Abstract
Exposure to excessive manganese (Mn) levels leads to neurotoxicity, referred to as manganism, which resembles Parkinson’s disease (PD). Manganism is caused by neuronal injury in both cortical and subcortical regions, particularly in the basal ganglia. The basis for the selective neurotoxicity of Mn is not yet fully understood. However, several studies suggest that oxidative damage and inflammatory processes play prominent roles in the degeneration of dopamine-containing neurons. In the present study, we assessed the effects of Mn on reactive oxygen species (ROS) formation, changes in high-energy phosphates and associated neuronal dysfunctions both in vitro and in vivo. Results from our in vitro study showed a significant (P<0.01) increase in biomarkers of oxidative damage, F2-isoprostanes (F2-IsoPs), as well as the depletion of ATP in primary rat cortical neurons following exposure to Mn (500 µM) for 2 hours. These effects were protected when neurons were pretreated for 30 min with 100 µM of an antioxidant, the hydrophilic vitamin E analog, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), or an anti-inflammatory agent, indomethacin. Results from our in vivo study confirmed a significant increase in F2-IsoPs levels in conjunction with the progressive spine degeneration and dendritic damage of the striatal medium spiny neurons (MSNs) of mice exposed to Mn (100 mg/kg, s.c.) 24 hours. Additionally, pretreatment with vitamin E (100 mg/kg, i.p.) or ibuprofen (140 µg/ml in the drinking water for two weeks) attenuated the Mn-induced increase in cerebral F2-IsoPs and protected the MSNs from dendritic atrophy and dendritic spine loss. Our findings suggest that the mediation of oxidative stress/mitochondrial dysfunction and the control of alterations in biomarkers of oxidative injury, neuroinflammation and synaptodendritic degeneration may provide an effective, multi-pronged therapeutic strategy for protecting dysfunctional dopaminergic transmission and slowing of the progression of Mn-induced neurodegenerative processes.
Keywords: Manganese, oxidative stress, medium spiny neurons, neurodegeneration, vitamin E, trolox, ibuprofen
INTRODUCTION
Manganese (Mn) is a naturally occurring trace metal commonly found in the environment. As constituent of important metalloenzymes, such as arginase, glutamine synthetase, pyruvate carboxylase and superoxide dismutase, Mn is essential for brain development (Lucchini et al., 2009) and for the regulation of numerous biochemical and cellular reactions (Takeda, 2003). However, elevated occupational exposure to Mn poses a significant health risk. Major sources of environmental contamination from Mn include the manufacturing of alloys, steel, iron, glass ceramics and fertilizers, as well as mining operations (Mergler et al., 1994; Aschner, 2000; Racette et al., 2001; Bowler et al., 2007). Increased levels of Mn have been also associated with the use of the fuel additive, methylcyclopentandienyl managanese tricarbonyl (MMT) (Bolte et al., 2004), with individuals ingesting contaminated well-water and parenteral nutrition therapy, and with patients with compromised liver functions (Iinuma et al., 2003; Dobson et al., 2004). These environmental, occupational and disease conditions lead to excessive Mn accumulation in the nervous system (Erikson et al., 1996). Mn accumulation in the basal ganglia structures, specifically in the striatum and globus pallidus (Shinotoh et al., 1997), is associated with neurodegenerative disorder, commonly referred to as manganism, a condition which shares many similarities with Parkinson’s disease (PD). Early symptoms of this disorder include anxiety and decreased cognitive functions (Mergler et al., 1994), which are followed by changes in motor function and symptoms akin PD (Cersosimo and Koller, 2006). Both conditions are associated with alterations in the integrity of dopaminergic (DAergic) neurons and dopamine (DA) neurochemistry, including decreased DA transport function and/or striatal DA levels. Nigrostriatal DA axons synapse onto striatal medium spiny neurons (MSNs), and these neurons have radially projecting dendrites that are densely studded with spines (Wilson and Groves, 1980). Since MSNs comprise more than 90% of striatal neurons (Deutch et al., 2007), alterations in their dendritic length and dendritic spine number may destabilize the structural basis of synaptic communication and thus compromise MSN function. The integrity of DAergic neurons in the substantia nigra pars compacta, neurons which are preferentially targeted in PD, is thought to be spared in Mn-induced neurotoxicity (Perl and Olanow, 2007).
