Abstract
The aircraft cabin and flight deck ventilation are supplied from partially compressed unfiltered bleed air directly from the engine. Worn or defective engine seals can result in the release of engine oil into the cabin air supply. Aircrew and passengers have complained of illness following such “fume events”. Adverse health effects are hypothesized to result from exposure to tricresyl phosphate mixed esters, a chemical added to jet engine oil and hydraulic fluid for its anti-wear properties. Our goal was to develop a laboratory test for exposure to tricresyl phosphate. The assay was based on the fact that the active-site serine of butyrylcholinesterase reacts with the active metabolite of tri-o-cresyl phosphate, cresyl saligenin phosphate, to make a stable phosphorylated adduct with an added mass of 80 Da. No other organophosphorus agent makes this adduct in vivo on butyrylcholinesterase. Blood samples from jet airplane passengers were obtained 24–48 hours after completing a flight. Butyrylcholinesterase was partially purified from 25 ml serum or plasma, digested with pepsin, enriched for phosphorylated peptides by binding to titanium oxide, and analyzed by mass spectrometry. Of 12 jet airplane passengers tested, 6 were positive for exposure to tri-o-cresyl phosphate that is, they had detectable amounts of the phosphorylated peptide FGEpSAGAAS. The level of exposure was very low. No more than 0.05 to 3% of plasma butyrylcholinesterase was modified. None of the subjects had toxic symptoms. Four of the positive subjects were retested 3 to 7 months following their last airplane trip and were found to be negative for phosphorylated butyrylcholinesterase. In conclusion, this is the first report of an assay that detects exposure to tri-o-cresyl phosphate in jet airplane travelers.
Keywords: aerotoxic syndrome, butyrylcholinesterase, mass spectrometry, tricresyl phosphate, CBDP, titanium oxide
Introduction
Air on military and commercial jet aircraft is taken directly from compressors in the engine compartments and following conditioning for temperature and humidity, circulates unfiltered in the cabin and flight deck (Murawski and Supplee, 2008). Occasionally, oil fumes from the hot engine leak into the air conditioning ductwork, through faulty oil seals. The concern about air quality in the cabin of commercial and military aircraft has grown over the past twenty years, to a level that the United States Congress has taken notice. Senator Dianne Feinstein introduced an amendment approved by the Senate on March 22, 2010 requiring the Federal Aviation Association to study air quality in the cabins of jet aircraft. The concern arose from the complaints of aircrew and jet airplane passengers who experienced stomach ache, muscle weakness, tremors, dizziness, nausea, disorientation, blurred vision, short-term memory loss, and cognitive dysfunction after jet engines released smoke or fumes into the cabin air (Montgomery et al., 1977; Cox and Michaelis, 2002; Winder et al., 2002; Ross, 2008). The symptoms are hypothesized to be associated with exposure to ortho isomers of tri-cresyl phosphate, a component of the triaryl phosphates used as anti-wear additives in jet-engine lubricants and hydraulic fluids (van Netten and Leung, 2001; Winder and Balouet, 2002; De Nola et al., 2008). Cabin air contamination by tricresyl phosphate and tri-ortho-cresyl phosphate has been confirmed by in-flight air sampling carried out in commercial and military aircraft (Kelso et al., 1988; Hanhela et al., 2005; van Netten, 2009; Crump et al., 2011).
The toxicity of tri-o-cresyl phosphate was first recognized during Prohibition in the United States. In 1930 thousands of Americans developed limb paralysis after drinking “Jamaica ginger” adulterated with tricresyl phosphate. Scientists at the National Institutes of Health identified tri-ortho-cresyl phosphate as the cause of paralysis resulting from consumption of adulterated “Jamaica ginger”. Later it was recognized that tri-ortho-cresyl phosphate undergoes metabolic activation in vivo (Aldridge, 1954) to the potent neurotoxic agent, cresyl saligenin phosphate (CBDP), the structure of which was reported (Eto et al., 1962).
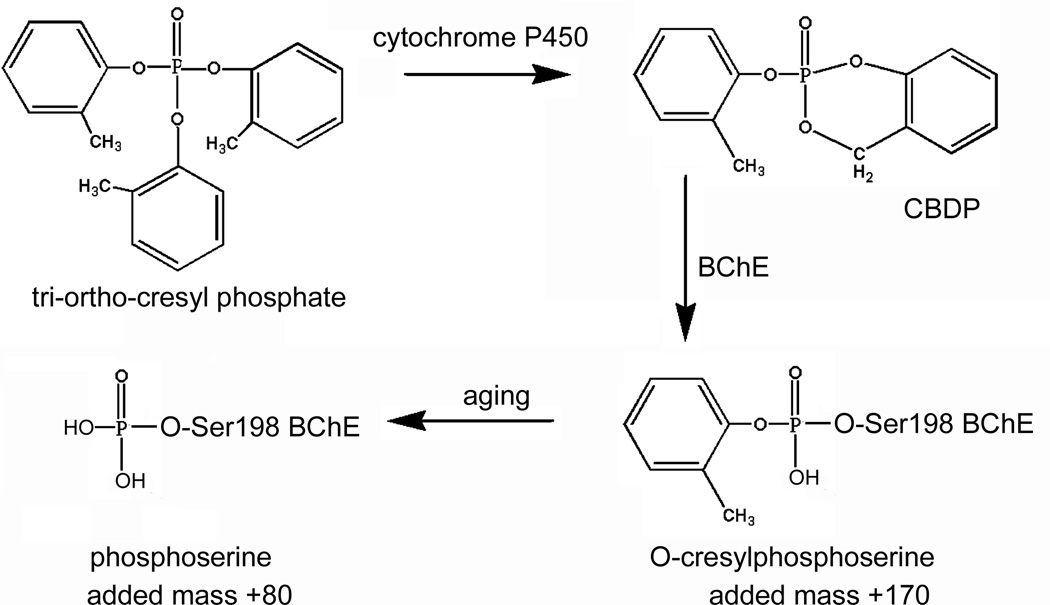
The illness hypothesized to be associated with exposure to fumes from jet oil is referred to as aerotoxic syndrome. This association is speculative since the link between jet oil exposure and aerotoxic syndrome is unproven and even contentious. To date there is no laboratory assay that proves a person was exposed to tri-o-cresyl phosphate and therefore no proof that the illness was actually caused by exposure to tri-o-cresyl phosphate. Our goal was to establish such an assay. We developed our assay after determining the type of adduct that butyrylcholinesterase forms with cresyl saligenin phosphate. Using mass spectrometry, X-ray crystallography, and kinetic analysis we found that CBDP reacts rapidly with butyrylcholinesterase to make a covalent adduct with the active site serine 198 (Schopfer et al., 2010; Carletti et al., 2011 in press). The initial adduct has an added mass of 170 Da from cresyl phosphate. This initial adduct releases the cresyl group to form a stable phosphoserine adduct with an added mass of 80 Da. See Figure 1. Butyrylcholinesterase is exquisitely sensitive to CBDP inhibition, forming an irreversible adduct with a bimolecular inhibition rate constant of 108 M−1 min−1, similar to the rate constant for reaction with nerve agents (Carletti et al., 2011 in press).
Figure 1.
Activation of tri-ortho-cresyl phosphate to the active metabolite cresyl saligenin phosphate (CBDP) by cytochrome P450; and reaction of CBDP with butyrylcholinesterase (BChE) to yield an adduct on the active-site serine 198. The initial adduct has an added mass of 170 Da from cresyl phosphate. This adduct ages to the phosphoserine adduct, where the added mass is 80 Da.
Cresyl saligenin phosphate is the only organophosphorus agent that makes a butyrylcholinesterase adduct with an added mass of 80 Da. Mipafox-inhibited acetylcholinesterase ages to a phosphate adduct (Kropp and Richardson, 2006), though mipafox inhibited butyrylcholinesterase does not (Kropp and Richardson, 2007). Care must be exercised over the interpretation of mass spectrometry data for adducts with phosphoramidates such as mipafox and tabun because the adducts can be easily hydrolyzed to their corresponding phosphates by acid catalyzed deamidation (Heath and Casapieri, 1951; Carletti et al., 2008; van der Schans et al., 2008). The low pH typical during sample preparation for mass spectrometry is sufficient to cause deamidation. We are confident that the phosphorylated butyrylcholinesterase generated by CBDP is not such an artifact because conversion of the cresyl phosphate adduct into the phosphate adduct has been documented to occur in the crystal by x-ray crystallography at neutral pH (Carletti et al., 2011 in press).
Our assay takes advantage of the unique phosphoserine adduct that results from the reaction of the active metabolite of tri-o-cresyl phosphate, CBDP, with butyrylcholinesterase. We purify butyrylcholinesterase from plasma, digest it with pepsin, enrich for phosphorylated peptides on titanium oxide beads, and analyze the peptides by mass spectrometry. In the present report we analyzed butyrylcholinesterase from 12 jet airplane passengers and found phosphoserine adducts in the blood of 6 travelers. Less than 1% of the butyrylcholinesterase was modified in 4 of these samples. None of the jet airplane passengers exhibited symptoms of toxicity. The phosphate adduct was absent from the blood of individuals following a 3 month period without jet airplane travel.
Materials and methods
Materials
2-(2-cresyl)-4H-1-3-2-benzodioxaphosphorin-2-oxide (CBDP) was a generous gift from Wolf-Dietrich Dettbarn (Vanderbilt Univ.) and David E. Lenz (US Army Medical Research Institute of Chemical Defense, Aberdeen Proving Ground, MD). The CBDP (99.5% pure) was custom synthesized by Starks Associates (Buffalo, NY, USA). CBDP is also known as cresyl saligenin phosphate, cyclic tolyl saligenin phosphate, and saligenin cyclic-o-tolyl phosphate (SCOTP). The CAS number is 1222-87-3. CBDP was dissolved in acetonitrile to 100 mM and stored at −80 °C.
The following were from Sigma-Aldrich, St. Louis, MO: porcine pepsin (P-6887), S-butyrylthiocholine iodide (B-3253), 5,5′-dithiobis(2-nitrobenzoic acid) (D-8130), glycolic acid (Fluka 50590), formic acid (Fluka 94318), and 2, 5-dihydroxybenzoic acid (DHB) (Fluka 85707 and Acros 165200050).
The following were from Fisher Scientific, Fair Lawn, NJ: sequencing grade modified trypsin (Promega V5113), trifluoroacetic acid (A11650) and acetonitrile (BP1170-4).
Sources of the following reagents and their catalog numbers are as follows. Titansphere bulk media 5 micron (GL Sciences, Inc. 1400B500), Pro-Q Diamond phosphoprotein gel stain (Invitrogen, P33301), Amicon stirred cell 10 ml capacity (Millipore model 8010) with YM30 membrane (Millipore 13712), and Q-Sepharose fast flow (Amersham Pharmacia Biotech 17-0510-04).
