Abstract
The variability in symptom control is a challenging feature of asthma that necessitates careful monitoring and the need to step-up and step-down individualized therapeutic regimens over time. This stepwise concept in asthma therapy can be considered in at least three contexts. For lack of control that is persistent over long periods of time, an increase in the overall medication or a “step-up long-term (SLT)” is indicated. A second approach, “step-up short-term (SST)”, may be utilized during a temporary loss of acceptable control, such as at the onset of a viral respiratory tract illness. In these cases, a step-up in therapy is usually terminated in 3–10 days once asthma control has been satisfactorily achieved. Finally, for treating symptoms related to the variability of asthma on a day to day basis, ICS used concomitantly with a beta agonist has been evaluated, though not currently approved in the United States. We will term this particular intervention as “step-up intermittent (SUI).” Here we summarize the existing data regarding these three approaches to step-up care, step-down management, as well as identify areas where more comparative studies are necessary to provide further guidance to clinicians regarding proper step-up and step-down strategies in the care of asthma.
Keywords: asthma, step-up, step-down, stepwise, guidelines, severity, control, step-up intermittent, step-up short-term, step-up long-term
INTRODUCTION
Asthma is characterized by chronic airway inflammation, variable airflow obstruction, airway hyper-responsiveness and recurrent symptoms (1). The variability in symptom control is a particularly challenging feature of asthma that necessitates careful monitoring and the need to step-up and step-down individualized therapeutic regimens over time. To provide guidance to clinicians regarding proper step-up and step-down strategies, both national [National Asthma Education and Prevention Program (NAEPP) that has published three Expert Panel Reports (EPR)] and international [Global Initiative for Asthma (GINA)] panels have continued to convene in order to review evidence and to provide structured recommendations based on both the published literature and expert opinion when scientific and clinical data have been lacking (1–4).
Crucial for the appropriate management of asthma are consistent measures to assess disease progression and response to therapy. As described in the EPR-3 report, the effective assessment and monitoring of asthma patients are closely linked to the concepts of severity, control, and responsiveness to treatment (5). Asthma severity is defined as the intrinsic intensity of the disease process. Severity is measured most easily and directly in a patient not receiving long-term-controller therapy. Asthma control is defined as the degree to which the manifestations of asthma (symptoms, functional impairments, and risks of untoward events) are minimized and the goals of therapy are met. Responsiveness to therapy is the ease with which asthma control is achieved by therapy. Both asthma severity and control are evaluated using the domains of current impairment and future risk. Impairment is the frequency and intensity of symptoms and functional limitations the patient is experiencing or has recently experienced. Risk is the likelihood of asthma exacerbations, progressive decline in lung function (or, for children, reduced lung growth), and/or risk of adverse effects from medication.
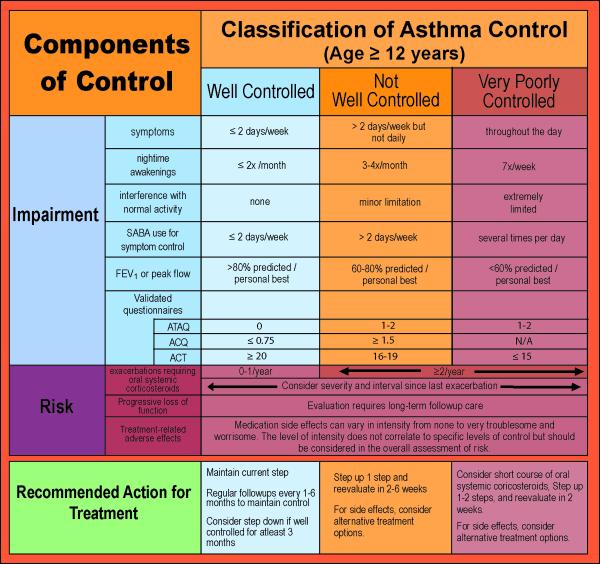
In terms of the assessment of current impairment, both the frequency and intensity of symptoms are measured. This can be assessed by further delineating asthma symptoms, such as the frequency of nighttime awakenings, need for short acting beta agonist (SABA) for the quick relief of symptoms, the number of work or school days missed, the effect on activities of daily living, as well as quality of life assessments. To help standardize the quantification of impairment, validated questionnaires like the Asthma Control Test (ACT) (6), the Childhood Asthma Control Test (7), the Asthma Control Questionnaire (8), the Asthma Therapy Assessment Questionnaire (ATAQ) control index (9), and others have been used more frequently in recent years. In addition to patient-reported symptoms, spirometry, as a means to measure lung function, can aid in classifying both severity and control (10).
The EPR-3 emphasizes the importance of periodic assessments at 1–6 month intervals to assess asthma control. The goal of asthma therapy is to maintain long-term control using the smallest amount of medication possible. Responsiveness to treatment is variable between patients, and to determine if goals of therapy are being met, follow-up assessments are critical (5). It is during these interval assessments that one must determine if the goals of therapy are being met and decide whether the dose, number of medications and/or frequency of administration should be increased, if necessary, and decreased when possible.
CHOOSING THE INITIAL STEP OF CARE
In patients naïve to asthma therapy, the initial step in choosing a therapeutic regimen is based on defining asthma severity (Figure 1). Patients with intermittent asthma are placed in the first of the six steps of the EPR-3 guideline-defined stepwise approach for managing asthma, as needed use of SABA (Figure 2). Should SABA use exceed 2 days per week, this would increase the patient's severity level to persistent asthma and a step-up would be recommended. Two exceptions to this guideline are exercised-induced bronchospasm (EIB) and viral respiratory infections. For viral-induced exacerbations occurring more than every 6 weeks, a step up (Step 2) in therapy is recommended (5).
