Summary
Interleukin (IL)-23 and CD4+ T helper-17 (Th17) cells are thought to be critical in the development of psoriasis. Here, we report that IL-23 predominantly stimulated dermal γδT cells to produce IL-17 that led to disease progression. Dermal γδT cells constitutively expressed the IL-23 receptor (IL-23R), RORγt, and various chemokine receptors. IL-17 production from dermal γδT cells was independent of αβT cells. The epidermal hyperplasia and inflammation induced by IL-23 were significantly decreased in T cell receptor δ deficient (Tcrd−/−) and IL-17 receptor deficient (Il17ra−/−) mice but occurred normally in Tcra−/− mice. Imiquimod-induced skin pathology was also significantly decreased in Tcrd−/− mice. Perhaps further promoting disease progression, IL-23 stimulated dermal γδT cell expansion. In psoriasis patients, γδT cells were also greatly increased in affected skin and produced large amounts of IL-17. Thus, IL-23-responsive dermal γδ T cells are the major IL-17 producers in the skin and may represent a novel target for the treatment of psoriasis.
INTRODUCTION
Psoriasis is one of the most common immune-mediated chronic inflammatory skin disorders characterized by hyperproliferative keratinocytes and massive infiltration of leukocytes (Schon and Boehncke, 2005). Although the pathogenesis of psoriasis is not fully understood, there is growing evidence that the interleukin (IL)-23-T helper 17 (Th17) cell axis and Th17 cell-related cytokines play critical roles in disease development (Clark, 2010; Di Cesare et al., 2009; Zaba et al., 2009).
Psoriatic skin lesions are reported to have increased gene and protein expression of IL-23, IL-21, IL-22, and IL-17 (Boniface et al., 2007; Caruso et al., 2009; Johansen et al., 2009; Lee et al., 2004). IL-23-induced changes in mouse skin share many characteristics with human psoriasis, including erythematosus, hyperplasia of the epidermis (acanthosis), parakeratosis, and leukocyte infiltration (Chan et al., 2006). The dermal inflammation and acanthosis induced by IL-23 are thought to be mediated by a Th17 cell cytokine, IL-22 (Zheng et al., 2007) and the chemokine receptor CCR6 expression is required as well (Hedrick et al., 2009). The importance of IL-23 is further demonstrated by the therapeutic efficacy of human mAb against the subunit of p40 of IL-12 and IL-23 in the treatment of psoriasis (Griffiths et al., 2010; Krueger et al., 2007). IL-23-induced skin inflammation has primarily been linked to the function of Th17 cells and related cytokines (Di Cesare et al., 2009; Harper et al., 2009; Steinman, 2010; Zaba et al., 2009). Therefore, IL-23 and Th17 cells may be key mediators of disease pathogenesis (Blauvelt, 2008). One caveat of these studies is that although elevated IL-17 and IL-22 production is observed in psoriatic skin (Harper et al., 2009; Lowes et al., 2008; Wilson et al., 2007; Zaba et al., 2007), it is not presently known whether these cytokines, e.g. IL-17, are directly secreted by Th17 cells. Thus, the primary IL-17-producing cells responsive to IL-23 stimulation in skin remain to be determined. Furthermore, the pathogenic role of these cells that induce skin inflammation and acanthosis in psoriasis needs to be established. These issues are significant because psoriasis is currently considered to be a CD4+ Th17-mediated disease (Di Cesare et al., 2009; Lowes et al., 2008; Steinman, 2010).
Murine epidermis contains large numbers of γδ T cells (Hayday and Tigelaar, 2003). These γδ T cells have a marked dendritic morphology and have been named dendritic epidermal T cells (DETCs) (Havran and Jameson, 2010). In addition, γδ T cells exist in both human and murine dermis. In this study, we found that innate dermal γδ T cells - but not epidermal γδ T cells or dermal γδ− T cells were the major IL-17-producing cells in the skin following IL-23 stimulation. Deficiency of γδ T cells or IL-17 receptor significantly decreased IL-23-induced epidermal thickness and neutrophil infiltration. This was also the case in the imiquimod (IMQ)-induced psoriasis-like model. Furthermore, IL-17-secreting γδ T cells were present in high frequency in human psoriatic skin lesions. These observations support the idea that IL-17-producing dermal γδ T cells are a key component in the pathogenesis of psoriasis.
RESULTS
IL-23 is mainly produced by dermal dendritic cells (DCs) and macrophages (Mϕ) which is critical for IL-17 production in the skin
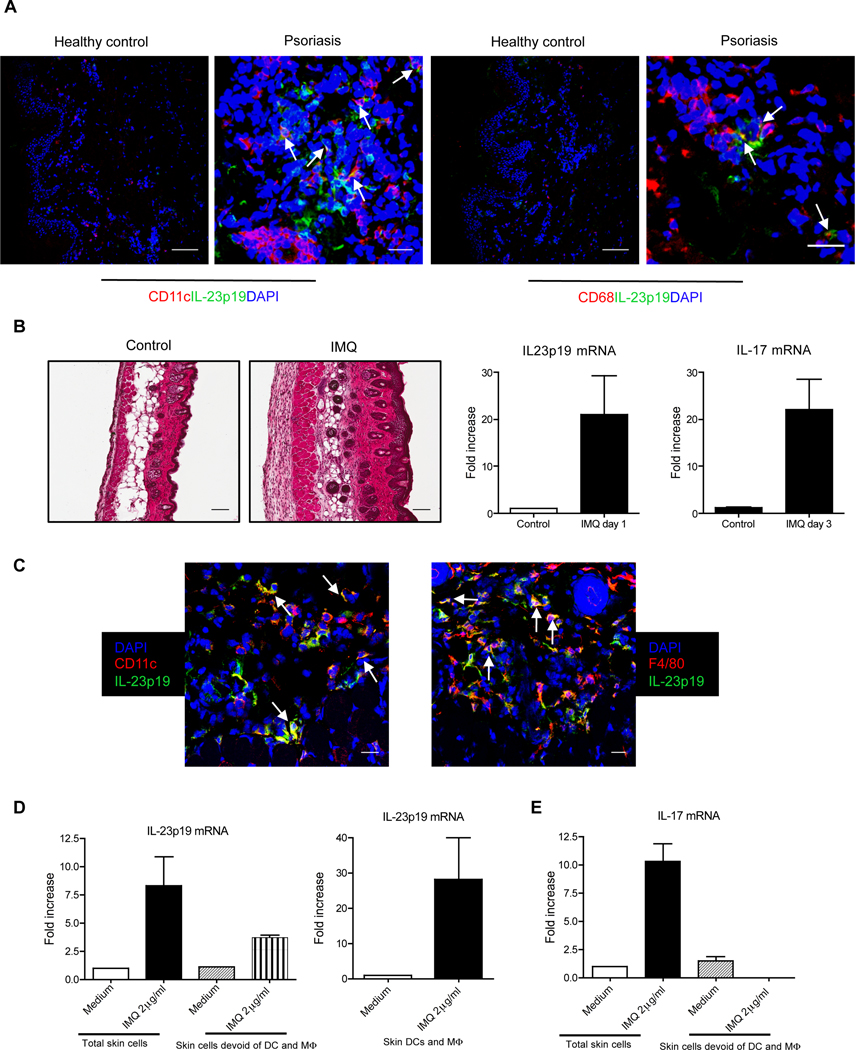
IL-23 has been clearly linked to the pathogenesis of psoriasis (Chan et al., 2006). Previous studies demonstrate that transcripts encoding IL-23p19 and IL-23p40 are increased during human psoriasis (Lee et al., 2004). To dissect the cellular source of IL-23, skin tissues from healthy individuals and patients with psoriasis were analyzed by immunofluorescent staining. We found that IL-23p19 protein was co-localized with CD11c+ DCs and CD68+ Mϕ (Figure 1A) in the lesional dermis. IL-23p19 protein was not present in skin from healthy donors. In addition, DC and Mϕ were increased in psoriatic lesions. We further established IMQ induced psoriasis-like model to examine IL-23-secreting cells in mice (van der Fits et al., 2009). IMQ is a toll-like receptor-7 and 8 (TLR7 and 8) ligand and can exacerbate psoriasis development in patients (Gilliet et al., 2004; Rajan and Langtry, 2006). IMQ topical treatment induced typical psoriasis-like manifestations including epidermal thickness, erythema, and inflammation (Figure 1B). IL-23 and IL-17 mRNAs were increased in the lesional skin. Analysis of lesional skin sections by immunofluorescent staining with IL-23p19 mAb demonstrated that IL-23p19 protein was co-localized with DC and Mϕ distributed throughout the dermis, suggesting that DCs and Mϕ are the predominant cells secreting IL-23 (Figure 1C).
