Abstract
The presenilin genes harbor approximately 90% of mutations linked to early-onset familial Alzheimer’s disease (FAD), but how these mutations cause the disease is still being debated. Genetic analysis in Drosophila and mice demonstrate that presenilin plays essential roles in synaptic function, learning and memory, as well as neuronal survival in the adult brain, and the FAD-linked mutations alter the normal function of presenilin in these processes. Presenilin has also been reported to regulate calcium homeostasis of intracellular stores, and presynaptic presenilin controls neurotransmitter release and long-term potentiation through modulation of calcium release from intracellular stores. Here we highlight recent advances in deciphering the role of presenilin in synaptic function, calcium regulation and disease, and pose key questions for future studies.
Presenilin in neurodegenerative diseases
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized clinically by progressive memory loss and cognitive decline. The neuropathological hallmarks of AD are neuronal and synaptic loss, accumulation of extracellular amyloid plaques consisting of 40- to 42-residue β-amyloid peptides (Aβ40 and Aβ42) and intra-neuronal fibrillary tangles composed of hyperphosphorylated forms of the microtubule-associated protein tau [1]. Synaptic loss is thought to provide the best correlate to the severity of the disease, followed by neurofibrillary tangles. The presenilin proteins (PS1 and PS2) harbor approximately 90% of familial AD (FAD) mutations identified to date, with most mutations identified predominantly in PS1. All of the mutations are dominantly inherited, and the vast majority of the mutations are missense mutations scattered across the coding sequences. In addition to FAD, three PS1 mutations, Leu113Pro, Gly183Val and insArg352, have been reported in families with frontotemporal dementia (FTD) [2–4]. FTD shares some common histopathological features with AD, including neurofibrillary tangles and neuronal degeneration, specifically in the frontal and temporal lobes of the brain; however, the distinguishing feature of FTD relative to AD is the absence of amyloid plaques [5]. Clinically, FTD is characterized predominantly by behavioral changes including emotional blunting, apathy, and the loss of initiative followed by language impairment whereas memory is relatively preserved at early stages of the disease [5]. Both the Leu113Pro and the Gly183Val PS1 mutations reside in exon/intron boundaries, suggesting that these mutations may affect proper splicing, and the Gly183Val mutation has been confirmed neuropathologically to have Pick’s tauopathy in the absence of amyloid deposition [3]. Neuropathological analysis of the patient carrying the insArg352 PS1 mutation revealed that the family also carries a loss of function mutation in progranulin [6], raising the question as to whether the insArg352 mutation is pathogenic.
A major challenge in studying neurodegenerative diseases is to determine the mechanisms of age-related neuronal cell death. A prevailing view of AD pathogenesis is that synaptic dysfunction precedes neurodegeneration. In particular, defects in presynaptic neurotransmitter release may represent a converging early pathogenic event leading to neurodegeneration. This hypothesis was prompted by genetic studies in adult mice where loss of presenilin function results in progressive synaptic and memory impairments prior to age-dependent neurodegeneration [7, 8]. For example, loss of presynaptic presenilin impairs glutamatergic neurotransmitter release by modulation of intracellular calcium release in presynaptic terminals and long-term potentiation (LTP, which is thought to be the cellular substrate of memory) [8]. In this review article, we will summarize and discuss the latest advances in determining the roles of presenilins in synaptic function, intracellular calcium homeostasis and their association with AD pathogenesis.
Normal functions of presenilin
Presenilins are ubiquitously expressed in all tissues, including the nervous system. Presenilin expression is developmentally regulated, and changes in expression are associated with neuronal differentiation and synaptogenesis. In neurons, the presenilin holoprotein is primarily localized in the endoplasmic reticulum (ER) where it undergoes endoproteolysis to produce both an amino-terminal and a carboxy-terminal fragment (~27–30 kDa and ~16–20 kDa, respectively) that are thought to be functional entities. Presenilins have also been observed to be localized to the plasma membrane, specifically in presynaptic and postsynaptic compartments of neurons. PS1 was found to associate with the postsynaptic NMDA receptor and is thought to function at synapses by facilitating the proper synaptic delivery and localization of NMDA receptors [7]. Presenilins have also been reported to interact via their loop regions with Rab11, a small GTPase involved in the vesicular transport [9].
Presenilins are evolutionarily conserved integral membrane proteins with nine-transmembrane domains [10]. Presenilin, along with Aph-1, Pen-2 and nicastrin are integral components of a multi-protein protease complex, termed γ-secretase, which is responsible for the intramembranous cleavage of both the amyloid precursor protein (APP) and Notch (reviewed in [11]). Many substrates of γ-secretase have been reported, with diverse cellular functions,, including cell adhesion (N- and E-cadherins, CD44), ion conductance (β2 subunit of the voltage-gated sodium channel), and receptor kinase activity (ErbB4), to name a few (reviewed in [12]). However, with the exception of Notch, it is unclear whether the substrates undergo γ-secretase cleavage to release signaling fragments to the nucleus. For example, γ-secretase cleavage of E-cadherin releases promotes disassembly of the E-cadherin-catenin adhesion complex and this cleavage is not known to produce a nuclear signaling molecule [13]. Furthermore, increasing evidence has shown that presenilins exert γ-secretase-independent activities including regulation of the Wnt/β-catenin signaling pathway, calcium release from the ER, and lysosomal proteolysis [14, 15]. PS1 was shown to operate as a negative regulator of the Wnt/β-catenin signaling by serving as a scaffold for β-catenin degradation in the cytoplasm [16, 17].
