Abstract
Background
To determine the effectiveness of pharmacologic prophylaxis on preventing clinically relevant venothromboembolic (VTE) events and deaths after surgery. Surgical Care Improvement Project recommends that VTE pharmacologic prophylaxis be given within 24 hours of the operation. The bulk of evidence supporting this recommendation uses radiographic endpoints.
Study Design
The Surgical Care and Outcomes Assessment Program (SCOAP) is a Washington State quality improvement initiative with data linked to hospital admission/discharge and vital status records. We compared the rates of death, clinically relevant VTE and a composite adverse event (CAE) in the 90-days after elective, colon/rectal resections, based on the receipt of pharmacologic prophylaxis (within 24 hours of surgery) at 36 SCOAP hospitals (2005-2009).
Results
Of 4,195 (61.1±15.6 yrs; 54.1% women) patients, 56.5% received pharmacologic prophylaxis. 90-day death (2.5% vs. 1.6%, p-value=0.03), VTE (1.8% vs. 1.1%, p-value=0.04), and CAE (4.2% vs. 2.5%, p-value=0.002) were lower in those who received pharmacologic prophylaxis. After adjustment for patient and procedure characteristics, the odds were 36% lower for CAE (OR 0.64, 95% CI 0.44-0.93) with pharmacologic prophylaxis. In any given quarter, hospitals where patients more often received pharmacologic prophylaxis (highest tertile of use) had the lowest rates of CAE (2.3% vs. 3.6%, p=0.05) compared to hospitals in the lowest tertile.
Conclusions
Using clinical endpoints this study demonstrates the effectiveness of VTE pharmacologic prophylaxis in patients having elective colorectal surgery. Hospitals that used pharmacologic prophylaxis more often had the lowest rates of adverse events.
Keywords: Venous thromboembolism, venous thromboembolism pharmacologic prophylaxis, Surgical Care Improvement Project
INTRODUCTION
Venous thromboembolism (VTE) is the second most common postoperative complication1 and one of the most common preventable causes of in-hospital death.2, 3,4 To prevent VTE, the American College of Chest Physicians generates evidence-based guidelines every 4 years.3, 5 Current guidelines recommend that unless otherwise contraindicated, heparin-based pharmacologic prophylaxis be administered to prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) in patients undergoing major abdominal surgery. This recommendation, with the specification that VTE pharmacologic prophylaxis be given within 24 hours of the operation has been adopted by the Surgical Care Improvement Project (SCIP) initiative as a “pay for performance” initiative. The bulk of evidence supporting this recommendation uses an endpoint of VTE “events” determined by radiolabelled fibrinogen uptake or venography rather than clinically relevant VTE (symptomatic DVT and symptomatic or fatal PE). In part because of the use of radiographic endpoints and concerns regarding bleeding risk there has been skepticism about the wider use of VTE pharmacologic prophylaxis in patients having surgery.6-8 Skeptics note that as many as 66% of patients who get a VTE have received appropriate pharmacologic prophylaxis9 and in at least one center, despite increasing use of pharmacologic prophylaxis the rate of symptomatic VTE on the surgical service actually increased over a 10-year period.10 These concerns may explain why the rate of use of pharmacologic prophylaxis is highly variable despite the SCIP mandate.11-14
Given the relative paucity of VTE studies using clinical endpoints and unclear effectiveness of pharmacologic prophylaxis in community practice settings, we performed an observational, comparative effectiveness evaluation across most Washington State hospitals. The study is based in Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP), a prospectively-gathered clinical registry and quality improvement (QI) activity now implemented at nearly all statewide hospitals where surgery is performed (n= 55).15 The purpose of this study was to evaluate the relationship between the use of pharmacologic prophylaxis and clinical VTE in patients having elective colorectal surgery and to determine the relationship between increasing hospital use of pharmacologic prophylaxis and outcomes.
METHODS
Study design
This study was approved by the University of Washington Human Subject Review Committee and the Washington State Department of Health. A prospective cohort study was conducted using the SCOAP in-hospital clinical registry linked to hospital administrative discharge database, and the state’s vital records system. SCOAP draws data from the medical record by trained, audited abstractors using standardized definitions (http://www.scoap.org/documents/index.html). The Washington State comprehensive health abstract reporting system (CHARS) includes administrative information on all hospitalizations and patient identifiers that allows for tracking of subsequent hospitalizations. SCOAP index cases were linked to CHARS to identify patients who were re-hospitalized at any center after a SCOAP index admission and to the vital status registry to determine if they had died. The CHARS dataset also contains International Classification of Diseases, Ninth Revision (ICD-9) procedure and diagnosis codes. Records of inpatient hospitalization between 4th quarter of 2005 and 1st quarter of 2009 at 36 SCOAP hospitals (Appendix 1, online only) were used to assess outcomes for patients undergoing elective colon/rectal resections.