Figure 1. DCs and Mϕ are the major cellular source of IL-23 in psoriatic skin.
(A) Frozen skin sections from patients with psoriatic lesions (scale bar, 25µm) and healthy controls (scale bar, 100µm) were stained with anti-human IL-23p19 (green), anti-human CD11c (red) or anti-human CD68 (red) and DAPI (blue) for immunofluorescent staining. (B) Representative H&E-stained sections of the back skin of C57BL/6 WT mice treated for 3 consecutive days with control cream or IMQ are shown (scale bar, 100µm). IL-17 and IL-23p19 mRNA concentrations were measured by qPCR. Data are shown as mean± SEM. (C) Frozen sections from 3 days of IMQ-treated mouse back skin were co-stained with anti-mouse IL-23p19 (green) and anti-mouse CD11c (red), or anti-mouse F4/80 (red) and DAPI (blue) for immunofluorescent staining. Scale bar, 25µm. (D, E) Whole mouse skin cells, skin cells devoid of DCs and Mϕ or purified skin DCs and Mϕ were stimulated with IMQ for 24 hr or 3 hr and IL-23p19 (D) and IL-17 (E) mRNA concentrations were measured by qPCR. Data are shown as mean± SEM.
We further used whole skin cells or skin cells devoid of DCs and Mϕ to detect IL-23p19 transcripts in vitro upon IMQ stimulation. The IL-23p19 mRNA was increased in total skin cells after IMQ stimulation. However, it was decreased when skin cells were devoid of DCs and Mϕ (Figure 1D). IMQ stimulated high amounts of IL-23p19 mRNA on skin DCs and Mϕ. Finally, we examined whether IL-23 secreted by DCs and Mϕwas responsible for skin IL-17 production. Whole skin cells stimulated with IMQ produced high amount of IL-17 mRNA while skin cells devoid of DCs and Mϕ did not transcribe appreciable amounts of IL-17 mRNA (Figure 1E). These data suggest that IL-23 secreted by skin DCs and Mϕ is essential for IL-17 production in the skin.
Dermal γδ T cells are the major source of IL-17 upon IL-23 stimulation in the skin
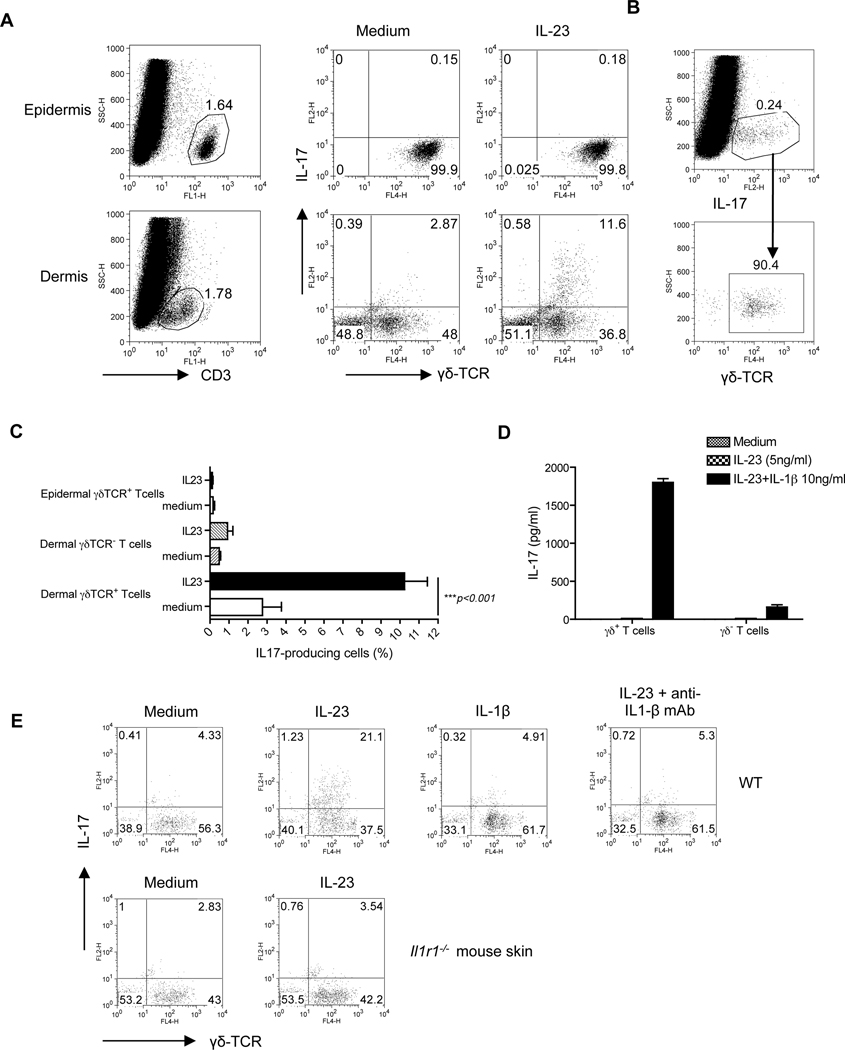
To detect IL-17-producing cells in the skin, single cell suspensions were prepared from both murine epidermis and dermis. The gating strategy is shown in Figure S1. Epidermal T cells were exclusively γδ T cells with high intensity of CD3 and T cell receptor γδ (TCR γδ) staining and did not produce any appreciable IL-17 in response to IL-23 stimulation (Figure 2A). In contrast, dermal T cells were approximately 50% γδ and 50% αβ T cells with intermediate intensity of CD3 and γδ TCR staining and IL-17 was mainly secreted by the dermal γδ T cells upon IL-23 stimulation (Figure 2A). Dermal γδ T cells constituted approximately 90% of IL-17-producing cells (Figure 2B). Minimal IL-17 production was observed from dermal γδ TCR negative T cells (Figure 2C). Since skin cell preparations contain many other cell subsets, dermal CD3+TCRγδ+ T cells and CD3+ TCRγδ− T cells were sorted and stimulated with IL-23 alone or in combination with IL-1β. As depicted in Figure 2D, IL-23 or IL-1β alone could not stimulate dermal γδ T cells for IL-17 production. However, the combination of IL-23 and IL-1 β stimulated dermal γδ T cells to produce large amounts of IL-17. Dermal γδ TCR− T cells also secreted detectable IL-17 upon IL-23 and IL-1β stimulation but the concentration was 10-fold lower compared to dermal γδ T cells. The requirement of IL-1β for IL-23-induced skin dermal γδ T cell IL-17 production was further confirmed by using neutralizing IL-1β mAb and IL-1 receptor deficient (Il-1r−/−) mouse skin cells (Figure 2E). Collectively, these data suggest that, in mice, dermal γδ T cells are the major IL-17-producing cells in the skin in response to IL-23 stimulation and the production of IL-17 by dermal γδ T cells requires endogenous IL-1β.
Figure 2. Dermal γδ T cells are the predominant IL-17 producers upon IL-23 stimulation in the skin.
Intracellular IL-17 production assessed by flow cytometry on epidermal and dermal cell suspensions from C57BL/6 WT mice that were stimulated with IL-23 for 18 hr. (A) Cells were gated on CD3+ T cells. (B) Dermal IL-17 producing cells were gated and calculated for γδ TCR expression. (C) Percentages of IL-17-producing cells in dermal CD3+γδ TCR+ cells, CD3+γδ TCR− cells and epidermal CD3+γδ TCR+ cells were analyzed from twelve independent experiments. Data are shown as mean± SEM. ***P<0.001 (unpaired Student’s t-test). (D) Dermal CD3+γδ TCR+ cells and CD3+γδ TCR− cells were sorted and then stimulated with IL-23 in the presence or absence of IL-1β for 2 days. IL-17 production was measured by ELISA. (E) Skin cells from WT or Il1r1−/− mice were stimulated with IL-23, IL-1β or IL-23 plus IL1β mAb or isotype control mAb. Intracellular IL-17 concentration was assessed by flow cytometry. Cells were gated on CD3+ T cells.