Figure 1. Classification of Asthma Severity.
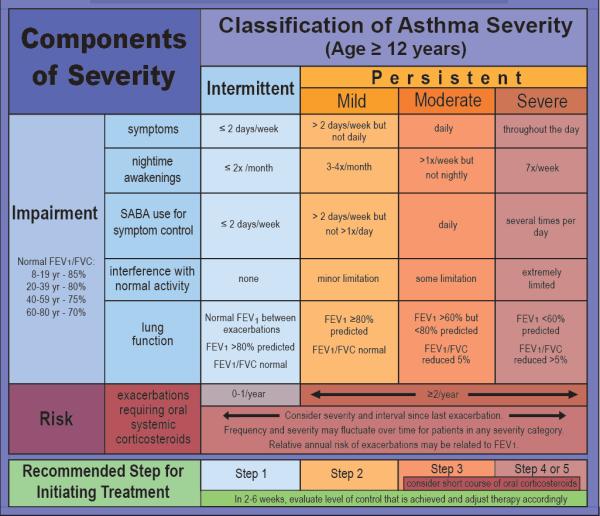
Adapted from the EPR-3. Expert panel report 3: guidelines for the diagnosis and management of asthma (EPR-3 2007) NIH Publication Number 08-5846. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, 2007.
Figure 2. Stepwise Approach for Managing Asthma.
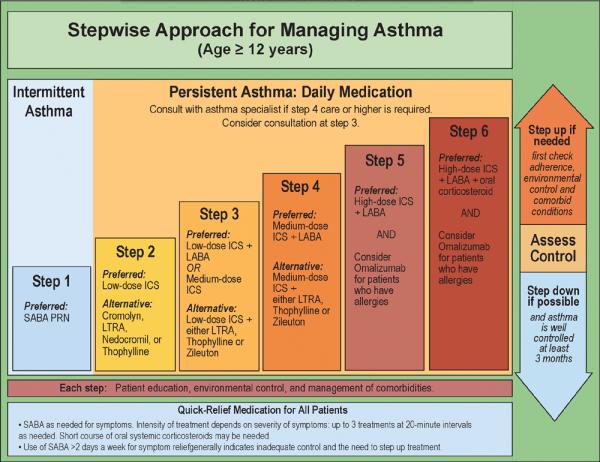
Adapted from the EPR-3. Expert panel report 3: guidelines for the diagnosis and management of asthma (EPR-3 2007) NIH Publication Number 08-5846. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, 2007.
For patients whose symptoms are not controlled by Step 1 intermittent SABA therapy, (e.g., daytime symptoms more than 2 days per week, nighttime symptoms more than 2 days per month, worsening lung function, greater than 2 exacerbation per year), Step 2 care should also be initiated. Inhaled corticosteroids (ICS) are the preferred treatment for Step 2 therapy across ages.
Once the initial step of care is chosen, the patient should be reevaluated periodically to determine whether their asthma control is satisfactory. If the current step of care is not controlling the patient's asthma based on the criteria defined in the EPR-3 guidelines (Figure 3), a step-up in care is initiated. Conversely, if the step of care has produced adequate control for prolonged periods of time, consideration should be given to step-down therapy based on the EPR-3 guideline recommendations (5).
Figure 3. Classification of Asthma Control.
Adapted from the EPR-3. Expert panel report 3: guidelines for the diagnosis and management of asthma (EPR-3 2007) NIH Publication Number 08-5846. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, 2007.
STRATEGIES FOR STEP-UP AND STEP-DOWN MANAGEMENT
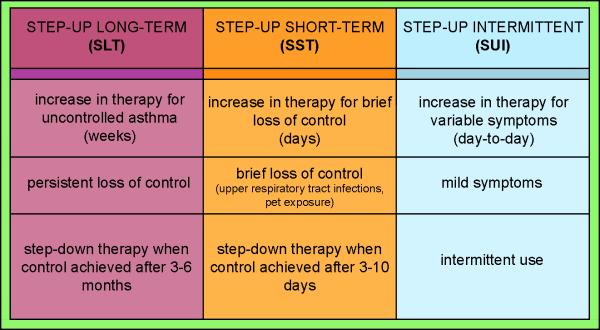
The concept of step-up and step-down in asthma therapy can be considered in at least three contexts (Figure 4). First, if lack of control is persistent over long periods of time (e.g., 2–3 weeks or longer), an increase in the overall medication regimen will be prescribed by moving up one or two steps as defined in the EPR-3 or GINA asthma guideline recommendations (4,5). We will term this particular intervention as “step-up long-term (SLT)”. This step-up in overall therapy is usually continued for 3–6 months to evaluate the ability and consistency of the new regimen to maintain adequate asthma control. At this point, consideration could be given to stepping down one absolute level with the stipulation that reevaluation should occur within the next 1–2 months to determine the consistency of control that this step-down regimen is able to maintain over time. A second approach to step-up therapy may occur in relationship to an anticipated brief loss of control (days), such as at the onset of a viral respiratory tract illness (11) or as a consequence of an acute short term exposure (e.g., a furred pet) that has been known to induce a temporary loss of acceptable asthma control (12). In most cases, this will entail a step-up in therapy consisting of more frequent short acting beta agonist (SABA) use and, potentially, an increase from baseline in the dose of inhaled corticosteroids (ICS) aimed at preventing a more significant exacerbation requiring oral corticosteroid treatment. This step-up in therapy is usually terminated in 3–10 days once asthma control has been satisfactorily achieved; at this point, a step-down to the baseline medication regimen is instituted. For the purposes of this review, we will term this particular intervention as “step-up short-term (SST)”. Finally, for treating symptoms related to the variability of asthma on a day to day basis, ICS used concomitantly with a SABA or a long acting beta agonist (LABA) has been evaluated. While this type of therapy has been studied in clinical trials and approved for use in many parts of the world, it is currently not approved in the United States. We will term this particular intervention as “ step-up intermittent (SUI).” Interestingly, this approach has been used in both step-up (using LABAs and SABAs) (13) and step-down (using SABA) treatment approaches (14) (15).
