Abstract
Background
The association between vitamin D status at birth and childhood allergic outcomes is uncertain. The desert climate of Tucson offers a unique setting for studying the health effects of higher exposure to vitamin D.
Objective
To assess relations between cord blood 25-hydroxyvitamin D (25[OH]D) levels and allergic outcomes through age 5 years.
Methods
Cord blood 25(OH)D levels were measured in 219 participants in the Tucson Infant Immune Study, a population-based birth cohort. Plasma total IgE and specific IgE to 6 aeroallergens were measured at 1, 2, 3 and 5 years. Skin-prick test (SPT) positivity (wheal ≥ 3mm), and physician-diagnosed active allergic rhinitis and asthma were assessed at age 5. Longitudinal models were used to assess relations between 25(OH)D and IgE outcomes. Logistic regression models were used to assess relations with SPT positivity, allergic rhinitis and asthma.
Results
The median cord blood 25(OH)D level was 64 nmol/L (interquartile range, 49 to 81 nmol/L). Relative to the reference group (50–74.9 nmol/L), both low (<50 nmol/L) and high (≥ 100 nmol/L) levels were associated with increased total IgE (coef.=0.27, P=0.006; and coef.=0.27, P=0.04, respectively) and inhalant specific IgE (OR=2.4, P=0.03; and OR=4.0, P=0.01, respectively) through age 5 years. High 25(OH)D levels also were associated with increased SPT positivity (OR=4.0, P=0.02). By contrast, 25(OH)D level was not significantly associated with allergic rhinitis or asthma.
Conclusion
Both low and high levels of cord blood 25(OH)D were associated with increased aeroallergen sensitization. The association between vitamin D status and actual allergic diseases merits further study.
Keywords: vitamin D, IgE, aeroallergen, allergic rhinitis, asthma, children
INTRODUCTION
Recent epidemiologic studies have shown an inverse association of maternal intake of vitamin D during pregnancy with childhood wheezing1, 2 and possibly childhood asthma.3, 4 Similarly, cross-sectional studies have found associations of low vitamin D status with both persistent wheezing in African American children5 and worse asthma severity among asthmatic children.6, 7These studies and others have led some investigators to propose that insufficient exposure to sunlight, with subsequent reduction of vitamin D, may be a cause of the asthma epidemic.8, 9
Complicating these studies, however, is the fact that most early wheezing is transient and not associated with classic atopic asthma,10 raising the question of whether vitamin D status influences risk for infectious wheezing or the allergic components of asthma. Indeed, recently published data from a New Zealand birth cohort showed that cord blood 25(OH)D levels were inversely associated with risk of respiratory infection and wheezing but had no association with incident asthma at age 5 years.11 Moreover, vitamin D supplementation was recently shown to reduce influenza A infection in a randomized controlled trial of Japanese school children.12
The relation of vitamin D to asthma pathogenesis is further complicated by studies suggesting that vitamin D levels may be associated with increased markers of atopy and increased risk ofallergic conditions.13–16 It has even been proposed that increased (not decreased) intake of vitamin D is responsible for the global epidemic of allergic disease.17
Using an established birth cohort in Tucson, Arizona, a desert city at 32°N latitude, we examined associations between cord blood levels of 25(OH)D with both atopy outcomes (total IgE, detectable inhalant IgE, and skin-prick test positivity) and childhood allergic diseases (allergic rhinitis and asthma) through age 5 years. Our objective was to study these associations in a population with high year-round exposure to direct sunlight and, consequently, higher levels of circulating 25(OH)D.
METHODS
Study design and population
The Infant Immune Study (IIS) is a prospective birth cohort study of immune system maturation and its relation to the development of asthma and allergic disease in childhood. Participants (N = 482) are healthy children born to pregnant women who planned to obtain care for their newborns from collaborating pediatricians, as previously described.8
Vitamin D status
Plasma levels of 25(OH)D were measured in cord blood specimens obtained at birth (n=241). Serum aliquots for each enrolled subject were shipped on dry ice to Massachusetts General Hospital (Boston, MA) for serum 25(OH)D measurement by liquid chromatography-tandem mass spectrometry (LC-MS).18 The method used was an isotope dilution, LC-MS assay optimized in the laboratory based on published procedures.19 The limit of detection is 5 nmol/L for D2 and 7.5 nmol/L for D3. The between-run CV for a quality control serum containing a total D104 vitamin D concentration of 57 nmol/L is 7.5%.
