play a role in the expression of relatively minimal cholinergic signs in adult rats following high dose acute CPF exposure.
We hypothesized that mice lacking the CB1 receptor would exhibit greater toxicity following exposure to CPF. Wild type (CB1+/+) and knockout (CB1−/−) littermates were treated acutely with CPF and classical signs of cholinergic toxicity were subsequently evaluated. AChE inhibition as well as ACh release in hippocampal and striatal slices were compared between wild type and knockout mice following CPF exposure.
Material and methods
Chemicals
Chlorpyrifos (CPF, O,O′-diethyl-O-(3,5,6-trichloro-2-pyridinyl-phosphorothioate, 99% purity), chlorpyrifos oxon (CPO, O, O′-diethyl-O-(3, 5, 6-trichloro-2-pyridinyl-phosphate, 99.1% purity) were purchased from Chem Service (West Chester, PA) and stored in a desiccator under nitrogen at 4°C. Acetylcholine iodide (acetyl-3H; specific activity = 76.0 mCi/mmol) and choline chloride (methyl- 3H; specific activity = 66.5 Ci/mmol) were purchased from Perkin Elmer (Boston, MA) and stored at −70°C. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and treatments
Two breeding pairs of homozygous CB1 nullizygotes were kindly provided by Dr. James Pickel at the National Institute of Neurological Disorders and Stroke (NINDS, Bethesda, MD). The CB1 gene was originally mutated by gene-targeted deletion of the sequence that encodes for amino acids 33 through 448 with PGK-neo cassette and effectively inactivates the locus as demonstrated by loss of ligand binding in the brains of the mutant mice (Zimmer et al., 1999). A homozygous CB1 receptor deficient male mouse in a harem mating with two C57BL/6NCrl (Charles River Laboratories) females was used to generate heterozygous receptor deficient mice that were subsequently intercrossed to generate CB1 receptor homozygous deficient mice and wild type littermate controls. Genotypes were confirmed by PCR of tail DNA. Male wild types (+/+, eight weeks of age) were first used in pilot studies to evaluate acute sensitivity to CPF. Male wild types (+/+) and male knockouts (−/−) were used for all subsequent studies. All procedures involving animals were in accordance with protocols of the NIH/NRC “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Laboratory Care and Use Committee (IACUC) of Oklahoma State University prior to use.
In vivo Studies
Pilot studies (n=5–6/treatment group) evaluated the acute toxicity of CPF (75, 150, or 300 mg/kg, sc, in peanut oil, 2 ml/kg) in male, wild type mice (8 weeks of age). The highest dosage of CPF (300 mg/kg) elicited marked, classical signs of cholinergic toxicity but no incidence of lethality, and was therefore selected for further studies. Wild type and CB1 knockout mice (n = 5–6/treatment group) were treated with vehicle (peanut oil, 2 ml/kg injection volume) or CPF (300 mg/kg) and functional signs of toxicity were evaluated for the following 48 h. Involuntary movements were scored as 1=normal, 2=slight tremor in head and neck region; 3=tremor extending into trunk, 4=whole body tremor. SLUD signs were scored as 1=normal, 2= slight secretions, 3=moderate, multiple secretions, 4=severe, multiple secretions. At the end of each study, body weights were recorded and mice were sacrificed by decapitation. Tissues (frontal cortex, cerebellum, whole heart) were collected, blotted to remove blood and frozen (−70° C) for later analysis of cholinesterase or were used fresh (hippocampus and striatum) to prepare slices for study of ACh release.
Cholinesterase assay
Cholinesterase activity was measured using a radiometric method (Johnson and Russell, 1975) using [3H]acetylcholine iodide (1 mM final concentration) as the substrate. Incubation times were selected to attain linear rates of substrate hydrolysis. Protein concentration was estimated using the Lowry method (Lowry et al., 1951) and data reported as nmol ACh hydrolyzed/min/mg protein.