Figure 4. Stepwise Management of Asthma in 3 strategies.
For persistent loss of control over long periods of time, Step-up long-term (SLT) is indicated for an increase in the overal medication regimen of 1–2 Steps per EPR-3 or GINA guidelines. Step-up short-term (SST) occurs with a brief loss of control, usually requiring more frequent SABA dosing and/or an increase in baseline ICS. Step-up intermittent (SUI) refers to intermittent use of combination ICS/LABA or ICS/SABA for the day-to-day treatment of variable asthma symptoms.
STEP-UP LONG-TERM (SLT) STRATEGY
The SLT strategy was initially evaluated in moving from Step 2 to Step 3 care in adult patients whose asthma was not well controlled on low doses of ICS. Studies by both Greening et al. (16) and Woolcock et al. (17) using the SLT approach demonstrated that, in patients not well controlled on a low dose of ICS, the addition of a LABA was superior to increasing the dose of ICS for many outcome measures in the impairment domain. These observations were subsequently extended to the risk domain in the Formoterol And Corticosteroid Establishing Therapy (FACET) study (18). This study evaluated the effect of adding a LABA to a fixed ICS dose, quadrupling the dose of ICS, or both, on reducing asthma exacerbations. For one year, patients were treated twice daily with 100 mcg of budesonide, 100 mcg of budesonide and 12 mcg of formoterol, 400 mcg of budesonide, or 400 mcg of budesonide and 12 mcg of formoterol. With the addition of LABA to lower dose ICS, severe exacerbations were reduced by 26% and mild exacerbations were reduced by 40% when compared to lower dose ICS alone. The higher dose of ICS alone reduced rates of severe exacerbations by 49% and mild exacerbations by 37%. When LABA was added to the higher dose of ICS, severe exacerbations were reduced by 63% and mild exacerbations were reduced by 62%. Both lung function and asthma symptoms improved with both higher ICS doses and formoterol, although the greatest improvements were achieved with formoterol.
The issue of which SLT intervention leads to better overall asthma control when choosing between more ICS versus adding a LABA was comprehensively studied by Batemen et al. (19) in the Gaining Optimal Asthma ControL (GOAL) study. This randomized, double-blind, parallel-group study assessed the ability of predefined, step-wise adjustments of fluticasone/salmeterol or fluticasone alone to achieve two predefined measures of asthma control. The definitions of control, included composite measures of peak expiratory flow (PEF), rescue medication use, daytime and nighttime symptoms, exacerbations, emergency visits and other adverse events, based on the GINA or National Institutes of Health (NIH) guidelines (4). Unlike previous studies of controller therapy that focused on improvements with fixed doses of medications, the GOAL study allowed for dose escalation, in a stepwise approach, to achieve more comprehensive, sustained control. Phase I of the study was the dose escalation phase. During this time, treatment was stepped up every 12 weeks until asthma was totally controlled or until the medication reached 500 mcg of fluticasone twice daily or fluticasone/salmeterol 500/50mcg twice daily. Once asthma control was totally achieved, or after 12 weeks of maximal medication dosing, the patients were advanced to phase II, the constant phase. During this double blind treatment period, patients remained on the same dose of controller medication for 1 year. Of note, in an effort to determine if there were incremental effects over time of remaining on these medications there was no step-down treatment plan during this phase. While the majority of patients achieved well controlled or totally controlled asthma in this study, approximately 9% of patients in the fluticasone alone group and 5% of patients in the fluticasone/salmeterol group did not achieve well controlled asthma in phase I or phase II, requiring escalation to the maximum treatment phase or oral corticosteroids and fluticasone/salmeterol 500/50mcg. Nevertheless, the GOAL study provided strong evidence that patients with uncontrolled asthma, from a varied range of severity, were able to achieve and sustain guideline-defined control. It also demonstrated that stepping up therapy to achieve guideline-defined control, even in patients who didn't achieve the most stringent definitions of “total control,” still had significant improvements in health status and rates of exacerbation. Bateman et al. did emphasize that many risks of sustained treatment may take years to become clinically significant, and further research into step-down therapy once control was achieved was indicated. Regardless, the possibility of achieving guide-line based total control through medication escalation provided solid evidence for a stepwise approach to asthma management.