Outcome measurements
Total and inhalant allergen specific IgE were measured from blood specimens obtained at approximately 1 year (1.1 ± 0.1 years; n=364), 2 years (2.1 ± 0.2 years; n=310), 3 years (3.2 ± 0.3 years; n=295), and 5 years (5.1 ± 0.4 years, n=277), using the Pharmacia AutoCAP assay (Pharmacia/Upjohn, Kalamazoo, Mich) before its discontinuation in 2006 and subsequently using Immulite 2000 (Siemens Medical Solutions, Los Angeles, Calif). Samples analyzed on both instruments (n=25) yielded a correlation coefficient of 0.995. Specific IgE levels were measured for 6 inhalant allergens (Alternaria species, Dermatophagoides farinae, Bermuda grass, careless weed, olive and mulberry tree). Each allergen-specific IgE was categorized as detectable or undetectable based on a cut-off of 0.25 IU/mL. Total IgE measures were log transformed (log 10) for all statistical analyses.
Skin-prick tests (SPT) were conducted at age 5 (5.1 ± 0.4 years, n=330), using extracts of 17 aeroallergens common in the Tucson area, including: hou sedust mix, Bermuda grass, olive, careless weed, Alternaria alternata, mequite, mulberry, Dermatophagoides pteronyssinus, cat hair, cat pelt, dog, cockroach, penicillium, cladosporium, cypress, western ragweed, and aspergillus. All tests were read at 20 minutes. Wheal size was recorded as the sum of two perpendicular diameters (in mm). A child with a wheal size of 3mm or greater after subtracting the negative control diameter sum for any allergen was considered SPT positive.
Parents completed questionnaires assessing doctor-diagnosed allergic rhinitis at 3 and 5 years, and asthma at 1, 2, 3 and 5 years. Given the uncertainty of allergic rhinitis and asthma diagnoses in early childhood,20 data from all years were incorporated into prevalence outcomes at age 5. Allergic rhinitis was defined as report of doctor-diagnosed rhinitis at 3 or 5 years, plus symptoms since the age of 4 years. Asthma was defined as report of doctor-diagnosed asthma at 1, 2, 3 or 5 years, plus either asthma symptoms or medication use since the age of 4 years.
Statistical analysis
Cord blood levels of 25(OH)D were categorized into 4 groups: less than 50 nmol/L; 50–74.9 nmol/L; 75–99.9 nmol/L; and greater than or equal to 100 nmol/L. This categorization is consistent with differing national standards for deficiency,21, 22 while reflecting the Gaussian distribution of values in our population. For all outcomes analysis, the reference group was 50–74.9 nmol/L, the largest group represented in this analysis.
Potential confounders in the relations between 25(OH)D levels and outcomes were determined by assessing relations between 25(OH)D levels and demographic and prenatal environmental exposure factors. These relations were assessed by Fisher's exact test for Categorical variables, and by one-way ANOVA for continuous variables. All factors that were at least marginally significant (P<0.10) were included in a multivariable model, and those that remained significant were considered potential confounders in subsequent analyses with outcomes. In addition, we conducted both forward and backward stepwise regression models with a probability threshold of 0.10 to determine the robustness of relations between 25(OH)D levels and each factor.
Longitudinal relations between cord blood 25(OH)D levels and log total IgE over 1, 2, 3 and 5 years were assessed using mixed models, treating each subject as a random effect to control for within-subject correlation. In each model, age at the time of total IgE measurement was included as a categorical variable since log total IgE increases with age in childhood but in a non-linear fashion. Regression coefficients were calculated, representing the change in log total IgE associated with each 25(OH)D category with respect to the reference group (50–74.9 nmol/L).