Acetylcholine release in hippocampal and striatal slices
Brain slices were prepared essentially as described before (Zhang et al, 2002) and ACh release studied as described by Dolezal and Tucek (1983). In brief, mice were sacrificed by decapitation and whole brain was immediately removed and dissected on ice to collect hippocampus and striatum by the method of Glowinski and Iversen (1966). Hippocampal and striatal slices (400 μm, unidirectional) were prepared using a McIlwain Tissue Slicer. Slices were collected and dispersed in Krebs Ringer bicarbonate buffer (KRB: 118 mM NaCl, 1.3 mM CaCl2, 1.2 mM KH2PO4, 4.7 mM KCl, , 1.2 mM MgSO4, 25 mM NaHCO3, and 11 mM d-glucose) by gentle trituration (4–5 x) with a Pasteur pipette. The slices were first pre-incubated for 20 min at 33°C under constant oxygenation in KRB. They were then washed with fresh KRB and then further incubated with 2 ml of KRB containing 15 μl of [3H]choline (113 nM final concentration, specific activity 66.5 Ci/mmol) for 30 min at 37°C. The slices were then transferred to a suprafusion apparatus (SF12/Brandel Inc., Gaithersburg, MD) and suprafused with KRB containing hemicholinium-3 (10 μM, 0.5 ml/min for 60 min, 37°C). ACh release was stimulated either once or twice for five min each (i.e., at 20 – 25 [S1] and at 60 – 65 [S2] min) with KRB containing hemicholinium-3 and either 25 mM KCl (for hippocampus) or 20 mM KCl (for striatum), with proportionately reduced sodium concentrations. Twenty 5-min fractions were collected. Release was reported as either the ratio of the size of the S2 peak (second depolarization peak) relative to S1 peak (i.e., S2/S1; for in vitro studies) or the size of the S1 peak relative to radioactivity in the first three baseline fractions (for ex vivo studies).
In vitro effects of chlorpyrifos oxon on acetylcholine release
Hippocampal and striatal slices from wild type and knockout mice were used to study the comparative effects of CPO on ACh release in the presence or absence of CB1. Preliminary concentration-response studies determined that 100 μM was needed to consistently reduce ACh release in both tissue types. Slices were thus exposed to either vehicle or a high concentration of CPO (100 μM) 20 min prior to the S2 depolarization. The direct cannabinoid receptor agonist, WIN 55,212-2 (1 μM) was also used under similar conditions to evaluate effects of direct cannabinoid receptor activation on ACh release in tissues from both genotypes.
In vivo effects of chlorpyrifos on acetylcholine release ex vivo
Wild type and knockout mice (n = 5–6/treatment group) were treated with either vehicle (peanut oil) or CPF (300 mg/kg, sc) as above and graded for functional signs of toxicity for the following 48 h. Mice were sacrificed by decapitation and brain slices (hippocampus and striatum) prepared and depolarization-induced ACh release (S1) evaluated as described above.
Statistical Analyses
Body weights and biochemical endpoints (cholinesterase, ACh release) were reported as mean ± standard error (SE) and analyzed using one-way ANOVA and post hoc analysis using Tukey’s test. Functional data (IM and SLUD signs) were reported as median ± interquartile range (IQR). Functional data were transformed (square root) and analyzed for statistical significance by two-way ANOVA. Post hoc analysis was performed with Bonferroni correction. For all statistical analyses the GraphPad Prism® version 4 statistical software was used. Statistical differences were considered significant at p < 0.05.