DAergic neurodegeneration in both PD and manganism is also accompanied by mitochondrial dysfunction, oxidative stress, aberrant signal transduction, protein aggregation and the activation of cell death pathways (Dobson et al., 2004; Kitazawa et al., 2005). Mn accumulates in mitochondria and inhibits the complexes of the electron transport chain (Zhang et al, 2004; Avila et al., 2010), thereby impairing oxidative phosphorylation (Gavin et al., 1992) and ATP production (Brouillet et al., 1993; Milatovic et al., 2007). The resulting decreased energy production alters mitochondrial permeability transition, leading to organelle swelling, disruption of the outer membrane and the release of numerous apoptogenic factors into the cytosol (Green and Reed, 1998). Mitochondrial-dependent proapoptotic factors associated with Mn neurotoxicity include cytochrome c release, caspase-3 activation, and DNA fragmentation (Latchoumycandane et al., 2005). Alterations in high-energy phosphates are also coupled with excessive Ca2+ influx and massive reactive oxygen species (ROS) production (Gunter et al., 2006; Milatovic et al., 2007). ROS induces the oxidation of membrane polyunsaturated fatty acids, yielding a multitude of lipid peroxidation products. One such family of products is the F2-isoprostanes (F2-IsoPs), prostaglandin-like molecules produced by the free radical-mediated peroxidation of arachidonic acid (Morrow and Roberts, 1999; Milatovic and Aschner, 2009). We have previously reported that Mn exposure induces a significant increase in these biomarkers of oxidative stress both in vitro and in vivo (Milatovic et al., 2007, 2009). Our results are in agreement with previous findings that antioxidants such as N-acetylcysteine (NAC), glutathione (GSH) and vitamin C prevented ROS production in mitochondrial preparations caused by a high concentration of Mn (Zhang et al., 2004). Other studies have also demonstrated that antioxidant treatments with an organotellurium compound and NAC are effective in protecting against the toxic effects of Mn in the rat brain and astrocytes, respectively (Hazzel et al., 2006; Avila et al., 2010). In addition to oxidative injury, our previous study also showed that Mn exposure is associated with inflammatory responses and the release of inflammatory mediators, including prostaglandins (Milatovic et al., 2009). Several studies have shown that neuroinflammation can lead to the overproduction and accumulation of various pro-inflammatory and neurotoxic factors, including cytokines, such as tumor necrosis factor-alpha (TNFα) and interleukin-1 beta (IL-1β), reactive nitrogen species (RNS), such as nitric oxide (NO), and lipid mediators that impel DA neurons to induce and/or exacerbate DA neurodegeneration (Liu et al., 2003; Filipov et al., 2005; Zhang et al., 2010). Therefore, the inflammatory response may cause irreversible tissue damage, and, in conjunction with oxidative stress, has been linked to the pathophysiology of several neurodegenerative diseases (Tansey et al., 2007).
In this study, we have employed biomarkers of oxidative damage (F2-IsoPs) and neuroinflammation (PGE2) to explore different pharmacological interventions to attenuate Mn-induced neurotoxicity in vitro and in vivo. We have also employed Golgi impregnation and Neurolucida-assisted neuronal tracings to investigate the extent to which such attenuation is accompanied by the protection of synaptodendritic degeneration in the striatal MSNs.
MATERIALS AND METHODS
Materials
Manganese chloride (MnCl2), indomethacin, trolox, vitamin E, ibuprofen (99% pure) and ATP standards were purchased from Sigma Chemical Company (St. Louis, MO). Dulbecco's Modified Eagle Medium (DMEM) with heat-inactivated horse serum, penicillin, streptomycin and cytosine arabinoside were purchased from Invitrogen (Carlsbad, CA). 15-F2α -IsoP-d4 (internal standard for F2-IsoPs that contains four deuterium atoms), PGE2 internal standard and prostaglandin F2, E2 and D2 methyl esters were purchased from Cayman Chemicals (Ann Arbor, MI).