Longitudinal relations between cord blood 25(OH)D levels and detectable inhalant IgE was assessed using Generalized Estimating Equations (GEE). Odds ratios were determined for each 25(OH)D category with respect to respect to the reference group (50–74.9 nmol/L). As with longitudinal models for total IgE, GEE models included age as a categorical variable.
Finally, cross-sectional logistic regression models were used to assess associations in terms of odds ratios between cord blood 25(OH)D levels and SPT positivity, allergic rhinitis and asthma at age 5 years.
All analyses were conducted using Stata version 10.0 (StataCorp, College Station, TX). This research was approved by the Institutional Review Board of the University of Arizona and informed consent was obtained for all subjects.
RESULTS
Children with both cord blood 25(OH)D data and at least one outcome measure are included in this analysis (n=219). Included children did not differ significantly from other children enrolled in the IIS (n=263) with respect to gender, maternal or paternal atopy, maternal or paternal asthma, household income or household smoking (all P>0.05). However, compared to excluded children, included children were more likely to have been born between May and October, and to have mothers who were older, non-Hispanic white, or had more years of education. A complete breakdown of these traits for included and excluded subjects is provided in a supplementary section (S-Table I).
Cord Blood 25(OH)D Levels
Cord blood levels of 25(OH)D ranged from 13 to 173 nmol/L, with 58 children (26.5%) having levels less than 50 nmol/L; 90 children (41.1%) with levels between 50 to 74.9 nmol/L; 47 children (21.5%) with levels between 75 to 99.9 nmol/L; and 24 children (11.0%) with levels greater than or equal to 100 nmol/L. The median cord level of 25(OH)D was 64 nmol/L (interquartile range, 49 to 81 nmol/L) (Figure 1).
Figure 1.
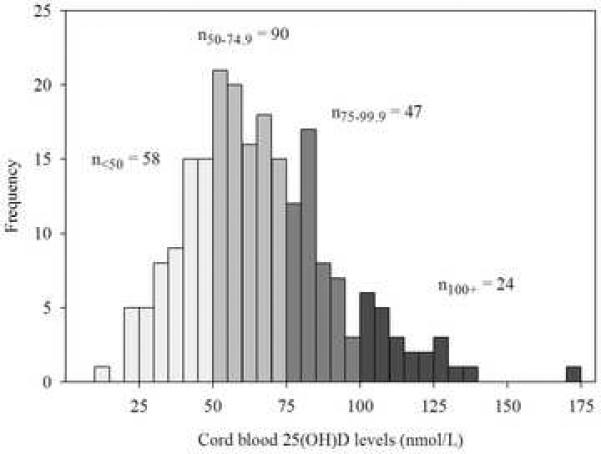
Histogram of cord blood 25(OH)D levels.
Demographic traits and prenatal exposures related to cord blood 25(OH)D levels included maternal ethnicity, maternal education, household income, birth season, and household smoking (Table I). When all of these factors were entered into a multivariable model of 25(OH)D level, only maternal ethnicity, birth season and household smoking remained statistically significant (data not shown). Both forward and backward stepwise regression modeling confirmed these same 3 factors as predictors of 25(OH)D levels, which were therefore considered as potential confounders in outcome analyses.
Table I.
Demographic traits and prenatal exposures, by cord blood 25(OH)D levels.