Butyrylcholinesterase, for limit of detection studies and for phosphoprotein staining, was purified from outdated human plasma as described (Lockridge et al., 2005). Double distilled water was from an in-house still. Procainamide Sepharose was synthesized with a 6-carbon spacer arm by Y. Ashani (Grunwald et al., 1997).
Blood from jet airplane passengers
Subjects were healthy adult volunteers who donated blood within 48 h of disembarking from a jet airplane. There were no other criteria for selection of subjects. The 9 females and 3 males ranged in age from 25 to 68 years. All were college educated, non-smokers, not obese, who worked at white collar jobs unrelated to the airline industry. Many of our subjects traveled on a jet airplane once every three months. Subjects completed a questionnaire about possible toxic symptoms associated with travel on their flight. They also provided information on the destination and duration of travel, and donated 50 ml (4 tablespoons) of blood under a protocol approved by The Institutional Review Board of the University of Nebraska Medical Center. Blood was drawn into 10 red cap tubes (no anticoagulant) or into 10 green cap tubes (heparin anticoagulant) or into 10 lavender cap tubes (EDTA anticoagulant). A total of about 25 ml serum or plasma was recovered. Subjects donated blood within 24 to 48 hours of disembarking from a jet airplane. Control subjects had not traveled on a jet airplane for 3 to 15 months at the time they donated blood.
Assay of butyrylcholinesterase activity
Butyrylcholinesterase activity was measured with 1 mM butyrylthiocholine and 0.5 mM dithiobisnitrobenzoic acid in 0.1 M potassium phosphate buffer pH 7.0 at 25 °C. The change in absorbance at 412 nm was monitored in a Gilford spectrophotometer in 1 cm quartz cuvettes. The extinction coefficient for the product was 13,600 M−1cm−1. One unit of butyrylcholinesterase activity was defined as the amount that hydrolyzes 1 µmol of butyrylthiocholine in 1 min.
Determination of butyrylcholinesterase protein concentration and purity
Protein concentration was estimated from absorbance at 280 nm. To calculate the purity of butyrylcholinesterase we used an absorbance at 280 nm of 1.8 for a solution containing 1 mg/ml protein and a value of 720 units/mg as the specific activity of 100% pure butyrylcholinesterase (Lockridge et al., 2005). Plasma butyrylcholinesterase is a tetramer of four identical subunits. The subunit molecular weight of human butyrylcholinesterase is 85,000 Da. The concentration of butyrylcholinesterase subunits in human plasma is about 4 mg per liter, or 50 nanomolar. The butyrylcholinesterase in plasma has to be purified about 12,000 fold to achieve a specific activity of 720 units/mg. The 12,000-fold purification requirement was calculated by using 3 units/ml as the average butyrylcholinesterase activity in plasma, and 50 mg/ml as the average protein concentration in plasma.
Purification of butyrylcholinesterase
Highly purified butyrylcholinesterase was prepared from 70 Liters of outdated human plasma as described (Lockridge et al., 2005). The highly purified butyrylcholinesterase was used for the experiments in Figures 2 and 6. It was also tested for the presence of adducts on butyrylcholinesterase.
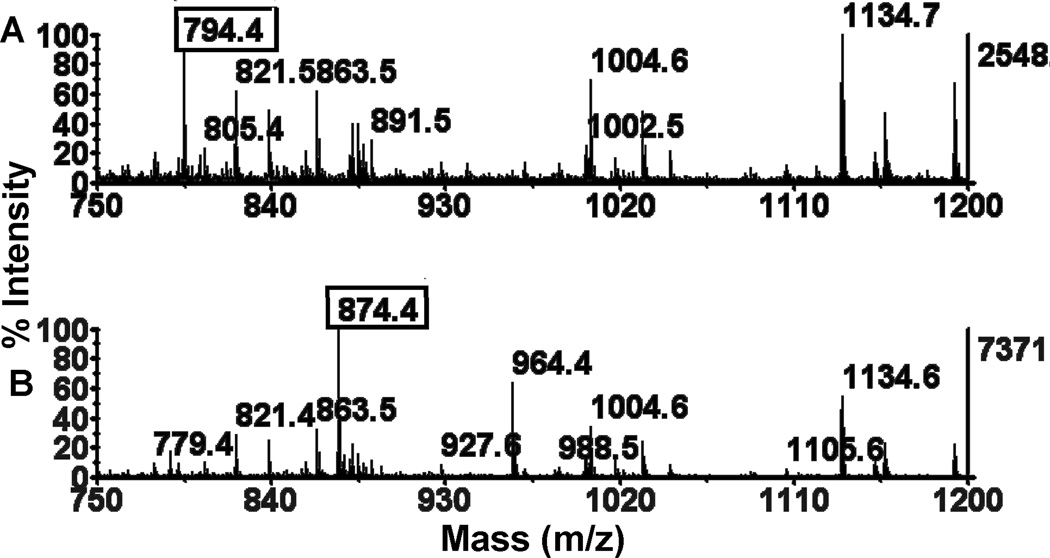
Figure 2.
MALDI-TOF mass spectra of control unmodified butyrylcholinesterase and CBDP-butyrylcholinesterase digested with pepsin. A) Highly purified human butyrylcholinesterase with a specific activity of 518 units/mg and a concentration of 1.7 mg/ml was digested with pepsin. The active site peptide had the sequence FGESAGAAS and a mass of 794.4 Da in negative mode. B) A second aliquot from the same butyrylcholinesterase stock was treated with a 50-fold molar excess of CBDP, which inhibited 100% of the butyrylcholinesterase activity, and was digested with pepsin. The active site peptide from this preparation had the sequence FGEpSAGAAS and a mass of 874.4 Da in negative mode. The added mass of 80 Da was from phosphorylation of serine 198 (accession # gi 34810860 for human butyrylcholinesterase protein). MS spectra were acquired in negative mode with DHB matrix at a laser voltage of 5000 volts.
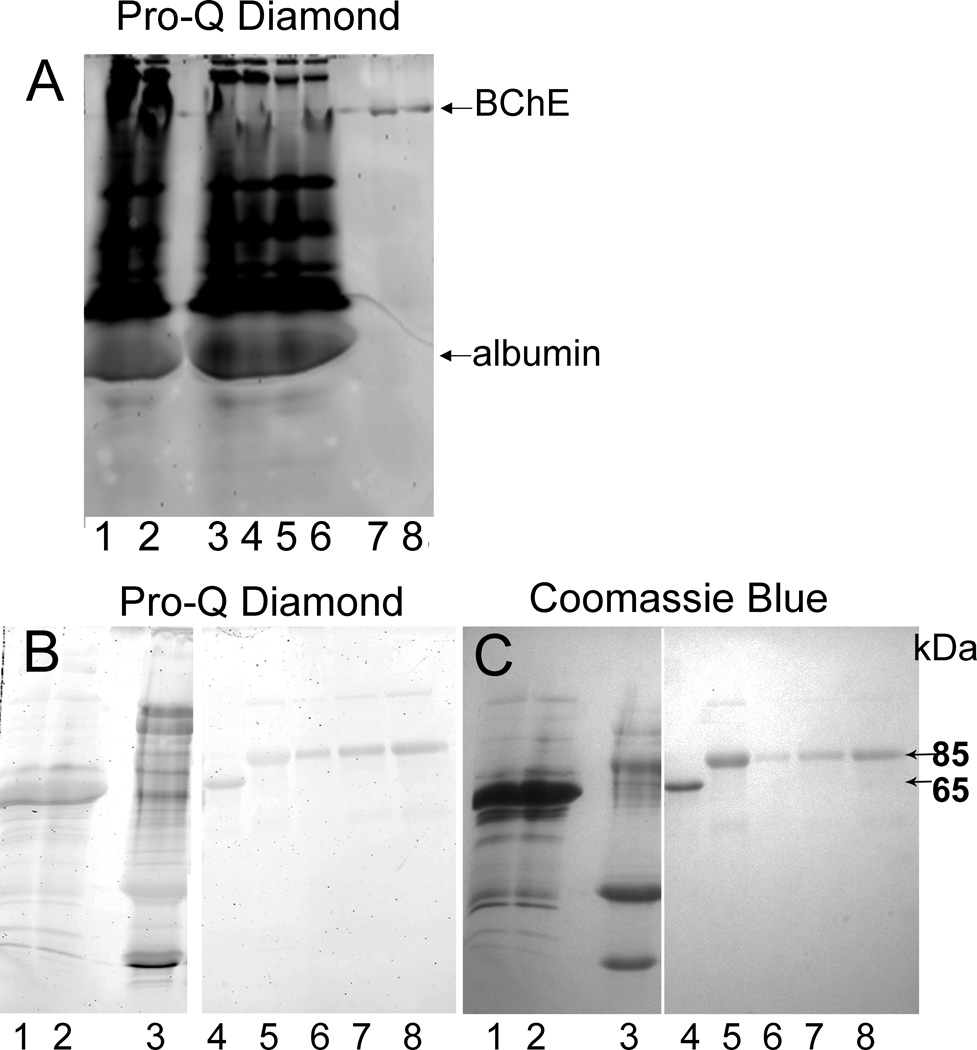
Figure 6.
Polyacrylamide gel staining for phosphoproteins and total protein. Panel A, nondenaturing polyacrylamide gel stained for phosphoproteins with Pro-Q Diamond: Lanes A1 and A2; 10 and 5 µl of human plasma treated with CBDP to inhibit 100% of the butyrylcholinesterase activity. Lanes A3 and A4; 10 µl of unlabeled, control human plasma. Ten microliters of human plasma contains 0.04 micrograms of butyrylcholinesterase. Lanes A5 and A6; 5 µl of control human plasma. Lane A7, 2.1 µg of 50% purified control human butyrylcholinesterase (with a specific activity of 360 units/mg). Lane A8, 0.4 µg of 50% purified control human butyrylcholinesterase. Panel B, SDS polyacrylamide gel stained for phosphoproteins with Pro-Q Diamond; and Panel C, SDS polyacrylamide gel counterstained for protein with Coomassie Blue R-250: Lanes B1 and C1; 0.8 µl control human plasma. Lanes B2 and C2; 0.8 µl of CBDP-treated human plasma (100% inhibition of butyrylcholinesterase). Lanes B3 and C3; 5 µg of 8% purified butyrylcholinesterase (with specific activity of 60 units/mg). Lanes B4 and C4; 5 µg of pure human albumin. Lanes B5 and C5; 5 µg of 50% purified untreated control human butyrylcholinesterase. Lanes B6 and C6; 0.9 µg of 50% pure butyrylcholinesterase treated with CBDP to inhibit 100% of the activity (CBDP-butyrylcholinesterase). Lanes B7 and C7; 2.2 µg of CBDP-butyrylcholinesterase. Lanes B8 and C8; 3.5 µg of CBDP-butyrylcholinesterase. The 85 kDa band is butyrylcholinesterase. The 65 kDa band is albumin.