Variable Definitions
Patient risk factors
SCOAP records were used to obtain sociodemographic characteristics, clinical comorbidities, and operative details. We used the Deyo modification of the Charlson Comorbidity Index to calculate a weighted index of comorbid conditions for each patient.16 Scores range from 0 to 3+, where 0 indicates the absence of comorbid conditions and the score was truncated at 3 and above.
Duration of operation
Anethesia record and operating room (OR) log was used to identify the OR incision and end times. Duration was measured from incision to final wound closure.
Type/Method of operation
Operation type was specified as right hemicolectomy, left hemicolectomy, low anterior resection, abdominal perineal resection, total abdominal colectomy, colostomy takedown, and perineal proctectomy. Method of operation was specified as laparoscopic, open, laparoscopic converted to open, and laparoscopic/hand-assited.
Use of pharmacologic prophlaxis
VTE pharmacologic prophylaxis administration was obtained by directed chart review of all patients. SCIP criteria were used to define the use of pharmacologic prophylaxis, specifically chemical agents administered 24 hours before or after the operative start time in a patient not otherwise contraindiacted for use.17 Acceptable pharmacologic prophylaxis included unfractionated heparin, low molecular weight heparin (enoxaparin, dalteparin, tinzaparin), and synthetic factor Xa inhibitor (fondaparinux). Use of warfarin was not counted as acceptable as defined by the SCIP criteria.18 Use of agents that did not conform to SCIP criteria (sequential pneumatic compression devices) was also recorded.
Outcomes
Given recent evidence that the risk of operation-related VTEs does not return to baseline for 12 weeks,19 the primary outcome was 90-day death rate, new VTE diagnosis or VTE-related intervention, as well as the composite of these adverse events (CAE). Complication potentially related to the use of VTE pharmacologic prophylaxis (intra-operative or post-operative red blood cell transfusions) was also recorded. Readmissions for VTE were defined as any hospital admission within 90 days of discharge from the index hospitalization. At index and subsequent hospitalizations, VTE diagnosis or VTE-related interventions were defined as either a documented new use of anticoagulation therapy (at therapeutic dose) for presumed/confirmed DVT or PE (from SCOAP), and/or specific ICD-9 codes as previously described related to VTE diagnosis and/or treatment (Appendix 2, online only).20 The 90-day mortality was defined as all-cause death ≤90 days of procedure as ascertained from Washington State Vital Records.
Analysis
Patient level analysis
Patient characteristics were summarized using frequency distributions for categorical variables, and means and standard deviations for continuous variables stratified by receipt of perioperative VTE pharmacologic prophylaxis. 90-day mortality, VTE events and CAE were summarized using frequency distributions stratified by use of pharmacologic prophylaxis. Pearson chi-square statistics were used to compare characteristics and unadjusted event rates. Logistic regression models were created to evaluate the association between the receipt of pharmacologic prophylaxis and outcomes adjusting for patient, clinical, and operative characteristics identified as statistically significant (p<0.05) on univariate evaluation or found to be important in previous studies. For sensitivity analysis, we calculated the propensity score for receipt of VTE pharmacologic prophylaxis among all patients without regard to the outcome using the same variables. Patients were divided into quartiles of propensity scores and within each stratum the 90-day CAE rates were calculated based on receipt of perioperative VTE pharmacologic prophylaxis.
Hospital level analysis
We evaluated the use of VTE pharmacologic prophylaxis at each hospital in each calendar quarter using descriptive statistics and multivariate adjustments (adjusting for patient history of VTE and comorbid conditions within each hospital for that quarter). Hospitals were divided in tertiles according to the frequency of use of VTE pharmacologic prophylaxis and the rates of VTE were calculated for each calendar quarter based on the level of use (highest, mid, lowest tertile) of prophylaxis
Not all 36 hospitals began data entry at the same time and quality improvement interventions were occuring during this evaluation period and for the hosptal-level analysis each hospital’s calandar quarter was considered as a separate unit of analysis.
STATA was used for all analyses (Version 11, STATACorp, College Station, TX).