IL-17-producing dermal γδ T cells are phenotypically unique
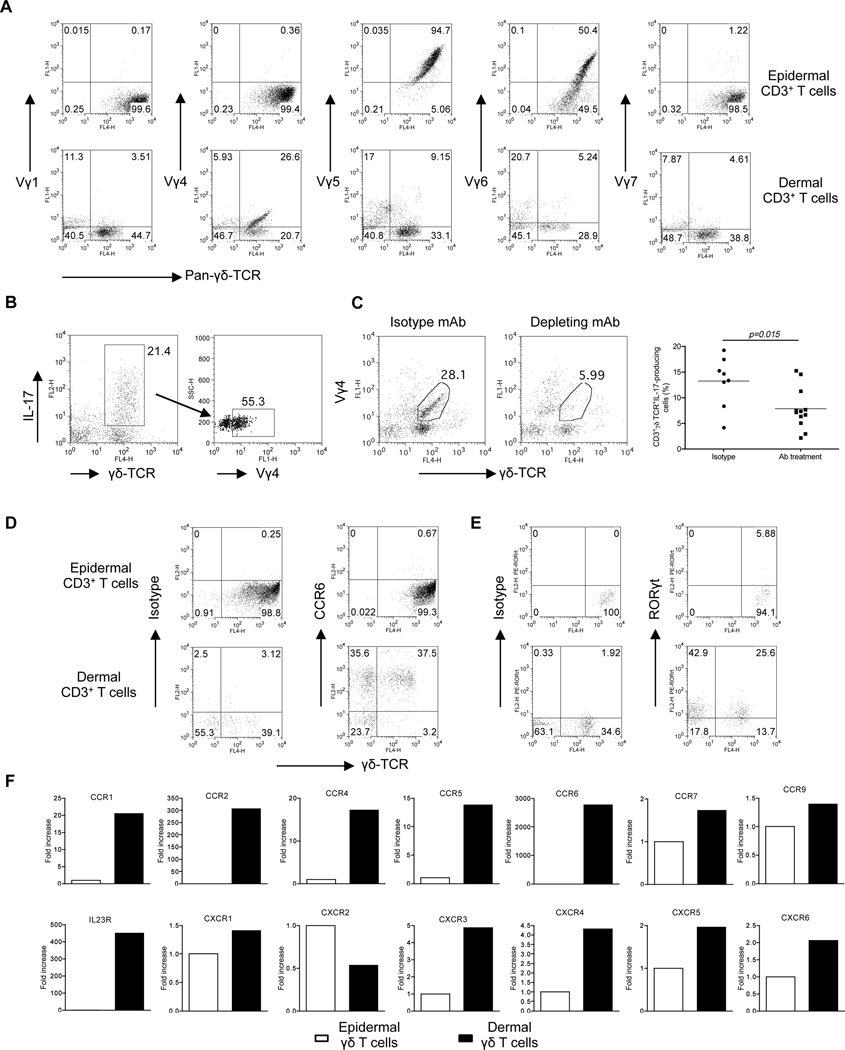
γδ T cells accounted for 0.5–1% of total dermal cells in naïve C57BL/6 mouse skin. To determine whether dermal γδ T cells used a unique γδ TCR, we measured the TCR Vγ usage using a panel of antibodies specific for various Vγ TCR segments including Vγ1, Vγ4, Vγ5, Vγ6, and Vγ7 (Heilig and Tonegawa, 1986). As depicted in Figure 3A, a fraction of dermal γδ T cells expressed Vγ4 whereas epidermal γδ T cells expressed exclusively Vγ5 and partly cross-reacted with Vγ6 antibody (Roark et al., 2004). Reverse transcriptase-Polymerase chain reaction (RT-PCR) analysis revealed that dermal γδ T cells could also use a Vγ2 gene segment (Figure S2C). Consistent with previous results (Born et al., 2010), splenic γδ T cells preferentially expressed Vγ1 and Vγ4 and both were capable of producing IL-17 (Figure S2). In addition, approximately 50% of IL-17-producing dermal γδ T cells expressed Vγ4 (Figure 3B). To further delineate the role of Vγ4 T cells in skin IL-17 production, mice were injected with Vγ4 depleting mAb. We found that IL-17 production from dermal γδ T cells was significantly decreased after Vγ4 T cell depletion (Figure 3C).
Figure 3. Phenotypic analysis of dermal γδ T cells versus epidermal γδ T cells.
(A) Epidermal and dermal cell suspensions were stained with a panel of different Vγ TCR antibodies (Vγ1, Vγ4, Vγ5, Vγ6 and Vγ7) and analyzed by flow cytometry. Flow plots gated on CD3+ cells are representative of two independent experiments with similar results. (B) Dermal cell suspensions were stimulated with IL-23 for 18 hr and analyzed for intracellular IL-17 expression by flow cytometry after staining with different Vγ TCR antibodies. Flow plots gated on CD3+ cells are representative of two independent experiments. (C) Dermal cells from C57BL/6 WT mice receiving mouse Vγ4 mAb or isotype control mAb for three days were stimulated with IL-23 and intracellular IL-17 expression was determined by flow cytometry. Percentages of IL-17+CD3+γδ TCR+ cells were analyzed from three independent experiments. Data are shown as mean ± SEM. *P<0.05 (unpaired Student’s t-test). (D, E) CCR6 (D) and RORγt (E) expression on epidermal and dermal γδ T cells were determined by flow cytometry. (F) Expression of chemokine receptors and IL-23R mRNA measured by qPCR in FACS-sorted epidermal or dermal γδ T cells. The figure shows fold changes of the indicated genes normalized for β-MG mRNA versus the epidermal γδ T cells.
The chemokine receptor CCR6 and transcriptional factor RORγt are associated with the development and recruitment of CD4+ Th17 cells and are also expressed on splenic γδ T cells (Martin et al., 2009). We found that dermal γδ T cells, but not epidermal γδ T cells, constitutively expressed CCR6 and RORγt (Figures 3D, E). We also used real-time PCR to further explore their chemokine receptor mRNA expression profiles and IL-23R expression. As shown in Figure 3F, dermal γδ T cells expressed constitutively IL-23R, along with the chemokine receptors CCR1, CCR2, CCR4, CCR5, CCR6, CXCR3, and CXCR4. These findings suggest that dermal γδ T cells display a unique TCR Vγ usage and chemokine receptor profile with IL-17 producing capability.
CD27 has recently been defined as a critical molecule that differentiates interferon-γ (IFN-γ)-versus IL-17-producing γδ T cells (Ribot et al., 2009; Wakita et al., 2010). Skin dermal and epidermal γδ T cells were exclusively CD27− and did not produce any appreciable IFN-γ (Figure S3A). In contrast, lymph node (LN) γδ T cells have both CD27+ and CD27− populations. Consistent with previous findings, only the CD27+ population produced IFN-γ (Figure S3A). To further investigate whether dermal γδ T cells produce other cytokines such as IL-22 and tumor necrosis factor-α (TNF-α), γδ T cells from skin or LNs were stimulated. Skin dermal γδ T cells produced large amounts of IL-17 and intermediate amounts of TNF-α and IL-22 (Figure S3B) while LN γδ T cells produced IL-17, IL-22, TNF-α and IFN-γ. Thus, dermal γδ T cells appear to be developmentally skewed toward IL-17-producing cells.
γδ T cells are required for IL-23- and IMQ-induced skin inflammation and acanthosis
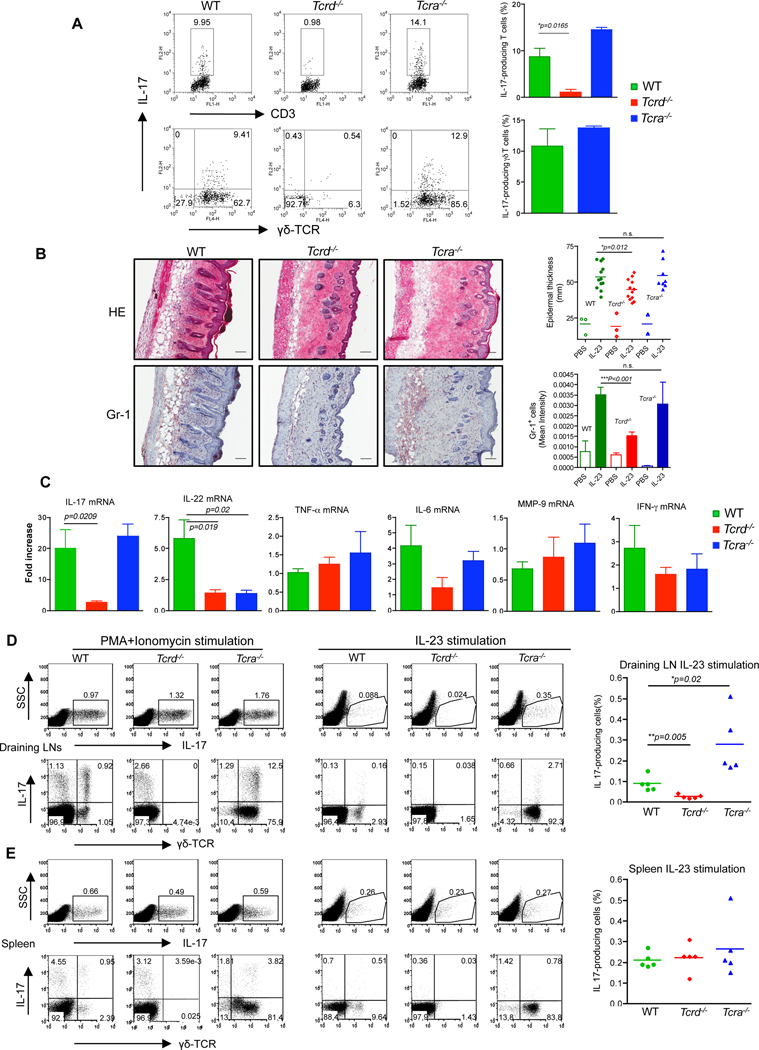
Having found that dermal γδ T cells are the major IL-17-producing cells upon IL-23 stimulation, we tested whether these cells might be involved in a model of IL-23-induced skin inflammation which resembles some features with human psoriasis. We used wildtype (WT) and Tcrd−/− mice to first assess IL-17 production from skin cells. Dermal T cells from WT mice produced significant amounts of IL-17 and dermal γδ T cells were the predominant IL-17 producers. In contrast, dermal T cells from Tcrd−/− mice secreted minimal IL-17 (Figure 4A). Previous studies demonstrated that autoreactive T cell IL-17 production requires interaction of αβ and γδ T cells (Cui et al., 2009). However, Tcra−/− skin cells produced similar amount of IL-17 (Figure 4A), predominantly from dermal γδ T cells, suggesting that the IL-17 production from dermal γδ T cells is independent of αβ T cells.