Butyrylcholinesterase from individual jet airplane travelers was purified from about 25 ml of serum or plasma. The first step was dialysis against 2 × 4 Liters of 20 mM sodium acetate, 1 mM EDTA pH 4.0 (pH 4 buffer) at 4°C. Dialysis was performed in 22 mm diameter dialysis tubing with 12 000 MW cutoff (Spectrapor 3787-D32). During dialysis a heavy precipitate appeared which was removed by centrifugation. After dialysis the volume of serum or plasma typically increased by 2 ml. All chromatography steps were conducted at room temperature and were completed in one day. The clarified serum or plasma (approximately 27 ml) was loaded onto a 5 ml column of Q-Sepharose Fast Flow packed in a 15 ml (10 × 195 mm) Pharmacia column. The ion exchanger had been equilibrated by overnight washing with 1 Liter of pH 4 buffer. Yellow color and a large quantity of protein eluted during sample loading. The column was washed with 150 to 200 ml of pH 4 buffer until the absorbance at 280 nm of the eluant was approximately 0.03. A blue band of ceruloplasmin was visible at the top of the column at the start of the buffer wash, but it eluted during washing with buffer. Butyrylcholinesterase eluted with a linear 100 ml gradient of zero to 0.2 M NaCl in pH 4 buffer. Three ml fractions were collected. The butyrylcholinesterase began to elute when the NaCl concentration was 0.05 M (Lockridge et al., 2005). The purest butyrylcholinesterase eluted early, in fractions 7–10, occupying a volume of 9 to 12 ml. Later fractions contained 10-fold less pure butyrylcholinesterase.
The second step of the purification was on a 2 ml procainamide Sepharose affinity column packed in a 7 ml (10 × 95 mm) Pharmacia column, equilibrated with 20 mM TrisCl, 1 mM EDTA pH 7.5 at room temperature. TrisCl was used rather than phosphate buffer because phosphate ions compete with phosphorylated peptides for binding to titanium oxide beads in the enrichment step that precedes mass spectrometry. Fractions from the ion exchange column containing the purest butyrylcholinesterase were pooled and the pH adjusted from 4 to 7 by adding 0.19 ml of 1 M NH4HCO3 per 3 ml butyrylcholinesterase. The sample was loaded onto the procainamide affinity gel. The affinity gel was washed with 20–30 ml of 20 mM TrisCl, 1 mM EDTA pH 7.5 and 20 ml of 0.2 M NaCl in pH 7.5 buffer. Butyrylcholinesterase was eluted with 1 M NaCl in pH 7.5 buffer. The purest BChE (15 to 20% pure) eluted in the first 4 ml, the next 2 ml typically contained 9% pure BChE, and the last 4 ml contained 3% pure BChE.
The purity of butyrylcholinesterase following ion exchange at pH 4 and procainamide affinity chromatography varied for each sample. With most samples we achieved an 1800-fold increase in specific activity to 15% purity, but the range varied from 2 to 30% purity. The highest purity was achieved when the serum or plasma volume was at least 30 ml and when the starting butyrylcholinesterase activity was 3 units per ml. A low 2% purity was achieved when the plasma volume was 17 ml and the starting plasma butyrylcholinesterase activity was 1.3 units per ml. Generally, about 30% of the starting activity could be recovered at 15% purity. In some cases the yield was 50%. Similar purification yields were obtained for serum, heparin plasma, and EDTA plasma. Percent purity was calculated from absorbance at 280 nm and activity per ml using the information that 100% pure BChE has a specific activity of 720 units/mg and a 1 mg/ml solution of BChE has an absorbance at 280 nm of 1.8 (Lockridge et al., 2005). For example, a 15% pure solution of BChE with an activity of 60 units/ml has an absorbance of 1.0 at 280 nm.
Butyrylcholinesterase dialysis and concentration
The two-step purified butyrylcholinesterase was concentrated in a 10 ml Amicon stirred cell with a YM30 membrane. The use of the YM30 membrane in the Amicon stirred cell resulted in no loss of butyrylcholinesterase activity, whereas other concentrating devices such as the Amicon centrifugal filter 10,000 MWCO (UFC901024) resulted in significant losses of activity. The buffer was changed to 10 mM NH4HCO3, 0.01 % (w/v) sodium azide, pH 8.1 by diluting and re-concentrating 3 times. Changing the buffer and removing the salt was necessary to promote ionization of the peptides in the mass spectrometer. The final volume of the concentrated butyrylcholinesterase was 0.1 ml and the activity was about 120 units/ml. The fact that we found phosphorylated butyrylcholinesterase peptide in our partially purified butyrylcholinesterase samples means that phosphorylated butyrylcholinesterase copurified with unmodified butyrylcholinesterase in our protocol. Thus, we do not expect the ratio of phosphorylated to unmodified butyrylcholinesterase to change after purification.
When only 0.05% of the butyrylcholinesterase was phosphorylated, about 12 units of partially purified butyrylcholinesterase (16.6 µg) were needed for mass spectrometry detection of phosphorylated butyrylcholinesterase. The final degree of purity was not critical, though it was important to remove albumin, the blood coagulation proteins, and proteins that precipitate at pH 2. The pH is lowered to 2 for pepsin digestion. Our protocol would work for smaller volumes of blood if the level of exposure were high. For example, we have detected other organophosphorus and carbamate adducts on butyrylcholinesterase using 1 or 2 ml plasma from suicide and murder victims (Li et al., 2009; Li et al., 2010). In those cases 60–80% of the butyrylcholinesterase was inhibited.
Stability of phosphorylated butyrylcholinesterase
Two of the partially purified butyrylcholinesterase samples from jet airplane passengers were stored at 4°C in 10 mM ammonium bicarbonate, 0.01% sodium azide pH 8.3 for one year before they were digested with pepsin. Both samples were found to be positive for the phosphorylated butyrylcholinesterase peptide. This demonstrates that the phosphorylated butyrylcholinesterase adduct is stable and that it does not spontaneously dephosphorylate. Phosphorylated butyrylcholinesterase was found in butyrylcholinesterase purified from serum as well as plasma, suggesting that phosphatases in blood do not dephosphorylate the butyrylcholinesterase adduct.
Pepsin digestion of butyrylcholinesterase
The pH of partially purified butyrylcholinesterase in 0.1 ml of 10 mM NH4HCO3, 0.01 % sodium azide was adjusted to pH 2 by adding 1 µl of 25% trifluoroacetic acid. A fresh solution of 5 mg/ml pepsin in 5% formic acid was prepared just before use. Pepsin dissolved with difficulty to make an opalescent solution. The butyrylcholinesterase was digested with 5 µl pepsin (25 µg) at 37 °C for 2 h. Digestion was stopped by inactivating the pepsin either by 5 min of incubation in a boiling water bath, or by adjusting the pH of the digest to 7.
The pepsin digestion step was the most difficult to optimize. When attempts were made to simplify the purification by omitting the pH 4 ion exchange step and purifying only on procainamide affinity gel, acidification prior to addition of pepsin caused the butyrylcholinesterase solution to solidify into an indigestible clot. A second problem was that pepsin partially precipitated out of solution at 1 mg/ml in 10 mM hydrochloric acid when stored frozen in 10 µl aliquots (a common method for preparing pepsin). The precipitate was not visible to the eye, but the effect on digestion was dramatic in that the target peptide was not generated. A third problem was pH. As noted above, the purity of butyrylcholinesterase preparations ranged from 2% to 30% depending on the volume and activity of the starting plasma and on the extent to which side-fractions were pooled with the main fractions. Less pure preparations required more acid to lower the pH to 2 and required more pepsin for proper digestion. The amount of pepsin to add was calculated as follows. Twelve units of butyrylcholinesterase = 16.6 µg butyrylcholinesterase protein. A 17% pure preparation therefore contains a total of 98 µg protein (16.6 µg/0.17 = 98 µg). The desired ratio of total protein to pepsin is 4 to 1 on a weight basis (98 µg/4 = 25). Therefore, 25 µg of pepsin were required to digest 12 units of 17% pure butyrylcholinesterase.
Enrichment of phosphorylated peptide by binding to titanium oxide
Phosphorylated butyrylcholinesterase peptide was enriched and concentrated by the method of Jensen and Larsen (Jensen and Larsen, 2007). TiO2 beads from GL Sciences Inc. (Torrance, CA, USA) were manually packed into empty pipette tips (that previously contained TopTip POROS R-2 1–10 µl part no TTIPR2.96 from the Glygen Corp., Columbus, MD, USA) to a bed height of 3 mm (2.2 mg TiO2). The Glygen tips come with an adapter that holds the packed pipette tip in a standard 1.5 ml microfuge tube, allowing solvent to be centrifuged through the tip. Sample and solvents were centrifuged through the TiO2 microcolumn at a speed of 4000 rpm in a Sorvall MC12V microfuge (1500 × g). Higher speeds extruded the beads out of the tip. The TiO2 microcolumn was conditioned with 75 µl of 75% acetonitrile, 1% trifluoroacetic acid and washed with 75 µl loading buffer (1 M glycolic acid in 80% acetonitrile, 5% trifluoroacetic acid). Before the sample was loaded onto the microcolumn, the pepsin-digested butyrylcholinesterase (100 µl) was diluted with an equal volume of loading buffer and centrifuged at 12,000 rpm to remove turbid material which could clog the beads. The clarified sample was centrifuged through the microcolumn at 4000 rpm. The microcolumn was washed with 75 µl loading buffer, followed by 3 × 75 µl of 75% acetonitrile, 1% trifluoroacetic acid. Phosphorylated peptides were eluted with 2 × 20 µl of 0.4 M ammonium hydroxide, 30% acetonitrile. Fractions were combined and the volume was reduced to about 1 µl in a SpeedVac. The sample was mixed with 2 µl of 2,5-dihydroxybenzoic acid matrix (20 mg/ml DHB in 50% acetonitrile, 0.1% trifluoroacetic acid, 1% phosphoric acid) and spotted on a Maldi plate. If no signal was obtained, the sample was dried completely, dissolved in 5 µl of 50% acetonitrile, 1% trifluoroacetic acid, and spotted on a MALDI plate. After the spot was dry, it was overlaid with DHB matrix.
Mass spectrometry
MALDI mass spectra were acquired on a MALDI–TOF/TOF 4800 mass spectrometer (Applied Biosystems, Framingham, MA, USA). Data collection was controlled by 4000 Series Explorer software (version 3.5). Mass spectra were taken in negative reflector mode using delayed extraction (625 nsec) and default calibration. The mass spectrometer was calibrated in positive mode against bradykinin (904.47 m/z), angiotensin 1 (1296.68 m/z), Glu-fibrinopeptide B (1570.68 m/z), adrenocorticotropic hormone (ACTH) 1–17 clip (2093.09 m/z), ACTH 18–39 clip (2465.20 m/z), and ACTH 7–38 clip (3657.96 m/z) (Cal Mix 5 from Applied Biosystems). Each spectrum was the average of 500 laser shots taken with the laser energy adjusted to 5000 volts. MS/MS fragmentation spectra were taken using postsource decay in positive mode at 1 kilovolt collision energy in the absence of collision gas and with metastable ion suppression on. Each spectrum consisted of 500 laser pulses taken with the laser energy adjusted to yield optimal signal-to-noise. MS/MS calibration used the fragmentation spectrum of angiotensin 1. Spectra were analyzed with Data Explorer Software.