A recent study completed by the Childhood Asthma Research and Education (CARE) Network has provided evidence to guide SLT in children uncontrolled on low doses of ICS and needing Step 3 care. The Best Add–on Therapy Giving Effective Responses (BADGER) trial was a double-blind, three-treatment, crossover trial, in children aged 6 to 17 years with asthma uncontrolled on 100 mcg twice daily of fluticasone (20). The ICS step-up consisted of 250 mcg of fluticasone twice daily, the LABA step-up was fluticasone/salmeterol 100/50 mcg twice daily and the leukotriene receptor antagonist (LTRA) step-up was fluticasone 100 mcg twice daily plus 5 or 10mg of montelukast daily. The primary outcome was the differential response to each therapy based on a composite outcome of three measures in both the risk (exacerbations requiring oral prednisone) and impairment (the number of asthma control days and forced expiratory volume in 1 second (FEV1)) domains of asthma control. Remarkably, a differential response to therapy was demonstrable in 98% of the patients and there were no differences in response patterns based on age. Overall, LABA step-up led to the greatest likelihood of best response; however, some children had better responses with one of the other two therapies. This study emphasized the importance of regularly monitoring each child's response to medication and providing appropriate adjustment of therapy. Moreover, the findings also demonstrate that in the event that a child is not responding to a specific Step 3 therapy, rather than escalate to Step 4 care, alternative Step 3 treatments should be considered.
Safety concerns regarding the chronic use of LABA (21) have provided impetus for evaluating additional options in moving from Step 2 to Step 3 care. In this regard, the efficacy of an inhaled anticholinergic medication (tiotropium bromide) in comparison to either doubling doses of ICS or the addition of a LABA was recently examined. Peters et al. (22) hypothesized that the addition of tiotropium bromide in patients with inadequately controlled asthma would be more effective than doubling the dose of ICS, and that the addition of the long acting anticholinergic would not be inferior to the addition of a LABA. Compared with doubling glucocorticoid dosing, the use of tiotropium resulted in a greater improvement in morning and evening peak expiratory flow, pre-bronchodilator FEV1, daily symptom scores, and a greater proportion of asthma control days. Tiotropium also was non-inferior compared to the addition of salmeterol in all outcomes assessed. While the duration of treatment was limited to 14 weeks in this study, and long-term safety issues or rates of exacerbations could not be taken into account fully, the results suggest that another efficacious step-up treatment option when moving from Step 2 to Step 3 care may be the addition of a long acting cholinergic antagonist.
Step-up and step-down strategies using various biomarkers felt to be relevant to asthma pathophysiology have also been evaluated. Biomarkers evaluated have included methacholine airway hyperresponsiveness (23), sputum eosinophils (24), and exhaled nitric oxide (25,26). While these approaches have shown promise in reducing exacerbations and/or improving asthma control, measurements of airway hyperresponsiveness and sputum eosinophil counts, although performed in many subspecialty practices and research centers, are not routinely available in most primary care practices. Exhaled nitric oxide levels are currently being measured in many outpatient settings, though their overall contribution to patient management, their cost effectiveness and specificity to asthma are still not definitively established (27,28).
Strategies for choosing SLT to Steps 4 through 6 have not been evaluated as rigorously. However, recent work using interventions with monoclonal antibodies directed at either the allergic (e.g., omalizumab) or immunoinflammatory (mepolizumab) response have provided new insights regarding which patients should be considered for step-up involving these treatments. Humbert et al. (29) examined the effect of monoclonal anti-immunoglobulin (Ig)E antibody (omalizumab) in patients whose severe allergic asthma was inadequately controlled despite high dose ICS and LABA therapy with a reduced lung function and a recent history of asthma exacerbations. In this randomized, double-blind, parallel-group, placebo controlled trial of 419 patients, patients were placed on omalizumab or placebo for 28 weeks. Following adjustment for baseline exacerbation history, there was a 26% reduction of clinically significant exacerbation rates in the omalizumab group compared to placebo. Omalizumab also improved morning peak expiratory flow, asthma symptoms scores, and asthma-related quality of life. Additionally, there were no significant side-effects associated with the omalizumab group, with the most common adverse event being a local injection site reaction. Therefore, Humbert et al. concluded that omalizumab should be considered as a step-up option in patients with poorly controlled asthma despite high-dose ICS and LABA therapy. A recent study by Busse et al. (30) comparing the addition of omalizumab vs. placebo to guideline-based care in patients age 6–20 has also demonstrated its effectiveness in preventing asthma exacerbations across steps of asthma severity, suggesting that omalizumab may be considered for SLT in patients with frequent exacerbations. Currently, omalizumab is FDA approved for patients age 12 and above with moderate to severe allergic asthma (5).
The efficacy of an anti-IL-5 monoclonal antibody (mepolizumab), known to decrease eosinophil recruitment and survival, has been recently studied as well. In an early study of anti-IL-5 in human subjects with mild asthma, anti-IL-5 treatment decreased sputum and blood eosinophils following inhaled allergen challenge, but had no effect on either the late phase response or post-allergen increase in airway responsiveness to histamine (31). In another randomized, double-blind, placebo controlled trial, patients with severe asthma and persistent eosinophilia were given anti-IL-5 monoclonal antibody for one year. The treatment group had significantly less exacerbations, lower eosinophil counts, but no significant changes from baseline in symptoms, FEV1, bronchodilator use or airway hyper-responsiveness (32). Similar findings were seen in a study of mepolizumab in patients with persistent eosinophilia and corticosteroid dependent asthma. In addition to a significant decrease in the number of exacerbations, the treatment group also had a lower corticosteroid burden (33).