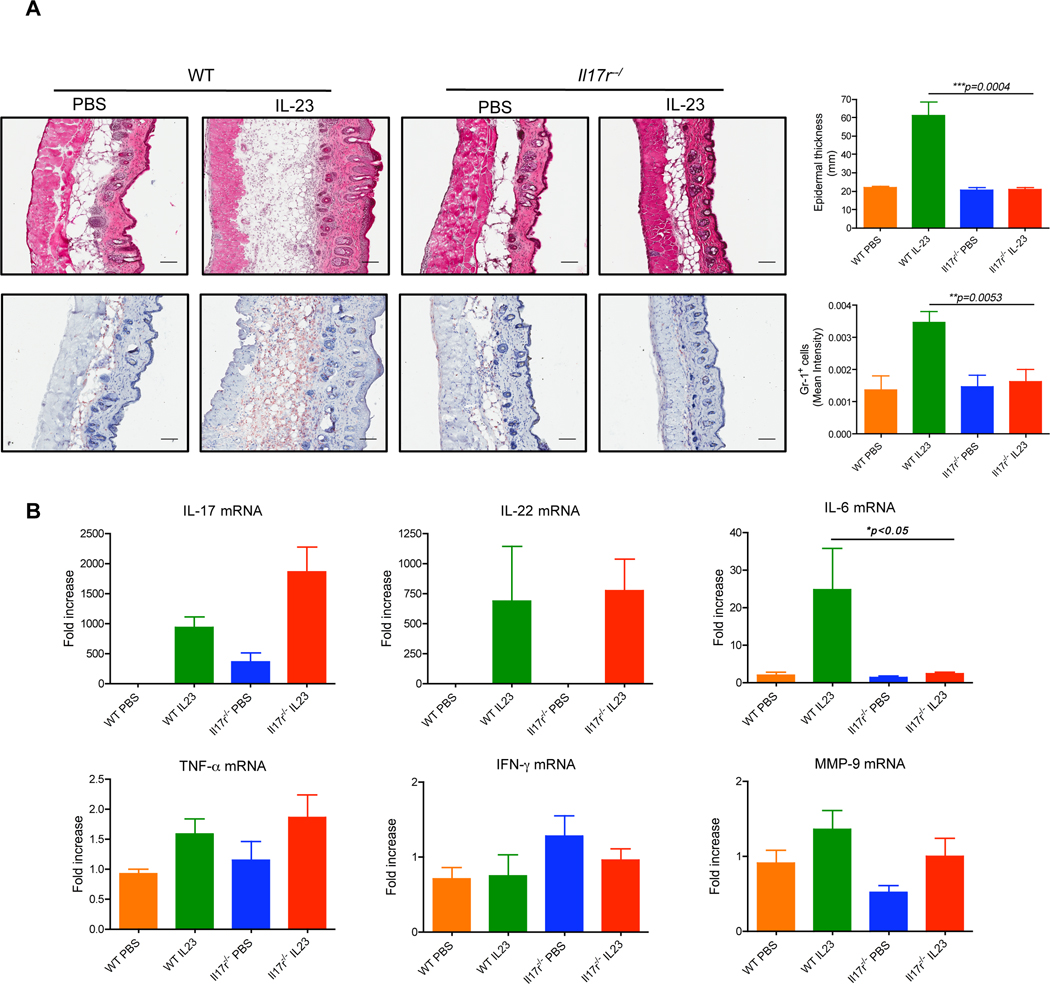
Figure 4. γδ T cells are critical in IL-23-induced skin inflammation and acanthosis.
(A) Dermal cell suspensions from C57BL/6 WT, Tcrd−/− and Tcra−/− mice were stimulated with IL-23 and analyzed for intracellular IL-17 expression by flow cytometry. Flow plots gated on CD3+ cells are representative of two independent experiments. (B) C57BL/6 WT (n=12), Tcrd−/− (n=12) and Tcra−/− (n=8) mice received daily intradermal injections with IL-23 or vehicle control for 4 days. Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Epidermal thickness and Gr-1 infiltration were measured at day 4. Scale bar, 100 µm. Data are combined from two independent experiments. *P<0.05, ***p<0.001; n.s., not significant (unpaired Student’s t-test). (C) IL-17, IL-22, IL-6, TNF-α, MMP-9 and IFN-γ mRNA concentrations were measured by qPCR. The figure shows fold changes normalized for β-MG mRNA versus IL-23-injected skin from Tcrd−/− mice. Data are representative of two independent experiments and shown as mean±SEM. (D, E) Skin draining LN cells (D) or splenocytes (E) from IL-23-treated mice were stimulated with PMA plus ionomycin or IL-23 and intracellular IL-17 expression was determined by flow cytometry. Upper panels are representative dot plots gated on the total cells. The lower panels are representative dot plots gated on the CD3+ T cells. *P<0.05, **P<0.01 (unpaired Student’s t-test).
IL-23 induced changes in mouse skin share many characteristics with human psoriasis (Chan et al., 2006). In agreement with a previous report (Chan et al., 2006), WT mice injected intradermally with IL-23 developed epidermal thickening and massive neutrophil infiltration (Figure 4B). These changes were significantly decreased in Tcrd−/− mice but were not significantly altered in Tcra−/− mice (Figure 4B). We used real-time PCR on skin tissues to assess the effect of IL-23 on mRNA expression of IL-17, IL-22, TNF-α, IL-6, matrix metalloprotease-9 (MMP-9), and IFN-γ. IL-17 and IL-22 mRNAs were significantly increased in WT mice receiving IL-23 injection and both were significantly decreased in Tcrd−/− mice compared to WT mice (Figure 4C). In Tcra−/− mice, IL-17 mRNA was similar to that in WT mice but IL-22 mRNA was significantly lower than that in WT mice (Figure 4C). We also examined IL-17 production in skin draining LNs (DLNs) and spleen from these mice to determine IL-23 effect on local and systemic IL-17 production. In skin DLNs, the frequency of IL-17-producing cells was not significantly changed in mice lacking αβ or γδ T cells upon phorobol myristate acetate (PMA) plus ionomycin stimulation (Figure 4D). Both γδ and αβ T cells produced IL-17. However, upon IL-23 stimulation, IL-17-producing cells in Tcrd−/− mice were significantly lower as compared to those in WT or Tcra−/− mice and IL-17 was predominantly produced by γδ T cells (Figures 4D). Tcra−/− mice in fact had the highest frequency of IL-17-producing cells. Splenic IL-17-producing cells were not significantly altered in these mice (Figures 4E). Together, these in vivo data corroborate our in vitro observations that IL-23 preferentially promotes IL-17 production from γδ T cells. These data further suggest that γδ T cells are required for IL-23-induced skin inflammation and acanthosis.