Animals
All experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committees. As used in our previous studies, C57Bl/6 female mice (obtained from Jackson laboratories, Bar Harbor, Maine) between 6 and 8 weeks of age, were housed at 21 ± 1 °C, humidity 50 ± 10%, and a light/dark cycle of 12 h/12 h, and the animals had free access to pelleted food (Rodent Laboratory Chow, Purina Mills Inc., St. Louis, MO) and water. Mice were exposed to 100 mg/kg Mn by a single subcutaneous (s.c.) injection at the scruff of the neck. Control mice received same volume of saline. Vitamin E (α-tocopherol, 100 mg/kg) was dissolved in mineral oil and administered intraperitoneally (i.p.) in three doses on days −2, −1 and 0 relative to the Mn injection. Ibuprofen was administered in the drinking water at 140 µg/ml continuously for 2 weeks. All groups (4–6 mice in each group) were euthanized 24 hours after the injection of Mn or saline. Cerebral hemispheres were immediately removed and processed for the evaluation of F2-IsoPs and PGE2 or were processed with Golgi impregnation for the evaluation of the synaptodendritic changes of striatal MSNs. The dose and route selections of Mn administration for this study are based on our previous study (Milatovic et al., 2009) as well as on observations by Dodd et al. (2005), showing that the Mn injection protocol produced a greater than six-fold increase in striatal Mn levels relative to vehicle control mice.
Primary neuronal cultures
Primary cultures of cortical neurons were obtained from 17- to 18-day-old fetal Sprague–Dawley rats. Briefly, the cerebral cortex was isolated and placed into Hank's balanced salt solution (HBSS) containing 0.125% trypsin. After removal of the meninges, the cerebral cortices were digested with bacterial neutral protease for 30 min at room temperature, followed by mechanical trituration with pipettes. Subsequently, cells were plated on glass coverslips placed in 6-well plates coated with poly-l-ornithine at a density of 6.7 × 105 cells/well. The neurons were grown in DMEM containing 10% heat-inactivated fetal bovine serum and F12 with penicillin (100 IU/ml) and streptomycin (100 µg/ml) at 37°C in a humidified atmosphere of 95% air–5% CO2. Two days after plating, non-neuronal cell proliferation was inhibited by the addition of cytosine arabinoside (10 µM), and the next day, the culture media was changed to NeuroBasal supplemented with B27, penicillin (100 IU/ml) and streptomycin (100 µg/ml). Experiments were carried out in 10- to 14-day-old cultures.
Mn concentrations (500 µM) in this study with primary cultures of cortical neurons were determined based on the relevant toxic Mn effect on mammalian cells as described in the literature. Weekly injections of Mn over a 3-month period (0, 2.25, 4.5, or 9 mg/kg) in monkeys have been shown to produce dose-related clinical signs, which are more severe in the higher dose ranges (Suzuki et al., 1975). While the basal ganglia represent the main target for Mn neurotoxicity, reflecting its preferential accumulation in this region (Dorman et al. 2006), it is also well known that Mn affects the cerebral cortex (Guilarte and Chen 2007), thus providing a rationale for examining cells derived from this brain region. The concentration-dependent neurotoxic effect of 100 µM, 500 µM or 1 mM Mn has also been confirmed in our previous study with primary neuronal and astrocytes cultures (Milatovic et al., 2007, 2009). Primary neuronal cultures were incubated with indomethacin or trolox (100 µM) for 30 min at 37°C, and F2-IsoPs and ATP levels were quantified at 2 hours following exposure to Mn.
Biochemical assays
Quantification of F2-IsoPs
Upon completion of the experiments, primary neuronal cultures and brains were rapidly harvested, flash-frozen in liquid nitrogen and stored at − 80°C until analysis. Total F2-IsoPs were determined with a stable isotope dilution method with detection by gas chromatography/mass spectrometry and selective ion monitoring as previously described (Morrow et al., 1999; Milatovic and Aschner, 2009). For F2-IsoPs evaluation from the neuronal cultures, cells were resuspended in 0.5 ml of methanol containing 0.005% butylated hydroxytoluene, sonicated and then subjected to chemical saponification using 15% KOH to hydrolyze-bound F2-IsoPs. The cell lysates were adjusted to a pH of 3, followed by the addition of 0.1 ng of 15-F2α-IsoP-d4 internal standard. F2-IsoPs were subsequently purified by C18 and silica Sep-Pak extraction and by thin layer chromatography. They were then analyzed by pentafluorobenzyl ester, a trimethylsilyl ether derivative, via gas chromatography, negative ion chemical ionization-mass spectrometry. Quantification of F2-IsoPs from brains of mice pretreated with vitamin E or ibuprofen and exposed to saline or Mn was carried out following the same procedures with exception that, prior to chemical hydrolyses with KOH, cerebral hemispheres were homogenized in Folch solution and lipids were extracted from the chloroform layer by evaporation (Milatovic and Aschner, 2009).