While omalizumab and mepolizumab have shown efficacy for SLT in patients with more severe asthma, comparison studies are needed to provide the clinician with evidence to guide individualized approaches to choosing SLT regimens at steps 4–6 of asthma care.
STEP-UP SHORT-TERM (SST) STRATEGY
A number of clinical trials have evaluated variations on the SST strategy for both step-up and step-down approaches to therapy. Noting the common practice of doubling ICS for the onset of exacerbations, Fitzgerald et al. (34) evaluated this approach for loss of control not responding to rescue SABA alone in a double-blind, randomized, controlled trial of 290 patients. Patients in the maintenance dose (MD) group received a maintenance dose of budesonide (100, 200 or 400 micrograms (mcg) twice daily) plus placebo inhaler to be used twice daily within 48 hours of the onset of an exacerbation. Patients in the double dose (DD) group received the same maintenance ICS dose plus an additional inhaler to be used twice daily for exacerbations that doubled the patient's ICS dose. The primary outcome measure was failure to regain control after developing symptoms of an impending exacerbation. The number of subjects with asthma exacerbations was not significantly different between the MD group (52) and the DD group (46) and treatment failure was equivalent in both study groups. Thus, doubling the dose of ICS was not effective in the management of impending asthma exacerbations.
Oborne et al (35) investigated if quadrupling the dose of ICS would be an effective option for attenuating impending exacerbations. In addition to their usual asthma treatment, patients were randomized to placebo or a quadrupled ICS dose in an inhaler used with a decline in PEF. The primary outcome, oral corticosteroid-requiring exacerbation, was less frequent in the treatment group but this was not statistically significant. However, in patients who were adherent to the study protocol (i.e., administered their study inhaler), exacerbations were significantly reduced by over fifty percent. Collectively, these data indicate that increased doses of ICS for SST may be effective in reducing oral corticosteroid exacerbations if the dose is high enough and the Boushey medication is started early enough.
Boushey et al. (36) approached SST using another trial design. They recruited patients with EPR guideline-defined mild persistent asthma who were well controlled on low doses of ICS and then randomized them into three groups: low dose ICS (200 mcg twice daily), a leukotriene receptor antagonist (zafirlukast 20 mg), and placebo. All three groups, when an a priori definition of lack of control was achieved, activated a symptom-based action plan, which consisted of open-label budesonide (800 mcg twice daily) for 10 days or prednisone (0.5 mg per kilogram of body weight per day) for 5 days if their asthma symptoms worsened. Thus, although labeled as the “placebo group”, these subjects received intermittent therapy that can be classified as an SST strategy. The three treatments produced similar increases in morning PEF and similar rates of asthma exacerbations. As compared with intermittent therapy or daily zafirlukast therapy, daily budesonide therapy produced greater improvements in pre-bronchodilator FEV1, bronchial reactivity, the percentage of eosinophils in sputum, exhaled nitric oxide levels, scores for asthma control, and the number of symptom-free days, but not in post-bronchodilator FEV1 or in the quality of life (P=0.18). Daily zafirlukast therapy did not differ significantly from intermittent treatment in any outcome measured. Based on these results, the authors concluded that it may be possible to treat mild persistent asthma with short, intermittent courses of inhaled or oral corticosteroids taken when symptoms worsen.
Another SST strategy, termed adjustable maintenance dosing (AMD), using combination therapy with an ICS and a LABA has been studied by a number of groups. Fitzergald et al. (13) compared fixed dosing with fluticasone/salmeterol (250/50 mcg twice daily) versus AMD with budesonide/formoterol (400/12 mcg twice daily). Following a run-in with each of these dosing strategies, patients in the budesonide/formoterol arm subsequently halved their dose and were then permitted to step-up or step-down numbers of puffs per day as indicated by the presence or absence of nocturnal awakenings due to asthma, frequency of rescue medication use, and changes in morning PEF. Following 48 weeks of therapy, stable dosing of fluticasone/salmeterol 250/50 mcg twice daily resulted in significantly greater increases in symptom-free days, days free of rescue medication, and morning PEF, as well as almost halving the exacerbation rate, compared with AMD with formoterol/budesonide. The authors concluded that there is a minimum daily amount of maintenance therapy necessary to prevent exacerbations in adults with persistent asthma.
In contrast, using similar combinations of ICS plus LABA, Aalbers et al. (37) found that AMD with budesonide/formoterol provided more effective asthma control by reducing exacerbations and reliever medication usage compared with fixed-dose fluticasone/salmeterol. Ind and colleagues (38) used a single combination product (formoterol/budesonide) to compare the degree of asthma control that AMD could achieve and maintain versus a fixed dosing strategy. In both groups, symptom control was maintained or improved in 85–86% of patients, and 94% experienced no treatment failures. The authors concluded that AMD with budesonide/formoterol in a single inhaler provides effective asthma control at reduced medication doses. Similar results favoring AMD strategies have been generated by other research groups as well (39).