Since IMQ-induced psoriasis-like murine model is also mediated by IL-23-IL-17 axis as described previously (van der Fits et al., 2009) as well as demonstrated in our study (Figure 1B), we examined whether dermal γδ T cells are also responsible for skin IL-17 production. Dermal γδ T cells spontaneously secreted a large amount of IL-17 in IMQ-treated skin cells (Figure S4). In addition, γδ T cells were increased in both skin and LNs from IMQ-treated mice and secreted large amount of IL-17. Consistent with the IL-23-injected psoriasis model, the frequency of splenic γδ T cells and IL-17 production from γδ T cells were not altered. To further assess the role of γδ T cells and αβ T cells in the IMQ-induced mouse model of psoriasis, WT, Tcra−/−, and Tcrd−/− mice were treated with or without IMQ cream. WT mice treated with IMQ had significantly increased epidermal hyperplasia and massive neutrophil infiltration (Figure S5). However, the epidermal thickening and neutrophil infiltration induced by IMQ were significantly decreased in Tcrd−/− mice. In contrast to IL-23-induced skin inflammation, IMQ-induced epidermal hyperplasia was also significantly decreased in Tcra−/− mice (Figure S5A). However, neutrophil infiltration induced by IMQ was not significantly different in Tcra−/− mice (Figure 5B). Furthermore real-time PCR analysis revealed that IL-17 mRNA was increased upon IMQ treatment. However, the IL-17 mRNA in Tcrd−/− mice was lower than that in WT mice and Tcra−/− mice (data not shown). Similarly, IL-22 mRNA was increased after IMQ treatment. There was no difference among WT, Tcra−/− and Tcrd−/− mice (data not shown). These data suggest that dermal γδ T cells are also the major IL-17 producing cells that are critical in an IMQ-induced psoriasis-like model.
Figure 5. IL-17R expression is essential for IL-23-induced epidermal hyperplasia.
C57BL/6 WT (n=5) and Il17ra−/ − (n=5) mice received daily intradermal injections with IL-23 or vehicle control for 4 days. (A) Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Epidermal thickness and Gr-1 infiltration were measured at day 4. Scale bar, 100 µm. Data are shown as mean± SEM. (B) IL-17, IL-22, IL-6, TNF-α, MMP-9 and IFN-γ mRNA concentrations were measured by qPCR. The figure shows fold changes normalized for β-MG mRNA versus control skin from WT mice. Data are shown as mean± SEM. **P<0.01, ***P<0.001 (unpaired Student’s t-test).
IL-17R expression is necessary for IL-23-induced epidermal hyperplasia and inflammation
The IL-17 cytokine family includes 6 members, IL-17A to F. IL-17A and IL-17F mediate inflammatory activities via the IL-17R complex, comprised of the IL-17RA and IL-17RC subunits (Gaffen, 2009). The IL-17R is expressed ubiquitously in hematopoietic tissues (Yao et al., 1995) as well as in psoriatic lesions (Johansen et al., 2009). As shown in Figure 5A, the epidermal hyperplasia induced by IL-23 was markedly decreased in Il17ra−/− mice compared to WT mice. Neutrophil infiltration was also significantly decreased in these mice. Real-time PCR indicated that mRNA concentrations of IL-17 and IL-22 were increased upon IL-23 treatment in both WT and Il17ra−/− mice. The mRNAs of TNF-α, MMP-9, and IFN-γ remained unchanged but IL-6 mRNA amount was significantly decreased in Il17ra−/− mice (Figure 5B). This suggests that downstream IL-17R signaling is critical in IL-23-induced epidermal hyperplasia.
IL-23 but not pathogen products stimulates in vitro expansion of dermal γδ T cells
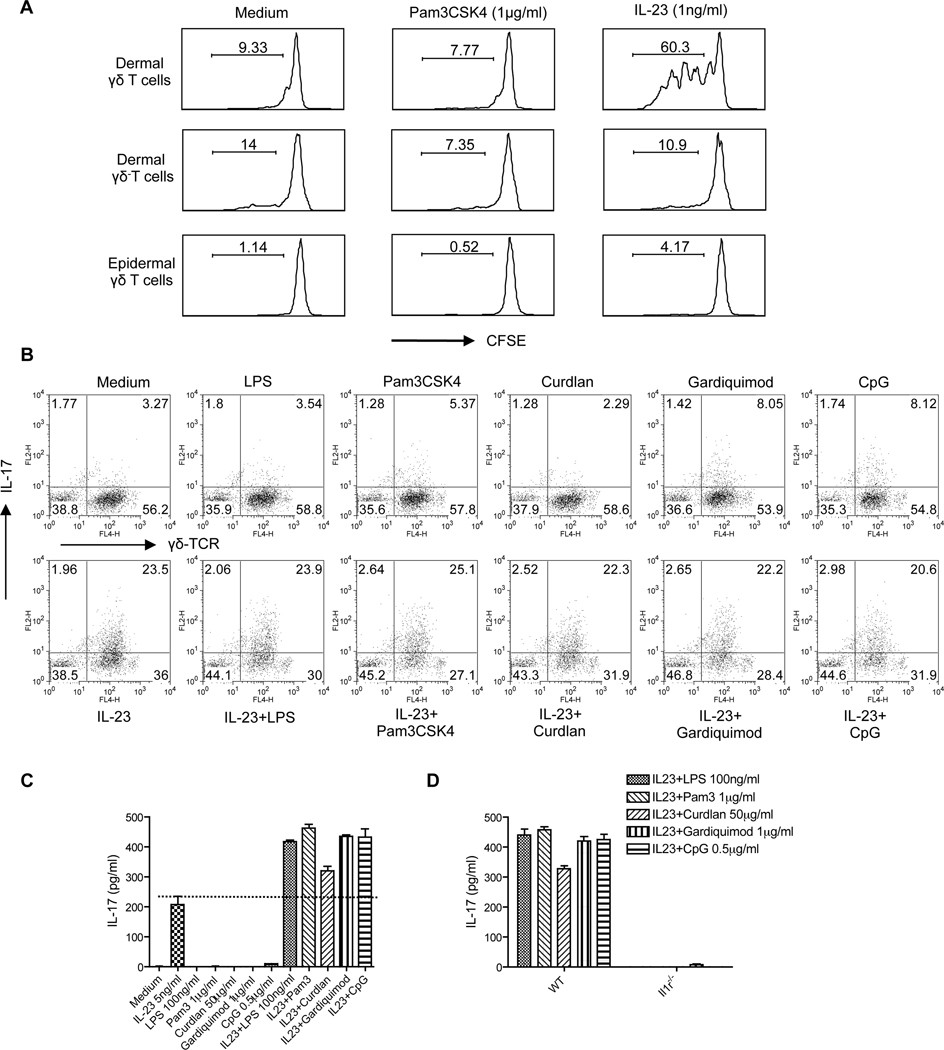
Microbial infection is thought to be important in the initiation of psoriasis (Gudjonsson et al., 2003) (Cai et al., 2009). Previous studies demonstrated that the TLR2 agonist Pam3CSK4, but not IL-23, stimulates splenic γδ T cell expansion in vitro (Reynolds et al., 2010). We therefore stimulated skin cells with different pathogen products or IL-23. Pam3CSK4 and other pathogen products alone did not stimulate dermal γδ T cell proliferation (Figure 6A and data not shown). However, IL-23 alone was found to be sufficient for driving dermal γδ T cell proliferation, but not for dermal γδ− T cells or epidermal γδ T cells (Figure 6A). In contrast, Pam3CSK4, but not IL-23, stimulated splenic γδ T cell proliferation (Figure S6), consistent with a previous report (Reynolds et al., 2010). These data suggest that IL-23 is essential for both maintaining dermal γδ T cell homeostasis and regulating its differentiation program.
Figure 6. Dermal γδ T cell in vitro expansion and IL-17 production stimulated by IL-23 combined with specific pathogenic products.
(A) Skin cell suspensions were labeled with CFSE and then stimulated with Pam3CSK4 or IL-23 for 3 days. Cells were harvested and stained with CD3 and γδ TCR mAbs. (B) Skin cells were stimulated with IL-23 alone, different pathogen products (LPS, Pam3CSK4, Curdlan, Gardiquimon, or CpG) or IL-23 plus different pathogenic products for 2 days. Intracellular IL-17 production was determined by flow cytometry. Cells were gated on CD3+ T cells. (C) Supernatants harvested from (B) were measured for IL-17 concentration by ELISA. Data are shown as mean± SEM. (D) Skin cells from WT and Il1r1−/− mice were stimulated with IL-23 plus different pathogen products for 2 days. Supernatants were harvested and IL-17 concentrations were determined by ELISA. Data are shown as mean± SEM. Data are representative of at least three independent experiments with similar results.
We next examined the effect of these pathogen products on dermal γδ T cell IL-17 production. TLR agonists Pam3CSK4 (TLR2), Gardiquimod (TLR7), and CpG (TLR9) but not lipopolysaccharide (LPS) (TLR4) or dectin-1 ligand curdlan (LeibundGut-Landmann et al., 2007) stimulated dermal γδ T cells to produce low amount of IL-17 as assessed by intracellular cytokine staining (Figure 6B). Combining pathogenic products with IL-23 did not increase the percentage of IL-17-producing cells. However, the mean fluorescent intensity of IL-17 production was increased. Furthermore, a synergistic effect on IL-17 production as assessed by ELISA was observed when cells were exposed to both IL-23 and pathogen products (Figure 6C). IL-17 production by γδ T cells from the peritoneum, lungs, and spleen requires both IL-23 and IL-1β stimulation (Duan et al., 2010; Sutton et al., 2009). Similarly, IL-17 production from dermal γδ T cells was completely abrogated in Il1r1−/− mice (Figure 6D), suggesting that IL-1R signaling pathway is essential for pathogen product-mediated IL-17 production in skin.
