Abstract
Aryl hydrocarbon receptor (AHR) ligands, including 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD), accelerate reproductive senescence and one proposed target is the early embryo. To discriminate between direct effects on the oocyte and early embryo and those mediated by complex ovarian interactions with TCDD, IVF was carried out in the presence of TCDD (10, 100 nM) and the aryl hydrocarbon antagonist CH-223191 (1 μM) combined factorially. TCDD-induced Cyp1a1 mRNA expression was absent in 2-cell embryos; however morulae exhibit dose-dependent Cyp1a1 expression. TCDD induced accumulation of sperm in the perivitelline space and displacement of blastomere nuclei. At 100 nM TCDD, aberrations in cytokinesis and nuclear positioning were observed 2-cell embryos and morula and these effects were reversed in the presence of CH-223191. Our data suggest that acute exposure to TCDD has direct effects on early development in the rat that permit discrimination of AHR- mediated and AHR-independent mechanisms through which environmental toxicants impair mammalian reproduction.
Keywords: TCDD, IVF, early development, preimplantation embryo, toxicology
Introduction
The aryl hydrocarbon receptor (AHR) pathway is an evolutionarily conserved, widely expressed orphan receptor pathway activated by many environmental toxicants [1, 2]. AHR mediates effects of environmental toxicants on fertility and may also serve as yet undefined role(s) in normal reproduction [3, 4, 5, 6]. Activation of the AHR by small coplanar molecules and subsequent signal transduction through the aryl hydrocarbon receptor nuclear translocator (ARNT) protein, heat shock protein 90, and xenobiotic-response elements (XREs) leads to changes in gene transcription in many tissues [7]. The most studied of these response genes include Cyp1a1, Cyp1a2 and Cyp1b1 loci that encode the xenobiotic-metabolizing monooxygenases central to the adaptive metabolic response [8]. Both AHR and Cyp1a1 mRNA are strongly expressed in mouse pre-implantation embryos. Interestingly, it was shown that immediately following fertilization, there is an increase in constitutive CYP1a1 mRNA levels in mouse embryos [9]. AHR ligands (such as dioxins and polychlorinated biphenyls) induce a spectrum of maldevelopmental and toxic responses [10]. It was shown that, in rodents, both acute and chronic exposure to the prototypic AHR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) accelerates reproductive senescence through ovarian targets [11, 12, 13]. To discriminate between direct effects on the oocyte versus those mediated by complex ovarian interactions with TCDD, we developed a rat IVF model to examine AHR related functions soon after fertilization and early cleavage. Our past in vivo studies showed that maternal exposure to TCDD disrupts compaction-stage embryonic development [13]. Most previous studies examining maternal dioxin exposure and subsequent fetal health have focused on late gestation, while peri-conceptional and pre-implantation stages of development remain largely unexplored. TCDD affects early embryogenesis during pre-implantation development, but it is still not clear if TCDD induces these reproductive effects during this period in a direct manner [14]. The goal of this study was to evaluate the direct effects of TCDD exposure during fertilization and early embryonic development on embryo quality in vitro.