Samples premixed with DHB matrix were spotted in 1 µl aliquots onto a 384-well Opti-TOF sample plate (cat. no. 1016491, Applied Biosystems, Foster City, CA, USA). MS spectra were acquired in negative mode at 5000 volts. MSMS spectra were acquired in positive mode. The amino acid sequences of the peptides were determined by manual inspection of the MSMS fragmentation spectra, with the aid of the MS-Product algorithm from Protein Prospector (http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msproduct) and the Proteomics Toolkit from DB Systems Biology (http://db.systemsbiology.net:8080/proteomicsToolkit/FragIonServlet.html).
Limit of detection of phosphorylated butyrylcholinesterase
Partially purified butyrylcholinesterase with a specific activity of 173 units/mg (24% pure) was used for the experiment to determine the limit of detection of phosphorylated butyrylcholinesterase. This purification level was selected because it was similar to the purity of butyrylcholinesterase processed from plasma of jet airplane passengers. An aliquot of this butyrylcholinesterase, in 10 mM NH4HCO3, 0.01% sodium azide with an activity of 120 units/ml, was treated with CBDP to inhibit 100% of the butyrylcholinesterase activity. The CBDP-butyrylcholinesterase was digested with pepsin. CBDP does not inhibit pepsin, so there was no need to remove excess CBDP. Control unmodified butyrylcholinesterase from the same lot was also digested with pepsin. The digested CBDP-butyrylcholinesterase was added to the digested control butyrylcholinesterase so that the proportion of CBDP-butyrylcholinesterase ranged from 0.05 to 5%. The total amount of butyrylcholinesterase in each mixture was 16.6 µg in a volume of 0.1 ml. The digests were enriched for phosphorylated peptides by binding to TiO2 and eluted with 0.4 M NH4OH, 30% acetonitrile. The eluted peptides were analyzed in the MALDI-TOF mass spectrometer. The phosphorylated, active-site, peptic peptide of butyrylcholinesterase (FGEpSAGAAS) has a mass of 874.3 Da in negative mode.
Phosphorylated proteins stained with Pro-Q Diamond
Polyacrylamide 4–30% gradient gels 0.75 mm thick were prepared in a Hoefer SE600 gel apparatus and run at a constant voltage for 3000 volt-hours (150 V for 20 h) at 4°C. Phosphorylated proteins were visualized by staining with Pro-Q Diamond followed by measurement of fluorescence on a Typhoon 9410 Imager (GE Healthcare Life Sciences) with 532 nm excitation and 580 nm longpass emission filters. Gels were counterstained with Coomassie Blue R-250.
Results
Mass spectrometry method for detection of phosphorylated butyrylcholinesterase
In our previous work (Schopfer et al., 2010; Carletti et al., 2011 in press) we determined that CBDP reacts with human butyrylcholinesterase to make a covalent bond on the active-site serine. The predominant adduct has an added mass of 80 Da from phosphate. The only other example of a +80 adduct on butyrylcholinesterase is an artifact produced by acid hydrolysis of butyrylcholinesterase inhibited by the chemical warfare agent, tabun (van der Schans et al, 2008). The majority of pesticides yield either a dimethoxyphosphate or diethoxyphosphate adduct with an added mass of +108 or +136 on the active site serine.
In the present report we used this information to develop an assay for in vivo exposure to tri-o-cresyl phosphate. The key step in our protocol was the use of titanium oxide to enrich for the phosphorylated butyrylcholinesterase peptide. Phosphopeptides bind to titanium oxide through the negative charge on the phosphate group. Our protocol requires partial purification of butyrylcholinesterase from serum or plasma, digestion with pepsin, enrichment of phosphopeptides by binding to titanium oxide, and detection of the phosphorylated butyrylcholinesterase peptide by MALDI-TOF/TOF mass spectrometry.
Previously, we used trypsin to produce the phosphorylated active-site butyrylcholinesterase peptide. However, we found that the 29-residue tryptic peptide was recovered in poor yield from titanium oxide. Fidder et al. as well as Sporty et al. have shown that butyrylcholinesterase digested with pepsin yields a 9-residue active-site peptide with the sequence FGESAGAAS (Fidder et al., 2002; Sporty et al., 2010). In Figure 2A we confirm that digestion of butyrylcholinesterase with pepsin yields a peptide with a mass of 794.4 Da (in negative mode) as is expected for FGESAGAAS. Fragmentation of this parent ion in positive mode (mass 796.4 Da) proved that this peptide has the sequence FGESAGAAS (data not shown). Figure 2B shows that butyrylcholinesterase completely inhibited by treatment with CBDP and digested with pepsin has a new peak at 874.4 Da and has no peak at 794.4 Da. The difference in mass between 794.4 and 874.4 is 80 Da, consistent with modification by phosphate.
A second, new mass appeared at 964.4 Da. This ion is consistent with the cresyl phosphate adduct of FGESAGAAS, with an added mass of 170 Da. We reported the appearance of a cresyl phosphate adduct previously (Schopfer et al., 2010; Carletti et al., 2011 in press). However, because there is no convenient method for selective enrichment of a cresyl phosphate adduct, it would be difficult to detect when in low abundance. Therefore, we have chosen to pursue the 874.4 Da mass.
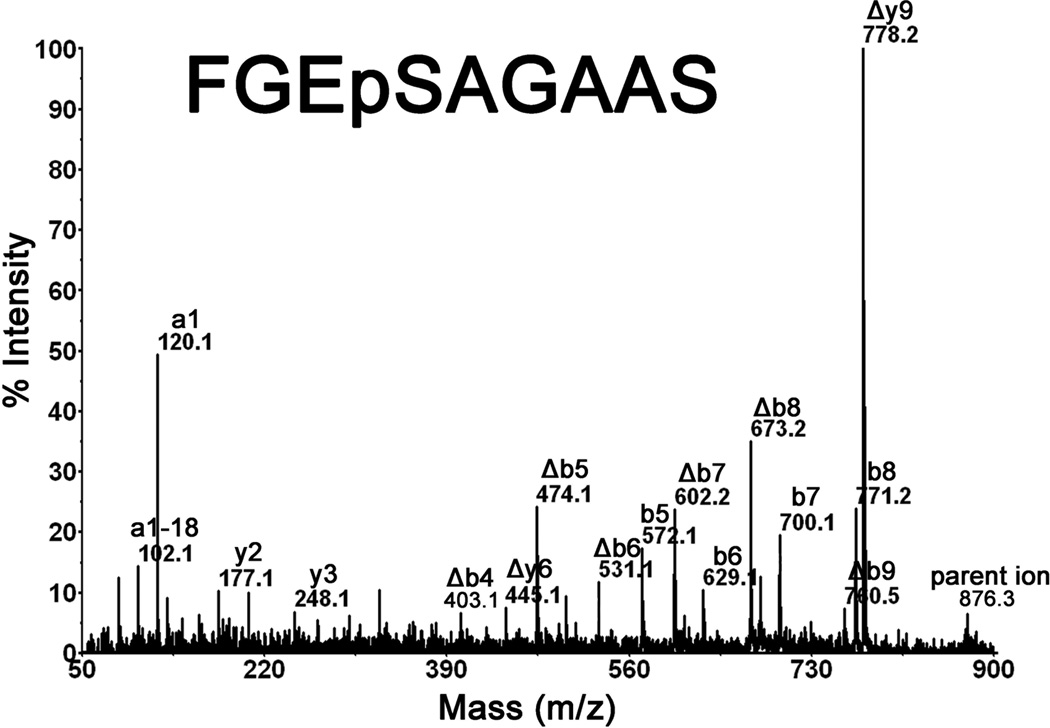
The sequence of parent ion, 876.4 Da in positive mode, was determined from the MSMS spectrum in Figure 3. The masses are consistent with y- and b-ions from the sequence FGEpSAGAAS where the phosphate is on serine, the 4th residue from the N-terminus. The most intense peak in Figure 3 has a mass of 778.2 Da. This mass represents a neutral loss of 98 Da from the parent ion, which is consistent with beta-elimination of the phosphate as well as a molecule of water. The beta-elimination reaction converts phosphoserine into dehydroalanine. Loss of 98 Da during fragmentation in the mass spectrometer is characteristic of phosphoserine-containing peptides.
Figure 3.
Identification of phosphorylated Ser198 of human butyrylcholinesterase. The MALDI MS/MS spectrum of parent ion 876.3 Da, in positive mode, shows major y- and b-ions of peptide FGEpSAGAAS. The fragment ion masses support phosphorylation of serine 198, the active site serine. The symbol Δ represents fragments which have undergone loss of phosphate and a molecule of water, thus converting phosphoserine into dehydroalanine.
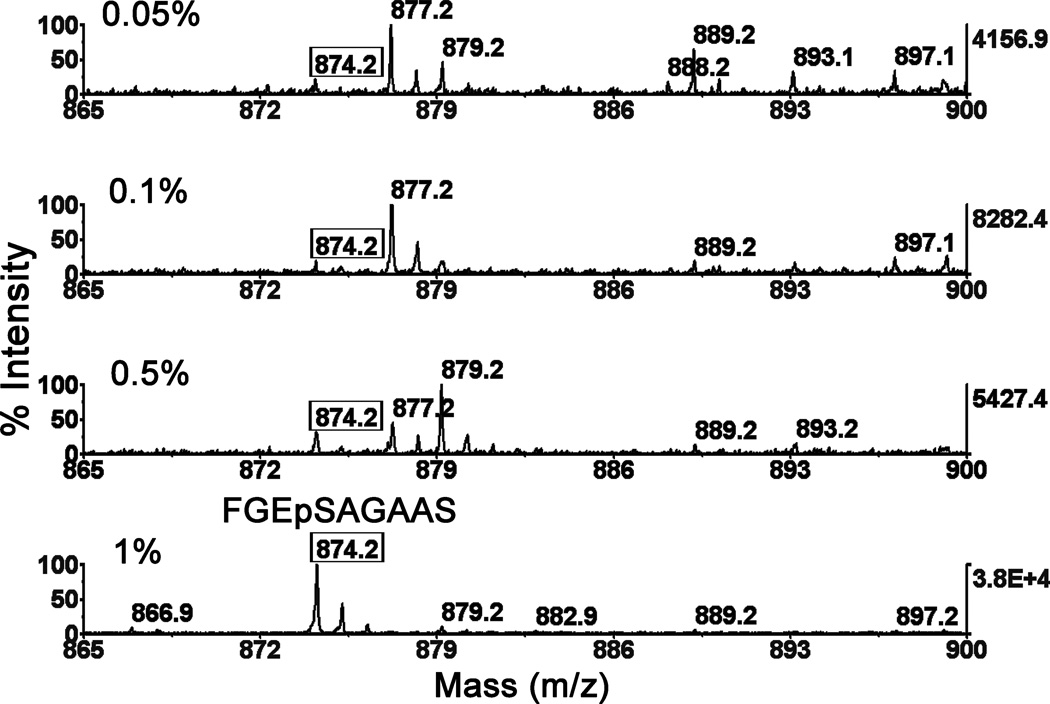
There are additional masses in the spectrum consistent with two b-ion series, one in which the phosphate is retained (b5 at 572.1 m/z through b8 at 771.2 m/z) and one without phosphate (Δb4 at 403.1 m/z through Δb9 at 760.5 m/z). Elimination of phosphate from a portion of the phosphoserine containing peptides is commonly observed. The fact that phosphate loss begins at b4 eliminates the possibility that phosphorylation occurred on Ser203 (the 9th residue from the N-terminus) and favors phosphorylation on Ser198 (the active-site serine). The limit of detection for the phosphorylated active-site peptide was determined in negative mode from samples containing known concentrations of CBDP-butyrylcholinesterase. Figure 4 shows that the phosphorylated butyrylcholinesterase peptide at 874.2 Da could be detected with signal-to-noise of about 5 when as little as 0.05% of butyrylcholinesterase (0.05% of 12 units or of 16.6 µg = 0.006 units or 0.0083 µg) were labeled by CBDP. At 1% labeling, the signal-to-noise was 38,000 to 1. Samples containing no added CBDP-butyrylcholinesterase were free of the 874.2 Da phosphorylated peptide. As can be noted, the signal was not linear with the level of phosphorylation, which can be explained by variability in ion signal intensity from sample to sample, a well-known characteristic of MALDI-TOF mass spectrometry.
