Abstract
Role of mannose binding lectin (MBL) complement activation pathway, an arm of innate immunity in multiple sclerosis (MS) was evaluated by analyzing the expression of MBL, MBL-associated serine protease -2 (MASP-2), and functional MBL/MASP-2 mediated C4 cleavage (fMBL) in 87 plasma and cerebrospinal fluid (CSF) samples from MS patients and non-MS controls. Median fMBL and MASP-2 plasma levels were higher in MS vs. non-MS cases. These associations remained in an analysis of subtypes of MS disease. These findings suggest a potential activation of MBL complement pathway in MS that may possibly alter the risk or progression of MS disease.
Keywords: multiple sclerosis, mannose-binding lectin, innate immunity, plasma, cerebrospinal fluid
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease characterized by demyelination of the central nervous system (CNS) (Petersen et al., 2009). About 1.1 million people worldwide and about 400,000 of them live with MS in the United States. MS is considered a multi-factorial disease including viral infection (e.g., herpes simplex virus), genetic factors, and an impairment of the immune system (Hogancamp et al., 1997). MS linked autoimmunity has been mostly attributed to adaptive immune system dysfunction (Kasper and Shoemaker, 2010). However, innate immune system may play a crucial role in the pathogenesis of MS (Waldner, 2009). MS associated demyelination can be induced by direct complement activation after its binding to the myelin, which may result in the damage and lysis of oligodendrocyte and also in the chemoattraction and infiltration of macrophages.
Mannose binding lectin (MBL) is primarily synthesized in the liver and is distributed throughout the body via the bloodstream. MBL binds to mannose residues present on the surface of a pathogen, activates the MBL-associated serine protease -2 (MASP-2) and leads to C3 activation, MBL-mediated opsonization and phoagocytosis of the pathogen (Garred, 2008). A critical injury of the CNS may also initiate complement activation in MS disease. A neuroinflammatory response to a CNS injury may also result in MS related neurodegeneration (Yong and Marks, 2010).
In this study, we determined the expression of MBL, MASP-2 and functional MBL (MBL/MASP-2 mediated C4 cleavage activity) in plasma and cerebrospinal fluids from multiple sclerosis patients and analyzed their association with the MS disease.
2. Material and Methods
2.1. Study population
20 each blood and cerebrospinal fluid samples from subjects with varying degrees of multiple sclerosis were obtained from Human Brain and Spinal Fluid Resource Center, Los Angeles, CA. 29 plasma and 7 CSF samples from control non-MS subjects were used. Subjects were on several medications including steroids, estrogen, betaseron, extavia, avonex, baclofen, copaxone, natalizumab (Tysabri), ibuprofen, multivitamins, etc.
Multiple sclerosis primary progressive (MS1 P) disease was classified as primary progressive MS disease with no relapses, while MS secondary progressive (MS2 P) disease was classified as secondary progressive MS with or without relapses. MS 2 progressive with relapse (MS2 PR) was classified as secondary progressive MS with relapses in the last 2 years; and MS PRPS was classified as progressive relapsing MS disease that started initially as primary progressive but later had relapses. MS Relapsing/Remitting in Remission (MS RR) was classified by relapse (attacks of symptom flare-ups) followed by remission (periods of recovery).
2.2. MBL, MASP-2 and functional MBL/MASP-2 ELISAs for plasma samples
ELISA kits for MBL (Kirkpatrick et al., 2006), functional MBL/MASP-2 (fMBL) (Petersen et al., 2001) and MASP-2 (Møller-Kristensen et al., 2003; Schlapbach et al., 2007) were used (Catalog #s HK323, HK327, HK326 respectively, Cell Sciences Inc. Canton, MA, USA).
2.3. MBL, MASP-2 and functional MBL/MASP-2 ELISAs for CSF samples
MBL pathway proteins were analyzed in the CSF by modified sensitive ELISA assays followed by biotinyl-tyramide based horseradish peroxidase signal amplification (Bobrow et al., 1989; Bobrow et al., 1991).
2.4. Statistical analyses
A comparison of the MBL, MASP-2, and fMBL levels was done between the MS and non-MS groups for plasma (N=20 and 29 samples) and CSF (N=20 and 7 samples) respectively. The unadjusted comparisons used Wilcoxon rank-sum test. The comparisons were adjusted for age and gender via multiple regression, if necessary, using a conservative cut-off level of 0.15 (two-sided). The variables were analyzed on the logarithmic scale, in order to improve symmetry and normality of the distributions. The significance of the MS group effect was considered at the 0.05 level, two-sided. The effect sizes were computed based on the linear regression model (adjusted for age and/or gender, if necessary).