2. Materials and Methods
2.1. In vitro fertilization
2.1.1. Animals
Female Sprague-Dawley rats (age, 28 days) were purchased from Charles River Laboratories (St. Louis, MO, USA) and housed under a 12L:12D photoperiod (0600-1800 h) and controlled temperature (23 ± 2°C) and humidity. Food (Purina Rodent Chow; Ralston Purina Co., St Louis, MO) and water were provided ad libitum. Exposure to AHR ligands was limited in these studies to the fertilization period to evaluate fertilization, first cleavage competence, and early embryo morphology. Accordingly, in vitro fertilization (IVF) was carried out in the presence of TCDD (0, 10, 100 nM [15,16]) and the aryl hydrocarbon antagonist CH-223191 (0, 1 μM [17]) combined factorially. TCDD (CAS 1746-01-6; MW: 391.9; purity > 99%) was obtained from Cambridge Isotope Laboratories, Inc (Lenexa, KS, USA) and CH-223191 was obtained from Calbiochem, Inc (La Jolla, CA, USA). CH-223191 has been demonstrated to specifically antagonize AhR-mediated actions of TCDD in vitro [17]. The following study was approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
2.1.2. Preparation of sperm suspension and capacitation
All IVF procedures were performed as described previously [18]. In our study we used a chemically defined medium for 1-cell rat embryos (mR1ECM) composed of 110 mM NaCl, 3.2 mM KCl, 0.5 mM MgCl2·6H2O, 2.0 mM CaCl2·2H2O, 25.0 mM NaHCO3, 7.5 mM d-glucose, 0.5 mM sodium pyruvate, 13.3 mM sodium lactate, 0.1 mM glutamine, 2% minimal essential medium (MEM) essential amino acid solution (Gibco BRL Grand Island, NY, USA), 1% MEM nonessential amino acid solution (Gibco BRL), 335 mOsm NaCl, 4 mg/ml bovine serum albumin (BSA). Based on previous results [19] and to assure successful fertilization and high rate of development, our mR1ECM medium was supplemented with NaCl (110 nM). Drops of fertilization and culture media (400 μl each) were covered with mineral oil (M-8410; Sigma, MO, USA) in polystyrene culture dishes (60×15 mm; Falcon 353004; Becton Dickinson, NJ, USA) and equilibrated overnight at 37°C in 5% CO2. Sperm obtained from adult Sprague-Dawley males as described previously [18] were diluted (1×106 spermatozoa/ml) in pre-equilibrated IVF medium (mRECM1) with or without CH-223191 at 37°C, in 5% CO2, 5% O2 for capacitation to occur.
2.1.3. Collection of cumulus-oocyte complexes, in vitro fertilization and assessment of sperm penetration
After 5 h of sperm capacitation, in vivo matured cumulus oocyte complexes were collected from oviducts of 31-day old female Sprague-Dawley rats (n=10 donors following superovulation with eCG and hCG [16]. IVF (n=6-10 COCs/treatment group) was undertaken in the presence of the aryl hydrocarbon agonist (TCDD; 0, 10, 100 nM) and antagonist (CH-223191; 0, 1 μM) and incubated at 37°C, in 5% CO2, 5% O2. The dose of aryl hydrocarbon receptor antagonist was chosen based on the studies of Kim et al. [20]. CH-223191 shows high specificity for AHR and in contrast to other known AHR antagonists has no agonist effect on AhR [20]. After 8 h of co-culture with sperm, oocytes were collected, washed, and examined for fertilization under a phase-contrast or differential interference contrast (DIC) optics (Zeiss Axiovert II, 40X, USA). Fertilization rates were calculated as the number of penetrated oocytes (two pronuclei, a sperm tail in the vitellus and emission of the second polar body) divided by the total number of oocytes. First cleavage rates were calculated from the number of 2-cell embryos divided by the number of fertilized embryos for each treatment group. Fertilized oocytes were washed and transferred to media containing appropriate treatments in addition to mRECM1, 300 mOsm NaCl, 4 mg/ml BSA. Cells were cultured for additional 14 hours at 37°C, in 5% CO2, 5% O2.
2.1.4. Embryo culture
After 14 h, 2-cell embryos (n=6/group) were transferred to mRECM1 rat ≥ 2-cell media. Embryos from each treatment group were either frozen for analysis of Cyp1a1 gene expression, or fixed for fluorescence microscopy analysis of DNA and f-actin. 96 h after insemination, multi-cellular embryos were transferred to mRECM1 rat ≥ 2-cell media supplemented with 10% FBS. Additional samples were frozen for gene expression analysis or fluorescence microscopy of embryos at 96 h and 120 h after insemination. As controls for IVF, we performed natural mating experiments to obtain embryos processed as described previously [13, 21, 22]. Female Sprague-Dawley rats (21 days, n= 5 per group) were purchased and housed as described in section 2.1.1. Proven males were introduced on evening of proestrus following superovulation with pregnant mare serum gonadotropin (PMSG) and hCG [13, 16]. Mating was confirmed by the presence of sperm on vaginal cytology the following morning. Pre-implantation embryos were collected in EmbryoMax® FHM Hepes Buffered Medium (Chemicon, CA, USA) pre-warmed to 37°C by flushing oviducts and uteri on 1 day after mating. Embryos were pooled and cultured as in experiment 1 (10-20 embryos per treatment group) in medium supplemented with TCDD (0, 100 nM) and aryl hydrocarbon antagonist CH-223191 (0, 1 μM) combined factorially for additional 72 hours. Exposure to AHR ligands was limited in these studies to the culture period to evaluate first cleavage competence, and embryo morphology. Embryos were counted at the 2-cell stages and fixed for fluorescence analysis at the 8-16 cell stages.