Presenilin plays essential roles in development, as mice lacking either PS1 or both presenilins exhibit perinatal and early embryonic lethality, respectively [18, 19]. The most important role of presenilin in neural development is to prevent neural progenitor cells from differentiating into postmitotic neurons [20, 21]. Presenilin is also required for normal neuronal migration and cortical lamination as well as survival of Cajal-Retzius neurons [22]. Notch is the key mediator of presenilin function during development, based on extensive genetic analysis of various presenilin and Notch mutant mice [23]. Presenilin appears to maintain the neural progenitor population primarily through the regulation of the Notch signaling pathway [20, 24].
Presenilin in synaptic function
A wealth of evidence has accumulated showing that presenilins play an important role in synaptic function in the adult cerebral cortex (Table 1). The first study to investigate this employed Cre/loxP technology to generate PS1 conditional knockout (cKO) mice. By crossing floxed PS1 mice with transgenic mice that expresses the Cre recombinase under the control of the αCaMKII promoter, PS1 was selectively deleted in the excitatory neurons of the forebrain. In these PS1 cKO mice, PS1 inactivation begins around postnatal day 18 [25]. In contrast to the striking developmental defects in PS1 germline knockout mice, the forebrain-specific PS1 cKO mice are viable and exhibit normal evoked field excitatory postsynaptic potentials (EPSPs) recorded in the Schaeffer-collateral pathway using acute hippocampal slices (Figure 1) [25]. Cultured cortical or hippocampal neurons derived from PS1 germline knockout embryos showed increased frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) [26]. This result may be explained by higher numbers of synapses in PS1 knockout cultured neurons, which may be compensatory changes due to developmental defects caused by the absence of PS1 (e.g., fewer neurons). This interpretation is consistent with the greater synaptic contacts revealed by presynaptic and postsynaptic markers in PS1 knockout cultured cortical neurons.
Table 1.
Role of presenilins in synaptic plasticity, learning and memory and neuronal survival in the adult cerebral cortex.
| Presenilin knockoutsa | Synaptic deficitsb | Cognitive deficitsc | Neuronal lossc | Refs. | |
|---|---|---|---|---|---|
| FB-PS1 cKO (3–6 months) | ↔I/O ↔PPF |
↔LTP (TBS) ↔LTP, L-LTP (HFS) ↔LTD (ppLFS) |
mild deficits in spatial learning and memory | ↔cortical volume ↔cortical neuronal number |
[25] |
| FB-PS cDKO (2 months) | ↔I/O ↓PPF ↓synaptic facilitation ↓Pr (unitary responses) |
↓NMDAR function ↓LTP (TBS, pairing) ↔LTD (ppLFS) |
impaired spatial and associative memory | ↔cortical volume ↔cortical neuronal number ↑apoptotic cells |
[7,33,36] |
| FB-PS cDKO (6 months) | ↓PPF ↓maximal fiber volley ↑I/O |
↓NMDAR function ↓LTP (TBS, pairing) ↔LTD (ppLFS) |
severely impaired spatial associative memory | ↓cortical volume ↓cortical neuronal number |
[7,32,33] |
| CA3-PS cDKO (2 months) | ↓PPF ↓synaptic facilitation ↓Pr (MK-801) |
↔NMDAR function ↓LTP |
N/A | N/A | [8] |
| CA1-PS cDKO (2 months) | ↔PPF ↔synaptic facilitation |
↔NMDAR function ↔LTP |
N/A | N/A | [8] |
CA1-PS cDKO, inactivation by conditional double knockout of presenilins in CA1 neurons; CA3-PS cDKO, inactivation by conditional double knockout of presenilins in CA3 neurons; FB-PS1 cKO, forebrain PS1 conditional knockout; FB-PS cDKO, forebrain PS conditional double knockout.
I/O, input-output; LTD (ppLFS), long-term depression (paired-pulse low frequency stimulation); L-LTP (HFS), late phase of long-term potentiation (high frequency stimulation); LTP (TBS), long-term potention (theta burst stimulation); NMDAR, N-Methyl-D-aspartic acid receptor; PPF, pair-pulse facilitation; Pr, release probability.
N/A, not available.
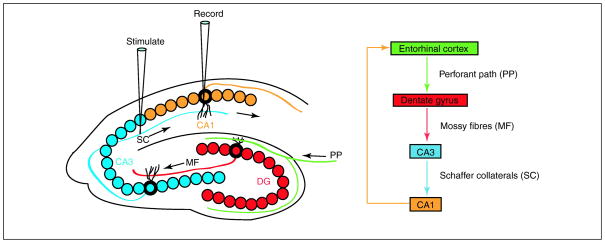
Figure 1. The hippocampal network.
The hippocampus forms a unidirectional network with input arising from the entorhinal cortex that forms synaptic connections with the dentate gyrus (DG) via the perforant path (PP). Axons from DG project to CA3 pyramidal neurons via the mossy fibres pathway (MF). Axons from CA3 project to CA1 pyramidal neurons via the Schaeffer collateral pathway (SC). These CA1 neurons in turn send the main output back to the entorhinal cortex. CA3 and CA1 neurons can also receive input directly from the perforant path.