| Cord 25(OH)D level (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|
| <50 | 50–74.9 | 75–99.9 | ≥100.0 | ||||
| N | %(n) | %(n) | %(n) | %(n) | P-value* | ||
| Total | 219 | 26.5 (58) | 41.1 (90) | 21.5 (47) | 11.0 (24) | - | |
| Gender | |||||||
| Male | 107 | 30.8 (33) | 43.0 (46) | 16.8 (18) | 9.3 (10) | ||
| Female | 112 | 22.3 (25) | 39.4 (44) | 25.9 (29) | 12.5 (14) | 0.24 | |
| Maternal Ethnicity | |||||||
| Non-Hispanic White | 168 | 20.2 (34) | 42.3 (71) | 23.8 (40) | 13.7 (23) | ||
| Hispanic | 39 | 48.7 (19) | 38.5 (15) | 10.3 (4) | 2.6 (1) | ||
| Other/Unknown | 12 | 41.7 (5) | 33.3 (4) | 25.0 (3) | 0.0 (0) | 0.004 | |
| Household Income | |||||||
| < $25,000 | 28 | 50.0 (14) | 35.7 (10) | 10.7 (3) | 3.4 (1) | ||
| $25,000–45,000 | 50 | 18.0 (9) | 44.0 (22) | 22.0 (11) | 16.0 (8) | ||
| > $45,000 | 127 | 22.8 (29) | 43.3 (55) | 24.4 (31) | 9.4 (12) | 0.06 | |
| Maternal Atopy | |||||||
| No | 70 | 32.9 (23) | 37.1 (26) | 20.0 (14) | 10.0 (7) | ||
| Yes | 143 | 23.8 (34) | 44.1 (63) | 23.1 (33) | 9.1 (13) | 0.52 | |
| Maternal Asthma | |||||||
| No | 169 | 26.6 (45) | 43.8 (74) | 19.5 (33) | 10.1 (17) | ||
| Yes | 43 | 25.6 (11) | 30.2 (13) | 27.9 (12) | 16.3 (7) | 0.25 | |
| Birth Season | |||||||
| Nov – Apr | 93 | 31.2 (29) | 46.2 (43) | 18.3 (17) | 4.3 (4) | ||
| May – Oct | 126 | 23.0 (29) | 37.3 (47) | 23.8 (30) | 15.9 (20) | 0.02 | |
| Househod smoking | |||||||
| No | 176 | 23.9 (42) | 41.5 (73) | 21.6 (38) | 13.1 (23) | ||
| Yes | 43 | 37.2 (16) | 39.5 (17) | 20.9 (9) | 2.3 (1) | 0.10 | |
| Maternal age (years) | |||||||
| mean (sd) | 219 | 28.7 (6.2) | 29.5 (6.4) | 30.7 (5.8) | 29.8 (6.5) | 0.42 | |
| Maternal education (years) | |||||||
| mean (sd) | 219 | 14.8 (2.7) | 15.7 (2.7) | 16.0 (2.5) | 15.5 (1.4) | 0.08 | |
P-values from Fisher's exact tests and one-way ANOVA (for maternal age and education).
Outcomes
Geometric mean total IgE levels increased with age (1 year: 8.9 IU/mL, n=179; 2 years: 13.9 IU/mL, n=157; 3 years: 15.0 IU/mL, n=162; and 5 years: 21.9 IU/mL, n=135), as did prevalence of detectable inhalant IgE (1 year: 4.5% [8/176]; 2 years: 13.2% [21/159]; 3 years: 21.8% [34/156]; and 5 years: 31.6 [43/136]). Skin-prick test positivity occurred in 42.4% (73/172) of children at age 5. At age 5 years, the prevalence of doctor-diagnosed active allergic rhinitis was 19.8% (38/192) and the prevalence of doctor-diagnosed active asthma was 14.9% (29/194).
Relation of Cord Blood 25(OH)D Levels to Outcomes
Total and Detectable Specific IgE
Longitudinal relations between cord blood 25(OH)D levels and total and specific IgE from ages 1 to 5 years are displayed in Table II, using regression coefficients and odds ratios, respectively. Children with cord blood levels of 25(OH)D less than 50 nmol/L or of ≥100 nmol/L had significantly higher total IgE levels through age 5 with respect to children in the reference group (i.e., those with 25(OH)D levels between 50 and 74.9 nmol/L). The total IgE of children with 25(OH)D levels between 75 and 99.9 nmol/L was similar to the reference group. Detectable inhalant specific IgE also showed non-linear associations to 25(OH)D levels in that children with cord blood levels of 25(OH)D less than 50 nmol/L or of ≥ 100 nmol/L had significantly greater risk of having detectable inhalant IgE through age 5 than children in the reference group. Adjustment for maternal ethnicity, household smoking and birth season did not substantially alter these results. Cross-sectional analyses of total and detectable specific IgE show that these trends were present at each age, though not statistically significant at most ages (S-Tables II and III).