Large numbers of IL-17-secreting γδ T cells in human psoriatic skin
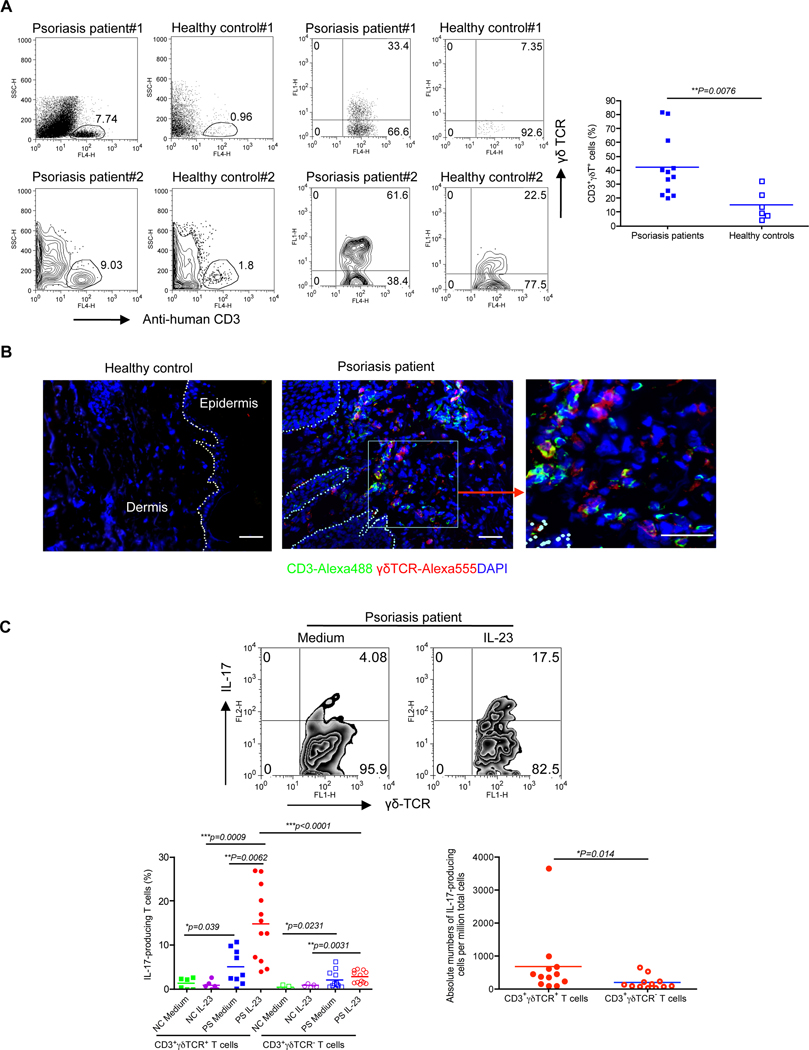
We next examined dermal γδ T cells in lesions from patients with psoriasis. Unlike the case in murine skin, γδ T cells do not exist in human epidermis but approximately 4% of human dermal leukocytes are CD3+γδ TCR+ T cells (Ebert et al., 2006). We found that CD3+ T cells were significantly increased in psoriatic lesions while CD3+ T cells were scarce in healthy control skins (Figure 7A). In addition, the frequency of γδ T cells in dermis was significantly increased in patients with psoriasis compared to healthy controls (Figure 7A). Confocal microscopy analysis revealed that infiltration of γδ T cells was readily seen in the dermis of psoriatic skin lesions (Figure 7B). We further analyzed IL-17 production from these cells. Healthy control dermal γδ T cells produced minimal IL-17 even when these cells were stimulated with IL-23, probably due to lower or absent endogenous IL-1β (Kryczek et al., 2008). In contrast, approximately 15% of γδ T cells in psoriatic lesions produced IL-17 upon IL-23 stimulation (Figure 7C). Furthermore, the percentage of γδ T cells secreting IL-17 was significantly higher than that of γδ− T cells secreting IL-17. Similarly, the absolute numbers of IL-17-secreting γδ T cells were significantly higher than those of IL-17-secreting γδ− T cells (Figure 7C). To further compare the amount of IL-17 production on a per cell basis, we examined the mean fluorescent intensity (MFI) of IL-17-producing cells. However, we did not see difference of MFI between IL-17-secreting dermal γδ T cells and γδ− T cells (data not shown). These data demonstrate that dermal γδ T cells in patients with psoriasis are greatly increased and produce substantial amounts of IL-17.
Figure 7. Skin lesions from psoriasis patients display high frequency of IL-17-secreting γδ T cells.
(A) Dermal cells from psoriatic lesions or healthy controls were analyzed for CD3 and γδ TCR expression by flow cytometry. Two donors from each group (both dot plots and contour plots) were shown. Flow plots gated on CD3+ cells are representative from 12 patients and 6 healthy controls. Percentage of CD3+γδ TCR+ cells is shown as mean± SEM. Statistical analysis was performed by using a two-tailed Mann Whitney test. **p<0.01. (B) Frozen sections from psoriatic lesion and healthy controls were stained with human γδ TCR mAb (red), CD3 mAb (green) and DAPI (blue) for immuno-fluorescent staining. Scale bar, 10 µm. (C) Dermal cell suspensions from psoriatic lesion (PS) and healthy control (NC) were stimulated with IL-23 and IL-17 expression was determined by flow cytometry. Cells were gated on CD3+γδ TCR+ or CD3+γδ TCR− cells. Percentage of IL-17+CD3+γδ TCR+ and IL-17+CD3+γδ TCR− cells is shown as mean ± SEM. Absolute numbers of IL-17-producing cells per million cells are shown from 12 patients. *p<0.05, **p<0.01, ***p<0.001 (a Mann Whitney test).
DISCUSSION
Our results demonstrate that murine innate dermal γδ T cells are the major source of IL-17 upon IL-23 stimulation in skin and these cells constitutively express RORγt, IL-23R, CCR6 and other chemokine receptors. Furthermore, in mice, IL-17 production by dermal γδ T cells appears to be independent of αβ T cells. Importantly, IL-23-induced skin inflammation and acanthosis are significantly attenuated in Tcrd−/− mice and Il17ra−/− mice. In addition, IMQ-induced skin inflammation and epidermal hyperplasia are also significantly decreased in Tcrd−/− mice. In vitro, IL-23 preferentially stimulates dermal γδ T cell proliferation, suggesting a possible feed-forward mechanism in the pathogenesis of psoriasis. Finally, in both mice and humans, dermal γδ T cells are critical IL-17-producing cells in the genesis of psoriasis.
A major role of IL-23 in autoimmunity is thought to be promotion of Th17 cell expansion and survival (Kikly et al., 2006). A recent study reveals that IL-23 together with IL-1β activates splenic γδ T cells to produce IL-17 (Sutton et al., 2009). Microbiota also regulate the production of IL-17 from γδ T cells (Duan et al., 2010). IL-23 has a central role in the pathogenesis of psoriasis (Krueger et al., 2007; Lee et al., 2004). The cellular sources of IL-23 in skin are mainly infiltrating DCs and monocytes in the dermis (Lee et al., 2004; Wilson et al., 2007), with keratinocytes and langerhans cells in the epidermis (Aliahmadi et al., 2009; Piskin et al., 2006). We found that IL-23 is mainly secreted by skin infiltrating DCs and Mϕ in both human psoriatic skin and IMQ-induced mouse psoriatic skin lesions. IL-23 not only promoted IL-17 production by dermal γδ T cells which was irrespective of γδ TCR stimuli but also drove their in vitro expansion. IL-17 production by dermal γδ T cells required endogenous IL-1β because sorted dermal γδ T cells could not secrete IL-17 upon stimulation with IL-23 alone. However, psoriatic skin keratinocytes and infiltrating inflammatory cells do secrete elevated amounts of IL-1β (Yoshinaga et al., 1995). IL-1β treatment results in an up-regulation of tight junction (TJ) proteins occludin and ZO-1, which resembles TJ protein alteration in early psoriasis (Kirschner et al., 2009). Thus, skin dermal γδ T cells, as well as γδ T cells from spleen, LNs, lungs, and peritoneum (Duan et al., 2010; Sutton et al., 2009) require both IL-23 and IL-1β cytokines for IL-17 production.