Measurement of adenosine 5′-triphosphate (ATP)
Levels of ATP were analyzed by an isocratic reversed-phase high performance liquid chromatography (HPLC) method (Yang et al., 2004). ATP was extracted from control, indomethacin or trolox pretreated and Mn-exposed neurons by adding 950 µl of ice-cold perchloric acid (0.2 M) containing Na-EDTA (1 mM) to the primary astrocyte culture plates immediately after the medium was removed. The cells were then scraped off the bottom of the plates, and the acid extract was transferred to a microcentrifuge tube. The acid extract was neutralized with 170 µl of potassium hydroxide (KOH; 2 M) and centrifuged at 9000 g for 5 min to remove fine precipitates of perchlorate (KCLO4). The supernatants were stored at −20°C before being subjected to ATP determination. The concentration of ATP was determined in a 15 µl sample extract injected into HPLC with UV detector and 0.1 M of ammonium dihydrogen phosphate (pH 6.0) containing 1% methanol as a mobile phase. Using the Symmetry Shield C-18 column and a flow rate of 0.6 ml/min, the peak of ATP was eluted at a retention time of 3.462 min and recorded at 206 nm.
Quantification of prostaglandin E2 (PGE2)
PGE2 was also measured by using a stable isotope dilution gas chromatographic/negative ion chemical ionization-mass spectrometric assay (Awas et al., 1996). Briefly, [4H2]-PGE2 (1.28 ng) was added to aqueous layer of brain homogenates after Folch extraction. The sample was then acidified to a pH of 3 with 1 N HCl and extracted on a C18 Sep-Pak. PGE2 was eluted with ethyl acetate:heptane and evaporated under a stream of N2. PGE2 in methoxylamine solution was extracted with ethyl acetate and evaporated with N2. The pentafluorobenzyl esters were purified by thin layer chromatography (PGE2 and PGD2 methyl esters are used as TLC standards), converted to O-methyloxime pentafluorobenzyl ester trimethylsilyl derivatives, and PGE2 was dissolved in undecane that is dried over a bed of calcium hydride. Gas chromatographic/negative ion chemical ionization-mass spectrometric analysis was performed as described previously with the M-181 ions for PGE2 (m/z 526) and the [4H2]-PGE2 as an internal standard (m/z 528).
Quantitative morphology of medium spiny neurons
Quantitative neuronal analysis was conducted on tissue stained with Golgi impregnation that was uniform throughout the section. The length of dendrites and spine number counts of MSN were evaluated in Golgi-impregnated 50 µm thick striatal sections from paraffin-embedded blocks prepared according to the manufacturer's specifications (FD Rapid GolgiStain Kit). MSN were recognized by their soma size and dendritic extensions. Six or more MSN with no breaks in staining along the dendrites were selected by an observer blinded to the experimental procedures, and spines were counted according to the published methods (Leuner et al., 2003; Zaja-Milatovic et al., 2005). Reconstruction of the three-dimensional dendritic tree by tracing each neuron in a two-dimensional plane and spine counting were done using a Neurolucida system at 60× magnification (MicroBrightField, VT).
