Abstract
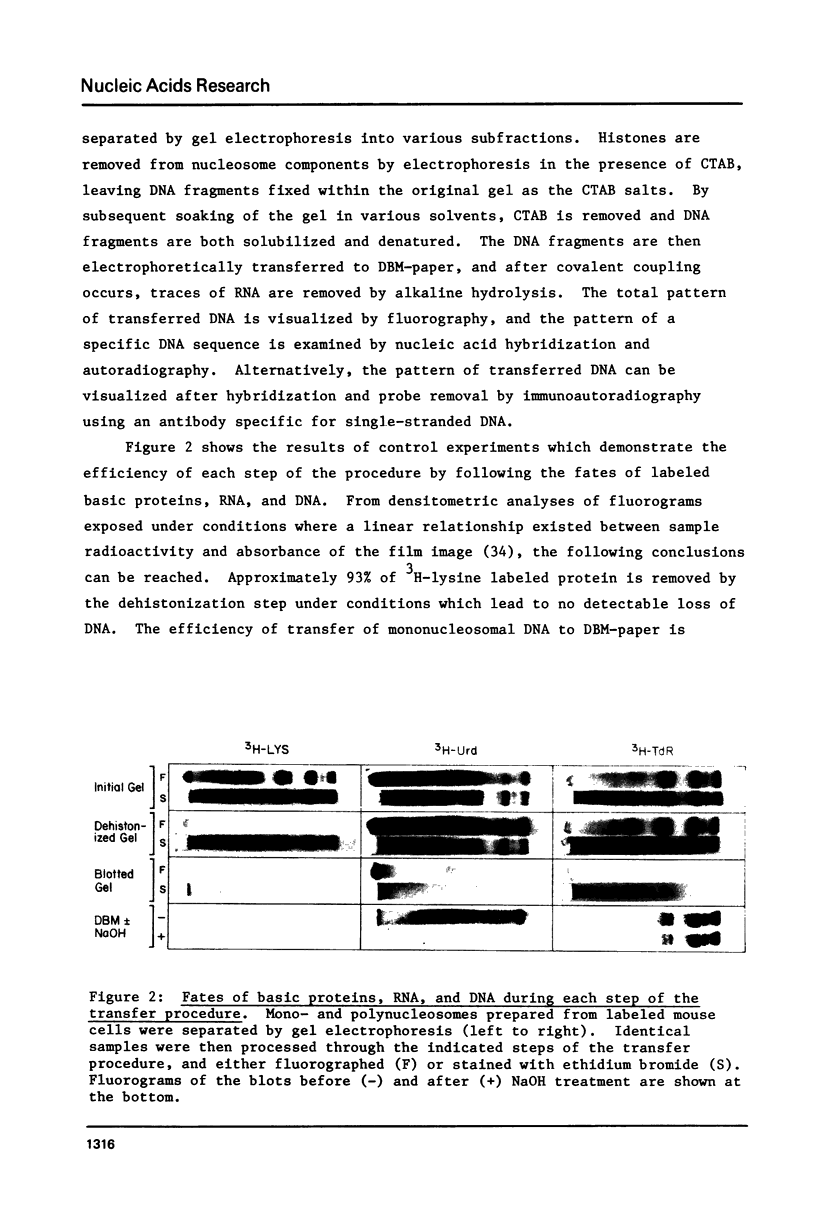
Electrophoresis fractionates nucleosomes which possess different protein compositions. We report here a procedure for transferring the DNA components of electrophoretically resolved nucleosomes to diazobenzyloxymethyl cellulose (DBM-paper). Histones are first removed from nucleosome components by electrophoresis in the presence of cetyltrimethylammonium bromide (CTAB), leaving DNA fragments fixed within the original gel as the CTAB salts. The DNA is then converted to the sodium salt, denatured, and electrophoretically transferred to DBM-paper. The overall pattern of DNA on the resulting blot is visualized either by fluorography or by immunoautoradiography. This DNA pattern is then compared with autoradiograms obtained after hybridizing the same blot with specific 32P-labeled probes. Using mouse satellite DNA as a hybridization probe, we illustrate the above techniques and demonstrate that nucleosomes carrying satellite sequences are compositionally heterogeneous. The procedures described here should also be useful in the analysis of the nucleic acid components associated with other nucleoprotein complexes.