2.1.5. Fluorescence Microscopy
Pre-implantation embryos from both IVF and natural mating experiments were processed for chromatin (DNA, Hoechst 33258, Invitrogen, USA) and f-actin (Alexa 568 phalloidin, Invitrogen, USA) staining and analyzed by fluorescence microscopy as previously described [ 13]. Embryos were fixed for 30 min in 4% PFA at 37°C and stored at 4°C in wash solution comprising PBS supplemented with 2% BSA, 2% skim milk powder, 2% normal goat serum, 100 mM glycine, 0.01% Triton-X-100 and 0.2% sodium azide until processing for microscopy. Pre-implantation embryos were extracted for 30 min at room temperature in 0.1% Triton-X-100 and incubated overnight at 4°C in wash solution. DNA was stained with Hoechst 33258 (1 μg/ml in wash solution) for 30 min and cytoskeletal integrity was analyzed by staining f-actin with rhodamine-labeled phalloidin (1unit/ml in wash solution; Molecular Probes, Invitrogen, USA) for 30 min. Pre-implantation embryos were mounted under cover slips without compression in medium containing 50% glycerol and 25 mg/ml sodium azide. Embryos were analyzed by whole mount fluorescence microscopy (Zeiss Instruments, Axiovert II, 40X oil immersion, N.A.0.95).
2.1.6. Real-time PCR
Quantitative real-time PCR was used to assess changes in Cyp1a1 gene expression in 2-cell and morula stage in vitro fertilized embryos as a marker of toxicant action. The induction of Cyp1a1 mRNA has long been used as an indicator of AHR activation in response to both endogenous and exogenous ligands [10, 22]. RNA was isolated from embryos using the RNAqueous-Micro kit (Ambion, TX, USA). A single round of linear amplification was performed using the MessageAmp II aRNA kit (Ambion, TX, USA). Amplified embryo RNA and universal reference RNA (Stratagene, CA, USA) were reverse transcribed to cDNA using Arrayscript Reverse Transcriptase (Ambion, TX, USA) and assayed in duplicate for β-actin (housekeeping gene) and Cyp1a1 gene expression using specific primer and probe sets and TaqMan chemistry [22-24]. Real-time PCR was performed in a 25 μl final reaction volume using TaqMan Gene Expression Assay and TaqMan® 2×Universal PCR Master Mix (Applied Biosystems, USA). The final concentration of each real-time PCR reaction component was: 1×TaqMan Universal PCR Master Mix, 250 nM for the probe, and 900 nM for each primer. Real-time PCR was carried out in the 7300 real-time PCR System (Applied Biosystems, USA). The non-template control (NTC) samples were included in each run. Additionally to exclude the amplification of contaminating genomic DNA, reactions without reverse transcriptase were performed. Assays were pre-validated by Applied Biosystems and used FAM as a reporter dye. Ct values were calculated for each endpoint and corrected for β-actin gene expression. Relative gene expression was calculated using a universal rat cDNA standard curve.
2.2. Statistical analysis
Data were tested to confirm normality and homogeneity of variances by the Kolmogorov-Smirnov normality test and Bartlett’s test respectively. Data were then analyzed by ANOVA followed by Fisher’s test as a post hoc test for comparison of means. The nonparametric Mann-Whitney U test was used to identify difference among treatment groups for endpoints that did not meet the above criteria. P-values of less than 0.05 were considered significant.