PS1 cKO mice also exhibited mild cognitive deficits in long-term spatial memory that appear to be independent of the Notch signaling pathway [25]. Interestingly, conditional inactivation of PS1 in APP transgenic mice effectively prevented the accumulation of Aβ peptides but failed to ameliorate the memory impairments in APP transgenic mice [27, 28]. Furthermore, inhibition of γ-secretase activity by PS1 inactivation caused an age-dependent accumulation of γ-secretase substrates APP carboxyl-terminal fragments at presynaptic terminals, which are likely detrimental to neuronal function [27].
Interestingly, several reports showed age-dependent effects of PS1 mutations on synaptic plasticity as well as learning and memory. PS1 knockin mice expressing the Met146Val mutation exhibited age-dependent impairment on LTP induction; LTP was increased at 6 months, unchanged at 9 months and decreased at 12 months of age [29]. These knockin mice also showed spatial memory deficits in the post-training probe trials in the Morris water maze at 3 and 9 months of age [30]. In transgenic mice, overexpression of the PS1 Leu286Val mutant caused a transient increase in N-Methyl-D-aspartic acid receptor (NMDAR)-mediated responses and LTP at 4 months of age, but decreases by 13 months of age [31].
Analysis of presenilin conditional double knockout mice (PS cDKO) lacking both PS1 and PS2 in the postnatal forebrain revealed striking deficits in hippocampal learning and memory as well as synaptic plasticity impairments prior to elevated levels of tau hyperphosphorylation and neurodegeneration that resembles key neuropathological features of AD [7]. As these mice aged, they developed progressive synaptic and neuronal loss (Figure 2) [7, 32]. Curiously, following presenilins inactivation there is a significant delay (~4 weeks) in the detection of apoptosis, and apoptotic cell death occurs in only approximately 0.1% of cortical neurons, despite the fact that presenilins are inactivated in most, if not all, cortical neurons [33]. The presence of human tau exacerbated the memory impairment and neurodegeneration observed in PS cDKO mice [34]. These results define essential roles of presenilins in synaptic plasticity, learning and memory, and neuronal survival in the adult cerebral cortex. Mild spatial and associative memory impairments, concomitant with alterations in both short-term and long-term synaptic plasticity in the hippocampal Schaeffer-collateral pathway, were seen as early as 2 months of age in PS cDKO mice (Figure 3). Specifically, pair-pulse facilitation, a measure of presynaptic short-term plasticity, was impaired in PS cDKO mice, and LTP, a measure of experience-dependent synaptic strengthening was also significantly lower [7]. However, another form of synaptic plasticity, the metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD) induced by paired pulse low frequency stimulations, was normal in PS cDKO hippocampal slices, indicating the specificity of synaptic defects caused by loss of presenilins. LTP deficits in PS cDKO mice were associated with a reduction in NMDAR-mediated responses, synaptic levels of NMDAR subunits (specifically NR1 and NR2A), and αCamKII (a downstream effector of NMDARs in LTP induction) [7]. These results suggest impairment of synaptic NMDAR activity in PS cDKO mice may be due to a defect in intracellular trafficking and synaptic delivery of NMDARs. Moreover, presenilin deficiency resulted in decreased levels of transcriptional coactivator CREB binding protein (CBP) and transcription of CREB/CBP target genes, most likely due to indirect regulation of presenilins in CREB-mediated transcription [7, 35]. Loss of presenilins resulted in presynaptic deficits in short-term plasticity prior to postsynaptic NMDAR dysfunction, suggesting that presenilins regulate postsynaptic NMDAR function in part by trans-synaptic mechanisms [36]. Consistent with these findings in mice, analysis of the neuromuscular junction in presenilin-null flies also revealed impaired presynaptic plasticity and associative learning [37].
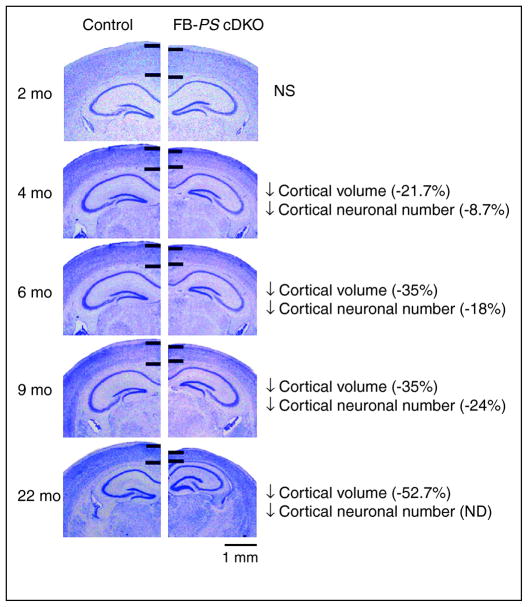
Figure 2. Age-dependent neurodegeneration in the forebrain of presenilin conditional double knockout mice (FB-PS cDKO).