Statistical analysis
Measurements of F2-IsoPs and ATP from primary neuronal cultures were conducted in duplicate or triplicate wells/experiment, and the mean from three to four independent experiments was used for statistical analysis. The data from in vivo experiments are presented as means ± S.E.M. of 4–6 mice in each group. The data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison tests with statistical significance set at p < 0.05. All analyses were carried out using GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Protection of Mn-induced oxidative stress and high-energy phosphate alterations in cultured neurons
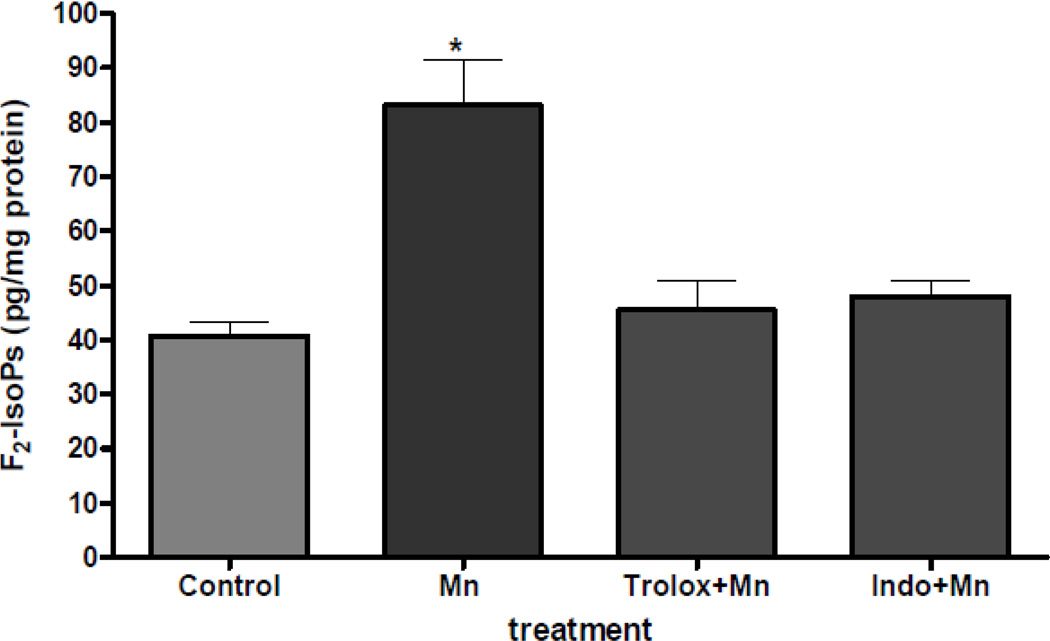
By measuring the levels of F2-IsoPs, a lipid peroxidation biomarker of oxidative injury, we have previously shown that Mn concentrations of 500 µM or 1 mM induced oxidative stress in primary cortical neuronal cultures (Milatovic et al., 2009). Our earlier study also showed that treatment of neurons with 500 µM Mn resulted in a time-dependent increase in the levels of the inflammatory biomarker, prostaglandin E2 (PGE2) (Milatovic et al., 2009). Therefore, our initial experiments sought to determine whether pretreatment with antioxidant and anti-inflammatory agents suppresses Mn-induced oxidative injury. Results from these experiments showed that 100 µM pretreatment with either the antioxidant, trolox (water soluble analog of vitamin E), or the non-steroidal anti-inflammatory drug, idomethacin, fully protects the Mn-induced increase in F2-IsoPs (Figure 1).