3. Results
3.1. Fertilization rate and 1st cleavage
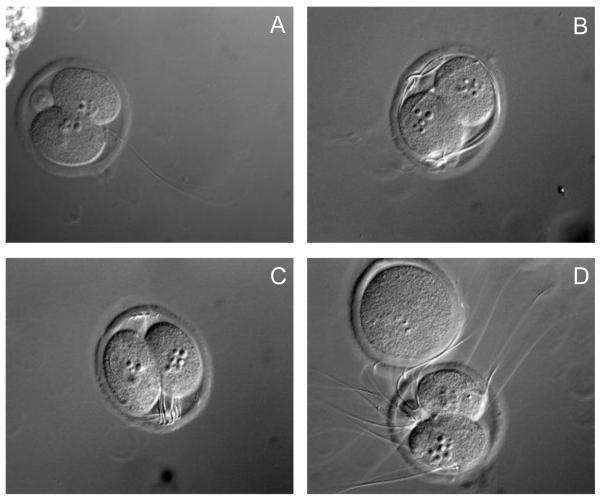
Both fertilization rate and 1st cleavage rate did not differ among IVF treatment groups (Table 1). Similarly, in embryos derived from natural mating first cleavage rate and general morphology were not affected by TCDD treatment (Table 2). Additionally, percentage of 2-cell embryos from control group fertilized in vitro (86%) is comparable with embryos fertilized in vivo (Table 1, 2). Thus, at the level of fertilization efficiency and ability to progress through the first embryonic cell cycle, TCDD had no effect. In contrast, analysis of zygotes by DIC optics (Figure 1), revealed two striking differences between TCDD exposed and control groups. Control oocytes had one sperm in the perivitelline space, while those fertilized in the presence of TCDD had multiple sperm. In addition, 2 cell embryos produced in the presence of TCDD exhibited cortically displaced nuclei in comparison to control embryo where nuclei are aligned at the first cleavage furrow. (Figure 1A-C).
Table 1.
The effect of TCDD on fertilization and 1st cleavage rates following IVF.
| % of oocytes examined |
||
|---|---|---|
| Treatment | Penetrateda | 2-cell stageb |
| Control | 65.9 ± 5.4 | 86.0 ± 5.2 |
| 1 μM CH-223191 | 55.9 ± 17.7 | 100.0 ± 9.4 |
| 10 nM TCDD | 59.3 ± 7.9 | 83.3 ± 0.0 |
| 10 nM TCDD, 1 μM CH-223191 | 56.0 ± 8.5 | 99.3 ± 14.8 |
| 100 nM TCDD | 65.1 ± 10.4 | 78.1 ± 11.2 |
| 100 nM TCDD, 1 μM CH-223191 | 57.1 ± 11.8 | 80.2 ± 11.2 |
Calculated as the number of oocytes with 2 pronuclei and sperm tail in the vitellus divided by the total number of oocytes observed per group (n=40-60/group).
Percentage is the portion that developed to the 2-cell stage from those that were considered fertilized after 22 h of culture.
Table 2.
The effect of TCDD on rate development of embryos derived from natural mating.
| Rate development |
||
|---|---|---|
| Treatment | 2-cell-stage | Normal morulaa |
| Control | 86.9 ± 2.7a,b | 76.2 ± 23.8 |
| 1 μM CH-223191 | 97.7 ± 2.3a | 90.0 ± 10.0 |
| 100 nM TCDD, 1 μM CH-223191 | 95.2 ± 2.8a | 83.6 ± 2.1 |
| 100 nM TCDD | 78.4 ± 9.6b | 67.8 ± 24.4 |
Percentage is calculated from fertilized 2-cell embryos (n=40-60/group). Abnormal morulae were fertilized, but had improper cell division or were fragmented. Values with different letters within the same column are significantly different (P< 0.05).
Figure 1.
Differential interference contrast images of representative 2-cell IVF embryos from control (A) or TCDD (B,C,D) treatment groups. N= 40-60 embryos per group from three replicate experiments; magnification=560X.
These results indicate that TCDD exposure permits monospermic penetration of the oocyte during IVF but impairs the block to polyspermy at the level of the zona pellucida. Moreover, while TCDD does not impede oocyte fertilization and progression of the first cell cycle, the failure to maintain nuclear alignment in two cell embryos is likely a consequence of cytoskeletal perturbation.