RESULTS
A total of 4,195 patients (mean age 61.1±15.6 yrs; 54.1% women) underwent elective colorectal resections. Patients who received perioperative VTE pharmacologic prophylaxis (n=2,369; 56.5%) and those who did not (n=1,826; 43.5%) were similar with respect to age, sex, smoking status, BMI, comorbidities such as hypertension and coronary artery disease, Charlson comorbidity indices, hospital factors such as length of stay and intraoperative duration, and indication for procedure (Table 1). Those who received perioperative VTE pharmacologic prophylaxis were more likely to have had previous history of DVT or PE (4.1% vs. 2.9%, p-value=0.04).
Table 1.
Patient and Clinical Characteristics Stratified by Receipt of Perioperative Venous Thromboembolism Pharmacologic Prophylaxis
| No perioperative VTE pharmacologic prophylaxis (n=1,826) |
Perioperative VTE pharmacologic prophylaxis (n=2,369) |
p Value | |
|---|---|---|---|
| Age, y, mean ± SD |
61.4 ± 15.7 | 60.9 ± 15.4 | 0.33 |
| Female sex, n (%) | 991 (54.3) | 1,277 (53.9) | 0.81 |
| Smoker, n (%) | 285 (15.8) | 391 (16.7) | 0.47 |
| BMI, n (%) <20 20-25 25-30 >30 |
147 (8.8) 506 (30.1) 590 (35.1) 437 (26.0) |
173 (7.8) 660 (29.8) 729 (32.9) 651 (29.4) |
0.1 |
| Hypertension, n (%) |
787 (43.2) | 1,033 (43.6) | 0.78 |
| Coronary artery disease, n (%) |
211 (11.6) | 258 (10.9) | 0.47 |
| Previous history of DVT/PE, n (%) |
52 (2.9%) | 96 (4.1%) | 0.04 |
| Length of stay, d, mean ± SD |
7.6 ± 6.8 | 7.8 ± 9.8 | 0.66 |
| OR time, min, mean ± SD |
156.0 ± 95.8 | 155.5 ± 92.1 | 0.85 |
| Charlson comorbididty index, n (%) 0 1 2 3+ Mean± SD |
733 (40.1) 163 (8.9) 381 (20.9) 549 (30.1) 0.33±0.65 |
977 (41.2) 225 (9.5) 548 (23.1) 619 (26.1) 0.40±0.70 |
0.07 |
| Indication for procedure, n (%) Malignancy IBD |
716 (39.2) 140 (7.7) |
916 (38.7) 208 (8.9) |
0.72 0.20 |
| Application of pneumatic compressions, n (%) |
1,641 (91.0) | 2,223 (94.4) | <0.001 |
VTE, venous thromboembolism.
The overall rates of 90-day death and VTE events were 2.0% and 1.4%, respectively. The unadjusted rates of 90-day death (2.5% vs. 1.6%, p-value=0.03), VTE events (1.8% vs. 1.1%, p-value=0.04) and CAE (4.2% vs. 2.5%, p-value=0.002) were lower among those who received VTE pharmacologic prophylaxis. The rate of intra- or post-operative transfusions were more common in those who did not receive the pharmacologic prophylaxis (7.0% prophylaxis vs.10.9% no prophylaxis, p-value<0.001). Only 31.7% of 90-day VTE events, 56.6% of deaths and 46.7% of CAE occurred during the initial in-patient hospital stay, while corresponding rates of 80%, 59.0% and 70.8% were identified at 30 days, respectively.
There was a 41% reduction in the unadjusted odds of 90-day CAE (OR 0.59, 95% CI 0.42-0.83) (Table 2). After adjustment for calendar year, history of VTE and other patient and procedure characteristics (such as duration of operation, and method and type of operation) the odds of 90-day CAE were 36% lower with VTE pharmacologic prophylaxis (OR 0.64, 95% CI 0.44-0.93) [adjusted odds of 30-day CAE (OR 0.54, 95% CI 0.35-0.83)]. 1,641 (39.5%) of patients had pneumatic compressions alone. When adjusting for the same covariates, there was no significant reduction in the odds of VTE based on the use of pneumatic compressions (OR 0.82, 95% CI 0.43-1.57). Sensitivity analysis using propensity quartile matching found that 90-day CAE rates were consistently lower if perioperative VTE pharmacologic prophylaxis was given. Adjusting for propensity score found a significant reduction in the odds of 90-day CAE when VTE pharmacologic prophylaxis was used (OR 0.61, 95% CI 0.43-0.89).
Table 2.