Table II.
Relation of cord blood 25(OH)D level to total IgE and detectable inhalant IgE from longitudinal models over ages 1, 2, 3, and 5 years.
| Log total IgE (n=207) | Detectable Inhalant IgE (n=208) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||
| 25(OH)D (nmol/L) | Coef (95% CI) | P-value | Coef (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| <50 | 0.27 (0.08, 0.46) | 0.006 | 0.27 (0.08, 0.47) | 0.007 | 2.4 (1.1, 5.5) | 0.03 | 2.8 (1.2, 6.6) | 0.02 |
| 50–74.9 | ref | - | ref | - | ref | - | ref | - |
| 75–99.9 | −0.01 (−0.21, 0.19) | 0.92 | 0.00 (−0.21, 0.20) | 0.99 | 2.0 (0.9, 4.7) | 0.10 | 2.1 (0.9, 4.7) | 0.08 |
| 100+ | 0.27 (0.01, 0.53) | 0.04 | 0.27 (−0.00, 0.54) | 0.054 | 4.0 (1.4, 11.6) | 0.01 | 3.6 (1.2, 10.5) | 0.02 |
Abbreviations: Coef (Coefficient), CI (confidence interval), OR (Odds Ratio)
Adjusted for maternal ethnicity, household smoking and birth season.
Skin-prick Test Positivity
Relations between cord blood 25(OH)D levels and skin-prick test positivity are displayed in the form of odds ratios in Table III. As was shown for total and specific IgE, 25(OH)D levels of 100 nmol/L or more were associated with significantly increased risk for skin-prick test positivity at age 5, relative to levels in the reference group. However, low levels of 25(OH)D showed no association with skin test response. Adjustment for potential confounders did not substantially alter these results.
Table III.
Prevalence and odds ratios for positive skin-prick test at age 5, by cord blood 25(OH)D level.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| 25(OH)D (nmol/L) | % (n+/N) | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Positive Skin-Prick Test (n = 172) | <50 | 37.5 (18/48) | 1.1 (0.5, 2.3) | 0.84 | 1.2 (0.5, 2.7) | 0.66 |
| 50–74.9 | 35.7 (25/70) | ref | - | ref | - | |
| 75–99.9 | 50.0 (19/38) | 1.8 (0.8, 4.0) | 0.15 | 2.0 (0.8, 4.5) | 0.12 | |
| 100+ | 68.8 (11/16) | 4.0 (1.2, 12.7) | 0.02 | 3.4 (1.0, 11.4) | 0.046 | |
Abbreviations: OR (odds ratio), CI (confidence interval)
Adjusted for maternal ethnicity, household smoking and birth season.
Allergic Rhinitis and Asthma
Relations between cord blood 25(OH)D levels and allergic rhinitis and asthma are displayed in the form of odds ratios in Table IV. Although there was a marginally significant increase in prevalence of allergic rhinitis among children with 25(OH)D of 100 nmol/L or more in the unadjusted and adjusted models (P=0.07 and P=0.11, respectively), there was no significant association between cord blood 25(OH)D level and asthma prevalence at age 5 (both P>0.60).
Table IV.
Prevalence and odds ratios for allergic rhinitis, and asthma at age 5, by cord blood 25(OH)D level.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| 25(OH)D (nmol/L) | % (n+/N) | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Allergic Rhinitis (n = 192) | <50 | 20.0 (10/50) | 1.1 (0.4, 2.6) | 0.86 | 1.1 (0.4, 2.8) | 0.81 |
| 50–74.9 | 18.8 (15/80) | ref | - | ref | - | |
| 75–99.9 | 12.5 (5/40) | 0.6 (0.2, 1.8) | 0.39 | 0.6 (0.2, 1.8) | 0.38 | |
| 100+ | 36.4 (8/22) | 2.5 (0.9, 7.0) | 0.09 | 2.4 (0.8, 7.3) | 0.11 | |
| Asthma (n = 194) | <50 | 10.0 (5/50) | 0.6 (0.2, 1.7) | 0.32 | 0.5 (0.2, 1.6) | 0.26 |
| 50–74.9 | 16.3 (13/80) | ref | - | ref | - | |
| 75–99.9 | 16.7 (7/42) | 1.0 (0.4, 2.8) | 0.95 | 1.1 (0.4, 3.1) | 0.84 | |
| 100+ | 18.2 (4/22) | 1.1 (0.3, 3.9) | 0.83 | 1.4 (0.4, 5.4) | 0.58 | |
Abbreviations: OR (odds ratio), CI (confidence interval)
Adjusted for maternal ethnicity, household smoking and birth season.