Full text
PDF





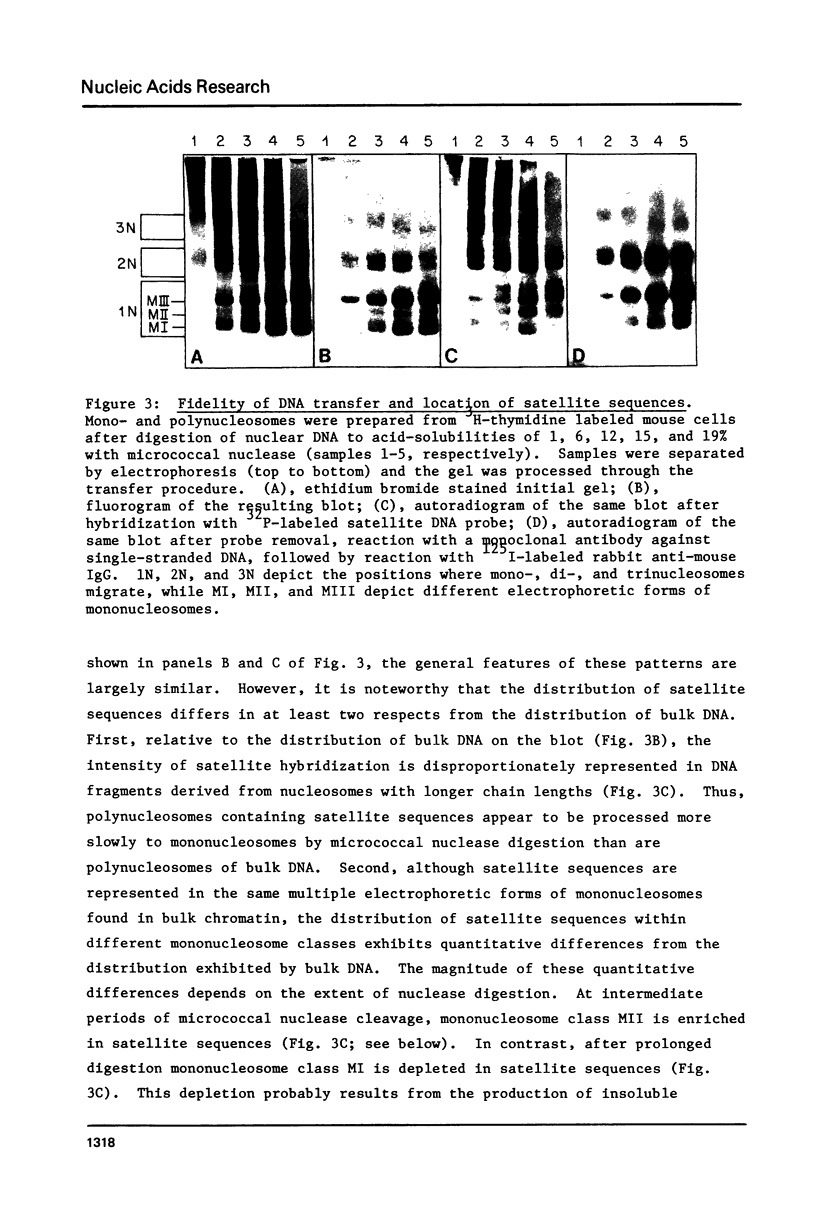







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanese I., Weintraub H. Electrophoretic separation of a class of nucleosomes enriched in HMG 14 and 17 and actively transcribed globin genes. Nucleic Acids Res. 1980 Jun 25;8(12):2787–2805. doi: 10.1093/nar/8.12.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright S. C., Nelson P. P., Garrard W. T. Histone molar ratios among different electrophoretic forms of mono- and dinucleosomes. J Biol Chem. 1979 Feb 25;254(4):1065–1073. [PubMed] [Google Scholar]
- Albright S. C., Wiseman J. M., Lange R. A., Garrard W. T. Subunit structures of different electrophoretic forms of nucleosomes. J Biol Chem. 1980 Apr 25;255(8):3673–3684. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski C., Jr, Stollar B. D., Lalor T. M., Schwartz R. S. Hybridoma autoantibodies to DNA. J Immunol. 1980 Mar;124(3):1499–1502. [PubMed] [Google Scholar]
- Bafus N. L., Albright S. C., Todd R. D., Garrard W. T. A method for mapping the distributions of modified and variant histones among mono- and polynucleosomes. J Biol Chem. 1978 Apr 25;253(8):2568–2574. [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Schmatchenko V. V., Georgiev G. P. Non-histone proteins in mononucleosomes and subnucleosomes. Eur J Biochem. 1978 Nov 2;91(1):291–301. doi: 10.1111/j.1432-1033.1978.tb20965.x. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Schmatchenko V. V., Georgiev G. P. Subnucleosome particles containing high mobility group proteins HMG-E and HMG-G originate from transcriptionally active chromatin. Nucleic Acids Res. 1979 Nov 24;7(6):1525–1540. doi: 10.1093/nar/7.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bokhon'ko A., Reeder R. H. The subunit structure of mouse satellite chromatin. Biochem Biophys Res Commun. 1976 May 3;70(1):146–152. doi: 10.1016/0006-291x(76)91120-7. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerksen J. D., Paul I. J. Satellite DNA sequence content of polylysine-titratable and nuclease-resistant fractions of mouse liver hepatoma chromatin. Nucleic Acids Res. 1976 Sep;3(9):2277–2291. doi: 10.1093/nar/3.9.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan P. A., Levy-Wilson B. Structure of transcriptionally active and inactive nucleosomes from butyrate-treated and control HeLa cells. Biochemistry. 1981 Jun 23;20(13):3695–3702. doi: 10.1021/bi00516a005. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., Walker P. M., McCallum M. Some properties of the single strands isolated from the DNA of the nuclear satellite of the mouse (Mus musculus). J Mol Biol. 1969 Mar 28;40(3):423–443. doi: 10.1016/0022-2836(69)90163-6. [DOI] [PubMed] [Google Scholar]
- Giri C. P., West M. H., Ramirez M. L., Smulson M. Nuclear protein modification and chromatin substructure. 2. Internucleosomal localization of poly(adenosine diphosphate-ribose) polymerase. Biochemistry. 1978 Aug 22;17(17):3501–3504. doi: 10.1021/bi00610a012. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Mathew C. G., Wright C. A., Venkov C. D., Johns E. W. Analysis of the high mobility group proteins associated with salt-soluble nucleosomes. Nucleic Acids Res. 1979 Dec 11;7(7):1815–1835. doi: 10.1093/nar/7.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Melton D. A. The length of nucleosome-associated DNA is the same in both transcribed and nontranscribed regions of chromatin. Nature. 1978 May 25;273(5660):317–319. doi: 10.1038/273317a0. [DOI] [PubMed] [Google Scholar]