Univariate and multivariate regression analysis of 90-day venous thromboembolism events (VTE) and composite adverse events (CAE)
| Crude Odds Ratio (95% CI) 90-d CAE |
Adjusted Odds Ratio (95% CI)* 90-d CAE |
Crude Odds Ratio (95% CI) 90-d VTE |
Adjusted Odds Ratio (95% CI)* 90- d VTE |
|
|---|---|---|---|---|
| Perioperative VTE pharmacologic prophylaxis |
0.59 (0.42-0.83) | 0.64 (0.44-0.93) | 0.58 (0.35-0.98) | 0.62 (0.36-1.06) |
| Age | 1.04 (1.03-1.06) | 1.04 (1.02-1.06) | 1.01 (0.99-1.03) | 1.01 (0.99-1.03) |
| Sex | 0.97 (0.69-1.37) | 1.03 (0.72-1.48) | 0.96 (0.58-1.61) | 0.88 (0.4-1.96) |
| Smoking | 1.10 (0.70-1.73) | 1.41 (0.86-2.32) | 0.90 (0.44-1.84) | 0.89 (0.4-1.96) |
| Previous history of VTE | 1.73 (0.83-3.61) | 1.49 (0.71-3.13) | 2.53 (0.99-6.42) | 2.32 (0.88-6.09) |
| Charlson Comorbidity Index 1 2 3+ |
1.10 (0.55-2.23) 1.59 (0.99-2.52) 1.98 (1.31-3.01) |
0.92 (0.45-1.89) 1.19 (0.72-1.97) 1.38 (0.87-2.21) |
0.84 (0.29-2.45) 1.41 (0.73-2.71) 1.33 (0.71-2.49) |
0.85 (0.29-2.5) 1.65 (0.84-3.25) 1.36 (0.68-2.71) |
| Pneumatic compression | 0.79 (0.43-1.44) | 0.82 (0.43-1.57) | 0.57 (0.26-1.27) | 0.54 (0.24-1.2) |
| Method of operation (open)† | 1.96 (1.23-3.11) | 1.52 (0.94-2.48) | 1.87 (0.94-3.7) | 1.57 (0.77-3.21) |
| Type of operationa‡ Left hemicolectomy Low anterior resection Abdominal perineal resection Total abdominal colectomy Colostomy takedown Perineal proctectomy |
0.82 (0.5-1.34) 0.63 (0.41-0.97) 0.78 (0.35-1.74) 1.2 (0.63-2.29) 0.82 (0.11-6.09) 1.05 (0.14-7.94) |
0.81 (0.48-1.38) 0.83 (0.52-1.3) 0.82 (0.37-1.83) 2.45 (1.25-4.82) 1.34 (0.15-12.05) 1.31 (0.17-10.18) |
1.37 (0.65-2.91) 1.21 (0.62-2.35) 0.37 (0.05-2.8) 2.69 (1.14-6.36) 2.78 (0.36-21.62) - |
1.3 (0.63-2.69) 1.42 (0.72-2.83) 0.35 (0.05-2.65) 3.84 (1.63-9.07) 4.43 (0.48-41.27) - |
| Year of operation 2006 2007 2008 2009 |
0.44 (0.21-0.88) 0.54 (0.28-1.05) 0.34 (0.18-0.67) 0.19 (0.07-0.48) |
0.39 (0.19-0.81) 0.49 (0.25-0.96) 0.36 (0.18-0.71) 0.20 (0.075-0.53) |
0.73 (0.24-2.25) 0.61 (0.21-1.84) 0.55 (0.19-1.61) 0.17 (0.03-0.92) |
0.63 (0.19-2.10) 0.68 (0.22-2.14) 0.63 (0.21-1.89) 0.17 (0.03-1.03) |
Adjusted for all other variables listed.
Compared to laparoscopic procedures.
Compared to right hemicolectomies.
CAE, composite adverse event; VTE, venous thromboembolism.