Results
Pilot toxicity studies
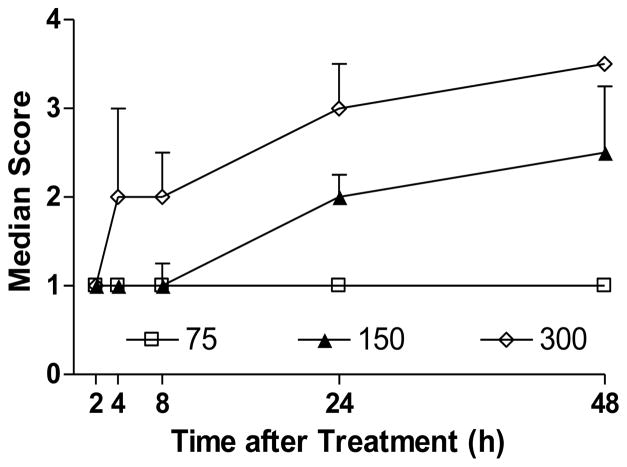
Preliminary studies evaluated dose-related toxicity in wild type mice following CPF (75, 150 or 300 mg/kg, sc). Figure 1 shows cholinergic signs (involuntary movements) of toxicity from 2–48 h after CPF exposure. The extent of cholinergic signs noted in the mice was markedly greater than in our previous studies in Sprague Dawley rats treated with similar high dosages of CPF (Pope et al., 1992, 1995; Liu and Pope, 1996, 1998). A CPF dosage that elicited marked signs of toxicity but no lethality (300 mg/kg) was therefore selected for further studies.
Figure 1. Effect of chlorpyrifos on cholinergic signs (involuntary movements) in wild type C57Bl/6 mice.
Mice (n = 5–6/group) were exposed to chlorpyrifos (75, 150 or 300 mg/kg, sc) and were graded for cholinergic signs of toxicity as described in Methods (reported as median ± interquartile range). Dose-related and time-dependent signs of toxicity were noted in mice treated with 150 or 300 mg/kg CPF.
Effects of chlorpyrifos on cholinergic toxicity and cholinesterase activity in wild type and CB1 nullizygotes
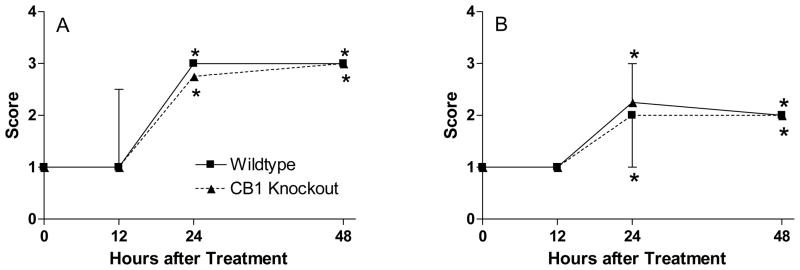
Male mice of both genotypes (+/+ and −/−) were treated with CPF (300 mg/kg, sc, n=5–6/treatment group) and observed for body weight changes and functional signs of cholinergic toxicity. A similar reduction in body weight was observed 48 h after dosing in both wild type and CB1 knockout mice (WT: 21 ± 2%; KO: 26 ± 3%). Figure 2A shows the effect of CPF on involuntary movements. Tremors were noted in both wild type and CB1 knockouts at 24 and 48 h after dosing. Relatively similar SLUD signs were also noted between genotypes (Figure 2B).
Figure 2. Effect of chlorpyrifos on A) involuntary movements and B) SLUD signs in wild type and CB1 knockout mice.
CB1+/+ and CB1−/− littermates (n = 5–6/group) were exposed to either vehicle (peanut oil, 1 ml/kg, sc) or chlorpyrifos (300 mg/kg, sc) and were graded for functional signs of toxicity as described in Methods (reported as median ± interquartile range). An asterisk indicates a significant difference between CPF-treated mice and the respective controls. No functional signs were seen in either control group (wild type or CB1 knockout).
Cholinesterase inhibition was evaluated in frontal cortex, cerebellum and heart 48 h following CPF treatment. Chlorpyrifos elicited extensive, similar degrees of inhibition in both wild type (WT) and CB1 knockout (KO) mice in all three tissues examined: frontal cortex (92 ± 1% in WT vs 95 ± 1% in KO), cerebellum (88 ± 1% in WT vs. 87 ± 2% in KO) and heart (83 ± 1% in WT vs. 82 ± 2% in KO). Control values for frontal cortex, cerebellum and heart (nmol/min/mg protein, mean ± SE) were WT: 35.5 ± 1.9, 27.7 ± 1.8 and 0.6 ± 0.1; KO: 40.6 ± 2.3, 24.9 ± 2.0 and 0.6 ± 0.1, respectively.