Adjustment for maternal asthma
Though the p-value associated with the relation between maternal asthma and 25(OH)D levels did not meet our threshold of significance for inclusion as a potential confounder in this analyses, we repeated all analyses adjusting for this variable as well. The inclusion of this variable did not materially alter the effect sizes reported above (S-Tables IV and V).
DISCUSSION
This birth cohort study shows a nonlinear relation between vitamin D status at birth and immune system markers of atopy through age 5 years. Both low and high levels of cord blood 25(OH)D were associated with higher levels of total IgE and inhalant specific IgE. In addition, we showed that high, though not low, levels of 25(OH)D at birth were significantly associated with increased SPT positivity in children at age 5. A similar pattern was seen for allergic rhinitis at age 5, although this relation was not statistically significant. In contrast, no relation was evident between cord blood 25(OH)D and physician diagnosed active asthma at age 5 years.
Several recent studies suggest that low vitamin D status at birth is associated with increased risk for childhood wheezing illness. Camargo et al.11 found an inverse relation of cord blood 25(OH)D to wheezing by age 15 months, 3 years and 5 years in a New Zealand birth cohort. Earlier studies are consistent with these 25(OH)D findings by showing that maternal vitamin D intake in pregnancy, through either foods or supplementation, is inversely related to wheeze2, 4 or recurrent wheeze1 in young children. In addition, recent studies have shown associations between vitamin D deficiency and asthma severity.6, 23 Given this body of research and the strong association between wheezing illness and IgE levels,24, 25 we were not surprised to find that low levels of 25(OH)D at birth were associated with elevated total and inhalant specific IgE.
We also found, however, that high levels of 25(OH)D at birth appear to convey risk for elevated markers of atopy, specifically increased total IgE, inhalant specific IgE, and positive skin test response prevalence at age 5 years. Other investigations have similarly suggested that vitamin D supplementation may be associated with increased risk for atopy, variously defined, and actual allergic diseases. In a historical cohort study, Hypponen et al. found that supplementation with high daily doses (≥2000 IU/d) of vitamin D in the first year of life was associated with increased SPT response and allergic rhinitis in more than 5000 subjects at age 31.15 Two small prospective studies showed 1) that vitamin D intake during infancy was positively associated with atopic dermatitis with and without asthma or rhinitis symptoms at age six;13 and 2) that maternal serum levels of 25(OH)D were positively associated with childhood eczema and asthma.14 A much larger prospective study found that supplementation during infancy with vitamins A and D was associated with increased risk of asthma and allergic sensitization at 4 years of age.16 Finally, a large cross-sectional study in over 7000 adults showed that both low and high serum 25(OH)D levels were associated with increased total IgE,26 a finding similar to that which we found in this longitudinal study of children. This evidence of a nonlinear relation with atopy suggests that it will be important to disentangle relations with immune system markers of atopy from those associated with actual allergic diseases to explicate the complex health effects of vitamin D.
The association between high levels of 25(OH)D at birth and subsequent markers of atopy was not consistently reflected in clinical outcomes in this population. Although statistical power was limited, there was a non-significant trend toward higher rates of physician-diagnosed allergic rhinitis at age 5 in association with elevated levels of 25(OH)D that mirrored our findings pertaining to IgE and skin test response. By contrast, we found no evidence of an association between cord blood levels of 25(OH)D and risk of childhood asthma. The literature regarding relations of vitamin D and allergic diseases is also inconsistent. Ekkola et al. showed an inverse relation of maternal dietary intake of vitamin D with both asthma and allergic rhinitis in their children to age 4.3 Other studies have reported a positive association between adult allergic rhinitis and concurrent serum 25(OH)D levels27 or vitamin D supplementation in the first year of life.15 Since both total IgE and inhalant allergen specific IgE are strongly associated with subsequent risk of allergic rhinitis and asthma,28 it will be important to continue to assess these associations as our population ages, and to address this complex issue in larger cohorts with greater statistical power.