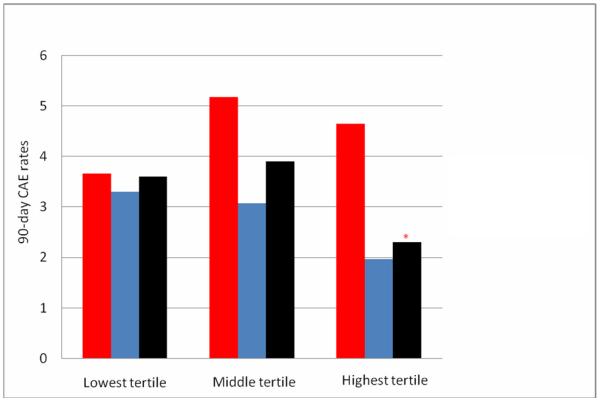
Over the course of the study period (Figure 1), the use of VTE pharmacologic prophylaxis increased (35.8% in Q0 to 70.4% in Q13), while overall rates of 90-day CAE decreased (4.3% in Q0 to 1.7% in Q13). Patients treated at hospitals in the top VTE pharmacologic prophylaxis “use tertiles” in a given quarter had a significantly lower 90-day CAE compared to patients at the lowest “use tertile” hospitals (2.3% vs. 3.6%, p=0.05) (Figure 2) and after adjustment for patient characteristics, the highest tertile use-hospitals (in a given quarter) had a 37% lower odds of CAE than lowest tertile use-hospitals (OR 0.63, 95% CI 0.40-0.99).
Figure 1.
Trend of (A) venous thromboembolism (VTE) pharmacologic prophylaxis use and (B) 90-d composite adverse events (CAE) over time.
Figure 2.
90-d composite adverse events (CAE) overall (black bars) and dependent upon receipt (blue bars) or non-receipt (red bars) of venous thromboembolism (VTE) pharmacologic prophylaxis shown by hospitals who use pharmacologic prophylaxis most frequently (highest tertile) to least frequently (lowest tertile). *p Value = 0.05 in comparing overall 90-d composite adverse event rates at the hospitals with highest VTE pharmacologic prophylaxis use practices vs lowest use tertile hospitals (2.3% vs 3.6%).
DISCUSSION
Our evaluation of statewide patients undergoing colorectal surgery with and without VTE pharmacologic prophylaxis found that VTE pharmacologic prophylaxis given within 24 hours of colorectal resection (SCIP criteria) was associated with significantly lower 90-day mortality and CAEs, despite the fact that patients receiving VTE pharmacologic prophylaxis were “higher risk” for VTE. VTE pharmacologic prophylaxis was not associated with rates of bleeding events and those hospitals where doctors used pharmacologic prophylaxis more often than others (highest tertile use) had the lowest rates of VTE or death. Almost all the prior studies used to support SCIP guidelines have used surrogate, radiographic endpoints. While studies looking only at fatal PEs7, 21 and meta-analysis pooling the results of many smaller studies have suggested a reduction in clinically relevant endpoints in the general surgery patient population, 8, 22 this is the first large-scale modern study evaluating the comparative effectiveness of SCIP VTE metrics using clinically relevant endpoints. Our finding, based on the clinical records of patients from nearly the entire State of Washington, across all types of hospital and communities reinforces the recommendation for adherence to existing VTE prevention guidelines.
Approximately 10 to 40% of inpatient general surgical patients have been found to have “radiographically determined” DVT.3 Autopsy studies have attributed 10% of all surgical hospital deaths to PE.23 Given its prevalence, a national campaign to encourage the use of pharmacologic prophylaxis has been led by the Centers for Medicare & Medicaid Services through the SCIP initiative. Furthermore, the National Quality Forum established a nationwide preventative performance measure standard for VTE,24 and Agency for Healthcare Research and Quality’s highest ranked safety practice was the “appropriate use of prophylaxis to prevent VTE.”25
Despite these efforts, use of VTE pharmacologic prophylaxis has been variable.26-28 In a cross-sectional study of the 11,613 surgical patients at risk, the 2008 ENDORSE study demonstrated that only 59% of surgical patients received the recommended pharmacologic prophylaxis.29 While self-reported rates of adherence to SCIP VTE prophylaxis criteria may be as high as 88% nationwide30, a recent study based on audits of actual performance demonstrated a 56% adherence.18 Physician resistance may be a component of the variable use since most of the supporting data for the recommendation have been based on radiographic endpoints and correlation to clinically relevant VTE reduction (outside of specific studies looking at fatal PEs and cancer patients7, 21, 31) has only been demonstrated in meta-analysis of underpowered studies.6, 8, 22 Unlike radiographic DVTs, there is less agreement about the effect of pharmacologic prophylaxis on clinically relevant outcomes.32, 33 The first study with a sufficient sample size to evaluate the clinical impact of VTE pharmacologic prophylaxis was the International Multicentre Trial (IMT). Clouded in controversy, the authors first reported in 197534 that patients receiving heparin had a 3-fold reduction in DVT and 8-fold decrease in fatal PE events. The results of the study were re-issued in 197735 because one of the sites36 withdrew its data suggesting that they observed the opposite effect. Almost all subsequent studies have been either underpowered or used radiographic endpoints. A meta-analysis of 46 randomized trials including more than 15,000 surgical patients demonstrated a greater than 60% reduction of DVT (diagnosed with radiolabelled fibrinogen), 40% reduction in PE and more than 60% reduction in fatal PE events.22 Mismetti et al., in their meta-analysis study, found an even greater reduction of 71-75% in the relative risk of symptomatic