Finally, in preschool children, intermittent therapy (SST) with ICS or LTRA for episodic disease was evaluated by Bacharier et al. in the Acute Intermittent Management Strategies (AIMS) study (40). Preschool aged children were randomized to either take budesonide 1 mg twice daily, montelukast 4 mg daily or placebo for 7 days at the first sign of a respiratory tract illness. The primary outcome was episode free days with a secondary outcome of symptom scores and oral corticosteroid use during illness. Among the three treatment groups, there was no significant difference in episode-free days, oral corticosteroid use, linear growth, health care use or quality of life. There were more significant differences in symptoms in both the LTRA and ICS groups compared to placebo in children with a positive modified asthma predictive index (mAPI) (41) or prior oral corticosteroid use. The findings suggested that episodic use of high-dose ICS or LTRA in children with intermittent asthma can decrease symptom burden during acute respiratory tract infections and most prominently in those with a positive mAPI (and future risk for asthma) or previous use of corticosteroids (propensity for greater illness severity).
STEP-UP INTERMITTENT STRATEGY
Due to the rapid onset of bronchodilation seen following administration of the LABA formoterol, various research groups have evaluated whether patients receiving combination therapy with formoterol/budesonide could use this medication not only for daily maintenance therapy but for the intermittent treatment of symptoms for which albuterol would normally be administered (SUI). The rationale for this approach was that, if this was shown to be therapeutic, patients would only need to possess one inhaler both for maintenance and reliever therapy. Moreover, the additional ICS that the patient would receive intermittently might further reduce risk for exacerbations.
The STAY study was a multicenter clinical trial involving 2,760 patients with asthma aged 4–80 years (FEV(1) 60–100% predicted) (42). The patients were randomized into one of three treatment arms: either terbutaline 0.4 mg as SABA with budesonide/formoterol 80/4.5 mug twice a day (budesonide/formoterol + SABA); or budesonide 320 mcg twice a day (budesonide + SABA); or budesonide/formoterol 80/4.5 mcg twice a day with 80/4.5 mcg as-needed (budesonide/formoterol maintenance + relief). Budesonide/formoterol maintenance + relief prolonged the time to first severe exacerbation, resulting in a 45–47% lower exacerbation risk compared to the other two treatment options. Budesonide/formoterol maintenance + relief also prolonged the time to the first, second, and third exacerbation requiring medical intervention, reduced severe exacerbation rates, and improved symptoms, awakenings, and lung function compared with both fixed dosing regimens.
Despite these efficacy data, in the United States concern regarding potential safety issues for LABAs has dampened enthusiasm by the Food and Drug Administration (FDA) to approve combination therapy for this type of SUI strategy (43). If clinical trials specifically addressing these safety concerns can be successfully conducted due to the large sample size needed to properly evaluate the outcomes of concern, it is possible that this step-up strategy may be reevaluated and ultimately approved in the United States if no safety concerns can be demonstrated (44).
These potential concerns related to safety of LABA administration have prompted alternative approaches to SUI. Two recent studies, one performed in adults by Papi et al. (14) and one in children by Martinez et al. (15), have taken the novel approach of stepping down therapy in patients with asthma well controlled on low doses of ICS (Step 2 care) and using a SABA (albuterol) plus a dose of ICS each time the patient feels a need for albuterol rescue. Thus, in one of the trial arms, patients have the option of using intermittent step-up therapy when required for symptom control and, when doing so, receive not only a bronchodilator but an anti-inflammatory medication as well.
In the adult study, patients with mild asthma controlled on beclomethasone 250 mcg twice daily, were randomly assigned to receive one of four inhaled treatments for 6 months: placebo twice daily plus 250 mcg of beclomethasone and albuterol in a single inhaler as needed (as-needed combination therapy); placebo twice daily plus albuterol as needed (as-needed albuterol therapy); 250 mcg of beclomethasone twice daily and albuterol as needed (regular beclomethasone therapy); or 250 mcg of beclomethasone and albuterol in a single inhaler twice daily plus albuterol as needed (regular combination therapy). Remarkably, the number of exacerbations during the 6-month treatment was significantly lower in the as-needed combination therapy group (SUI) than in the as-needed albuterol therapy group, but were not significantly different from those in the groups receiving regular beclomethasone therapy or regular combination therapy. Further, the cumulative dose of inhaled beclomethasone was lower in the as-needed combination therapy group (SUI) than in the groups receiving regular beclomethasone therapy or regular combination therapy (14).
The trial design of the pediatric study was similar but the ICS and albuterol were both in separate inhalers (as opposed to the adult study in which both ICS and albuterol were in a single inhaler). In this 44 week trial, 288 children and adolescents aged 5–18 years with mild persistent asthma were assigned to 1 of 4 parallel treatment groups. The daily ICS group received beclomethasone diproprionate 40 mcg twice daily and albuterol as a rescue medication. The rescue ICS (SUI) group received no daily controller and used beclomethasone 40 mcg and albuterol as a rescue medication. The combined group received both beclomethasone twice daily with albuterol and beclomethasone as a rescue. The placebo group received no daily controller and used albuterol alone as a rescue. The study's primary outcome was time to first exacerbation. While the frequency of exacerbations was 49% in the placebo group, the frequency of exacerbation was 28% in the daily ICS group, 31% in the combined group and 35% in the rescue ICS group. Linear growth was less in patients in the daily and combined ICS groups, but not in the rescue group when compared to placebo. With exacerbation frequencies significantly less than placebo and comparable to the other treatment groups, patients in the rescue ICS group (SUI) received only 15–25% of the daily ICS that those in the combined and daily ICS groups received. Martinez et al. concluded that, in children with mild-persistent asthma, rather than a complete discontinuation of daily ICS therapy altogether, the use of ICS as a rescue medication may be an alternative step-down strategy. Thus, intermittent step-up with ICS plus albuterol may be an option for children and adults whose asthma is on the border between Step 1 and Step 2 care (15).