Comparison of nissl-stained coronal brain sections of age-matched control (left) and FB-PS cDKO (right) from 2 to 22 months of age. The nissl-stain dyes for cell bodies and is useful to examine cell sizes, numbers and overall morphology of the brain. No detectable differences were found at 2 months of age; however, there was a gradual decrease in cortical thickness with subsequent ages in FB-PS cDKO brains. Black bars delineate the cerebral cortex. Scale bar: 1 mm. (Adapted from Wines-Samuelson et al. [33])
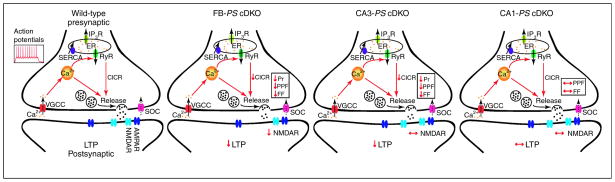
Figure 3. Models depicting how presenilins regulate synaptic function.
Upon depolarization, intracellular calcium levels are elevated due to calcium influx through voltage-gated calcium channels (VGCC), store-operated calcium channels (SOC), and calcium induced calcium release (CICR) from intracellular endoplasmic reticulum (ER) stores that is mediated through the ryanodine (RyR) and the inositol-1,4,5-triphosphate receptors (IP3R). Cytosolic calcium into the ER lumen is mediated through sarco-ER calcium ATPases (SERCAs) pumps. Inactivation of presynaptic presenilins in FB-PS cDKO and CA3-PS cDKO mice alters presynaptic release machinery through its control of calcium release from RyR in the ER thus reducing CICR. The reduction in calcium impairs the probability of neurotransmitter release (Pr), pair-pulse facilitation (PPF) and frequency facilitation (FF) which causes postsynaptic LTP impairments. In addition, inactivation of presynaptic and postsynaptic presenilins in FB-PS cDKO mice decreases NMDAR functions thus contributing to LTP deficits. Meanwhile, inactivation of postsynaptic presenilins in CA1-PS cDKO mice did not display any changes in short- and long-term plasticity.
Recent studies have shown that presenilins are essential for regulating neurotransmitter release during synaptic transmission. By genetically engineering mice with specific deletion of both presenilin genes in either presynaptic (CA3) or postsynaptic (CA1) neurons of the hippocampal Schaeffer-collateral pathway, it was shown that presynaptic, but not postsynaptic inactivation of presenilins, decreased LTP induced by theta burst stimulation [8] (Figure 2). Moreover, inactivation of presynaptic presenilins altered short-term plasticity and synaptic facilitation [8]. LTP deficits were not due to changes in postsynaptic NMDA or AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors but were instead attributed to a decreased probability of glutamate release, measured by the open channel blocker NMDAR antagonist MK-801 [8]. Remarkably, depletion of ER calcium internal stores by thapsigargin or inhibition of calcium release from intracellular stores by ryanodine receptor (RyR) inhibitors mimicked and occluded the effect of presynaptic presenilin inactivation, suggesting that presenilins modulate calcium-induced calcium release through RyRs on the ER [8]. Interestingly, there has been increasing evidence supporting a presynaptic role for intracellular calcium stores in regulating neurotransmitter release and modulating presynaptic plasticity [38, 39]. For example, axonal localization of RyR2 enables use dependent calcium release from intracellular stores within the mossy fibers and thereby facilitates presynaptic plasticity at the mossy fiber-CA3 synapse [38, 39]. Altogether, these studies demonstrate that loss of presenilin impairs LTP induction and glutamate neurotransmitter release by altering calcium release from intracellular stores and raised the possibility that impaired presynaptic mechanisms may play a role in AD pathophysiology.
Presenilins promote memory and neuronal survival in a γ-secretase-dependent mechanism, which is consistent with findings showing that conditional inactivation of nicastrin, another component of the γ-secretase complex, in the adult cerebral cortex, also resulted in progressive memory impairment and age-dependent neurodegeneration [40]. These results provide important insight into γ-secretase activity in learning, memory, and neuronal survival. The challenge is to identify the molecular targets downstream of γ-secretase activity that are involved in synaptic function and neuronal survival in the aging brain. Many substrates of γ-secretase-mediated intramembrane proteolysis have been reported, but the physiological significance of this cleavage is often unclear; Notch and APP are the confirmed physiological substrates [11]. Notch is clearly a key mediator of presenilin function during development [23], but its role in the adult brain has not been established. Similar to Notch, the intracellular domain of APP generated by γ-secretase-mediated cleavage has also been reported to have transcriptional transactivation activities [41, 42]; however, it is unclear whether presenilins promote memory and neuronal survival through the APP family. Recently, EphA4, an Ephrin receptor family member, was identified as a substrate of γ-secretase, and its proteolysis was enhanced by synaptic activity [43]. Overexpression of the EphA4 intracellular domain increases the number of dendritic spines though activation of the Rac signaling pathway [43]. This finding is consistent with the report showing that γ-secretase inhibitors reduce spine density in vivo [44]. Identification of the molecular targets of presenilin and γ-secretase, which are responsible for synaptic dysfunction and neurodegeneration caused by loss of presenilin, will provide novel therapeutic targets for AD treatment.