and clinical VTEs concordant with reduction in asymptomatic DVTs detected radiographically.8 However, outside of studies looking specifically at immediate postoperative fatal PEs, no study of appropriate size has reproduced the results from the IMT. One problem is that the incidence of PE is so rare that to evaluate an intervention that might reduce the risk by half (2% to 1%) might require randomization of 6,600 total patients. Secondly, identification of all clinically relevant DVTs and PEs is a challenge. Fatal PEs are usually found through autopsy, and other clinically symptomatic DVTs and PEs that do not lead to death are hard to identify. The risks also continue for some time19 making accurate numbers of VTE-related events difficult to obtain. Another challenge to determining the comparative effects of SCIP criteria for VTE prevention is because of concerns about bleeding8, 22, 35, 37 and/or ethical concerns about randomizing patients to non-guideline recommended care. Clinicians also remain skeptical about the disconnect between evidence-based process measures and outcome in real-world settings. For example, a recent study demonstrated a lack of association between a hospital’s use of SCIP process-of-care measures to prevent surgical infection and postoperative infection rates.38 On the contrary, our study demonstrates that greater use of one of the SCIP measures for VTE prophylaxis was associated with a reduction in clinically relevant endpoints. We not only found outcome improvements at the patient level, but also when considered at the hospital level, suggesting the value of QI interventions around this metric.
Our study is limited by several aspects of the data collection and VTE identification process. A recent study evaluating the risk of VTEs using the University HealthSystem Consortium (UHC) database found that the rates of VTE were lowest among patients not receiving the pharmacologic prophylaxis (0.0% to 0.9%).39 While there was an increase in VTE pharmacologic prophylaxis use from 2003/2004 to 2007/2008 (74% to 89%), there was increased number of VTEs (2.4% to 3.2%) in colorectal resections. The UHC study reported raw numbers of VTEs in the two different time periods and is only risk adjusted for patient’s severity of illness score. Other risk factors (such as previous history of VTEs, type and method of the operations, and temporal trends) associated with the use of VTE pharmacologic prophylaxis and VTEs were not adjusted for. More importantly, this study did not distinguish whether the anticoagulation medication was used for prophylaxis or therapy. Given that this study looked only at the postoperative period, they may have identified patients who had a diagnosis of VTEs and was started on VTE pharmacologic therapy. Our study was limited by a lack of information on duration of VTE pharmacologic prophylaxis, in and out of hospital. SCOAP has more recently included these metrics, but this was not available for this analysis. Clinicians within and between hospital may have variable approaches to evaluating patients at risk for VTE. Clinicians who had a lower threshold for diagnostic testing for VTE among symptomatic patients may also be more likely to adhere to SCIP VTE prevention measures. If so, this may have limited the finding that pharmacologic prophylaxis decreases the risk of VTE. Use of VTE pharmacologic prophylaxis by staff at a hospital can also be a marker for better use of other process measures that help reduce morbidity and mortality. We could not disentangle these from the effect of VTE pharmacologic prophylaxis. We used all-cause mortality-both alone and in combination with VTE-because most deaths that are directly caused by an acute PE occur before a timely diagnosis and treatment can be implemented.40 The use of “all cause” mortality may have included some patients who died of causes unrelated to VTE. For this reason, we performed analyses with and without death as an endpoint and found similar findings. Some of the limitations of this study arise from the use of administrative data (CHARS) to evaluate post-discharge outcomes by its design (retrospective), and the way health conditions and interventions are defined (using ICD-9 diagnostic and procedural codes). Because of this we did not have any information on how the diagnoses of VTEs were made. Also we could not separate out whether the transfusion was given intra-op or post-op. Given that the rate of transfusion was higher in the no prophylaxis group, it may well be instead that the intra-op bleeding led to lack of prophylaxis. Lastly, studies have demonstrated that patients discharged from the hospital have continual risk of VTEs.41-45 With recent studies demonstrating equivalent results of outpatient management of VTEs, fewer VTE-related hospitalizations may have occurred over time.46 The detection scheme used in this study would have missed VTEs that were diagnosed and treated in the outpatient setting, those occurring beyond 90 days, or diagnoses that were misclassified, but it seems unlikely that such misclassification would be associated with the receipt of VTE pharmacologic prophylaxis.