3. Results
3.1. Characteristics of Subjects
In the plasma samples (N=49), both the MS and no-MS groups had similar proportions of males (70% and 72.4% respectively, p-value=1.0), and the MS group (N=20) had similar median age (median = 47 years vs. 45 years) vs. the no-MS group (N=29), p=0.71. Similarly, in the CSF samples (N=27) there were 45% and 57.1% males in the MS (N=20) and no-MS groups (N=7) (p=0.68), and the MS group was slightly younger (median = 64.5 and 75 years, p-value=0.046). Of the 49 plasma samples, 44 were from Whites, 4 from Black and 1 from Hispanic subjects; while all of the 27 CSF samples studied were from White subjects. Median duration of the MS disease was 28.5 years.
3.2. Association of MBL, fMBL and MASP-2 expression in the plasma samples with MS disease
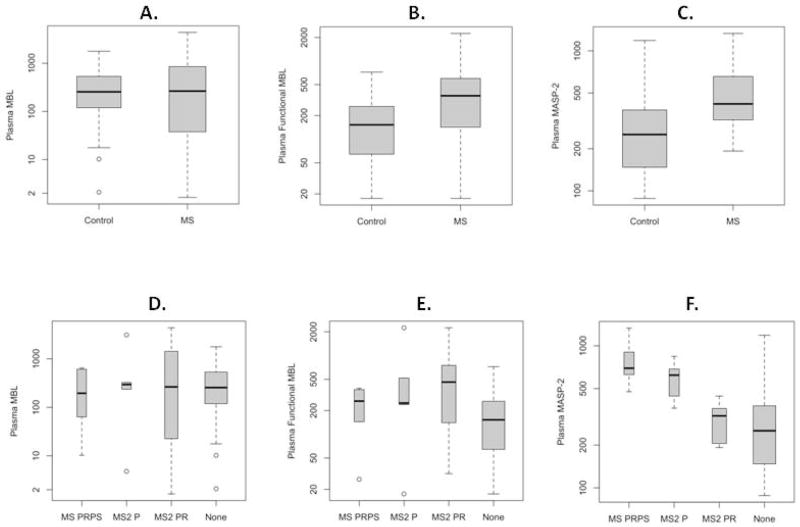
Total MBL levels in MS plasma samples were similar to those in the controls (MS vs. non-MS medians 264.7 vs. 255.4 ng/ml, p-value=0.89) (Figure 1A and Table 1). MASP-2 levels were higher in MS compared to non-MS plasmas (418.4 vs. 252.5 ng/ml respectively, p = 0.004) (Figure 1B, Table 1). The levels of fMBL were higher in MS vs. non-MS plasmas (359.4 vs. 152.7 U/ml respectively, p=0.029) (Figure 1C, Table 1).
Figure 1. Comparison of plasma levels of total MBL (ng/ml), MASP-2 (ng/ml), and functional MBL (U/ml), on logarithmic scale, between the MS and non-MS groups using Wilcoxon rank-sum test.
Dark middle line represents the median value, the box includes the middle 50% of the data, and the “whiskers” extend to the middle 95% of the data. Total MBL levels in plasma did not differ in MS vs. non-MS (control) samples while there were significantly higher levels of functional MBL and MASP-2 in MS vs. control samples. When analyzed by MS subtypes, levels of total MBL in plasma did not differ in MS progressive, relapsing and progressive or MS relapsing/remitting (MS2 PRPS) disease, MS secondary progressive (MS2 P), or MS secondary progressive with relapse (MS2 PR) disease compared to MS negative control samples (none) (D). However, functional MBL (E) and MASP-2 (F) were significantly higher in MS subtypes than the MS negative controls (none) (see text for details).
Table 1.