Presenilins in calcium homeostasis
Emerging evidence suggests a central role of presenilins in calcium homeostasis [reviewed [45, 46]. Calcium is an essential and tightly regulated cellular second messenger that orchestrates dynamic changes in neuronal function (Box 1). While extracellular calcium can enter the cell through voltage-gated calcium channels (VGCCs) and store-operated calcium channels, the ER is a major source of intracellular calcium and acts as a major regulator of intracellular calcium homeostasis. Cytosolic calcium is pumped into the ER by sarco-ER calcium ATPases (SERCAs), and calcium release from the ER into the cytosol occurs via two types of ligand-gated calcium channels, the RyR and the inositol-1,4,5-triphosphate receptor (IP3R). It was proposed that presenilins could form calcium leak channels in the ER and were integral components of intracellular calcium homeostasis via passive leak properties from the ER stores that is independent of γ-secretase activity [14].
Box 1. Calcium regulation.
Sources
Extracellular calcium can enter the cell through voltage-gated calcium channels (VGCCs), store-operated calcium channels (SOC), and various ligand-gated channels such as AMPA and NMDA receptors.
The endoplasmic reticulum (ER) is a major source of intracellular calcium and acts as a major regulator of intracellular calcium homeostasis.
Calcium release from the ER into the cytosol occurs via two types of ligand-gated calcium channels, the ryanodine receptor (RyR) and the inositol-1,4,5-triphosphate receptor (IP3R).
Intracellular targets
Cytosolic calcium binds to a large number of calcium binding proteins, such as calmodulin, that serve as a molecular effector to initiate the activation of several kinase-dependent signaling cascades.
Influx of calcium into the presynaptic terminal regulates neurotransmitter release via binding to synaptotagmin.
Influx of calcium into the postsynaptic terminal initiates activation of several kinase-dependent signaling cascades leading to CREB activation that is important for synaptic plasticity and long-term potentiation (LTP).
Removal mechanisms
Calcium can be transported from the cytosol to the extracellular space by the plasma membrane calcium ATPase (PMCA) and the sodium calcium exchanger (NCX).
Cytosolic calcium is pumped into the ER by the sarco-ER calcium ATPase (SERCA).
Cytosolic calcium is taken up by mitochondria through the mitochondrial calcium uniporter (MCU), rapid mode of uptake (RaM) and ryanodine receptor (RyR).
Cytosolic calcium can also be reduced via calcium binding proteins such as calbindin, which serves as calcium buffers.
In addition to a possible role for presenilins as calcium leak channels, presenilins have been reported to act at multiple sites in the regulation of intracellular calcium levels. Deficiency in PS1 leads to increases in calcium currents mediated by L- and P-type VGCCs in cortical neuronal cultures derived from PS1 knockout embryos [47], though conditional inactivation of presenilins in hippocampal CA3 neurons did not affect VGCC currents in acute slices [8]. In addition, capacitative calcium entry, a refilling mechanism for depleted intracellular calcium stores that triggers influx of extracellular calcium from store-operated calcium channels on the plasma membrane, is potentiated in cultured cells lacking PS1 or expressing FAD mutants [48, 49]. PS1 has also been shown to be involved in the regulation of ER calcium homeostasis; it can interact with and regulate SERCA, and can modulate calcium release through both the IP3R and RyR [50–52]. Additional investigation is needed to resolve how presenilins can functionally interact with all three major calcium regulators in the ER as well as potentially form a leak channel.
Large numbers of studies have suggested that FAD mutations in presenilins alter intracellular calcium signaling pathways. FAD mutations in PS1 have been shown to enhance ER calcium release via IP3R and RyR [51–53]. In contrast to these results, another study found that FAD-linked PS1 mutations reduced calcium release from ER intracellular stores [54]. Also, reports showing that FAD, but not FTD, mutations in PS1 abolished the ER calcium leak function, leading to excessive ER calcium overload and subsequent receptor-mediated release, made the apparent involvement of presenilins in calcium homeostasis even more complicated [14, 55]. Furthermore, PS1-deficient cells were reported to display reduced ER calcium levels due to presenilin interaction and positive regulation of SERCA activity [50]. At the present time, it is difficult to reconciliate these seemingly contradictory findings. The experimental systems employed in these studies are varied; some used immortalized PS-deficient murine embryonic fibroblasts [14, 50], which appear to have general defects, such as in transcription of housekeeping genes, independent of presenilin inactivation [35]. Others have used immortalized cell lines or primary cells that overexpress mutant PS1 [51]. The overexpression approach is problematic because the effect of mutant PS1, which causes a varying degree in the loss of its function dependent on the specific mutation, can be compensated by overexpression [56]. The neurogenesis and gliogenesis defects caused by PS1 deficiency also make primary cortical neurons derived from PS1 germline knockout embryos quite unsuitable for the study of PS1 function in the regulation of calcium homeostasis. Interestingly, genetic analysis in Drosophila showed that expression of FAD-linked mutant presenilin affects intracellular calcium stores and a loss of function mutant in calmodulin, an effector of intracellular calcium, can suppress presenilin-induced calcium deficits [57].
Given the central role of calcium in the regulation of synaptic function and neuronal survival, it will be crucial to determine the precise site of action by presenilins in the regulation of calcium homeostasis: VGCCs or store-operated calcium channels on the plasma membrane; SERCA, IP3R, RyRs, or leak channels on the ER; or a combination of these channels. Because calcium levels and fluxes are controlled by these calcium regulatory proteins, it is important to elucidate the mechanism by which FAD-linked presenilin mutations alter calcium homeostasis. From the earlier studies, it seems that a more physiologically relevant experimental system(s) should be employed, and calcium imaging studies should be coupled with electrophysiological or cell death analysis so that any changes caused by the mutations (FAD or deficiency) can be confirmed using multiple approaches. For example, the study by Zhang et al. employed a multidisciplinary approach (genetics, imaging, electrophysiology and pharmacology) to elucidate the role of presenilins in synaptic function and calcium homeostasis using two physiologically relevant and complementary experimental systems (acute hippocampal slices and primary hippocampal neuronal cultures in which presenilins were inducibly inactivated to circumvent the requirement of presenilin for embryonic neural development) [8].