The dermal IL-17-producing γδ T cells share many characteristics with other IL-17-producing cell subsets (Hedrick et al., 2009), including constitutive expression of RORγt, CCR6, and IL-23R. The dermal γδ T cells also constitutively express a number of chemokine receptors including CCR1, CCR2, CCR4, CCR5, CXCR3, and CXCR4, which may be involved in their trafficking. In addition, dermal γδ T cells express lower intensity of CD3 and γδ TCR as compared to epidermal γδ T cells. It is worth noting that there is a small percentage of a CD3hipopulation existing in the dermal preparations. These CD3hi γδ T dermal cells are probably contaminating epidermal γδ T cells because they express a unique Vγ5 (data not shown) gene segment, which is a hallmark of epidermal γδ T cells. However, it is also possible that these cells are bona fide dermal residents. Compared to the previous studies indicating that γδ T cells expressing Vγ1 and Vγ4 gene segments are predominantly IL-17-producing γδ T cells (Cui et al., 2009; Martin et al., 2009), we found that the dominant dermal IL-17-producing γδ T cells are Vγ4+; as depletion of Vγ4 T cells in the skin significantly decreased IL-17 production from dermal γδ T cells. We also found that dermal γδ T cells express the Vγ2 gene segment. Although dermal γδ T cells share many features with other IL-17-producing γδ T cells, they bear some unique characteristics. First, dermal γδ T cells are uniformly CCR6+, whereas γδ T cells from LNs or spleen contain CCR6+ and CCR6− populations (Martin et al., 2009). Similarly, dermal γδ T cells do not express CD27 while γδ T cells from other anatomical sites have both CD27+ and CD27− populations. In addition, dermal γδ T cells do not produce IFN-γ whereas γδ T cells from other anatomical sites produce both IFN-γ and IL-17. This may imply that dermal γδ T cells are ‘professional’ IL-17-producing cells. Second, IL-23 is capable of stimulating the in vitro expansion of dermal γδ T cells whereas IL-23 has no effect on splenic γδ T cell proliferation (Reynolds et al., 2010).
The pathogenic importance of γδ T cell response in murine psoriasis models is emphasized by significant decreases of acanthosis and skin inflammation in Tcrd−/− mice. In contrast Tcra−/− mice respond to IL-23 just as do WT mice and dermal γδ T cells from Tcra−/− mice produce similar amounts of IL-17. Consistent with in vitro data, Tcrd−/− mice have lower IL-17 mRNA. Notably, IL-22 mRNA in Tcra−/− mice was lower than in WT mice, yet these mice still developed acanthosis and skin inflammation. It is possible that the IL-22 concentration in Tcra−/− mice, while low, is sufficient to induce biological effects. Alternatively, IL-22 may not be absolutely required for IL-23-induced skin inflammation and acanthosis. This notion is further supported by the observation in Il17ra−/− mice in which IL-22 mRNA was increased upon IL-23 injection but acanthosis and skin inflammation were completely abrogated. It is worth noting that Tcrd−/− mice did show minor epidermal hyperplasia and neutrophil infiltration compared to PBS-treated WT mice. This may be related to the IL-23-mediated T cell-independent inflammatory process (Buonocore et al., 2010; Hedrick et al., 2009). In the current study, we also observed that the epidermal hyperplasia induced by IL-23 was completely abrogated in Il17ra−/− mice despite similar IL-17 mRNA amount whereas an earlier report indicated that IL-23-induced acanthosis was independent of IL-17A (Chan et al., 2006). A recent study using both Il17a−/− mice and neutralizing IL-17A mAb clearly demonstrated that IL-17A is a pathogenic cytokine in IL-23-induced skin inflammation and acanthosis (Rizzo et al., 2010). In addition, an initial phase II double-blind, randomized trial has demonstrated the therapeutic efficacy of a fully human IL-17A mAb (AIN457) in psoriasis treatment (Miossec et al., 2009).
We further demonstrated that dermal γδ T cells secreted large amount of IL-17 in an IMQ-induced psoriasis model. IMQ cream topical treatment induces exacerbated psoriasis in patients with a well-controlled psoriasis pathology (Rajan and Langtry, 2006). Interestingly, IMQ-induced dermatitis is diminished in mice treated with CD3 mAb or in Rag2−/− γc−/− mice (van der Fits et al., 2009). Although Th17 cells were marginally increased, dermal γδ T cells were increased in skin and DLNs and produced large amounts of IL-17 in IMQ-treated skin lesion. Furthermore, IMQ-induced skin inflammation and epidermal hyperplasia were significantly decreased in Tcrd−/− mice. In contrast to IL-23-induced psoriasis-like model, IMQ-induced epidermal hyperplasia was also significantly decreased in Tcra−/− mice, suggesting both αβ T cells and γδ T cells contribute to IMQ-induced skin pathology. However, IMQ-induced neutrophil infiltration was only significantly decreased in Tcrd−/− mice but not in Tcra−/− mice, suggesting that dermal γδ T cells are the major IL-17-producing cells in skin. Furthermore, IL-22 mRNA was not different among WT, Tcra−/−, and Tcrd−/− mice. Thus, dermal γδ T cells also play a critical role in natural stimuli-induced psoriasis. These data also suggest that the roles of IL-17, IL-22, γδ T cells and αβ T cells may vary in their degree of contribution to skin pathology depending on whether IL-23 or IMQ is used to induce skin inflammation.
In support of possible bacterial initiation of psoriasis, we did find that pathogen products have a stimulatory effect on dermal γδ T cell IL-17 production. TLR and dectin-1 ligands promoted IL-17 production from dermal γδ T cells in combination with IL-23. The synergistic effect was dependent on the IL-1R signaling pathway. CD44hiCCR6+ γδ T cells express TLR1, TLR2 and dectin-1 (Martin et al., 2009); thus these pathogen products may directly stimulate dermal γδ T cells to produce IL-17. In addition, pathogen products may stimulate IL-1β production that synergizes with IL-23 to induce potent IL-17 production.
Although IL-23- or IMQ-induced psoriasis-like skin lesions in mice share some clinical and histological characteristics with human psoriasis (Chan et al., 2006), there are differences. Most notably, human skin does not have DETCs while T cells bearing αβ TCR exist predominantly in human dermis. Thus, the importance of dermal γδ T cells as the major IL-17-producing T cells needs to be tested in human psoriasis patients. In healthy controls, CD3+ T cells are scarce and are predominantly αβ T cells. γδ T cells only constitute 5–10% of total dermal leukocytes. We found that γδ T cells were significantly increased in psoriatic skin consistent with a previous pathology report (Seung et al., 2007). In addition, both percentage and absolute numbers of IL-17-producing γδ T cells were significant more than those of γδ− T cells. The increased γδ T cell infiltration implies that these cells may expand locally. Alternatively, these γδ T cells may also migrate from the periphery into the skin dermis via chemotaxis. Local chemokine CCL20 production is also increased in psoriatic skin (Kryczek et al., 2008). Although γδ T cells are conventionally considered to be innate immune cells that provide early and rapid responses including high amounts of effector cytokines such as IFN-γ and IL-17 in the models of infection, tumor, and exposure to injury (Gao et al., 2003; Hamada et al., 2008; Matsubara et al., 2009), the increased frequency of dermal γδ T cells in psoriatic skin suggests that γδ T cells are also crucial in the form of chronic inflammation. This may be not very surprising as marked expansion of γδ T cells occurs in relatively late stages of infection and chronic inflammation (Roark et al., 2007; Simonian et al., 2006). Thus, dermal γδ T cells may engage in immune responses both early and late. Taken together, our results emphasize the importance of dermal γδ T cells on innate IL-17 production. Dermal γδ T cells represent a major source of the IL-17 that promotes the development and progression of skin inflammation such as psoriasis.
EXPERIMENTAL PROCEDURES
Mice
WT, Tcrd−/−, Tcra−/− and Il1r1−/− mice on C57BL/6 background were purchased from Jackson Laboratory. C57BL/6 Tcrd−/− mice do not have dermatitis (Girardi et al., 2002). Il17ra−/− mice have been previously described (Ye et al., 2001). All animals were housed and treated in accordance with institutional guidelines and approved by the IACUC at the University of Louisville.
Human subjects
Patients with psoriasis vulgaris were diagnosed based on the clinical and histopathologic criteria. Skin biopsies were collected from the lesional site of 12 patients and 6 normal volunteers. All patients had not been treated on systemic therapy for at least 4 weeks prior to the study entry. The study was approved by the Shanghai Jiaotong University School of Medicine Research Ethics Committee. All the participants gave their written informed consent.