Figure 4.
Limit of detection of phosphorylated butyrylcholinesterase. Partially purified butyrylcholinesterase was digested with pepsin and mixed in known ratios with 100%-labeled CBDP-butyrylcholinesterase that also had been digested with pepsin. The phosphorylated peptides were purified on titanium oxide, eluted with ammonium hydroxide, reduced in volume, and spotted on a MALDI plate with DHB matrix. MS spectra were acquired in negative mode at 5000 volts. Spectra represent phosphorylated peptide from 12 units of butyrylcholinesterase (16.6 micrograms) containing 0.05 to 1% CBDP-treated butyrylcholinesterase. As little as 0.05% phosphorylated butyrylcholinesterase is detectable as a peptide of mass 874.2 Da, in negative mode.
Jet airplane passengers
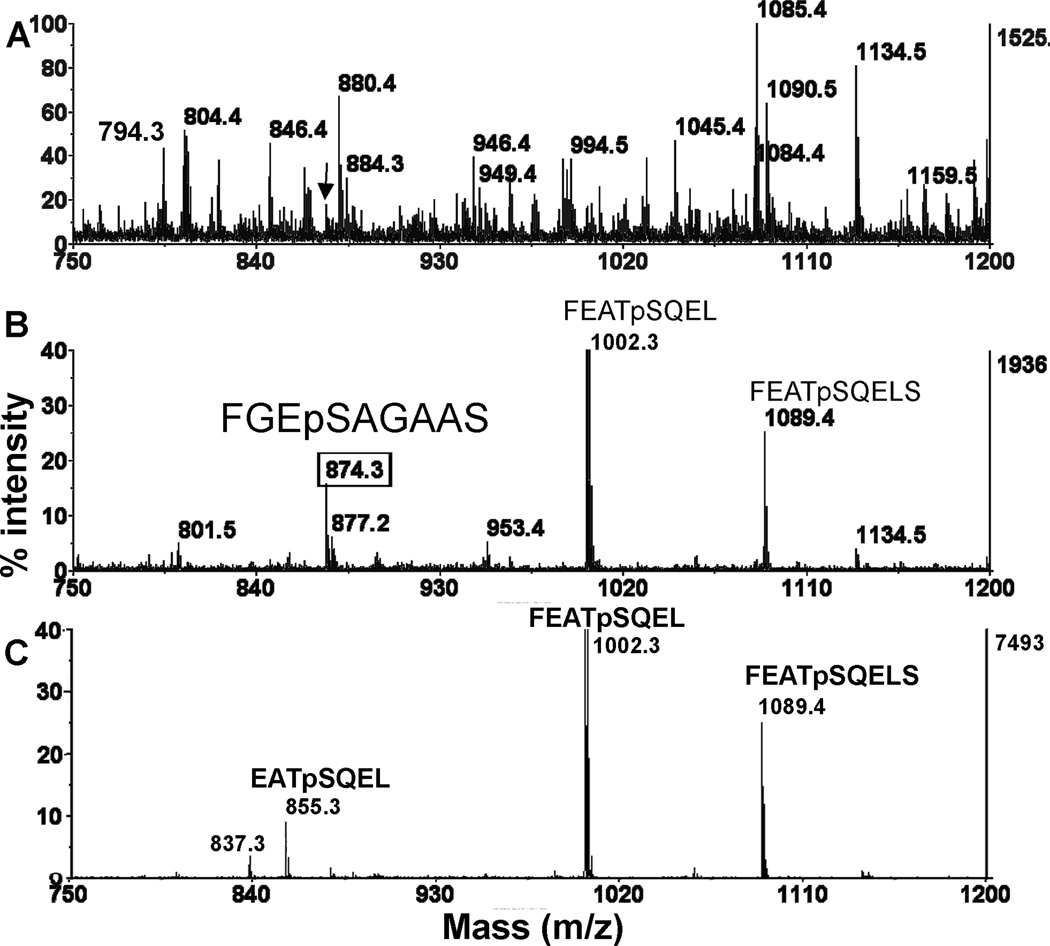
Blood from twelve randomly selected jet airplane passengers was tested for the presence of phosphorylated butyrylcholinesterase. Six out of 12 passengers were positive for phosphorylated butyrylcholinesterase. None of these passengers had symptoms of toxicity, such as dizziness, upset stomach, nausea, blurred vision, disorientation, shaking, or tingling sensations and none perceived smoke, haze, or fumes in the cabin of their aircraft. They did not notice the dirty sock smell that is associated with exposure to toxic jet oil fumes. Their levels of exposure were very low, averaging 0.05 to 1% phosphorylated butyrylcholinesterase. The passenger with the highest level of phosphorylated butyrylcholinesterase was estimated to have less than 3% phosphorylation. The MS spectra in Figure 5 are from this passenger. A small peak at 874.3 Da (indicated by the arrow) is visible in Figure 5A for pepsin digested butyrylcholinesterase before enrichment on titanium oxide. Samples from other positive passengers who had a lower level of phosphorylation showed a peak at 874.3 Da only after phosphopeptides were purified on titanium oxide.
Figure 5.
Presence of phosphorylated butyrylcholinesterase in the blood of a jet airplane passenger. The jet airplane passenger had no symptoms of toxicity. Butyrylcholinesterase purified from the passenger’s blood contained about 3% phosphorylated butyrylcholinesterase. The panels show MALDI-TOF MS spectra in negative mode. A) A peptic digest of 12 units of 15.5% pure butyrylcholinesterase (specific activity 112 units/mg) from a jet airplane passenger. The arrow points to a small peak at 874.3 Da. B) MS spectrum of the same digest after enrichment of phosphopeptides on titanium oxide. The mass at 874.3 Da corresponds to the phosphorylated peptide FGEpSAGAAS which is the active-site peptide of butyrylcholinesterase. Porcine pepsin (accession # P00791) is phosphorylated on Ser68 by endogenous kinases (Sielecki et al., 1990). The mass at 1002.3 Da is the phosphorylated peptide FEATpSQEL from porcine pepsin. A missed cleavage yields a second phosphorylated porcine pepsin peptide with the sequence FEATpSQELS and a mass of 1089.4 Da. C) MS spectrum of 20 µg pepsin enriched for phosphorylated peptides under the same conditions as the digest in panel B, but in the absence of butyrylcholinesterase. The three phosphorylated peptides are all from pepsin. The amino acid sequence and site of phosphorylation were determined for each phosphorylated peptide by analysis of MSMS spectra (data not shown).
The 3% phosphorylation estimate is made from the observation that at 3% phosphorylation (and higher) there is a visible peak at 874.3 Da in the pepsin digest before it has undergone the titanium oxide enrichment step. Samples in which only 1% of the butyrylcholinesterase is phosphorylated (0.166 µg phosphorylated butyrylcholinesterase), do not show a peak at 874.3 Da in pepsin-digested butyrylcholinesterase, though they do show a strong peak after enrichment on titanium oxide.
Four of the six positive passengers donated blood for our study 3 to 7 months after their last airplane flight. Their butyrylcholinesterase had no detectable adducts after this passage of time. All traces of phosphorylated butyrylcholinesterase are expected to be cleared after one month, because butyrylcholinesterase has a half-life of about 12 days in the circulation. If phosphorylated butyrylcholinesterase is found at much later times after exposure (as we are finding in mice; unpublished results) the explanation could be that tri-o-cresyl phosphate is stored in fat depots and is slowly released to undergo activation to CBDP which inhibits newly synthesized butyrylcholinesterase.
The low levels of exposure we have detected by mass spectrometry cannot be detected by butyrylcholinesterase activity assays. Quadruplicate assays using 0.02 ml of serum or plasma in a 2 ml reaction volume can vary by 1 to 5%. Individuals in our study, with no evidence of butyrylcholinesterase phosphorylation, showed butyrylcholinesterase activity levels between 1.3 and 3.4 units/ml (individuals #12 and #5, respectively, see Table 1). Table 1 also shows that the individuals with the lowest activities (#9a and #12 with 1.7 and 1.3 units/ml, respectively) had no detectable butyrylcholinesterase adducts. A second sample of blood from four of the positive passengers (1a, 3a, 4a and 6a) was drawn 6 months (1b), 3 months (3b and 4b), and 7 months (6b) after their last flights. The samples were negative for phosphorylated butyrylcholinesterase. For passenger #3 the butyrylcholinesterase activity was lower in the negative (3b) than in the positive sample (3a). These examples make clear that butyrylcholinesterase activity does not provide a reliable means for determining low-level exposure to tri-o-cresyl phosphate. In view of the wide range in normal activities, reliable determination of even 50% adduct formation would be questionable.
Table 1.