The apparent nonlinear relation between vitamin D status and allergic/asthmatic outcomes observed both in this and previous studies may result from two opposing mechanisms operating simultaneously. On one hand, there is evidence from epidemiologic studies that vitamin D deficiency is a risk factor for respiratory infection. For example, Camargo et al. showed an inverse association of cord vitamin D levels and respiratory infections in almost 1000 New Zealand infants.11 Similarly, a randomized clinical trial among Japanese school children showed that supplementation with 1200 units of vitamin D was associated with significantly reduced risk of incidence of influenza A infection, and, among asthmatics, a reduced frequency of asthma attacks.12 On the other hand, the positive association between high levels of 25(OH)D levels at birth with total and inhalant specific IgE may be attributable to a different mechanism. Vitamin D is an immune system regulator, involved in both cell-mediated and humoral antibody responses, and supports a Th2-mediated anti-inflammatory cytokine profile.29 In a murine model of experimental allergic asthma, mice that lack a critical vitamin D receptor fail to develop airway inflammation, eosinophilia, or airway hyperresponsiveness, despite high IgE concentrations and elevated Th2 cytokines.30 Similarly, mice challenged with ovalbumin who received injections of 25(OH)D showed elevations in production of Th2 cytokines (IL-4 and 13) and total IgE, while the inflammatory response in the lungs was concurrently reduced, as indicated by eosinophils and IL-5 in BAL fluid and lung tissue.31 These studies suggest that increased levels of 25(OH)D might lead to sustained Th2 skewing in immune system markers in circulation, while simultaneously creating opposing, anti-inflammatory effects in the lung. Additional research into these complex relationships is clearly warranted.
This study has several strengths. First, it is one of the few birth cohort studies to have measured 25(OH)D levels in cord blood as opposed to estimating infant levels based on 25(OH)D levels in pregnant women 14 or maternal intake of vitamin D during pregnancy.1–4 Secondly, we collected outcome data at multiple ages, providing more robust measures of potential health effects than single time-point outcomes could provide. Finally, our study population was unique with respect to high annual sun exposure (i.e. 350 days of sunshine annually), providing a natural setting for assessing effects of higher 25(OH)D levels. Indeed, cord blood 25(OH)D levels in our population were considerably higher than those recently reported in a New Zealand study,32 and were higher than levels in women during late pregnancy in the United Kingdom.14 The large range of 25(OH)D levels in our population is a substantial strength given that our goal was to assess health effects of vitamin D in a population with high sun exposure.
This study also had several potential limitations. First, we cannot be sure that birth levels of 25(OH)D are causally linked with the outcomes studied. High levels of 25(OH)D at birth may be indicative of a lifestyle of increased sun exposure or increased supplementation, and thus children with high levels of 25(OH)D at birth may have high levels throughout childhood, which may be the true driver of the observed associations. Unfortunately, as this vitamin D analysis was not planned when this study was begun in 1997, our questionnaires did not specifically ask about vitamin D supplementation. Second, the number of subjects with allergic rhinitis (n=38) or asthma (n=29) in this analysis was relatively small, which limits our power to see relations with these outcomes, either positive or negative, with cord blood levels of 25(OH)D. Third, subjects were followed only to age 5 years, at which age asthma is still evolving from being infection-related to being predominantly related to allergy. For example, in an older cohort we have shown similar null effects between frequent wheeze at this age and day care, which was positively and negatively associated with frequent wheeze before and after that age, respectively.33 Finally, our sample included a disproportionate number of children born between May and October, months of higher sun exposure. However, our goal was to assess associations with higher levels of cord 25(OH)D which were evident in these children, and we adjusted for birth season in all analyses.