It will be important to elucidate the exact mechanism by which presenilins regulate RyR function. Moreover, it is not yet clear whether γ-secretase activity is required for presenilin-mediated neurotransmitter release and synaptic plasticity. The simplest explanation would be that γ-secretase activity is required for memory formation and neuronal survival, as inactivation of nicastrin or presenilins, two components of the γ-secretase complex, causes memory impairment followed by age-dependent neurodegeneration [7, 40]. Another central question that needs to be addressed is whether synaptic defects caused by loss of presynaptic presenilins are a cellular precursor to neurodegeneration and other related AD pathology. If so, searching for presenilin targets and identifying molecular alterations that underlie synaptic dysfunction would be a viable alternative approach to delay or prevent the development of AD.
Concluding remarks
This review discussed several important implications for presenilins in synaptic function and disease. First, presenilins play a critical role in neurons as shown by the fact that loss of presenilins results in synaptic dysfunction and age-dependent neurodegeneration [7, 33, 58]. Second, the loss of presenilins causes impairment in neurotransmitter release, which may be the earliest pathogenic change prior to neurodegeneration and dementia. Defects in neurotransmitter release could be a common theme in neurodegenerative disease, as pathogenic mutations in five genes that are linked to familial Parkinson’s disease all impair dopamine release [59–64]. Therefore, it is reasonable to test whether impaired neurotransmitter release is a pathogenic precursor to neurodegeneration. Third, the importance of presenilins in the regulation of calcium homeostasis has been established, but due to the use of a large variety of experimental systems the findings are often incompatible. Better and more reliable experimental systems should be used to elucidate the detailed mechanisms by which presenilins regulate calcium homeostasis and how FAD mutations affect this process. Fourth, the molecular mechanisms by which presenilins control calcium homeostasis and regulate synaptic function are unknown. Last, and perhaps most importantly, the molecular targets of presenilin in the promotion of neuronal survival have not been identified. The fact that only 0.1% of cortical neurons lacking presenilin undergo apoptotic cell death makes it very difficult to dissect the molecular mechanisms underlying presenilin-dependent neuronal survival. Imaging tools may need to be developed to reveal molecular changes occurring in these cortical neurons before apoptosis. A number of outstanding questions raised by these points are discussed in Box 2. Elucidation of how presenilins regulate calcium homeostasis, synaptic function and age-related neurodegeneration may provide clues to AD pathogenesis, and identification of molecular targets of presenilin in neuronal survival may be used as novel therapeutic targets to combat neurodegeneration in AD.
Box 2. Outstanding questions.
Synaptic function of presenilin
Is presynaptic impairment caused by loss of presenilin function a pathogenic precursor to neurodegeneration?
How do presenilins regulate NMDA receptor function?
What is the molecular basis of presenilin-mediated presynaptic and postsynaptic function?
Do presenilins regulate neurotransmitter release in a manner that is dependent or independent of γ-secretase?
Can synaptic mechanisms regulated by presenilins be targeted for therapeutic intervention?
Calcium regulation by presenilin
Where do presenilins act on the regulation of calcium homeostasis, at the plasma membrane or the ER?
How do presenilins control ryanodine receptor-mediated calcium release from the ER?
Is calcium dysregulation an early pathogenic event in AD?
Are ryanodine receptors involved in AD pathogenesis?
Do presenilins regulate calcium homeostasis in a manner that is dependent or independent of γ-secretase?
Can restoring calcium homeostasis be therapeutically beneficial?
Molecular targets of presenilin
What are the molecular targets of presenilin in promotion of neuronal survival?
Are there γ-secretase substrates or proteins that interact with presenilin?
Acknowledgments
A.H. and J.S. are supported by grants from the NIH (K01 AG027311 to A.H.; NS041783 and NS042818 to J.S.) and the Alzheimer’s Association (to A.H. and to J.S.). We would like to thank Dr. Bei Wu for her critical reading and review of the manuscript and Dr. Mary Wines-Samuelson for Figure 1.