Figure 1.
Protection by trolox and indomethacin of Mn-induced changes in F2-IsoPs formation. Rat primary neuronal cultures were incubated with trolox or indomethacin (100 µM) for 30 min at 37°C, and F2-IsoPs levels were quantified at 2 hours following exposure to MnCl2 (500 µM). Data represent the mean ± S.E.M. from three independent experiments. * p<0.01 versus control by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
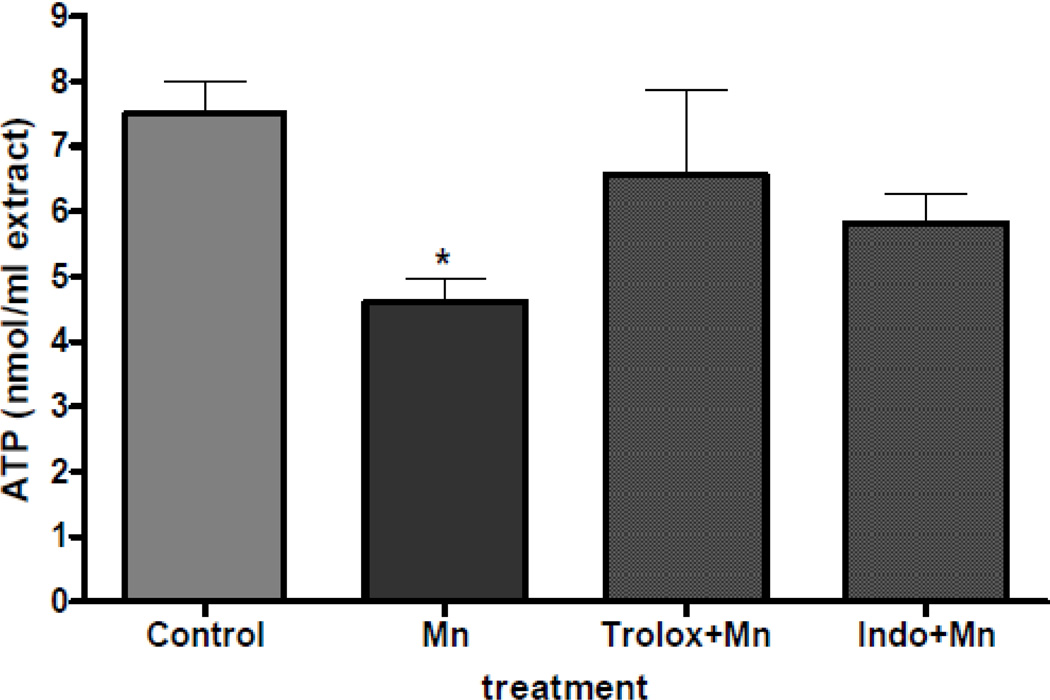
Analogous to the changes in biomarkers of oxidative stress, our previous experiment also showed that a 2 hour exposure to Mn induced a concentration-dependent decrease in neuronal ATP levels. In the current study, we tested the ability of antioxidant and anti-inflammatory agents to protect Mn-induced ATP depletion in primary neuronal cultures. Results from our study showed that depletion of ATP did not reach statistical significance following exposure to 500 µM of MnCl2 if primary neuronal cultures were pretreated with either trolox or intomethacin (100 µM) for 30 min (Figure 2).
Figure 2.
Protection by trolox and indomethacin of Mn-induced changes in ATP formation. Rat primary neuronal cultures were incubated with trolox or indomethacin (100 µM) for 30 min at 37°C, and ATP levels were quantified at 2 hours following exposure to MnCl2 (500 µM). Data represent the mean ± S.E.M. from three independent experiments. * p<0.01 versus control by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