Expression of MBL, functional MBL and MASP-2 in plasma and CSF.
| Compartment | Variable | MS group (median) | No MS group (median) | Ratio of means, MS vs. No MS (and 95% CI) | p-value |
|---|---|---|---|---|---|
| Plasma (N-49) | MBL (ng/ml) | 264.7 | 255.4 | 0.84 (0.28, 2.55) | 0.89 |
| Functional MBL (U/ml)* | 359.4 | 152.7 | 2.04 (1.00, 4.16) | 0.029 | |
| MASP-2 (ng/ml) | 418.4 | 252.5 | 1.75 (1.19, 2.57) | 0.004 | |
| CSF (N=27) | MBL (ng/ml) | 4.18 | 1.76 | NA** | 0.48 |
| Functional MBL (U/ml) | 11.2 | 11.8 | 1.00 (0.70, 1.43) | 0.99 | |
| MASP-2 (ng/ml) | 33.9 | 33.1 | 1.03 (0.94, 1.13) | 0.51 |
Functional MBL shows quantitation measurement of the active MBL in the plasma through the measurement of the ability of MBL/MASP-2 complex to initiate C4 cleavage when it is bound to mannan.
For MBL, 23 of the 27 subjects (85%) had levels below the limit of detection (1.64); therefore, the ratio of means could not be computed.
3.3. Association of MBL, fMBL and MASP-2 expression in the plasma samples with MS types
Of the 20 MS subjects with plasma samples, 5 (25%) were MS secondary progressive (MS2 P), 10 (50%) were MS secondary progressive with relapse (MS2 PR), and 5 (25%) were MS progressive, relapsing and progressive or MS relapsing/remitting (MS2 PRPS). The three groups did not differ in their MBL (p=0.97) (Figure 1D and Table 1), or fMBL levels (p=0.72) (Figure 1E and Table 1). However, there were significant differences in the MASP-2 levels among the subtypes (p<0.001), with the MS2 PR group having lower median values (321.1ng/ml) than the MS2 P (621.6ng/ml) or MS2 PRPS (695.1ng/ml) groups (Figure 1F and Table 1).
3.4. Association of MBL, fMBL and MASP-2 expression in the CSF samples with MS or MS disease subtypes
The expression of MBL, fMBL and MASP-2 in cerebrospinal fluid samples did not show any association with MS (MBL, p = 0.48; fMBL, p=0.99; MASP-2, p=0.51) (Table 1). Of note, fMBL analysis adjusted by gender showed significant association with MS disease (p=0.003). For MBL, 23 of the 27 subjects (85%) had levels below the limit of detection (1.64), hence their means and analyses could not be computed.
Of the 20 CSF subjects with MS, 4 (20%) were of type MS RR, 5 (25%) were of type MS1 P, 10 (50%) were MS2 P, and one was of unclassified MS type. The three types did not differ in their MBL, MASP-2, or fMBL levels (p=0.44, 0.41, and 0.69) (Table 1).
4. Discussion
Limited studies have evaluated the role of MBL complement pathway in the context of innate immune response in MS cases only with human endogenous retrovirus infection (Christensen et al., 2007) or related interferon-β therapy (Petersen et al., 2009).
Our results showed that the expressions of MBL and MASP-2, important components of MBL complement pathway were higher in plasma samples from MS subjects. We observed significantly higher fMBL levels in the plasma of MS vs. non-MS cases suggesting that overall expression of fMBL is increased during MS. Although, higher levels of total MBL were reported to be associated with the slower progression of the MS disease in human endogenous retrovirus infection (Christensen et al., 2007), our results showed that total MBL levels did not differ significantly in a comparison of MS vs. non-MS plasma samples. In particular, our results show that functional MBL estimations provide more accurate levels of MBL/MASP-2 mediated C4 cleavage activity in the lectin complement pathway. Analyzing the fMBL is important because the presence of MBL2 genetic variants can alter expression or function of MBL (Singh et al., 2008). Furthermore, we found that the levels of MASP-2 were significantly higher in MS vs. non-MS plasma samples. Plasma expression of MASP-2 is generally observed in excess to MBL expression. Paradoxically, high MASP-2 levels could be linked to lower MBL levels in MS disease, suggesting a redundancy inherent in the immune system (Christensen et al., 2007). Increased levels of plasma functional MBL and MASP-2 could potentially facilitate and proliferate a cellular immune response by lectin complement pathway activation after initial tissue injury (Bouwman et al., 2006).