Skin cell preparation and stimulation
Mouse back skin or human skin was incubated in dispase to separate epidermis and dermis. Epidermal cell suspensions were prepared by incubating epidermis with trypsin-EDTA. A buffer containing collagenase IV, hyaluronidase, and DNase-I was used to obtain dermal cell suspensions. Mouse cells were stimulated with varying concentrations of mouse rIL-23 (eBioscience), 100 ng/ml LPS, 1µg/ml Pam3CSK4, 50 µg/ml Curdlan, 1 µg/ml Gardiquimod, or 0.5 µg/ml CpG for 2 days. The supernatants were harvested for IL-17 measurement by ELISA (Biolegend). Cells were also stimulated with IL-23 (50 ng/ml) for 18 hr in the presence of GolgiPlug (BD Bioscience) for intracellular IL-17 staining. In IL-1β blocking experiment, neutralizing IL-1β mAb (2 µg/ml, eBioscience) or isotype mAb was added in the culture before IL-23 stimulation. Additionally, skin DCs and Mϕ were purified from whole skin cells by positive selection using anti-mouse CD11c and CD11b microbeads (Miltenyi Biotec). Whole skin cells, skin cells devoid of DCs and Mϕ, or purified skin DCs and Mϕ were stimulated with IMQ (2 µg/ml) at different time points. For human studies, dermal cells were stimulated with human rIL-23 (100ng/ml) for 18 hr in the presence of GolgiPlug for intracellular IL-17 staining. To calculate the absolute numbers of IL-17-producing cells, we used the formula: absolute IL-17-producing cell numbers=% of CD3+ cells × % of γδ+ or γδ− T cells × % of IL-17 positive cells × total cell numbers. To normalize the baselines for all samples, we used per million cells as baseline.
Flow cytometry analysis and intracellular staining
Mouse β TCR, CD3, IL-17A mAbs were obtained from BD Biosciences. Mouse γδTCR, RORγt, CD27, human γδTCR, CD3 and IL-17A mAbs were purchased from eBioscience. Mouse CCR6, IL-22 mAbs were obtained from R&D system. All mouse TCR Vγ antibodies were kindly provided by Dr. R. O’Brien (National Jewish Health, Denver, CO). For intracellular staining, cells were first stained with different cell surface Abs and then fixed, permeabilized and stained intracellularly for IL-17 IL-22, TNF-α, IFN-γ, or RORγt. The relevant isotype control mAbs were also used. Samples were harvested with a BD FACS Calibur and analyzed with FlowJo software (TreeStar).
Cell sorting
The whole skin cell suspensions were stained with mouse CD3 and γδTCR mAbs. Epidermal and dermal γδ T cells were sorted by MoFlow high-speed sorter. In addition, dermal cell suspensions were stained with mouse CD3 and γδTCR mAbs and dermal γδ+ and γδ− T cells were sorted.
In vivo mouse Vγ4 T cell depletion
Mouse Vγ4 Ab (250 µg) or isotype control mAb was intravenously injected into mice for 3 days. Mice were sacrificed and dermal cell suspensions were stimulated with IL-23 for intracellular IL-17 staining.
Establishment of psoriasis-like mouse models
IL-23-induced psoriasis-like mouse model was established as previously described (Chan et al., 2006). Briefly, IL-23 (1µg) or vehicle control was daily intradermally injected on the back skin of WT, Tcrd−/−, Tcra−/− or Il17ra−/− mice for 4 days. IMQ-induced psoriasis-like model was described previously (van der Fits et al., 2009). Mice were treated with a daily topical dose of commercially available IMQ cream (5%) (Aldara; 3M Pharmaceuticals) on the shaved back for 3 or 5 consecutive days. Mice were sacrificed and the skin samples were embedded and froze in OCT for H&E and immunohistochemistry (IHC) staining. In some experiments, skin DLNs and spleens were also isolated for intracellular cytokine staining.
Skin histology and IHC staining
Skin sections were stained with H&E and the epidermal thickness was determined by measuring the average interfollicular distance under the microscope in a blinded manner. For IHC staining, skin cryosections were fixed, blocked and then stained with rat-anti-mouse Gr-1 followed by goat-anti-rat IgG secondary Ab (Southern Biotech). Slides were developed with 3-amino-9- ethylcarbazole (AEC) substrate solution (Vector Laboratories) and then counterstained with hematoxylin. Images were acquired at ×200 magnification using Aperio ScanScope digital scanners and Gr-1 expression was quantitatively analyzed as mean intensity using Image-pro software (Media Cybernetics Inc.).
Immunofluorescence staining
Human skin samples were fixed, cryosectioned, blocked, and then stained with the following primary Abs: mouse anti-human γδ TCR and CD3 (eBioscience), rabbit anti-human or mouse IL-23p19 (Abcam), biotinylated mouse anti-human CD11c (eBioscience) or CD68 (Biolegend). For mouse skin samples, frozen sections were stained with primary Abs of rabbit anti-human or mouse IL-23p19 (Abcam) and APC-hamster anti-mouse CD11c (eBioscience) or APC-rat anti-mouse F4/80 (eBioscience). Images were acquired by Leica TCS SP5 confocal microscope system.
RNA extraction and real-time quantitative PCR (qPCR)
RNAs were isolated using a Qiagen RNeasy kit (Qiagen). After reverse transcription into cDNA with a Reverse Transcription Kit (Bio-Rad), qPCR was then performed on MyiQ single color RT-PCR detection system using SYBR Green Supermix (Bio-Rad) and gene-specific primers were summarized in the Supplemental Table I. We normalized gene expression amount to β-2 microglobulin (β-MG) housekeeping gene and represented data as fold differences by the 2−ΔΔCt method, where ΔCt=Cttarget gene−Ctβ-MG and ΔΔCt=ΔCtinduced−ΔCtreference.
Statistical analysis
All quantitative data are shown as mean± SEM unless otherwise indicated. All samples were compared using 2-tailed, unpaired Student’s T test or a Mann Whitney test as indicated. A P value less than 0.05 was considered significant. Statistical analysis was performed with GraphPad Prism software.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Rebecca O’Brien (National Jewish Health, Denver, CO) for providing TCR Vγ mAbs. We also thank Dr. John W. Eaton for critical reading of this manuscript. This work was supported by the National Institutes of Health (J.Y.), National Natural Science Foundation of China (30872278/H1103) and Science and Technology Commission of Shanghai Municipality (08JC1415700, J.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. doi: 10.1002/eji.200838742. [DOI] [PubMed] [Google Scholar]
- Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128:1064–1067. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born WK, Yin Z, Hahn YS, Sun D, O'Brien RL. Analysis of gammadelta T cell functions in the mouse. J Immunol. 2010;184:4055–4061. doi: 10.4049/jimmunol.0903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YH, Lu ZY, Shi RF, Xue F, Chen XY, Pan M, Yuan WR, Xu H, Li WP, Zheng J. Enhanced proliferation and activation of peripheral blood mononuclear cells in patients with psoriasis vulgaris mediated by streptococcal antigen with bacterial DNA. J Invest Dermatol. 2009;129:2653–2660. doi: 10.1038/jid.2009.153. [DOI] [PubMed] [Google Scholar]
- Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, Giustizieri ML, Pacciani V, Mazzotta A, Campione E, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15:1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Conrad C, Geiges M, Cozzio A, Thurlimann W, Burg G, Nestle FO, Dummer R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, Guzzo C, Xia Y, Zhou B, Li S, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530–534. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M, Blauvelt A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kirschner N, Poetzl C, von den Driesch P, Wladykowski E, Moll I, Behne MJ, Brandner JM. Alteration of tight junction proteins is an early event in psoriasis: putative involvement of proinflammatory cytokines. Am J Pathol. 2009;175:1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Takeda K, Jin N, Okamoto M, Matsuda H, Shiraishi Y, Park JW, McConville G, Joetham A, O'Brien RL, et al. Vgamma1+ T cells and tumor necrosis factor-alpha in ozone-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2009;40:454–463. doi: 10.1165/rcmb.2008-0346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- Rajan N, Langtry JA. Generalized exacerbation of psoriasis associated with imiquimod cream treatment of superficial basal cell carcinomas. Clin Exp Dermatol. 2006;31:140–141. doi: 10.1111/j.1365-2230.2005.01938.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-Mediated Psoriasis-Like Epidermal Hyperplasia Is Dependent on IL-17A. J Immunol. 2010 doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O'Brien RL. Subset-specific, uniform activation among V gamma 6/V delta 1+ gamma delta T cells elicited by inflammation. J Leukoc Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ, Cho HJ, Park HR. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol. 2007;34:903–911. doi: 10.1111/j.1600-0560.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O'Brien RL, Fontenot AP. Regulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11:41–44. doi: 10.1038/ni.1803. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga Y, Higaki M, Terajima S, Ohkubo E, Nogita T, Miyasaka N, Kawashima M. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158–164. doi: 10.1007/BF01262325. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, Krueger JG. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.