- Hutcheon T., Dixon G. H., Levy-Wilson B. Transcriptionally active mononucleosomes from trout testis are heterogeneous in composition. J Biol Chem. 1980 Jan 25;255(2):681–685. [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B., Pollock J. M., Jr, Rill R. L. Chromatin fractionation procedure that yields nucleosomes containing near-stoichiometric amounts of high mobility group nonhistone chromosomal proteins. Biochemistry. 1979 Aug 21;18(17):3739–3748. doi: 10.1021/bi00584a015. [DOI] [PubMed] [Google Scholar]
- KIT S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J Mol Biol. 1961 Dec;3:711–716. doi: 10.1016/s0022-2836(61)80075-2. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A. M., Martinson H. G. Structure of nucleosome core particles containing uH2A (A24). Nucleic Acids Res. 1981 Jun 11;9(11):2423–2431. doi: 10.1093/nar/9.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinger L., Barsoum J., Varshavsky A. Two-dimensional hybridization mapping of nucleosomes. comparison of DNA and protein patterns. J Mol Biol. 1981 Mar 5;146(3):287–304. doi: 10.1016/0022-2836(81)90389-2. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. High-resolution fractionation of nucleosomes: minor particles, "whiskers," and separation of mononucleosomes containing and lacking A24 semihistone. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3244–3248. doi: 10.1073/pnas.77.6.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. A simplified method for preparation of mouse satellite DNA. Anal Biochem. 1977 Apr;78(2):561–568. doi: 10.1016/0003-2697(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Mardian J. K., Paton A. E., Bunick G. J., Olins D. E. Nucleosome cores have two specific binding sites for nonhistone chromosomal proteins HMG 14 and HMG 17. Science. 1980 Sep 26;209(4464):1534–1536. doi: 10.1126/science.7433974. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Rae M. M., Franke W. W. The interphase distribution of satellite DNA-containing heterochromatin in mouse nuclei. Chromosoma. 1972;39(4):443–456. doi: 10.1007/BF00326177. [DOI] [PubMed] [Google Scholar]
- Reudelhuber T. L., Boulikas T., Garrard W. T. A nonamer of histones in chromatin. J Biol Chem. 1980 May 25;255(10):4511–4515. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rill R. L., Nelson D. A. Histone organization in chromatin: comparison of nucleosomes and subnucleosomal particles from erythrocyte, myeloma, and yeast chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):475–482. doi: 10.1101/sqb.1978.042.01.050. [DOI] [PubMed] [Google Scholar]
- Sager R., Grabowy C., Sano H. The mat-1 gene in Chlamydomonas regulates DNA methylation during gametogenesis. Cell. 1981 Apr;24(1):41–47. doi: 10.1016/0092-8674(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmatchenko V. V., Varshavsky A. J. A technique of low-pH gel electrophoresis of chromosomal proteins which does not require preliminary removal of DNA. Anal Biochem. 1978 Mar;85(1):42–46. doi: 10.1016/0003-2697(78)90271-3. [DOI] [PubMed] [Google Scholar]
- Sibatani A. Precipitation and counting of minute quantities of labeled nucleic acids as cetyltrimethylammonium salt. Anal Biochem. 1970 Feb;33(2):279–285. doi: 10.1016/0003-2697(70)90298-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Dahlberg A. E. Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM) - paper. Nucleic Acids Res. 1980 Jan 25;8(2):299–317. doi: 10.1093/nar/8.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Overall pathway of mononucleosome production. J Biol Chem. 1979 Apr 25;254(8):3074–3083. [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2A, a heteromorphous family of eight protein species. Biochemistry. 1980 Jul 8;19(14):3238–3245. doi: 10.1021/bi00555a022. [DOI] [PubMed] [Google Scholar]