Glossary
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)
An ionotropic glutamate receptor that mediates fast synaptic transmission in the central nervous system
- Amyloid plaques
Extracellular accumulation of insoluble fibrous proteins composed of 40–42 amino acid peptides derived from intramembranous cleavage of the amyloid precursor protein
- Amyloid precursor protein (APP)
A type I integral protein that is ubiquitously expressed in various tissues. Proteolysis of APP produces amyloid-β peptides
- Calcium-dependent adhesion proteins (cadherins)
Family of type I transmembrane proteins that mediate cell adhesion in a calcium-dependent manner. Their cytoplasmic domain is associated with catenins
- Cajal-retzius neurons
Pioneer neurons located in the marginal zone that secrete the extracellular glycoprotein Reelin, a guidance signal for neuronal migration in the developing neocortex
- Calcium leak channels
A mechanism of unknown molecular nature that allows calcium to be leaked from the ER at a slow rate
- β-Catenin
A cytoplasmic protein that binds to the cadherin cytoplasmic tail and mediates the linkage between cadherins and actin microfilaments. Various signaling pathways, including the Wnt pathway, can induce accumulation of β-catenin, which can then be translocated to the nucleus where it interacts with transcription factors to modulate gene expression
- Cortical lamination
The laminated structure of the neocortex that is composed of 6 layers of cortical neurons
- cAMP response element-binding (CREB)
A transcription factor that binds a cAMP response element in the promoter region of its target genes via a conserved basic leucine zipper motif and plays an important role in protein synthesis-dependent late phase LTP
- CREB binding protein (CBP)
A transcriptional co-activator of many transcription factors, including CREB, that has intrinsic histone acetyltransferase activity, allowing it to modulate the chromatin structure of target genes
- Ephrin type-A receptor 4 (EphA4)
A subfamily of receptor tyrosine kinases involved in cell signaling pathways mediating a variety of neurodevelopmental events, such as axon guidance
- Excitatory postsynaptic potential (EPSP)
A temporary depolarization of postsynaptic membrane potential caused by the flow of positively charged ions into the excitatory postsynaptic neuron as a result of opening of ligand-sensitive channels
- Excitatory postsynaptic current (EPSC)
The flow of positively charged ions that causes an EPSP
- Fibrillary tangles
Intracellular aggregates of hyperphosphorylated forms of the microtubule-associated protein tau
- Frequency facilitation (FF)
A form of short-term plasticity induced by repetitive stimulation and the resulting accumulation of residual calcium in the presynaptic terminal
- Inositol-1,4,5-triphosphate receptor (IP3R)
A calcium channel activated by the second-messenger IP3 in the endoplasmic reticulum to release calcium
- Long-term depression (LTD)
An activity-dependent reduction in synaptic strength that lasts for one hour or more
- Long-term potentiation (LTP)
An activity-dependent increase in synaptic strength that lasts for one hour or more
- Metabotropic glutamate receptors
A family of G protein-coupled glutamate receptors characterized by their seven-transmembrane topology
- Nissl stain
A cresyl and violet histological stain that labels neurons by virtue of their high ribosomal content
- N-Methyl-D-aspartic acid receptor (NMDAR)
A type of ionotropic glutamate receptor that displays ligand- and voltage-gated calcium permeability
- Notch
A type I membrane receptor that is highly conserved throughout the animal kingdom and plays important roles in cell-fate decisions during development
- Pair-pulse facilitation (PPF)
A form of short-term plasticity induced by two closely spaced stimuli and the resulting accumulation of residual calcium in the presynaptic terminal
- Parkinson’s disease
A neurodegenerative movement disorder characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta region of the brain
- Pick’s tauopathy
A neurodegenerative disorder characterized by frontotemporal lobar degeneration and intraneuronal inclusions (Pick’s bodies) composed of hyperphosphorylated tau
- Rac signaling pathway
A subfamily of the Rho family of small GTPases involved in the control of cell growth, actin cytoskeleton reorganization, and activation of protein kinases
- Ryanodine receptor (RyR)
A calcium channel in the endoplasmic reticulum that mediates calcium-induced calcium release from intracellular stores
- Sarco-ER calcium ATPase (SERCA)
A calcium pump residing in the endoplasmic reticulum that translocates calcium from the cytosol to the ER at the expense of ATP hydrolysis
- Schaeffer-collateral pathway
Afferent projections of CA3 pyramidal neurons that form synapses on the dendrites of CA1 neurons of the hippocampus
- Store-operated calcium channel (SOC)
A calcium channel localized in the plasma membrane that is responsible for replenishing intracellular calcium in the endoplasmic reticulum via the capacitive calcium entry mechanism
- γ-Secretase
A multi-subunit protease complex containing presenilin-1 or presenilin-2, nicastrin, aph-1 and pen-2. This protease complex is responsible for intramembranous cleavage of type I membrane proteins, including the amyloid precursor protein and Notch
- Synaptic plasticity
Use-dependent changes in synaptic strength
- Theta burst stimulation
Trains of stimuli in which afferent axons are activated by high frequency stimulation to mimic the hippocampal theta rhythm that is often used to induce LTP
- Voltage-gated calcium channel (VGCC)
A family of calcium channels that are activated by membrane depolarization and mediate influx of calcium into the cell
- Wnt signaling pathway
A signaling cascade initiated by Wnt binding to cell surface receptors of the Frizzled family, that leads to stabilization of cytoplasmic β-catenin and modulated expression of target genes
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Scheff SW, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Amtul Z, et al. A presenilin 1 mutation associated with familial frontotemporal dementia inhibits gamma-secretase cleavage of APP and notch. Neurobiol Dis. 2002;9:269–273. doi: 10.1006/nbdi.2001.0473. [DOI] [PubMed] [Google Scholar]
- 3.Dermaut B, et al. A novel presenilin 1 mutation associated with Pick’s disease but not beta-amyloid plaques. Ann Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 4.Raux G, et al. Dementia with prominent frontotemporal features associated with L113P presenilin 1 mutation. Neurology. 2000;55:1577–1578. doi: 10.1212/wnl.55.10.1577. [DOI] [PubMed] [Google Scholar]