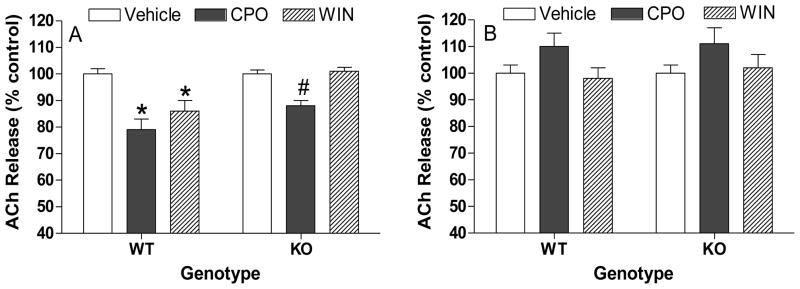
In vitro effects of chlorpyrifos oxon and WIN 55,212-2 on acetylcholine release in hippocampal and striatal slices from wild type and CB1 knockout mice
Figure 3 shows in vitro effects of CPO and WIN 55,212-2 on ACh release in A) hippocampal and B) striatal slices from wild type and CB1 knockout mice. Chlorpyrifos oxon (CPO, 100 uM) significantly reduced release in hippocampal slices from both groups, but the degree of reduction was significantly lower in slices from knockouts (21% vs 12%). There was a trend toward increased ACh release (10–11%) in striatal slices, but no difference was apparent between tissues from the different genotypes. WIN 55,212-2 reduced ACh release in hippocampal slices from wild type mice but had no effect on release in hippocampal slices from CB1 knockout mice or in striatal slices from either genotype.
Figure 3. In vitro effects chlorpyrifos oxon and WIN 55,212-2 on hippocampal and striatal ACh release in slices from wild type (WT) and CB1 knockout (KO) mice.
Hippocampal (A) and striatal (B) slices were incubated with [3H]choline to label endogenous acetylcholine. Prelabelled slices were then loaded into a suprafusion apparatus and perfused with physiological buffer. Release was stimulated twice (S1 and S2) by exposing the slices to a depolarizing buffer containing high concentration of KCl (25 mM for hippocampus; 20 mM for striatum). CPO or WIN 55,212-2 was added 20 min before the second pulse of potassium and the peak ratio of S2/S1 was used to evaluate effects on ACh release. An asterisk indicates a significant difference compared to respective controls while a pound sign indicates a significant difference in release between tissues from wild type and CB1 knockout mice. Data represent values from three independent assays.
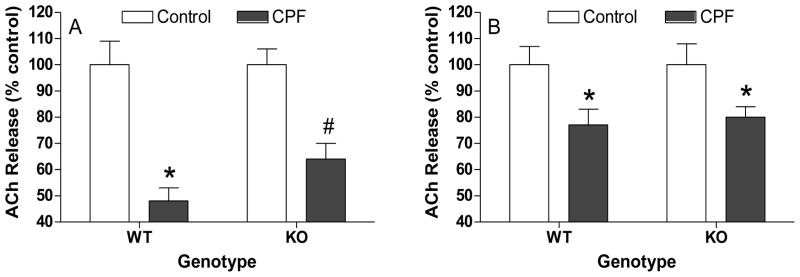
In vivo effects of chlorpyrifos on ACh release ex vivo in hippocampal and striatal slices from wild type or CB1 knockout mice
Figure 4 shows the effects of CPF on ACh release ex vivo 48 h after dosing in A) hippocampal and B) striatal slices from wild type and CB1 receptor knockout mice. Chlorpyrifos reduced hippocampal ACh release in tissues from both wild types and CB1 knockouts (WT: 52% vs. 36%) but there was significantly lesser reduction in tissues from CB1 knockouts. In striatum, there was a similar reduction in ACh release 48 h after CPF exposure, with no differences noted between the genotypes (20% vs 23% reduction).