In conclusion, VTE pharmacologic prophylaxis was associated with significantly lower rates of 90-day mortality, clinical interventions for VTE, and composite adverse events. VTE pharmacologic prophylaxis was not associated with higher intra-op or post-op transfusion rates. This is the first time in recent decades that the impact of VTE pharmacologic prophylaxis on clinically relevant VTE endpoints in an appropriately sized cohort has been demonstrated. Our findings support the universal use pharmacologic prophylaxis in colorectal operations consistent with SCIP guidelines.
Acknowledgments
SCOAP is supported by a grant from Washington State’s Life Science Discovery Fund
Appendix 1
Hospitals involved in the data collection
| Allenmore | Overlake |
| Central Washington | PeaceHealth St John |
| Evergreen | Providence Everett |
| Good Samaritan | Sacred Heart |
| Grays Harbor | Samaritan Healthcare |
| Group Health Eastside | Skagit Valley |
| Harborview | Stevens Healthcare |
| Highline | Sunnyside |
| Holy Family | Swedish – First Hill |
| Island Hospital | Tacoma General |
| Jefferson General | United General |
| Kadlec | University of Washington |
| Kittitas Valley | Valley Medical Center |
| Legacy Good Sam (Portland) | Virginia Mason |
| Morton General | Wenatchee Valley |
| Mt Carmel | Whidbey General |
| Northwest | Yakima Regional & Heart Medical Center |
| Olympic | Yakima Valley |
Appendix 2
Specific ICD-9 codes related to venous thromboembolism diagnosis and/or treatment
| ICD-9 coding | Description |
|---|---|
| Pulmonary Embolism | |
| 415.1 | Pulmonary embolism and infarction |
| 38.7 | Interruption of the vena cava |
| Deep Venous Thrombosis | |
| 451.1 | Phlebitis and thrombophlebitis of deep vessels of lower extremities |
| 451.2 | Phlebitis and thrombophlebitis of lower extremities, unspecified |
| 451.8 | Phlebitis and thrombophlebitis of other sites |
| 451.9 | Phlebitis and thrombophlebitis of unspecified site |
| 453.2 | Other venous embolism and thrombosis of vena cava |
| 453.40-453.42 | Venous embolism and thrombosis of deep vessels of lower extremity |
| 453.8 | Other venous embolism and thrombosis of other specified veins |
| 453.9 | Other venous embolism and thrombosis of unspecified site |
| 997.2 | Peripheral vascular complications (phlebitis or thrombophlebitits during or resulting from procedure) |
| 999.2 | Other vascular complications (phlebitis/thrombophlebitits/thromboembolism following infusion, perfusion, or transfusion) |
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abstract presented at the American College of Surgeons 96th Annual Clinical Congress, Surgical Forum, Washington, DC, October 2010.
References
- 1.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. The Worcester DVT Study A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 3.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:S338–S400. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 4.Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001;43:1–668. [PMC free article] [PubMed] [Google Scholar]
- 5.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:S381–S453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 6.Prentice CR. Are symptomatic endpoints acceptable in venous thromboprophylactic studies? Haemostasis. 1998;28:S109–S112. doi: 10.1159/000022387. [DOI] [PubMed] [Google Scholar]
- 7.Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost. 2005;94:814–819. doi: 10.1160/TH02-10-0189. [DOI] [PubMed] [Google Scholar]
- 8.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 9.Arcelus JI, Monreal M, Caprini JA, et al. Clinical presentation and time-course of postoperative venous thromboembolism: Results from the RIETE Registry. Thromb Haemost. 2008;99:546–551. doi: 10.1160/TH07-10-0611. [DOI] [PubMed] [Google Scholar]
- 10.Shackford SR, Rogers FB, Terrien CM, Bouchard P, Ratliff J, Zubis R. A 10-year analysis of venous thromboembolism on the surgical service: the effect of practice guidelines for prophylaxis. Surgery. 2008;144:3–11. doi: 10.1016/j.surg.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm. 2007;64:69–76. doi: 10.2146/ajhp060115. [DOI] [PubMed] [Google Scholar]
- 12.Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5:1610–1616. doi: 10.1111/j.1538-7836.2007.02650.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–155. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132:936–945. doi: 10.1378/chest.06-2993. [DOI] [PubMed] [Google Scholar]