- 5.Lee VM, et al. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 6.Boeve BF, et al. Frontotemporal dementia and parkinsonism associated with the IVS1+1G->A mutation in progranulin: a clinicopathologic study. Brain. 2006;129:3103–3114. doi: 10.1093/brain/awl268. [DOI] [PubMed] [Google Scholar]
- 7.Saura CA, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, et al. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumanchin C, et al. Presenilins interact with Rab11, a small GTPase involved in the regulation of vesicular transport. Hum Mol Genet. 1999;8:1263–1269. doi: 10.1093/hmg/8.7.1263. [DOI] [PubMed] [Google Scholar]
- 10.Laudon H, et al. A nine-transmembrane domain topology for presenilin 1. J Biol Chem. 2005;280:35352–35360. doi: 10.1074/jbc.M507217200. [DOI] [PubMed] [Google Scholar]
- 11.De Strooper B, Annaert W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annu Rev Cell Dev Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi T, De Strooper B. Presenilins: members of the gamma-secretase quartets, but part-time soloists too. Physiology (Bethesda) 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- 13.Marambaud P, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu H, et al. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang DE, et al. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 17.Soriano S, et al. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, et al. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 19.Donoviel DB, et al. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handler M, et al. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- 21.Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wines-Samuelson M, et al. Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev Biol. 2005;277:332–346. doi: 10.1016/j.ydbio.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 26.Pratt KG, et al. A novel role for {gamma}-secretase: selective regulation of spontaneous neurotransmitter release from hippocampal neurons. J Neurosci. 2011;31:899–906. doi: 10.1523/JNEUROSCI.4625-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saura CA, et al. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewachter I, et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auffret A, et al. Progressive age-related impairment of the late long-term potentiation in Alzheimer’s disease presenilin-1 mutant knock-in mice. J Alzheimers Dis. 2010;19:1021–1033. doi: 10.3233/JAD-2010-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, et al. Hippocampal spatial memory impairments caused by the familial Alzheimer’s disease-linked presenilin 1 M146V mutation. Neurodegener Dis. 2005;2:6–15. doi: 10.1159/000086426. [DOI] [PubMed] [Google Scholar]
- 31.Auffret A, et al. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2009;29:10144–10152. doi: 10.1523/JNEUROSCI.1856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng R, et al. Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1 and presenilin-2. Proc Natl Acad Sci U S A. 2004;101:8162–8167. doi: 10.1073/pnas.0402733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wines-Samuelson M, et al. Characterization of age-dependent and progressive cortical neuronal degeneration in presenilin conditional mutant mice. PLoS One. 2010;5:e10195. doi: 10.1371/journal.pone.0010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peethumnongsin E, et al. Convergence of presenilin- and tau-mediated pathways on axonal trafficking and neuronal function. J Neurosci. 2010;30:13409–13418. doi: 10.1523/JNEUROSCI.1964-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe H, et al. Indirect regulation of presenilins in CREB-mediated transcription. J Biol Chem. 2009;284:13705–13713. doi: 10.1074/jbc.M809168200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D, et al. Inactivation of presenilins causes pre-synaptic impairment prior to post-synaptic dysfunction. J Neurochem. 2010;115:1215–1221. doi: 10.1111/j.1471-4159.2010.07011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight D, et al. Presynaptic plasticity and associative learning are impaired in a Drosophila presenilin null mutant. Dev Neurobiol. 2007;67:1598–1613. doi: 10.1002/dneu.20532. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, et al. Use-dependent amplification of presynaptic Ca2+ signaling by axonal ryanodine receptors at the hippocampal mossy fiber synapse. Proc Natl Acad Sci U S A. 2008;105:11998–12003. doi: 10.1073/pnas.0802175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nizami S, et al. Presynaptic roles of intracellular Ca(2+) stores in signalling and exocytosis. Biochem Soc Trans. 2010;38:529–535. doi: 10.1042/BST0380529. [DOI] [PubMed] [Google Scholar]
- 40.Tabuchi K, et al. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J Neurosci. 2009;29:7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, et al. Mediator is a transducer of amyloid-precursor-protein-dependent nuclear signalling. EMBO Rep. 2011;12:216–222. doi: 10.1038/embor.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 43.Inoue E, et al. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bittner T, et al. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 46.Mattson MP. ER calcium and Alzheimer’s disease: in a state of flux. Sci Signal. 2010;3:pe10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook DG, et al. Presenilin 1 deficiency alters the activity of voltage-gated Ca2+ channels in cultured cortical neurons. J Neurophysiol. 2005;94:4421–4429. doi: 10.1152/jn.00745.2005. [DOI] [PubMed] [Google Scholar]
- 48.Leissring MA, et al. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo AS, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 50.Green KN, et al. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheung KH, et al. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stutzmann GE, et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung KH, et al. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zatti G, et al. Presenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium. 2006;39:539–550. doi: 10.1016/j.ceca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Nelson O, et al. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michno K, et al. Intracellular calcium deficits in Drosophila cholinergic neurons expressing wild type or FAD-mutant presenilin. PLoS One. 2009;4:e6904. doi: 10.1371/journal.pone.0006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beglopoulos V, et al. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem. 2004;279:46907–46914. doi: 10.1074/jbc.M409544200. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 60.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J Neurochem. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 62.Tong Y, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 64.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]