Figure 4. Effects of chlorpyrifos on ACh release ex vivo in hippocampal and striatal slices from wild type (WT) and CB1 knockout (KO) mice.
Mice (n = 5–6/group) were exposed to either vehicle or chlorpyrifos and sacrificed 48 h later. Hippocampal (A) and striatal (B) slices were incubated with [3H]choline to label endogenous acetylcholine. Prelabelled slices were then loaded into a suprafusion apparatus and perfused with physiological buffer. Release was stimulated by exposing the slices to buffer containing a high concentration of KCl (25 mM for hippocampus; 20 mM for striatum) as described in Methods. Data (mean ± standard error) represent depolarization-induced ACh release (S1) and are expressed as percent of control values. An asterisk indicates a significant difference compared to respective controls while a pound sign indicates a significant difference in release between tissues from wild type and CB1 knockout mice.
Discussion
The classic mechanism of acute OP insecticide toxicity is initiated by inhibition of AChE, leading to accumulation of ACh, prolonged/excessive activation of cholinergic receptors, and subsequent signs of cholinergic toxicity. Activation of postsynaptic muscarinic M1 receptors in the CNS with extensive AChE inhibition would be expected to increase both synthesis and release of eCBs (Kim et al, 2002; Ohno-Shosaku et al, 2003). Increased release of eCBs would be further anticipated to enhance activation of pre-synaptic CB1 receptors on cholinergic neuron terminals and decrease ACh release in selected brain regions/signaling pathways (Gessa et al, 1998; Gifford et al, 1997, 2000; Kathmann et al, 2001; Tzavara et al., 2003), serving a potential neuromodulatory role in the expression of anticholinesterase toxicity. We therefore hypothesized that genetic deletion of the CB1 receptor would increase acute toxicity following OP anticholinesterase exposure by disrupting eCB-mediated inhibition of ACh release. Indeed, preliminary findings suggested that genetic deletion of the CB1 receptor would enhance cholinergic signs of toxicity following CPF exposure (Pope et al., 2008). These preliminary studies were conducted without littermate controls, however. In the present studies, we evaluated the comparative sensitivity to acute CPF in wild type and CB1 knockout littermates. Surprisingly, CB1 deletion had little apparent effect on toxicity following CPF exposure, with relatively similar body weight reductions, cholinergic signs, and cholinesterase inhibition compared to wild types. Moreover, in contrast to responses seen in male Sprague Dawley rats following acute subcutaneous exposure to CPF, both genotypes of mice showed classical signs of cholinergic toxicity following CPF.
We previously reported reductions in acute functional and long-term behavioral changes following OP anticholinesterase intoxication in rats following co-exposure to direct or indirect cannabinoid receptor agonists (Nallapaneni et al, 2006, 2008; Wright et al., 2010). The studies reported herein suggest however relatively little role for CB1 receptor-mediated signaling in the expression of CPF toxicity in mice. Differences in these studies could be due to use of different species (rats vs. mice) or different OP toxicants. In the previous reports, paraoxon and diisopropylphosphorofluoridate were used, which are both direct-acting anticholinesterases that elicit a rapid onset of cholinergic signs. In the present study, CPF was used which requires metabolic bioactivation and thus leads to a more delayed onset and prolonged duration of signs. In addition, studies with rats used either direct or indirect cannabinomimetics (administered at the same time as the OP toxicant) to enhance eCB signaling, while the studies reported herein used CB1 receptor gene knockout mice to study the role of CB1-mediated eCB signaling in OP anticholinesterase toxicity.