Protection of Mn-induced alterations in biochemical and structural parameters of neurotoxicity in vivo
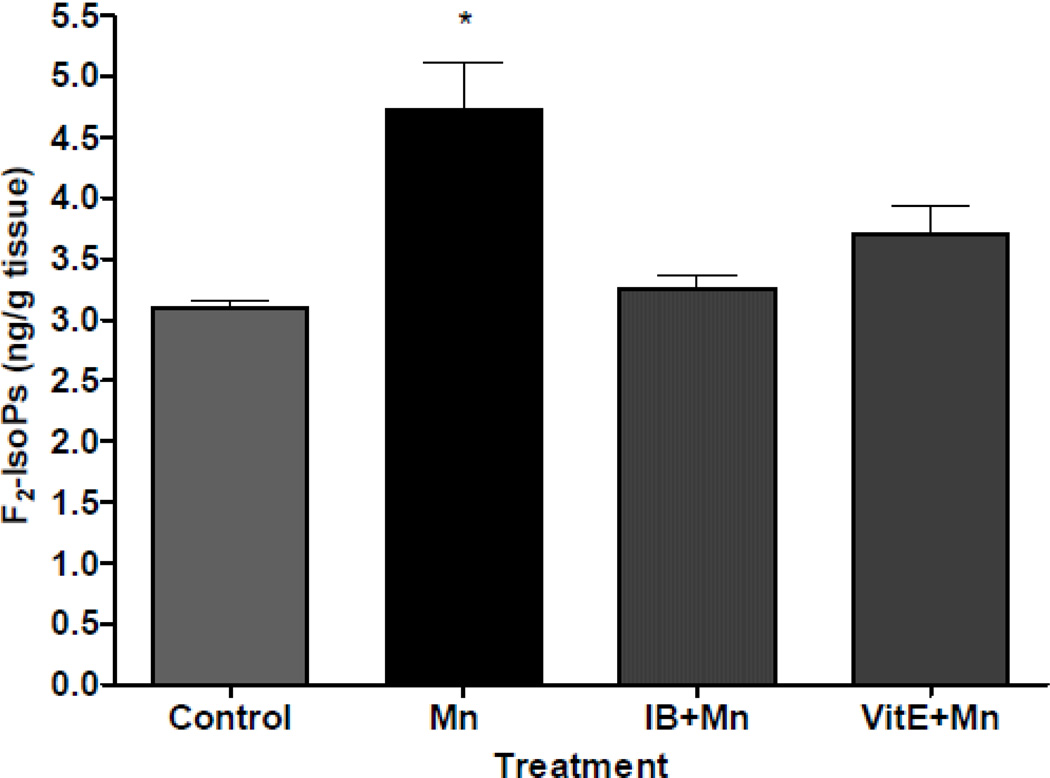
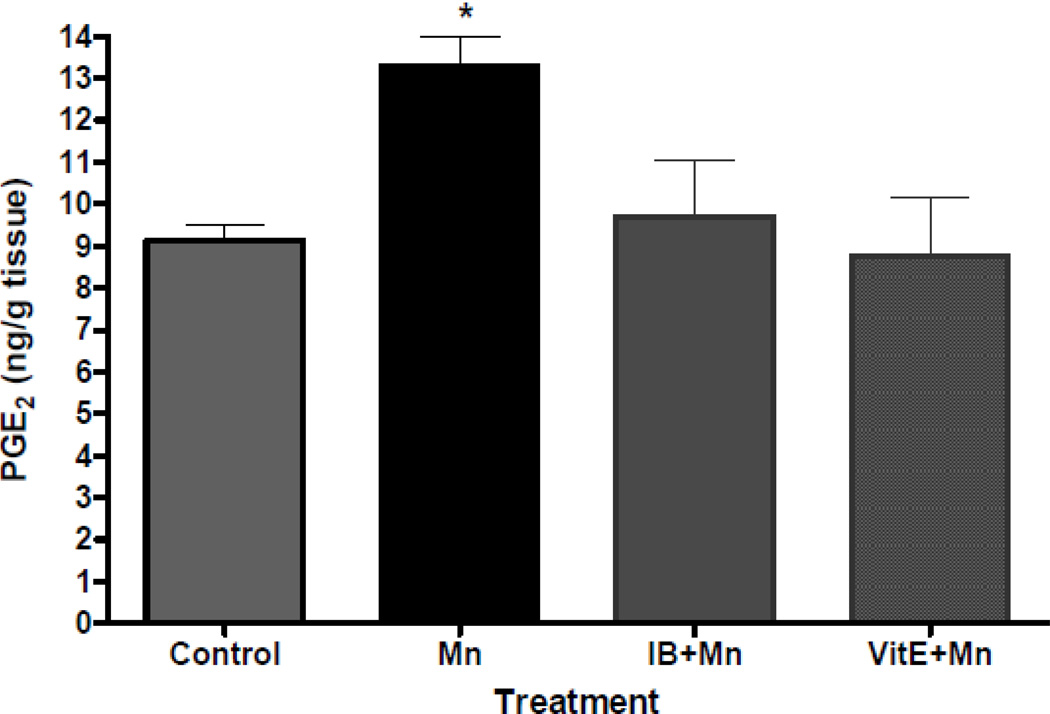
Initial in vivo experiments investigated the extent of cerebral oxidative damage and neuroinflammation in C57Bl/6 mice exposed to Mn. Analysis of cerebral biomarkers of oxidative damage and neuroinflammation revealed that the injection of 100 mg/kg MnCl2 leads to significant increases in F2-IsoPs and PGE2 in adult mouse brains, respectively (Milatovic et al., 2009). In the next series of experiments, we sought to determine whether cerebral lipid peroxidation and neuroinflammation associated with Mn-induced neurotoxicity can be suppressed by pretreatment with antioxidants and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Doses of neuroprotectants were based on results from previous research in the model of activated innate immunity (Milatovic et al., 2003, 2004) and excitotoxicity (Zaja-Milatovic et al., 2008, 2009). These doses were previously shown to have no neurotoxic effect on saline-exposed animals. Pretreatment with the antioxidant, vitamin E, significantly protected F2-IsoPs and PGE2 generation following 24 hours of Mn exposure (Figure 3 and 4). In addition, NSAID pretreatment was similarly effective in attenuating oxidative damage and neuroinflammation in this neurotoxicity model. As shown in Figures 3 and 4, ibuprofen (140 µg/ml) fully protected the Mn-induced increases in both F2-IsoPs and PGE2.