Jet airplane passengers
| passenger number |
passenger sex |
passenger race |
phosphorylated BChE |
BChE activity, units/ml |
number of flights in the trip |
Flight duration, hours |
destination |
|---|---|---|---|---|---|---|---|
| 1a | female | White | positive | 2.7 | 2 | 6 | domestic USA |
| 1b | female | White | negative | 3.2 | 0 | 0 | no airplane 6 months |
| 2 | male | White | positive | 3.0 | 2 | 10.5 | transcontinental |
| 3a | female | White | positive | 2.4 | 8 | 25 | transcontinental |
| 3b | female | White | negative | 2.2 | 0 | 0 | no airplane 3 months |
| 4a | female | White | positive | 2.2 | 2 | 5 | domestic USA |
| 4b | female | White | negative | 2.3 | 0 | 0 | no airplane 3 months |
| 5 | female | White | positive | 3.4 | 4 | 10 | domestic USA |
| 6a | female | Asian | positive | 2.2 | 2 | 12.5 | transcontinental |
| 6b | female | Asian | negative | 3.3 | 0 | 0 | no airplane 7 months |
| 7a | female | Asian | negative | 2.6 | 4 | 9 | domestic USA |
| 7b | female | Asian | negative | 2.6 | 0 | 0 | no airplane 15 months |
| 8 | female | White | negative | 2.5 | 2 | 2.5 | domestic USA |
| 9a | male | White | negative | 1.7 | 5 | 9 | domestic USA |
| 9b | male | White | negative | 2.0 | 0 | 0 | no airplane 4 months |
| 10 | male | White | negative | 2.9 | 3 | 3 | domestic USA |
| 11 | female | White | negative | 2.7 | 8 | 17 | transcontinental |
| 12 | female | White | negative | 1.3 | 4 | 22.5 | transcontinental |
White passengers were non-Hispanic. Positive samples contain 0.05% to 3% phosphorylated butyrylcholinesterase (BChE). Negative samples contain less than 0.05% phosphorylated butyrylcholinesterase. Butyrylcholinesterase activity was tested in quadruplicate; the standard deviation for activity assays ranged from 1 to 5%. Four positive and two negative passengers were retested 3 to 15 months after their last jet airplane trip; results for the retested samples are in rows indicated by the letter “b”.
Table 1 lists the number and duration of flights taken by the jet airplane passengers in our study. The highest concentration of tri-o-cresyl phosphate contamination in cockpit air occurs during ground engine starts (Hanhela et al., 2005). This suggests that a person who has taken more flights is more likely to have been exposed to tri-o-cresyl phosphate. “Fume events” have been reported on most aircraft types including BAE 146, Airbus 320, Boeing 737, Boeing 757, Canadair CL600, Cessna 750, Douglas DC9, Embraer RJ-145, and McDonnell Douglas MD80 (Murawski and Supplee, 2008; Ross, 2008). The number of samples in Table 1 is too low to make a correlation between exposure to tri-o-cresyl phosphate and duration or number of flights responsible for the positive readings.
No adduct on butyrylcholinesterase purified from pooled plasma
It has been suggested that common environmental chemicals could be responsible for the phosphorylated butyrylcholinesterase that we detected in jet airplane passengers. To test this hypothesis we examined butyrylcholinesterase purified from 70 Liters of pooled plasma, donated by over 200 individuals. The highly purified butyrylcholinesterase from this large-scale preparation was tested for the presence of phosphorylated adduct and was found to be negative. If a common environmental chemical were responsible for phosphorylation of the active site of butyrylcholinesterase, we would have expected to see that adduct in butyrylcholinesterase purified from pooled plasma. Thus, reaction of butyrylcholinesterase with common environmental chemicals does not explain the phosphorylated butyrylcholinesterase adduct found in jet airplane passengers. To date we have found the phosphoserine adduct of butyrylcholinesterase only in jet airplane passengers.
Visualization of phosphoproteins on polyacrylamide gels
It was of interest to determine whether phosphorylated butyrylcholinesterase could be visualized and quantified by polyacrylamide gel electrophoresis. The Pro-Q Diamond phosphoprotein stain (Invitrogen) detects phosphorylated proteins in polyacrylamide gels. Plasma and purified butyrylcholinesterase samples with and without CBDP treatment were examined (see Figure 6). Lanes on the non-denaturing gel in Figure 6A were loaded with a relatively large volume of plasma, 10 and 5 µl, because albumin (65 kDa) separates well from butyrylcholinesterase tetramers (340 kDa) in a nondenaturing gel. In contrast, lanes on the SDS denaturing gel (see Figures 6B and 6C) were loaded with less than 1 µl plasma to avoid overlap between the albumin (65 kDa) and butyrylcholinesterase monomer (85 kDa) bands.
The plasma samples on the nondenaturing gel in Figure 6A show many bands that stain heavily for phosphoproteins, consistent with the fact that human plasma contains 443 phosphorylated proteins http://www.plasmaproteomedatabase.org/. A band for butyrylcholinesterase is not visible in plasma treated with CBDP to a final concentration of 5 mM (which inhibits 100% of the butyrylcholinesterase activity). See lanes A1 and A2 in Figure 6A.
Unexpectedly, 50% pure untreated control butyrylcholinesterase in lanes A7 and A8 of the nondenaturing gel (Figure 6A) stains with Pro-Q Diamond. Similarly, 50% pure untreated control butyrylcholinesterase in lane B5 of the SDS gel stains with Pro-Q Diamond. The control butyrylcholinesterase had no detectable phosphorylation on the active site serine. To determine why control butyrylcholinesterase stains with Pro-Q Diamond, we counterstained the Pro-Q Diamond gel with Coomassie Blue (Figure 6C). The 85 kDa gel band was excised from lane C5, digested with trypsin, and the proteins identified by MALDI-TOF/TOF mass spectrometry. It was found that in addition to butyrylcholinesterase, the band at 85 kDa contained small amounts of diacylglycerol kinase (accession # gi119617256), arginyl tRNA synthetase (accession # gi1217668), ribonuclease ZC3H12C (accession # gi148886668), and vinculin (accession # gi4507877). Three of these contaminating proteins are phosphoproteins, thus explaining the background phosphoprotein staining from highly purified butyrylcholinesterase.
It was concluded that low levels of phosphorylated butyrylcholinesterase in partially purified preparations of butyrylcholinesterase could not be confidently determined by staining for phosphoproteins with Pro-Q Diamond. The butyrylcholinesterase would have to be purified to 100% purity, or a method would have to be developed that separates butyrylcholinesterase from contaminating phosphoproteins, before staining with Pro-Q Diamond would be useful for detecting low dose exposure to tri-o-cresyl phosphate.
However, the SDS gel in Figure 6B provides visual proof that butyrylcholinesterase is phosphorylated by treatment with CBDP. The intensity of the Pro-Q Diamond stained bands in lanes B6, B7, and B8 progressively increases as the CBDP-butyrylcholinesterase content of the lanes increases (from 0.9, to 2.2, to 3.5 µg CBDP-butyrylcholinesterase). In all three of these lanes, the Pro-Q Diamond staining intensity is greater than the intensity of the 5 µg untreated control butyrylcholinesterase in lane B5. The SDS gel counterstained with Coomassie Blue in Figure 6C demonstrates that the amount of butyrylcholinesterase protein in the control butyrylcholinesterase lane, C5, is greater than the amounts of CBDP-butyrylcholinesterase protein in lanes C6, C7, and C8. These observations support the conclusion that butyrylcholinesterase is phosphorylated by CBDP. Thus, staining with Pro-Q Diamond could be used to visualize high dose exposure to tri-o-cresyl phosphate.
Partially purified (8%) untreated control butyrylcholinesterase in lane B3 contained several phosphoprotein bands. This control butyrylcholinesterase was not treated with CBDP but it had a phosphoprotein band at the position of butyrylcholinesterase. This illustrates and confirms the conclusion that staining for phosphoprotein cannot be used with partially purified butyrylcholinesterase if the goal is to demonstrate low dose exposure to tri-o-cresyl phosphate.
Discussion
Phosphorylated butyrylcholinesterase as a biomarker for exposure to tri-ortho-cresyl phosphate
Poisoning by tri-ortho-cresyl phosphate is known to inhibit the activity of butyrylcholinesterase in the plasma, spinal cord, and brain of chickens and rabbits (Earl and Thompson, 1952; Aldridge, 1954). Tri-ortho-cresyl phosphate becomes an inhibitor only after metabolic activation by cytochrome P450 to cresyl saligenin phosphate (Eto et al., 1962). Reaction of cresyl saligenin phosphate with butyrylcholinesterase results in a unique phosphorylated butyrylcholinesterase adduct with an added mass of 80 Da (Schopfer et al., 2010; Carletti et al. 2011 in press). Thus, phosphorylated butyrylcholinesterase serves as a biomarker for exposure to tri-o-cresyl phosphate, as demonstrated in the present report.
Other potential biomarkers include acyl peptide hydrolase in red blood cells and neuropathy target esterase in lymphocytes and platelets (Dudek and Richardson, 1982; Bleecker et al., 1983; Quistad et al., 2005; Kim et al., 2010). Acetylcholinesterase, though appealing as a biomarker, is 100-fold less sensitive to inhibition by CBDP compared to butyrylcholinesterase (Earl and Thompson, 1952; Carletti et al., 2011 in press). The bimolecular inhibition rate constant for human acetylcholinesterase is 106 M−1 min−1 whereas for human butyrylcholinesterase it is 108 M−1 min−1 (Carletti et al., 2011 in press). Therefore acetylcholinesterase would not be suitable for detection of low dose exposure.
Our assay for tri-o-cresyl phosphate exposure and aerotoxic syndrome
Over the past 20 years, a growing list of reports from passengers and aircrew on jet airplanes documents the occurrence of adverse symptoms following exposure to fumes from the engines. The term “aerotoxic syndrome” has been coined to describe these events. Winder defines aerotoxic syndrome: “features of this syndrome are that it is associated with air crew exposure at altitude to atmospheric contaminants from engine oil or other aircraft fluids, temporarily juxtaposed by the development of a consistent symptomalogy including short-term skin, gastro-intestinal, respiratory and nervous system effects, and long-term central nervous and immunological effects; this syndrome may be reversible following brief exposures, but features have emerged of a chronic syndrome following significant exposures.” (Winder, 2006). The principal toxic constituents of pyrolized jet engine oil are tricresyl phosphates, carbon monoxide, and N-phenyl-L-naphthylamine (Winder, 2006). The consensus of opinion among researchers in this area is that tri-cresyl phosphates containing at least one ortho-cresyl moiety are most likely to be the causative agents in this syndrome. Mono-o-cresyl phosphate and di-o-cresyl phosphate have been found to be more toxic than tri-o-cresyl phosphate when administered to chickens (tricresyl phosphates composed entirely of meta- and para-cresyl moieties are non-toxic) (Henschler, 1958). Baker and Furlong have shown that Durad 125, a commercial mixed-isomer additive for jet engine oils, is readily converted into an inhibitor for butyrylcholinesterase by either human or rat liver microsomes, despite the fact that it has a very low level of the tri-o-isomer (unpublished data). These observations strongly suggest that the tri-o-isomer is the least toxic of the ortho-cresyl phosphates, nevertheless the focus of this report is on tri-o-cresyl phosphate. The reasons for choosing to focus attention on tri-o-cresyl phosphate are: 1) Most of the basic, scientific studies have been conducted on tri-o-cresyl phosphate meaning that the pathways for activation of this compound and the consequences of exposure are well established. In contrast, it is not known whether mono-o-cresyl phosphate and di-o-cresyl phosphate are metabolically activated to CBDP. 2) The activated toxin from tri-o-cresyl phosphate, CBDP, is available for study. 3) The details of the reaction of CBDP with butyrylcholinesterase and the ultimate formation of phosphorylated butyrylcholinesterase are well documented. 4) The reactions of CBDP with other esterases and with proteins in general have been investigated. 5) The airlines industry, regulatory agencies and the manufactures of tricresyl phosphates all have focused their attention on tri-o-cresyl phosphate. 6) Results obtained from tri-o-cresyl phosphate/CBDP studies can be extrapolated more easily to the results expected from the more toxic cresyl phosphates, whereas the converse would be difficult to defend.