We studied ACh release in tissues from wild type and CB1 knockout mice following either in vitro (CPO) or in vivo (CPF) OP toxicant exposure. In vitro, CPO decreased ACh release in hippocampal slices from both wild types and knockouts (Figure 3A), but the degree of reduction was statistically greater in tissues from wild type mice. In contrast, in striatal slices, CPO did not decrease ACh release (there was a trend towards increased ACh release) in tissues from either wild type or CB1 knockout mice (Figure 3B). Similar to previous publications (Gifford et al., 1997; Kathmann et al., 2001), the direct cannabinoid receptor agonist WIN 55,212-2 (1 μM) reduced ACh release in hippocampal slices from wiltdype mice, but had no effect on release in hippocampus from CB1 knockouts, or in striatal slices from either genotype (Figure 3A and 3B). These findings thus provide further evidence for a brain regional-selective role of CB1-mediated eCB signaling in the regulation of ACh release, i.e., while ACh release is regulated by eCB signaling in hippocampus, striatal ACh release is not sensitive to modulation by eCB signaling. Moreover, the greater reduction in hippocampal ACh release in slices from wild type mice suggested changes in ACh release following CPO exposure may be modulated by eCB signaling. Thus, in hippocampus with viable CB1 receptors, extensive AChE inhibition may lead to ACh accumulation, stimulation of M1 receptors, enhanced release of eCBs, and finally activation of CB1 receptors to reduce net ACh release.
The use of 100 μM CPO to probe changes in ACh release in vitro was based on pilot studies that indicated high concentrations of CPO were needed to consistently reduce ACh release in mouse hippocampal slices (data not shown). This concentration of CPO is orders of magnitude higher than typically needed to inhibit AChE activity in tissue homogenates (Mortensen et al., 1998). It must be noted, however, that previous studies reported CPO was orders of magnitude less potent as a cholinesterase inhibitor when added to rat striatal slices in a suprafusion apparatus than when added to rat striatal tissue homogenates, using relatively similar conditions of preincubation time and temperature (Liu et al, 2002).
Chlorpyrifos significantly reduced hippocampal ACh release ex vivo in slices from both wild type and CB1 knockouts, but the extent of reduction was significantly greater in tissues from the wild types (Figure 4A). These findings were in general agreement with results obtained in hippocampal slices exposed in vitro to CPO (Figure 3A). In the striatum, a significant (yet lesser) reduction in ACh release ex vivo was observed in tissues from both wild type and CB1 knockouts as well (Figure 3B), with essentially no difference in the extent of response between the genotypes. Along with results from the in vitro studies, these findings suggest that eCB signaling via CB1 receptors modulates changes in ACh release following CPF exposure in a brain regional-manner.
As noted above, activation of postsynaptic muscarinic (M1) receptors and potentially other receptor types during OP anticholinesterase intoxication may trigger synthesis of eCBs in cholinergically innervated cells. OP anticholinesterase exposure in both wild type and CB1 knockout mice should therefore lead to enhanced eCB synthesis and release, regardless of the presence or absence of CB1 receptors. In the CNS, CB1 appears to be the main type of cannabinoid receptor involved in regulating neurotransmitter release (Herkenham et al, 1990; Matsuda et al, 1993; Tsou et al, 1998; Coutts et al, 2001). CB2 appears prominent in immune cells including microglia, but the presence/absence of CB2 receptors in neurons still remains unclear (De Filippis et al., 2009; Atwood and Mackie, 2010; Stella, 2010). Moreover, non-CB1/non-CB2 cannabinoid receptors have been postulated (Begg et al., 2005; Kreitzer and Stella, 2009; Stella, 2010). Some studies have also shown that eCBs interact directly with pre-synaptic voltage gated calcium channels, potentially regulating the release of neurotransmitters in a receptor-independent manner (Kofalvi et al., 2007; Nemeth et al., 2008). Thus, neurochemical changes via eCB signaling mediated through other (non-CB1) cannabinoid receptors or through receptor-independent mechanisms may occur following CPF exposure.