Similar to above results, we found higher plasma levels of fMBL and MASP-2 in subtypes of MS disease: MS secondary progressive (MS2 P), MS secondary progressive with relapse (MS2 PR) or MS progressive, relapsing and progressive or MS relapsing/remitting (MS2 PRPS) disease. When a comparison was made within the MS subtypes, only the association of higher MASP-2 with MS subtypes was significant. This suggests that higher MASP-2 levels may play an important role in overall immune activation through MBL complement activation pathway during MS disease. Finally, we found low levels of MBL, fMBL or MASP-2 in the CSF and none of these markers were associated with the MS disease.
In summary, increased functional MBL/MASP-2 mediated C4 cleavage activity and higher MASP-2 expression in the plasma from MS patients suggest that there is an activation of MBL complement pathway during MS disease. Further studies will be required to substantiate these findings.
Acknowledgments
This work was supported in part by NIMH/NIH (5R01MH085608). Blood and cerebrospinal fluid samples were obtained from Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Healthcare Center, Los Angeles, CA 90073 which is sponsored by NINDS/NIMH (NIH), National Multiple Sclerosis Society, and the Department of Veterans Affairs. This paper is subject to the NIH Public Access Policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen T, Møller-Larsen A, Thiel S, Brudek T, Hansen TK, Christensen T. Effects of interferon-beta therapy on innate and adaptive immune responses to the human endogenous retroviruses HERV-H and HERV-W, cytokine production, and the lectin complement activation pathway in multiple sclerosis. J Neuroimmunol. 2009;215:108–116. doi: 10.1016/j.jneuroim.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Hogancamp WE, Rodriguez M, Weinshenker BG. The epidemiology of multiple sclerosis. Mayo Clin Proc. 1997;72:871–878. doi: 10.4065/72.9.871. [DOI] [PubMed] [Google Scholar]
- 3.Kasper LH, Shoemaker J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology. 2010;74(Suppl 1):S2–S8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 4.Waldner H. The role of innate immune responses in autoimmune disease development. Autoimmun Rev. 2009;8:400–404. doi: 10.1016/j.autrev.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Garred P. Mannose-binding lectin genetics: from A to Z. Biochem Soc Trans. 2008;36:1461–1466. doi: 10.1042/BST0361461. [DOI] [PubMed] [Google Scholar]
- 6.Yong VW, Marks S. The interplay between the immune and central nervous systems in neuronal injury. Neurology. 2010;74(Suppl 1):S9–S16. doi: 10.1212/WNL.0b013e3181c97d04. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick BD, Huston CD, Wagner D, Noel F, Rouzier P, Pape JW, Bois G, Larsson CJ, Alston WK, Tenney K, Powden C, O’Neill JP, Sears CL. Serum mannose-binding lectin deficiency is associated with cryptosporidiosis in young Haitian children. Clin Infect Dis. 2006;43:289–294. doi: 10.1086/505396. [DOI] [PubMed] [Google Scholar]
- 8.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 9.Møller-Kristensen M, Jensenius JC, Jensen L, Thielens N, Rossi V, Arlaud G, Thiel S. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–167. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach LJ, Aebi C, Otth M, Leibundgut K, Hirt A, Ammann RA. Deficiency of mannose-binding lectin-associated serine protease-2 associated with increased risk of fever and neutropenia in pediatric cancer patients. Pediatr Infect Dis J. 2007;26:989–994. doi: 10.1097/INF.0b013e31811ffe6a. [DOI] [PubMed] [Google Scholar]
- 11.Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal. amplification. Application to immunoassays. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 12.Bobrow MN, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991;137:103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- 13.Christensen T, Petersen T, Thiel S, Brudek T, Ellermann-Eriksen S, Møller-Larsen A. Gene-environment interactions in multiple sclerosis: innate and adaptive immune responses to human endogenous retrovirus and herpesvirus antigens and the lectin complement activation pathway. J Neuroimmunol. 2007;183:175–88. doi: 10.1016/j.jneuroim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Singh KK, Lieser A, Ruan PK, Fenton T, Spector SA. An age-dependent association of mannose-binding lectin-2 genetic variants on HIV-1-related disease in children. J Allergy Clin Immunol. 2008 Jul;122(1):173–80. 180.e1–2. doi: 10.1016/j.jaci.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouwman LH, Roep BO, Roos A. Mannose-binding lectin: clinical implications for infection, transplantation, and autoimmunity. Hum Immunol. 2006 Apr–May;67(4–5):247–56. doi: 10.1016/j.humimm.2006.02.030. [DOI] [PubMed] [Google Scholar]