The proposal that aerotoxic syndrome is caused by ortho-cresyl phosphates is based on the knowledge that engine oil contains ortho-cresyl phosphates and that ortho-cresyl phosphates are known to cause neuropathy. However, heretofore there has been no direct evidence to support the contention that there is enough ortho-cresyl phosphate in the cabin air of jet aircraft to cause any detectable, physiological changes in passengers or crew. The purpose of our study has been to test that proposal. In other words, to answer the question: is there enough tri-o-cresyl phosphate in the cabin air of a jet airplane to cause detectable protein-adduct formation in the passengers? The answer is yes. We have found phosphorylated butyrylcholinesterase in the blood of freshly disembarked airline passengers where those same passengers showed no phosphorylated butyrylcholinesterase several months after their air travel. Phosphorylated butyrylcholinesterase is diagnostic for exposure to tri-o-cresyl phosphate (though other ortho-cresyl phosphates may also create this adduct). Therefore, exposure of airplane passengers to contaminants from jet engine oil can result in protein adduct formation. Recently, tricresyl phosphate levels were measured on board BAe-146-300 aircraft. The background levels (with no overt “fume event”) ranged from 31 to 83 nanograms/m3 (van Netten, 2009). It follows that even such a low tricresyl phosphate concentration in the ambient air in jet airplanes (or perhaps even in the airline terminals) contains sufficient tri-o-cresyl phosphate to cause protein adduct formation that is detectable in our assay.
Jet airplane travel is safe for infrequent flyers
Half of the blood samples from the jet airplane travelers that we tested had detectable levels of phosphorylated butyrylcholinesterase, which means these passengers were exposed to tri-ortho-cresyl phosphate. The adduct levels were very low and no toxic symptoms were observed for any individual in our study group. None of our travelers reported a “fume event” in the airplane where release of engine oil into the bleed air could be detected. We were able to obtain a second blood sample from four individuals who tested positive, 3 to 7 months after their flights. The second samples were negative for phosphorylated butyrylcholinesterase. It follows that jet airplane travel is generally safe for the infrequent flyer. However, we suggest that pilots and aircrew are frequently exposed to small doses of tri-o-cresyl phosphate when they fly. Turnover of butyrylcholinesterase in the blood has a half-life of about 12 days. It follows that phosphorylated butyrylcholinesterase would accumulate in their blood. Adducts of any other proteins/enzymes that react with CBDP would likewise accumulate. Such accumulations would be compounded if individuals were to experience a “fume event” where they would be exposed to a large dose of tri-o-cresyl phosphate. The US commercial fleet is estimated to have 0.86 “fume events” per day (Murawski and Supplee, 2008). It is expected that pilots and aircrew will have significantly higher levels of butyrylcholinesterase adducts than the passengers in our study group.
The next step
The next research question is whether the level of tri-o-cresyl phosphate exposure correlates with the symptoms associated with aerotoxic syndrome. Do people with aerotoxic syndrome have higher amounts of phosphorylated butyrylcholinesterase in their blood? To answer this question we aim to extend our research to pilots, crew members and airport workers. It will be necessary to recruit a large population. A high-throughput sample screening technique that uses only 1 or 2 ml of plasma would be preferred. An immunomagnetic method for purification of butyrylcholinesterase from 0.5 ml plasma has been developed by Sporty et al. (Sporty et al., 2010). The drawback of this method is the high cost of the antibody. The sensitivity of our assay would be improved by employing liquid chromatography mass spectrometry (LC-MS) instead of MALDI-TOF mass spectrometry. Use of LC-MS is expected to require a lower volume of plasma than the 25 ml in our present protocol.
Possible mechanism of toxicity
Is CBDP (i.e. the bioactivated metabolite of tri-o-cresyl phosphate) responsible for the symptoms associated with aerotoxic syndrome? This question has no answer with the data currently available. The symptoms for aerotoxic syndrome are rather general. They can be divided into two categories, short-term symptoms: dizziness, confusion, difficulty breathing, upset stomach, blurred vision, disorientation, shaking, tingling sensations, increased heart rate, watery eyes, and runny nose; and long-term symptoms: numbness, sleep disorders, memory loss, tremors, salivation, diarrhea, itching, fatigue, weakness, and joint pain; to name a few (Winder, 2006). For the record, short-term symptoms such as dizziness, headache, and blurred vision are consistent with carbon monoxide exposure. On the other hand, symptoms that persist for months or years suggest neurodegeneration. It would not be unreasonable to suggest that aerotoxic syndrome is a consequence of exposure to a number of toxicants, only one of which is tri-o-cresyl phosphate. But, if we allow that CBDP contributes to the aerotoxic syndrome symptomalogy, how is that contribution manifested?
Since CBDP reacts readily with butyrylcholinesterase, it is tempting to speculate on butyrylcholinesterase as the target responsible for the symptoms. However, butyrylcholinesterase activity has largely recovered before signs of tri-o-cresyl phosphate poisoning appear in chickens (Earl and Thompson, 1952). In addition, inhibition of butyrylcholinesterase with many potent organophosphorus agents does not lead to neuropathy. These observations together with the fact that butyrylcholinesterase has no known function make it unlikely that inhibition of butyrylcholinesterase will explain neurotoxicity (Abou-Donia, 1993).
Inhibition of acetylcholinesterase would be another obvious candidate for the source of the neurotoxicity. However, there is very little inhibition of acetylcholinesterase at doses of tri-o-cresyl phosphate that cause clear neuropathy in chickens and rabbits (Earl and Thompson, 1952; Aldridge, 1954). There are potent organophosphorus inhibitors for acetylcholinesterase that do not cause neuropathy. Therefore it is unlikely that inhibition of acetylcholinesterase is responsible for tri-o-cresyl phosphate neurotoxicity.
Neurotoxic esterase is an appealing candidate because all organophosphorus agents that are known to cause neuropathy react well with neuropathy target esterase. However, it is unclear how inhibition of neuropathy target esterase would lead to aerotoxic syndrome.
The hypothesis we favor is based on the work of Abou-Donia and co-workers. Patton et al. reported that poisoning with tri-ortho-cresyl phosphate results in increased phosphorylation by gamma 32P-ATP of proteins in the brain and spinal cord of hens (Patton et al., 1983). The hyperphosphorylated proteins are tubulin, microtubules, microtubule-associated protein 2, neurofilament proteins, calmodulin kinase II, myelin basic protein, cyclin-dependent kinase 5 and its activator p35/p25 (Suwita et al., 1986; Lapadula et al., 1991; Wang et al., 2006). Other organophosphorus agents also increase phosphorylation of brain proteins (Abou-Donia et al., 1993). For example, the CREB transcription factor is hyperphosphorylated in primary cultures of cortical and hippocampal neurons treated with chlorpyrifos (Schuh et al., 2002). In the face of these observations, the question arises: what do organophosphorus agents do to cause hyperphosphorylation? The answer may lie in direct organophosphorylation of proteins other than esterases.
We have found organophosphorylated tubulin in the brains of mice treated with nontoxic doses of chlorpyrifos (Jiang et al., 2010). The organophosphorylated tubulin does not dephosphorylate but rather forms a stable adduct (Jiang et al., 2010). We hypothesize that organophosphorylation may have an effect similar to that of phosphorylation on proteins that change activity states in response to phosphorylation. If for example, the organophosphorylated protein is a kinase, and if the kinase is activated by organophosphorylation, then the organophosphorylated kinase would become overactive leading to hyperphosphorylation of its targets. Hyperphosphorylation of cytoskeletal proteins observed by Patton et al. could be explained by an overactive kinase. Consistent with this hypothesis is the finding that calmodulin kinase II is overactive in the brains of tri-o-cresyl phosphate treated hens (Lapadula et al., 1991).
The consequences of hyperphosphorylation can be severe. Hyperphosphorylation disrupts the cycle of phosphorylation and dephosphorylation that is essential for proper functioning of the nervous system. Cytoskeletal proteins that are hyperphosphorylated do not transport cell components down the axon to the nerve terminals at a normal rate (Gupta et al., 1997). The consequence of axonal transport dysregulation is loss of connections between neurons and a slow dying back of synapses (Morfini et al., 2009). The result of synapse disappearance is a slow neurodegeneration.
More simply stated, we propose a mechanism for tri-o-cresyl phosphate induced neurodegeneration in which low dose exposure to tri-o-cresyl phosphate causes disruption of axonal transport (Terry et al., 2007). The axonal transport disruption initiates irreversible degradation of neurons and loss of neuronal function. This process could also be caused by other organophosphorus agents. In this context, phosphorylation of butyrylcholinesterase observed in our study cannot explain toxicity of tri-o-cresyl phosphate, but rather indicates that the formation of adducts does occur in vivo and may involve multiple targets.
Highlights.
Travel on jet airplanes is associated with an illness, aerotoxic syndrome.
A possible cause is exposure to tricresyl phosphate in engine lubricating oil.
A blood test for exposure to tri-o-cresyl phosphate is reported.
Acknowledgement
We thank the travelers who donated blood for this project. Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
Funding
Supported by U.S. Army Medical Research and Materiel Command (W81XWH-07-2-0034), the NIH (U01 NS058056, P30CA36727, R01ES09883, and P42ES04696), and funding from pilot and flight attendant unions, the Royal Australian Air Force, the Norwegian Union of Energy Workers (SAFE), and NYCO S.A. Graduate studies for ML were supported by a fellowship from the Department of Environmental, Agricultural and Occupational Health, College of Public Health, University of Nebraska Medical Center. Financial support to FN from the Direction Générale de l’Armement (Contract 08ca501) is acknowledged.
abbreviations
- BChE
butyrylcholinesterase
- CBDP
cresyl saligenin phosphate
- DHB
2,5-dihydroxybenzoic acid
- MALDI-TOF
matrix assisted laser desorption ionization-time of flight mass spectrometry
- MS
mass spectrum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
Contributor Information
Mariya Liyasova, Email: mliyasov@unmc.edu.
Bin Li, Email: binli@unmc.edu.
Lawrence M. Schopfer, Email: lmschopf@unmc.edu.