3.2. IVF derived 2-cell embryos and morulae
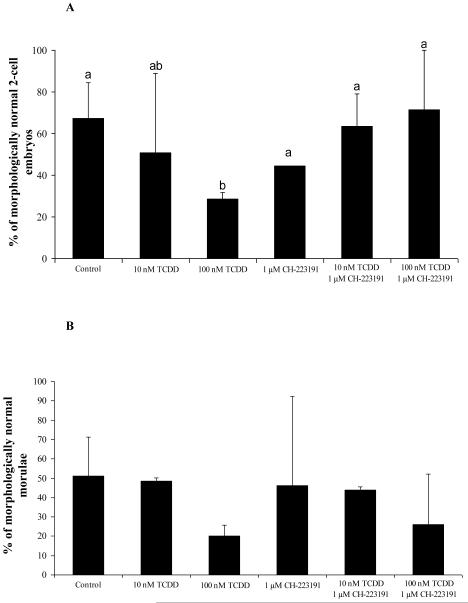
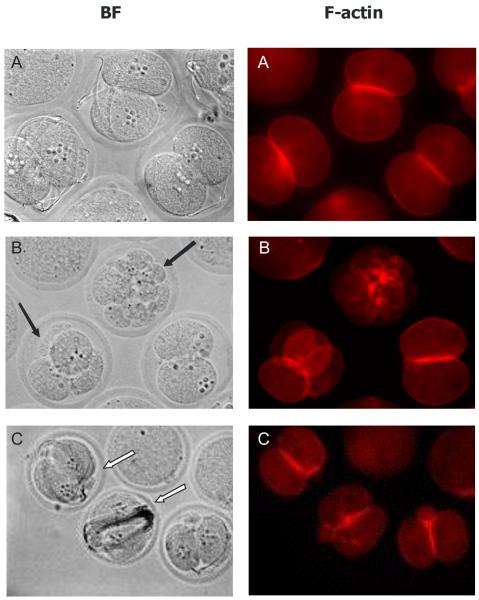
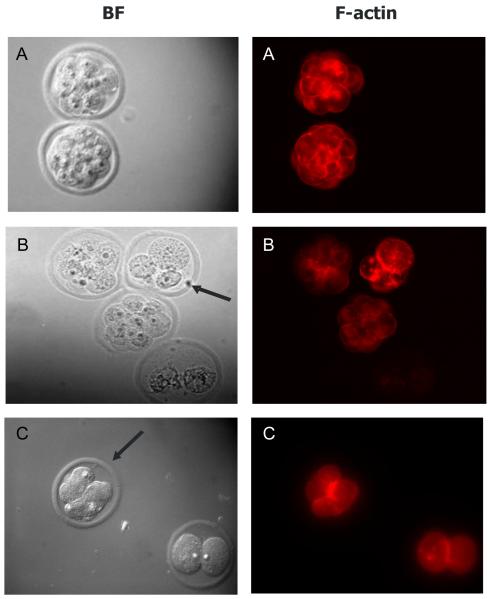
The percentage of morphologically normal (blastomere nuclei, second polar body, and 1-3 sperm visible) 2-cell embryos was calculated for each treatment group after 14 hours of culture. Percentage of normal morulae was calculated from fertilized 2-cell embryos. Embryos showing signs of blastomere fragmentation, asymmetric cytokinesis, or displaced nuclei were scored as abnormal. In control IVF 2-cell embryos, 67% appeared morphologically normal (Figure 2A, 3). While treatment with 10 nM TCDD did not affect the percentage of morphologically normal 2-cell embryos, 100 nM TCDD decreased (P<0.05) the number of normal 2-cell embryos. For IVF derived morulae, morphology and progression were similar between TCDD-exposed and non-exposed groups (Figure 2B, 4). However, while there were no significant differences in morphology of IVF derived morulae between groups, unequally-sized blastomeres, both nucleated and anucleated, and diminished phalloidin staining at sites of cell contact were observed after TCDD exposure. Interestingly, while the AHR antagonist, CH-223191, prevented these disturbances in 2-cell embryos, morula derived from IVF in the presence of 100 nM TCDD and CH-223191 were similar to morula exposed to TCDD alone (Figure 2). These results suggest that sensitivity to TCDD may increase during the course of early development and prompted examination of markers of AHR activation at the 2-cell and morula stages.
Figure 2.
The effect of TCDD on fertilization and first cell cycle in IVF derived 2-cell embryos (A) and morulae (B). Bars with different letters denote significant differences among treatment groups (P< 0.05)
Figure 3.
Effect of TCDD on 2-cell IVF embryos: A-control; B-10 nM TCDD; C-100 nM TCDD. BF, bright field optics; F-actin, Alexa568 phalloidin. N=40-60 embryos from three replicates, magnification = 560X.
Figure 4.
Effect of TCDD on IVF-derived morulae: A-Control; B-10 nM TCDD; C-100 nM TCDD. Arrows point at examples of fragmentation or abnormal cell division. DNA was stained with Hoechst 33258 (images not shown) and f-actin was labelled with Alexa 568labeled phalloidin. BF, bright field optics; N=40-60 embryos from three replicates, magnification =560X.