STEP DOWN
As the goal of asthma therapy is to maintain long-term control using the smallest amount of medication possible, EPR-3 guidelines recommend consideration of stepping down therapy if asthma remains well controlled for at least 3 months to reach a minimum effective dose(5). While the Papi and Martinez studies in both adults and children provide novel approaches of stepping down therapy to intermittent dosing using SABA and ICS concomitantly, research comparing other step-down strategies at various levels of asthma severity is clearly needed across all age groups. Indeed, recently the FDA proposed stepping down off LABA in patients who are well controlled on LABA + ICS combination therapy (Step 3 to Step 2 care) (21). However, 3 separate studies found that stepping off LABAs after the establishment of acceptable asthma control resulted in the patient's asthma becoming less well controlled (45–47). Although confirmatory studies are needed, these initial results do not support the FDA's proposed recommendation that the step down be withdrawal of LABAs.
CONCLUSION
The step-wise approach to asthma management must be considered in multiple contexts, as loss of asthma control has variable presentations. Although we have summarized much of the existing literature using three different forms of step-up strategies (Figure 4), it is likely that other approaches will be found to be useful clinically as well. It is important to emphasize, however, that the most important factor in the successful utilization of a stepwise approach to asthma management that will achieve the greatest degree of control with the least amount of medication possible is scheduled follow-up care rather than crisis management alone.
Acknowledgments
Supported by NIH grants: 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), U10HL098090, and P01 HL70831.
Footnotes
Abbreviations: SLT-step-up long-term, SST-step-up short-term, SUI- step-up intermittent, NAEPP- National Asthma Education and Prevention Program, EPR- Expert Panel Reports, GINA- Global Initiative for Asthma, SABA- short acting beta agonist, ACT- Asthma Control Test, ATAQ- Asthma Treatment Assessment Questionnaire, ICS- inhaled corticosteroid, LABA- long acting beta agonist, FACET- Formoterol and Corticosteroid Establishing Therapy, mcg- microgram, GOAL- Gaining Optimal Asthma Control, NIH- National Institutes of Health, BADGER- The Best Add-on Therapy Giving Effective Responses, LTRA- leukotriene receptor antagonist, FEV1- forced expiratory volume in 1 second, Ig- Immunoglobulin, IL- Interleukin, DD- double dose, MD- maintenance dose, PEF- peak expiratory flow, AMD- adjustable maintenance dosing, AIMS- Acute Intermittent Management Strategies, mAPI- modified asthma predictive index, FDA- Food and Drug Administration
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).EPR. Expert panel report: guidelines for the diagnosis and management of asthma (EPR 1991) U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; Bethesda, MD: 1991. NIH Publication No. 91-3642. [Google Scholar]
- 2).EPR-2. Expert panel report 2: guidelines for the diagnosis and management of asthma (EPR-2 1997) U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; Bethesda, MD: 1997. NIH Publication No. 97-4051. [Google Scholar]
- 3.EPR-Update 2002. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics 2002 (EPR-Update 2002) U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; Bethesda, MD: Jun, 2003. NIH Publication No. 02-5074. [Google Scholar]
- 4).Global Initiative for Asthma (GINA) Pocket guide for asthma management and prevention. National Institutes of Health; National Heart, Lung, and Blood Institute; Bethesda: 1998. Publication No. 95–3659B. [Google Scholar]
- 5.EPR-3. Expert panel report 3: guidelines for the diagnosis and management of asthma (EPR-3 2007) U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; Bethesda, MD: 2007. NIH Publication Number 08-5846. [Google Scholar]
- 6).Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7).Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817–25. doi: 10.1016/j.jaci.2006.12.662. Epub March 2007. [DOI] [PubMed] [Google Scholar]
- 8).Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999b;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 9).Vollmer WM, Markson LE, O'Connor E, Sanocki LL, Fitterman L, Berger M, Buist AS. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1647–52. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- 10).Fuhlbrigge AL, Weiss ST, Kuntz KM, Paltiel AD. Forced expiratory volume in 1 second percentage improves the classification of severity among children with asthma. Pediatrics. 2006;118(2):e347–e355. doi: 10.1542/peds.2005-2962. [DOI] [PubMed] [Google Scholar]
- 11).Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol. 2002;2(2):132–8. doi: 10.1038/nri725. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Van Metre TE, Jr, Marsh DG, Adkinson NF, Jr, Fish JE, Kagey-Sobotka A, Norman PS, Radden EB, Jr, Rosenberg GL. Dos of cat (felis domesticus) allergen 1 (Fel d 1) that induces asthma. J Allergy Clin Immunol. 1986 Jul;78(1 Pt 1):62–75. doi: 10.1016/0091-6749(86)90116-8. [DOI] [PubMed] [Google Scholar]
- 13).Fitzgerald JM, Boulet LP, Follows RM. The CONCEPT trial: a 1-year, multicenter, randomized,double-blind, double-dummy comparison of a stable dosing regimen of salmeterol/fluticasone propionate with an adjustable maintenance dosing (AMD) regimen of formoterol/budesonide in adults with persistent asthma. Clin Ther. 2005 April;27(4):393–406. doi: 10.1016/j.clinthera.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14).Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, Crimi N, Vignola AM, Morelli P, Nicolini G, Fabbri LM. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007 May 17;356(20):2040–52. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- 15).Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, Strunk RC, Szefler SJ, Bacharier LB, Bade E, Covar RA, Friedman NJ, Guilbert TW, Heidarian-Raissy H, Kelly HW, Malka-Rais J, Mellon MH, Sorkness CA, Taussig L. Use of beclomethasone diproprionate as a rescue treatment for children with mild persistent asthma (TREXA): a randomized, double-blind, placebo-controlled trial. Lancet. 