Florian Nachon, Email: fnachon@nachon.net.
Patrick Masson, Email: pmasson@unmc.edu.
Clement E. Furlong, Email: clem@uw.edu.
Oksana Lockridge, Email: olockrid@unmc.edu.
References
- Abou-Donia MB. The cytoskeleton as a target for organophosphorus ester-induced delayed neurotoxicity (OPIDN) Chem Biol Interact. 1993;87:383–393. doi: 10.1016/0009-2797(93)90066-8. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Viana ME, Gupta RP, Anderson JK. Enhanced calmodulin binding concurrent with increased kinase-dependent phosphorylation of cytoskeletal proteins following a single subcutaneous injection of diisopropyl phosphorofluoridate in hens. Neurochem Int. 1993;22:165–173. doi: 10.1016/0197-0186(93)90009-t. [DOI] [PubMed] [Google Scholar]
- Aldridge WN. Tricresyl phosphates and cholinesterase. Biochem J. 1954;56:185–189. doi: 10.1042/bj0560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker ML, Maroni M, Sepanski M. A biologic marker for organophosphate exposure: neurotoxic esterase activity in human lymphocytes and platelets. Dev Toxicol Environ Sci. 1983;11:507–512. [PubMed] [Google Scholar]
- Carletti E, Li H, Li B, Ekstrom F, Nicolet Y, Loiodice M, Gillon E, Froment MT, Lockridge O, Schopfer LM, Masson P, Nachon F. Aging of cholinesterases phosphylated by tabun proceeds through O-dealkylation. J Am Chem Soc. 2008;130:16011–16020. doi: 10.1021/ja804941z. [DOI] [PubMed] [Google Scholar]
- Carletti E, Schopfer LM, Colletier JP, Froment MT, Nachon F, Weik M, Lockridge O, Masson P. Reaction of cresyl saligenin phosphate, the organophosphorus implicated in the aerotoxic syndrome, with human cholinesterases: mechanistic studies employing kinetics, mass spectrometry and x-ray structure analysis. Chem Res Toxicol. 2011 doi: 10.1021/tx100447k. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L, Michaelis S. A survey of health symptoms in BAe 146 aircrew. J Occup Health Safety - Aust NZ. 2002;18:305–312. [Google Scholar]
- Crump D, Harrison P, Walton C. Aircraft cabin air sampling study. Institute of Environment and Health, Cranfield University, UK. 2011 [Google Scholar]
- De Nola G, Kibby J, Mazurek W. Determination of ortho-cresyl phosphate isomers of tricresyl phosphate used in aircraft turbine engine oils by gas chromatography and mass spectrometry. J Chromatogr A. 2008;1200:211–216. doi: 10.1016/j.chroma.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Dudek BR, Richardson RJ. Evidence for the existence of neurotoxic esterase in neural and lymphatic tissue of the adult hen. Biochem Pharmacol. 1982;31:1117–1121. doi: 10.1016/0006-2952(82)90351-3. [DOI] [PubMed] [Google Scholar]
- Earl CJ, Thompson RH. Cholinesterase levels in the nervous system in tri-ortho-cresyl phosphate poisoning. Br J Pharmacol Chemother. 1952;7:685–694. doi: 10.1111/j.1476-5381.1952.tb00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M, Casida JE, Eto T. Hydroxylation and cyclization reactions involved in the metabolism of tri-o-cresyl phosphate. Biochem Pharmacol. 1962;11:337–352. doi: 10.1016/0006-2952(62)90056-4. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anticholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Grunwald J, Marcus D, Papier Y, Raveh L, Pittel Z, Ashani Y. Large-scale purification and long-term stability of human butyrylcholinesterase: a potential bioscavenger drug. J Biochem Biophys Methods. 1997;34:123–135. doi: 10.1016/s0165-022x(97)01208-6. [DOI] [PubMed] [Google Scholar]
- Gupta RP, Abdel-Rahman A, Wilmarth KW, Abou-Donia MB. Alteration in neurofilament axonal transport in the sciatic nerve of the diisopropyl phosphorofluoridate (DFP)-treated hen. Biochem Pharmacol. 1997;53:1799–1806. doi: 10.1016/s0006-2952(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Hanhela PJ, Kibby J, DeNola G, Mazurek W. Organophosphate and amine contamination of cockpit air in the Hawk, F-111 and Hercules C-130 aircraft. Australian Govt Defence Science and Technology Organisation. 2005:1–21. DSTO-RR-0303 online. [Google Scholar]
- Heath DF, Casapieri P. Hydrolysis of dimethylamides of phosphoric acids. Trans. Faraday Soc. 1951;47:1093–1101. [Google Scholar]
- Henschler D. Tricresylphosphate poisoning; experimental clarification of problems of etiology and pathogenesis. Klin Wochenschr. 1958;36:663–674. doi: 10.1007/BF01488746. [DOI] [PubMed] [Google Scholar]
- Jensen SS, Larsen MR. Evaluation of the impact of some experimental procedures on different phosphopeptide enrichment techniques. Rapid Commun Mass Spectrom. 2007;21:3635–3645. doi: 10.1002/rcm.3254. [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Tox Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso AG, Charlesworth JM, McVea GG. Contamination of environmental control systems in Hercules aircraft. Department of Defence, Defence Science and Technology Organisation Salisbury, Melbourne, Australia. 1988 MRL-R-1116. [Google Scholar]
- Kim JH, Stevens RC, Maccoss MJ, Goodlett DR, Scherl A, Richter RJ, Suzuki SM, Furlong CE. Identification and characterization of biomarkers of organophosphorus exposures in humans. Adv Exp Med Biol. 2010;660:61–71. doi: 10.1007/978-1-60761-350-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp TJ, Richardson RJ. Aging of mipafox-inhibited human acetylcholinesterase proceeds by displacement of both isopropylamine groups to yield a phosphate adduct. Chem Res Toxicol. 2006;19:334–339. doi: 10.1021/tx050342o. [DOI] [PubMed] [Google Scholar]
- Kropp TJ, Richardson RJ. Mechanism of aging of mipafox-inhibited butyrylcholinesterase. Chem Res Toxicol. 2007;20:504–510. doi: 10.1021/tx600310y. [DOI] [PubMed] [Google Scholar]
- Lapadula ES, Lapadula DM, Abou-Donia MB. Persistent alterations of calmodulin kinase II activity in chickens after an oral dose of tri-o-cresyl phosphate. Biochem Pharmacol. 1991;42:171–180. doi: 10.1016/0006-2952(91)90696-3. [DOI] [PubMed] [Google Scholar]
- Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Masson P, Lockridge O. Dichlorvos, chlorpyrifos oxon, and aldicarb adducts of butyrylcholinesterase detected by mass spectrometry in human plasma following deliberate overdose. J Appl Toxicol. 2010;30:559–565. doi: 10.1002/jat.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ricordel I, Tong L, Schopfer LM, Baud F, Megarbane B, Maury E, Masson P, Lockridge O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J Appl Toxicol. 2009;29:149–155. doi: 10.1002/jat.1392. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J Med CBR Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MR, Wier GT, Zieve FJ, Anders MW. Human intoxication following inhalation exposure to synthetic jet lubricating oil. Clin Toxicol. 1977;11:423–426. doi: 10.3109/15563657708988205. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski JTL, Supplee DS. An attempt to characterize the frequency, health impact, and operational costs of oil in the cabin and flight deck supply air on U.S. commercial aircraft. J of ASTM International. 2008;5:1–15. [Google Scholar]
- Patton SE, O'Callaghan JP, Miller DB, Abou-Donia MB. Effect of oral administration of tri-o-cresyl phosphate on in vitro phosphorylation of membrane and cytosolic proteins from chicken brain. J Neurochem. 1983;41:897–901. doi: 10.1111/j.1471-4159.1983.tb04826.x. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol Sci. 2005;86:291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Ross SM. Cognitive function following exposure to contaminated air on commercial aircraft: a case series of 27 pilots seen for clinical purposes. J Nutr Environ Med. 2008;17:111–126. [Google Scholar]
- Schopfer LM, Furlong CE, Lockridge O. Development of diagnostics in the search for an explanation of aerotoxic syndrome. Anal Biochem. 2010;404:64–74. doi: 10.1016/j.ab.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Sielecki AR, Fedorov AA, Boodhoo A, Andreeva NS, James MN. Molecular and crystal structures of monoclinic porcine pepsin refined at 1.8 A resolution. J Mol Biol. 1990;214:143–170. doi: 10.1016/0022-2836(90)90153-D. [DOI] [PubMed] [Google Scholar]
- Sporty JL, Lemire SW, Jakubowski EM, Renner JA, Evans RA, Williams RF, Schmidt JG, van der Schans MJ, Noort D, Johnson RC. Immunomagnetic separation and quantification of butyrylcholinesterase nerve agent adducts in human serum. Anal Chem. 2010;82:6593–6600. doi: 10.1021/ac101024z. [DOI] [PubMed] [Google Scholar]
- Suwita E, Lapadula DM, Abou-Donia MB. Calcium and calmodulin stimulated in vitro phosphorylation of rooster brain tubulin and MAP-2 following a single oral dose of tri-o-cresyl phosphate. Brain Res. 1986;374:199–203. doi: 10.1016/0006-8993(86)90412-9. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- van der Schans MJ, Fidder A, van Oeveren D, Hulst AG, Noort D. Verification of exposure to cholinesterase inhibitors: generic detection of OPCW Schedule 1 nerve agent adducts to human butyrylcholinesterase. J Anal Toxicol. 2008;32:125–130. doi: 10.1093/jat/32.1.125. [DOI] [PubMed] [Google Scholar]
- van Netten C. Design of a small personal air monitor and its application in aircraft. Sci Total Environ. 2009;407:1206–1210. doi: 10.1016/j.scitotenv.2008.07.067. [DOI] [PubMed] [Google Scholar]
- van Netten C, Leung V. Hydraulic fluids and jet engine oil: pyrolysis and aircraft air quality. Arch Environ Health. 2001;56:181–186. doi: 10.1080/00039890109604071. [DOI] [PubMed] [Google Scholar]
- Wang YP, Mou DL, Song JF, Rao ZR, Li D, Ju G. Aberrant activation of CDK5 is involved in the pathogenesis of OPIDN. J Neurochem. 2006;99:186–197. doi: 10.1111/j.1471-4159.2006.04027.x. [DOI] [PubMed] [Google Scholar]
- Winder C. Hazardous chemicals on jet aircraft: case study - jet engine oils and aerotoxic syndrome. Curr Top Toxicol. 2006;3:65–88. [Google Scholar]
- Winder C, Balouet JC. The toxicity of commercial jet oils. Environ Res. 2002;89:146–164. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- Winder C, Fonteyn P, Balouet JC. Aerotoxic syndrome: a descriptive epidemiological survey of aircrew exposed to in-cabin airborne contaminants. J Occup Health Safety - Aust NZ. 2002;18:321–338. [Google Scholar]