Clearly, vitamin D deficiency is undesirable from multiple standpoints, particularly bone health, and thus improving the vitamin D status of those who are deficient could have substantial health benefits. Indeed, most studies suggesting a protective effect of supplementation on childhood respiratory health1–3, 33 have been conducted in populations at northern latitudes with limited sun exposure, conditions associated with a greater prevalence of vitamin D deficiency. However, our findings of elevated atopy outcomes in a population of children with relatively high cord levels of 25(OH)D raise concerns about “one size fits all” interventions involving vitamin D supplementation of pregnant women. If such trials are conducted in a population with high baseline cord levels of 25(OH)D, vitamin D supplementation may actually contribute to increased atopy in the child. The findings from the present study suggest that it may be important to monitor prenatal levels of 25(OH)D in order to provide supplementation in a dose-appropriate manner with the goal of preventing vitamin D deficiency, rather than universally supplementing pregnant women, regardless of baseline level.
In summary, this study in a population with high levels of annual sun exposure showed that both low and high levels of cord blood 25(OH)D were associated with increased total IgE and aeroallergen sensitization. Although statistical power was limited for clinical outcomes, vitamin D status at birth was not statistically associated with risk of allergic rhinitis or asthma at age 5 years. This nonlinear relation with immune system markers of atopy may help to bridge seemingly contradictory findings from earlier studies involving relations of vitamin D with atopy and allergic disease.
Supplementary Material
Clinical Implications.
Both low and high levels of cord blood 25(OH)D ware associated with increased risk of aeroallergen sensitization. These findings suggest caution in prescribing large doses of vitamin D to pregnant women.
ACKNOWLEDGMENTS
The authors thank the study nurses Heidi Erickson, Lydia de la Ossa, Nicole Pargas and Jody Mallie for data collection on study subjects; David Spies and Bruce Saul for database management; and all of the IIS study subjects and families for their participation.
This work was funded by NIH grants AI 42268 and AI 61811 (Bethesda, MD); and the Massachusetts General Hospital Center for D-receptor Activation Research (Boston, MA)
Abbreviations
- IgE
Immunoglobulin E
- nmol/L
nanomoles per liter
- 25(OH)D
25-hydroxyvitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Camargo CA, Jr., Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 3.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 4.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 35:1228–34. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 5.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 156:948–52. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9:202–7. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss ST, Litonjua AA. Childhood asthma is a fat-soluble vitamin deficiency disease. Clin Exp Allergy. 2008;38:385–7. doi: 10.1111/j.1365-2222.2007.02920.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr., Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 12.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 13.Back O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89:28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 14.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 16.Kull I, Bergstrom A, Melen E, Lilja G, van Hage M, Pershagen G, et al. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J Allergy Clin Immunol. 2006;118:1299–304. doi: 10.1016/j.jaci.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54:757–9. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 18.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–9. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 19.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–98. [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 126:52–8. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau S, Nickel R, Niggemann B, Gruber C, Sommerfeld C, Illi S, et al. The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS) Paediatr Respir Rev. 2002;3:265–72. doi: 10.1016/s1526-0542(02)00189-6. [DOI] [PubMed] [Google Scholar]
- 25.Sunyer J, Anto JM, Castellsague J, Soriano JB, Roca J. Total serum IgE is associated with asthma independently of specific IgE levels. The Spanish Group of the European Study of Asthma. Eur Respir J. 1996;9:1880–4. doi: 10.1183/09031936.96.09091880. [DOI] [PubMed] [Google Scholar]
- 26.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613–20. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 27.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 28.Brockow I, Zutavern A, Hoffmann U, Grubl A, von Berg A, Koletzko S, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. 2009;19:180–7. [PubMed] [Google Scholar]
- 29.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–23. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 30.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol. 2004;173:3432–6. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 31.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 32.Camargo CA, Ingham T, Wickens K, Thadhani RI, Silvers KM, Epton MJ, et al. Vitamin D status of newborns in New Zealand. Br J Nutr. :1–7. doi: 10.1017/S0007114510001674. [DOI] [PubMed] [Google Scholar]
- 33.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