- 15.Flum DR, Fisher N, Thompson J, Marcus-Smith M, Florence M, Pellegrini CA. Washington State’s approach to variability in surgical processes/Outcomes: Surgical Clinical Outcomes Assessment Program (SCOAP) Surgery. 2005;138:821–828. doi: 10.1016/j.surg.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770. doi: 10.1007/s11606-007-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deitelzweig SB, Lin J, Hussein M, Battleman D. Are surgical patients at risk of venous thromboembolism currently meeting the Surgical Care Improvement Project performance measure for appropriate and timely prophylaxis? J Thromb Thrombolysis. 2010;30:55–66. doi: 10.1007/s11239-009-0393-4. [DOI] [PubMed] [Google Scholar]
- 19.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164:1541–1545. doi: 10.1001/archinte.164.14.1541. [DOI] [PubMed] [Google Scholar]
- 21.Pezzuoli G, Neri Serneri GG, Settembrini P, et al. STEP-Study Group Prophylaxis of fatal pulmonary embolism in general surgery using low-molecular weight heparin Cy 216: a multicentre, double-blind, randomized, controlled, clinical trial versus placebo (STEP) Int Surg. 1989;74:205–210. [PubMed] [Google Scholar]
- 22.Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 23.Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78:849–852. doi: 10.1002/bjs.1800780725. [DOI] [PubMed] [Google Scholar]
- 24.Surgical Care Improvement Project [Accessed October 15];A National Quality Partnership. 2010 Available at: http://www.qualitynet.org/dcs/ContentServer?c=MQParents&pagename=Medqic/Content/ParenShellTemplate&cid=1122904930422&parentName=Topic.
- 25.The Joint Commission [Accessed May 15];National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE) 2010 Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm.
- 26.Geerts W. Prevention of venous thromboembolism: a key patient safety priority. J Thromb Haemost. 2009;7:S1–S8. doi: 10.1111/j.1538-7836.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 27.Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475. doi: 10.1001/archinte.167.14.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services [Accessed December 03];Hospital Compare. 2010 Available at: http://www.hospitalcompare.hhs.gov.
- 31.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell JR. Can we really prevent postoperative pulmonary emboli? Br Med J. 1979;1:1523–1524. doi: 10.1136/bmj.1.6177.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terblanche J, Benatar SR, Immelman EJ. Prophylaxis against fatal postoperative pulmonary embolism. Surgery. 1982;91:534–536. [PubMed] [Google Scholar]
- 34.Prevention of fatal postoperative pulmonary embolism by low doses of heparin. An international multicentre trial. Lancet. 1975;2:45–51. [PubMed] [Google Scholar]
- 35.Kakkar VV, Corrigan TP, Fossard DP, Sutherland I, Thirwell J. Prevention of Fatal Postoperative pulmonary embolism by low doses of heparin. Reappraisal of results of international multicentre trial. Lancet. 1977;1:567–569. [PubMed] [Google Scholar]
- 36.Gruber UF, Duckert F, Fridrich R, Rem J, Torhorst J. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. Lancet. 1977;1:898. doi: 10.1016/s0140-6736(77)91213-2. [DOI] [PubMed] [Google Scholar]
- 37.van Ooijen B. Subcutaneous heparin and postoperative wound hematomas. A prospective, double-blind, randomized study. Arch Surg. 1986;121:937–940. doi: 10.1001/archsurg.1986.01400080083015. [DOI] [PubMed] [Google Scholar]
- 38.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 39.Qadan M, Polk HC, Jr, Hohmann SF, Fry DE. A reassessment of needs and practice patterns in pharmacologic prophylaxis of venous thromboembolism following elective major surgery. Ann Surg. 2011;253:215–220. doi: 10.1097/SLA.0b013e3181f6fe09. [DOI] [PubMed] [Google Scholar]
- 40.Riedel M. Acute pulmonary embolism 1: pathophysiology, clinical presentation, and diagnosis. Heart. 2001;85:229–240. doi: 10.1136/heart.85.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergqvist D, Benoni G, Bjorgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335:696–700. doi: 10.1056/NEJM199609053351002. [DOI] [PubMed] [Google Scholar]
- 42.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 43.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge. An underestimated risk. Arch Surg. 1992;127:310–313. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 44.Scurr JH. How long after surgery does the risk of thromboembolism persist? Acta Chir Scand Suppl. 1990;556:22–24. [PubMed] [Google Scholar]
- 45.Planes A, Vochelle N, Darmon JY, Fagola M, Bellaud M, Huet Y. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet. 1996;348:224–228. doi: 10.1016/s0140-6736(96)01453-5. [DOI] [PubMed] [Google Scholar]
- 46.Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378:41–48. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]