3.3. Aryl hydrocarbon receptor activation
Cyp1a1 gene expression was measured in 2-cell and morula stage embryos as a biomarker indicating activation of AHR in response to TCDD. TCDD-induced Cyp1a1 mRNA expression was minimal in 2-cell embryos (data not shown). However, morulae stage embryos exhibited dose-dependent expression of Cyp1a1 that was blocked when CH-223191 was included during IVF and early cleavage stages of development (Figure 3). These findings are consistent with a requirement for zygotic gene activation before TCDD is able to elicit AHR mediated transcriptional activation [24,25]. Whether this delayed developmental response is related to the availability of maternal AHR or requires zygotic expression remains unknown [23].
4. Discussion
In the present study, TCDD exposure during fertilization resulted in multiple sperm in the perivitelline space despite monospermic penetration, indicating a failure to establish the zona block to polyspermy. Notably, second polar body extrusion and pronuclear development seemed to occur on schedule in the presence of TCDD. However, TCDD treated 2-cell embryos did have nuclear displacement from a central to peripheral location suggesting that cytoskeletal remodeling during the first cell cycle was perturbed by the dioxin. Interestingly, no significant differences were found among treatment groups when embryos were monitored for gross defects at the morula stage. While more subtle effects were observed in the morulae, it seems likely that direct effects of TCDD on the oocyte during fertilization are manifest only until the time of compaction, as seen in our previous studies [13,22].
Our results are consistent with past studies in the mouse in which TCDD concentrations below 10 nM had no effect on embryo viability or development [14]. Similarly, Blankenship et al. reported that TCDD accelerated differentiation of the blastocyst but did not affect the embryo viability and cell number [26]. Our previous in vivo study has documented that both chronic and acute maternal exposure to TCDD induces nuclear and cytoskeletal defects in pre-implantation embryo morphogenesis without impairing embryo survival. Again in this study, TCDD associated abnormalities at the 8-16 cell stage were not observed at blastocyst stage [13] implying that the compaction-related crisis was resolved as the embryo advanced to implantation. Mammalian oocytes and pre-implantation embryos are direct targets for TCDD, given that AHR is expressed in these cells [14, 22]. To confirm AHR involvement in TCDD action in the oocyte we used CH-223191, which was previously shown to act as an aryl hydrocarbon antagonist [20]. We found that CH-223191 prevented TCDD-induced increase of abnormal 2-cells embryos.
We used Cyp1a1 gene expression as a biomarker for dioxin action [10, 22-24]. In the rat IVF experiment Cyp1A1 mRNA expression was not detectable in 2-cell embryos. This is consistent with past studies using mouse embryos in which 10 nM TCDD did not result in Cyp1A1 expression in 1-, 2- , or 8-cell embryos, and was not detectable until embryos reached the blastocyst stage [14]. This result is also consistent with the fact that most maternal mRNAs undergo degradation during meiotic maturation or fertilization. In our study on IVF produced embryos, TCDD-induce Cyp1a1 gene expression occurs at the morula stage, and appears to be dose dependent. This delay in expression is most likely due to the transition from maternal to embryonic control of gene expression, which occurs late in the 2-cell stage in the rat [27, 28-29]. Interestingly, AHR and ARNT gene expression in mouse embryos is detectable in 1-cell embryos, decreases at the 2- to 8-cell stage, and increases again at the blastocyst stage, even when exposed to TCDD [14]. Again this is most likely due the maternal zygotic transition [28]. On first consideration, the lack of Cyp1a1 expression in 2 cell embryos in combination with significant effects on morphology suggest AHR-independent actions of the dioxin. However, the AHR antagonist CH-223191 did reverse the effects of TCDD on morphology consistent with a role for the AHR during TCDD effects at the 2 cell stage. This may indicate that non-genomic actions of the AHR are at work. Of course, the clear upregulation of Cyp1a1 at the morula stages indicates that the AHR is functional in these embryos, but this genomic functionality may be stage dependent. Additionally, others have shown that TCDD may affect regulation of gene expression and alter the genomic DNA methylation status of imprinted genes in preimplantation mouse embryos without altering gross morphology [14].