While CB1 deletion appeared to influence the effects of CPF on ACh release in the hippocampus, this neurochemical response was not associated with a difference in the expression of cholinergic toxicity between genotypes. The hippocampus has extensive cholinergic and cannabinergic signaling but is unlikely to play a major role in the expression of classical cholinergic signs of toxicity following anticholinesterase exposures. Other functional/neurobehavioral endpoints that rely more on hippocampal cholinergic signaling (e.g, water maze performance) may be differentially affected, however, in wild type and CB1 knockout mice exposed to CPF or other OP toxicants. Future studies should focus on cholinergic-cannabinergic interactions in hippocampal-dependent neurobehavioral functions.
The complexity of the nervous system can obscure understanding of selective signaling alterations following neurotoxicant exposures. Endocannabinoids are known to act as neuromodulators regulating the release of a variety of neurotransmitters including ACh, dopamine, glutamate, GABA and others in a brain-regional manner. Knowledge of the interactions between cholinergic and cannabinergic signaling pathways could be important in the etiology and treatment of a variety of neurological conditions.
Highlights.
C57Bl/6 mice showed dose-related cholinergic toxicity following subcutaneous chlorpyrifos exposure
Wild type and cannabinoid CB1 receptor knockout littermates responded similarly to the toxic effects of chlorpyrifos
Changes in depolarization-induced acetylcholine release following chlorpyrifos (ex vivo) or chlopyrifos oxon (in vitro) exposure appeared sensitive to modulation by CB1-mediated endocannabinoid signaling in a brain-regional manner
Acknowledgments
This research was supported by grant R01ES009119 from NIEHS and by the Oklahoma State University Board of Regents. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS.
Footnotes
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;157–158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, Piesla MJ, Zhang MY, Bingham B, Uveges A, Kowal D, Garbe D, Kouranova EV, Ring RH, Bates B, Pangalos MN, Kennedy JD, Whiteside GT, Samad TA. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- De Filippis D, Steardo A, D’Amico A, Scuderi C, Cipriano M, Esposito G, Iuvone T. Differential cannabinoid receptor expression during reactive gliosis: a possible implication for a nonpsychotropic neuroprotection. ScientificWorldJournal. 2009;9:229–235. doi: 10.1100/tsw.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Köfalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, Cunha RA, Nomikos GG. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol. 2006;70:1236–1245. doi: 10.1124/mol.106.024661. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Dolezal V, Tucek S. The effects of 4-aminopyridine and tetrodotoxin on the release of acetylcholine from rat striatal slices. Naunyn Schmiedebergs Arch Pharmacol. 1983;323:90–95. doi: 10.1007/BF00634254. [DOI] [PubMed] [Google Scholar]
- Eto M. Organophosphorus pesticides: organic and biological chemistry. CRC Press; Cleveland, Ohio: 1974. p. 387. [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Casu MA, Carta G, Mascia MS. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur J Pharmacol. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Tang Y, Gatley SJ, Volkow ND, Lan R, Makriyannis A. Effect of the cannabinoid receptor SPECT agent, AM 281, on hippocampal acetylcholine release from rat brain slices. Neurosci Lett. 1997;238:84–86. doi: 10.1016/s0304-3940(97)00851-3. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Volkow ND. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br J Pharmacol. 2000;131:645–650. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides industry sales and usage: 2006 and 2007 market estimates. Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention. U.S. Environmental Protection Agency; Washington, DC: 2011. [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol Sci. 2000;58:282–289. doi: 10.1093/toxsci/58.2.282. [DOI] [PubMed] [Google Scholar]