2011;377:650–57. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994 Jul 23;344(8917):219–24. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 17).Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996 May;153(5):1481–8. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 18).Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 19).Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 20).Lemanske RF, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, Covar RA, Guilbert TW, Larsen G, Morgan WJ, Moss MH, Spahn JD, Taussig LM. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Chowdhury BA, Dal PG. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med. 2010 April 1;362(13):1169–71. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- 22).Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Craig T, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Sont JK, Willems LN, Bel EH, Van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999 April;159(4 Pt 1):1043–51. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 24).Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002 December 30;360(9347):1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 25).Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005 May 26;352(21):2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 26).Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, Kattan M, Pongracic JA, Teach SJ, Bloomberg GR, Eggleston PA, Gruchalla RS, Kercsmar CM, Liu AH, Wildfire JJ, Curry MD, Busse WW. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008 September 20;372(9643):1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Smith AD, Cowan JO, Taylor DR. Exhaled nitric oxide levels in asthma: Personal best versus reference values. J Allergy Clin Immunol. 2009 October;124(4):714–8. doi: 10.1016/j.jaci.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 28).Jackson DJ, Virnig CM, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Burton RM, Salazar LP, DaSilva DF, Shanovich KM, Tisler CJ, Gern JE, Lemanske RF., Jr. Fractional exhaled nitric oxide measurements are most closely associated with allergic sensitization in school-age children. J Allergy Clin Immunol. 2009 November;124(5):949–53. doi: 10.1016/j.jaci.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedgecock S, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 30).Busse WW, Morgan WJ, et al. Randomized trial of omalizumab for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 32).Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl.J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl. J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 34).Fitzgerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J. Doubling the dose of budesonide versus mainteance treatment in asthma exacerbations. Thorax. 2004;59:550–556. doi: 10.1136/thx.2003.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroids to prevent asthma exacerbations. Am J Respir Crit Care Med. 2009;180:598–602. doi: 10.1164/rccm.200904-0616OC. [DOI] [PubMed] [Google Scholar]
- 36).Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, Chinchilli VM, Craig TJ, DiMango EA, Deykin A, et al. National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352(15):1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 37).Aalbers R, Backer V, Kava TT, Omenaas ER, Sandstrom T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Curr Med Res Opin. 2004;20(2):225–40. doi: 10.1185/030079903125002928. [DOI] [PubMed] [Google Scholar]
- 38).Ind PW, Haughney J, Price D, Rosen JP, Kennelly J. Adjustable and fixed dosing with budesonide/ formoterol via a single inhaler in asthma patients: the ASSURE study. Respir Med. 2004 May;98(5):464–75. doi: 10.1016/j.rmed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 39).Stallberg B, Olsson P, Jorgensen LA, Lindarck N, Ekstrom T. Budesonide/formoterol adjustable maintenance dosing reduces asthma exacerbations versus fixed dosing. Int J Clin Pract. 2003 October;57(8):656–61. [PubMed] [Google Scholar]
- 40).Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, Sorkness CA, Bloomberg GR, Morgan WJ, Paul IM, Guilbert T, Krawlec M, Cover R, Larsen G, Mellon M, Moss MH, Chinchilli VM, Taussig LM, Strunk RC. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127–1135. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr., Sorkness C, Szefler SJ, Larsen G, Spahn JD, Zeiger RS, Heldt G, Strunk RC, Bacharier LB, Bloomberg GR, Chinchilli VM, Boehmer SJ, Mauger EA, Mauger DT, Taussig LM, Martinez FD. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004 June;25(3):286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 42).O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu YJ, Ekström T, Bateman ED. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 43).Kramer JM. Balancing the benefits and risks of inhaled long-acting beta-agonists--the influence of values. N Engl J Med. 2009 April 16;360(16):1592–5. doi: 10.1056/NEJMp0810561. [DOI] [PubMed] [Google Scholar]
- 44).Sears MR. Safety of long-acting beta-agonists: are new data really required? Chest. 2009 August;136(2):604–7. doi: 10.1378/chest.09-1214. [DOI] [PubMed] [Google Scholar]
- 45).Bateman ED, Jacques L, Goldfrad C, Atienza T, Mihaescu T, Duggan M. Asthma control can be maintained when fluticasone propionate/salmeterol in a single inhaler is stepped down. J Allergy Clin Immunol. 2006;117:563–70. doi: 10.1016/j.jaci.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 46).Godard P, Greillier P, Pigearias B, Nachbaur G, Desfougeres JL, Attali V. Maintaining asthma control in persistent asthma: comparison of three strategies in a 6-month double-blind randomised study. Respir Med. 2008;102:1124–31. doi: 10.1016/j.rmed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 47).Koenig SM, Ostrom N, Pearlman D, Waitkus-Edwards K, Yancey S, Prillaman BA, et al. Deterioration in asthma control when subjects receiving fluticasone propionate/salmeterol 100/50 mcg Diskus are “stepped-down”. J Asthma. 2008;45:681–7. doi: 10.1080/02770900802168695. [DOI] [PubMed] [Google Scholar]