IVF has been largely unsuccessful using the Sprague-Dawley rats in the past [27] and a major goal of this work was to optimize a system that would permit toxicological assessments at early stages of embryonic development including fertilization in this strain. Jiang and Tsang published an IVF protocol for oocytes of the Sprague-Dawley rat [30]. They used an IVF medium based on IVF-20 supplemented with 30 mM NaCl. In the present study we chose mR1ECM supplemented with 110 mM NaCl following initial optimization studies. The rate of control-treated oocyte penetration and development in the current study was 65%. This is considerably higher than what Jiang and Tsang observed (15%) using similar media, but comparable to what they observed (75%) in IVF-20 supplemented with 30 mM NaCl. They also suggest that IVF-20 medium may include some components for successful fertilization in Sprague-Dawley rats. Unfortunately, the identity of these components remains unclear. In turn, our results are consistent with study of Oh et al. [18] who also showed that successful sperm penetration was achieved in mR1ECM supplemented with high NaCl concentration. Our successful results provide well-defined IVF model for Sprague-Dawley rats.
In conclusion, TCDD alters sperm penetration and early embryonic organization in the rat through the AHR pathway. Our findings reinforce the idea that perturbations in chromatin and cytoskeletal remodeling at early stages of development have a long-lasting impact on embryo quality that could underlie problems in offspring health without directly compromising embryonic progression. This work was made possible by an optimized rat IVF model, which will aid in identifying specific targets of TCDD in the pre-embryo that link cell cycle control with cytoskeletal and nuclear remodeling.
*Research Highlights.
Aryl hydrocarbon receptor (AHR) ligands, including 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD), accelerate reproductive senescence and one proposed target is the early embryo.
Polyspermy, aberrations in cytokinesis and nuclear positioning were observed embryos and following rat IVF.
Acute exposure to TCDD has direct effects on early development in the rat that permit discrimination of AHR-mediated and AHR-independent mechanisms through which environmental toxicants impair mammalian reproduction.
Footnotes
This study was supported by the United States National Institutes of Health (NIH/NIEHS ES012916, BKP), the NIH Center for Reproductive Sciences, University of Kansas Medical Center, the Hall Family Foundation (DFA), the ESHE Fund (DFA) and the University of Virginia NIH Center for Research in Reproduction Ligand Analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
REFERENCES
- [1].Birnbaum LS. Developmental effects of dioxin. Environ Health Perspect. 1995;103:89–94. doi: 10.1289/ehp.95103s789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hahn ME. Aryl hydrocarbons receptors: diversity and evolutions. Chem Biol Interact. 2002;141:131–60. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- [3].Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70(4):613–22. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- [4].Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56(2):382–88. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- [5].La Provost F, Riedlinger G, Yim SH, Benedict J, Gonzales FJ, Flaws J, Henninghausen L. The aryl hydrocarbon receptor (AhR) and its nuclear translocator (Arnt) dispensable for normal mammary gland development but are required for fertility. Genesis. 2002;32:231–39. doi: 10.1002/gene.10037. [DOI] [PubMed] [Google Scholar]
- [6].Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77(4):547–59. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pocar P, Fisher B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129(4):379–89. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- [8].Schrenk D. Impact of dioxin-type induction of drug metabolizing enzymes on the metabolism of endo - and xenobiotics. Biochem Pharmacol. 1998;55(8):1155–62. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- [9].Dey A, Nebert DW. Markedly increased constitutive CYP1A1 mRNA levels in the fertilized ovum of the mouse. Biochem Biophys Res Commun. 1998;251:657–61. doi: 10.1006/bbrc.1998.9519. [DOI] [PubMed] [Google Scholar]