- Karanth S, Liu J, Mirajkar N, Pope C. Effects of acute chlorpyrifos exposure on in vivo acetylcholine accumulation in rat striatum. Toxicol Appl Pharmacol. 2006;216:150–156. doi: 10.1016/j.taap.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Zimmer A, Schlicker E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB(1) receptor-deficient mice. Br J Pharmacol. 2001;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube A. Pesticides Industry Sales and Usage, 2000 and 2001 Market Estimates. Office of Prevention, Pesticides, and Toxic Substances, U.S. Environmental Protection Agency; Washington, DC: 2004. [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi A, Pereira MF, Rebola N, Rodrigues RJ, Oliveira CR, Cunha RA. Anandamide and NADA bi-directionally modulate presynaptic Ca2+ levels and transmitter release in the hippocampus. Br J Pharmacol. 2007;151:551–563. doi: 10.1038/sj.bjp.0707252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer FR, Stella N. The therapeutic potential of novel cannabinoid receptors. Pharmacol Ther. 2009;122:83–96. doi: 10.1016/j.pharmthera.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pope CN. Effects of chlorpyrifos on high-affinity choline uptake and [3H]hemicholinium-3 binding in rat brain. Fundam Appl Toxicol. 1996;34:84–90. doi: 10.1006/faat.1996.0178. [DOI] [PubMed] [Google Scholar]
- Liu J, Pope CN. Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion. J Toxicol Environ Health A. 1998;53:531–544. doi: 10.1080/009841098159123. [DOI] [PubMed] [Google Scholar]
- Liu J, Chakraborti T, Pope C. In vitro effects of organophosphorus anticholinesterases on muscarinic receptor-mediated inhibition of acetylcholine release in rat striatum. Toxicol Appl Pharmacol. 2002;178:102–108. doi: 10.1006/taap.2001.9326. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Brimijoin S, Hooper MJ, Padilla S. Comparison of the in vitro sensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: what do tissue IC50 values represent? Toxicol Appl Pharmacol. 1998;148:46–49. doi: 10.1006/taap.1997.8287. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorophosphate. Neurotoxicology. 2008;29:1037–1043. doi: 10.1016/j.neuro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212-2. Toxicology. 2006;227:173–183. doi: 10.1016/j.tox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Németh B, Ledent C, Freund TF, Hájos N. CB1 receptor-dependent and -independent inhibition of excitatory postsynaptic currents in the hippocampus by WIN 55,212-2. Neuropharmacology. 2008;54:51–57. doi: 10.1016/j.neuropharm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pope CN, Liu J, Nallapaneni A, Wright LKM, Baireddy P, Parsons L. The modulation of neurotoxicity by endocannabinoids. Abstracts, 25th International Neurotoxicology Conference; Rochester, NY. October 12–16, 2008..2008. [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD. Long-term neurochemical and behavioral effects induced by acute chlorpyrifos treatment. Pharmacol Biochem Behav. 1992;42:251–256. doi: 10.1016/0091-3057(92)90523-i. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chaudhuri J, Chakraborti TK. Organophosphate-sensitive cholinergic receptors: possible role in modulation of anticholinesterase-induced toxicity. In: Balasubramanian AS, Doctor BP, Taylor P, Quinn DM, editors. Enzymes of the Cholinesterase Family. Plenum; New York: 1995. pp. 305–312. [Google Scholar]
- Pope C, Mechoulam R, Parsons L. Endocannabinoid signaling in neurotoxicity and neuroprotection. Neurotoxicology. 2010;31:562–571. doi: 10.1016/j.neuro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol Appl Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol Appl Pharmacol. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- Steffens M, Szabo B, Klar M, Rominger A, Zentner J, Feuerstein TJ. Modulation of electrically evoked acetylcholine release through cannabinoid CB1 receptors: evidence for an endocannabinoid tone in the human neocortex. Neuroscience. 2003;120:455–465. doi: 10.1016/s0306-4522(03)00318-x. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LK, Liu J, Nallapaneni A, Pope CN. Behavioral sequelae following acute diisopropylfluorophosphate intoxication in rats: comparative effects of atropine and cannabinomimetics. Neurotoxicol Teratol. 2010;32:329–335. doi: 10.1016/j.ntt.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu J, Pope CN. Age-related effects of chlorpyrifos on muscarinic receptor-mediated signaling in rat cortex. Arch Toxicol. 2002;75:676–684. doi: 10.1007/s00204-001-0309-3. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]