- [10].Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619(3):263–68. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- [11].Franczak A, Nynca A, Valdez KE, Mizingo KM, Petroff BK. Effect of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescense in female Sprague-Dawley rats. Biol Reprod. 2006;74:125–30. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- [12].Shi Z, Valdez KE, Ting AY, Franczak A, Gum S, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol Reprod. 2007;76:198–02. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- [13].Hutt KJ, Shi Z, Albertini DF, Petroff BK. The environmental toxicant 2,3,7,8-teterachlorodibenzo-p-dioxin disrupts morphogenesis of the rat pre-implantation embryo. BMC Dev Biol. 2008;8:1–12. doi: 10.1186/1471-213X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu Q, Ohsako S, Baba T, Miyamoto K, Tohyama C. Effects of 2,3,7,8-teterachlorodibnenzo-p-dioxin (TCDD) on preimplantation mouse embryos. Toxicology. 2002;174:119–29. doi: 10.1016/s0300-483x(02)00047-1. [DOI] [PubMed] [Google Scholar]
- [15].Moran FM, Lohstroh P, VandeVoort CA, Chen J, Overstreet JW, Conley AJ, Lasley BL. Exogenous steroid substrate modifies the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estradiol production of human luteinized granulosa cells in vitro. Biol Reprod. 2003;58:244–51. doi: 10.1095/biolreprod.102.007161. [DOI] [PubMed] [Google Scholar]
- [15].Jiang JY, Tsang BK. Optimal conditions for successful in vitro fertilization and subsequent embryonic development in Sprague-Dawley rats. Biol Reprod. 2004;71:1974–79. doi: 10.1095/biolreprod.104.032839. [DOI] [PubMed] [Google Scholar]
- [16].Petroff BK, Croutch CR, Hunter DM, Wierman ME, Gao X. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) stimulates gonadotropin secretion in the immature female Sprague-Dawley rat through a pentobarbital- and estrogen-sensitive mechanism but does not alter gonadotropin-releasing hormone (GnRH) secretion immortalized GnRH neurons in vitro. Biol Reprod. 2003;68:2100–06. doi: 10.1095/biolreprod.102.010439. [DOI] [PubMed] [Google Scholar]
- [17].Zhao B, DeGroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (dioxin) receptor. Reprod Toxicol. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oh SH, Miyoshi K, Funahashi H. Rat oocytes fertilized in modified rat 1-cell embryo culture medium containing a high sodium chloride concentration and bovine serum albumin maintain developmental ability to the blastocyst stage. Biol Reprod. 1998;59:884–89. doi: 10.1095/biolreprod59.4.884. [DOI] [PubMed] [Google Scholar]
- [19].Miyoshi K, Kono T, Niwa K. Stage-dependent development of rat 1-cell embryos in a chemically defined medium after fertilization in vivo and in vitro. Biol Reprod. 1997;56:180–85. doi: 10.1095/biolreprod56.1.180. [DOI] [PubMed] [Google Scholar]
- [20].Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–7. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- [21].Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assesment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17(4):1006–16. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- [22].Hutt KJ, Shi Z, Petroff BK, Albertini DF. The environmental toxicant 2,3,7,8-teterachlorodibenzo-p-dioxin disturbs the establishment and maintenance of cell polarity in preimplantation rat embryos. Biol Reprod. 2010;82:914–20. doi: 10.1095/biolreprod.109.081109. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and (Ah) gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59(1):65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- [24].Valdez KE, Shi F, Ting AY, Petroff BK. Effect of chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats on ovarian gene expression. Reprod Toxicol. 2009;28:32–37. doi: 10.1016/j.reprotox.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assesment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17(4):1006–16. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- [26].Blankenship AL, Suffia MC, Matsumura F, Walsh KJ, Wiley LM. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) accelerates differentiation of murine preimplantation embryos in vitro. Reprod Toxicol. 1993;7:255–61. doi: 10.1016/0890-6238(93)90232-v. [DOI] [PubMed] [Google Scholar]
- [27].Bone W, Jones NG, Kamp G, Yeung CH, Cooper TG. Effect of ornidazole on fertility of male rats: inhibition of glycolysis-related motility pattern and zona binding required for fertilization in vitro. J Reprod Fertil. 2000;118(1):127–135. doi: 10.1530/jrf.0.1180127. [DOI] [PubMed] [Google Scholar]
- [28].Davis TE. Interstitial cell adenoma in rat testis: why is it such a problem in toxicology studies using Sprague-Dawley rats? Toxicol Pathol. 1995;23:83–86. doi: 10.1177/019262339502300110. [DOI] [PubMed] [Google Scholar]
- [29].Zernicka-Goetz M. Activation of embryonic genes during preimplantation rat development. Mol Reprod Dev. 1994;28(1):30–5. doi: 10.1002/mrd.1080380106. [DOI] [PubMed] [Google Scholar]
- [30].Jiang JY, Tsang BK. Optimal conditions for successful in vitro fertilization and subsequent embryonic development in Sprague-Dawley rats. Biol Reprod. 2004;71:1974–9. doi: 10.1095/biolreprod.104.032839. [DOI] [PubMed] [Google Scholar]