Figure 3.
Cerebral F2-IsoPs concentrations following saline- (control) or Mn- (100 mg/kg, s.c.) exposed mice with or without pretreatment with ibuprofen (IB, 140 µg/ml in drinking water for 2 weeks) or vitamin E (Vit E, α-tocopherol, 100 mg/kg, i.p., for 3 days). Brains from mice exposed to Mn were collected 24 hours post injections. Values of F2-IsoPs represent mean ± S.E.M. (n=4–6). *Significant difference between control mice and Mn-treated mice (p<0.05).
Figure 4.
Cerebral PGE2 concentrations following saline- (control) or Mn- (100 mg/kg, s.c.) exposed mice with or without pretreatment with ibuprofen (IB, 140 µg/ml in drinking water for 2 weeks) or vitamin E (Vit E, α-tocopherol, 100 mg/kg, i.p., for 3 days). Brains from mice exposed to Mn were collected 24 hours post injections. Values of PGE2 represent mean ± S.E.M. (n=4–6). *Significant difference between control and Mn-treated mice (p<0.05).
Next, we investigated morphological correlates of pretreatment and Mn injection by determining the structural integrity of the striatal dendritic system in mice. Using Golgi impregnation and Neurolucida-assisted morphometry (Figure 5), results from our previous studies showed that oxidative damage and neuroinflammation is accompanied by spine degeneration (total number of spines per neuron) and a reduction in the dendrite length of striatal MSNs in mice exposed to a single or multiple injections of MnCl2 (Milatovic et al., 2009). In the present experiments, we directly tested the role of antioxidants and NSAIDs in preserving the integrity of the dendritic system in this model. Our results show that pretreatment with vitamin E or ibuprofen completely protects both the spines (Figure 6) and the dendrite length (Figure 7) of MSNs from the degenerative consequences of Mn at 24 hours post injection.
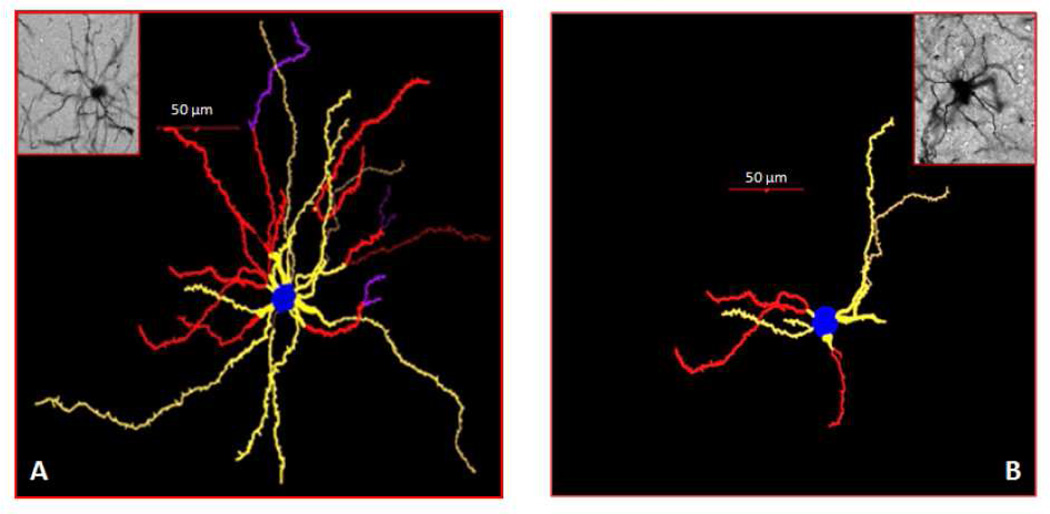
Figure 5.
Representative tracings of striatal medium spiny neurons (MSNs) with photomicrographs of mouse striatal sections from mice treated with saline (control) (A) or MnCl2 (100 mg/kg, s.c.) (B). The brain from mice exposed to MnCl2 was collected 24 hours after the injection. Treatment with Mn induced the degeneration of the striatal dendritic system as well as a decrease in the total number of spines and the length of the dendrites of MSNs. Tracing and counting were done using a Neurolucida system at 60× magnification (MicroBrightField, VT). Colors indicate the degree of dendritic branching (yellow=1°, red=2°, purple=3°).
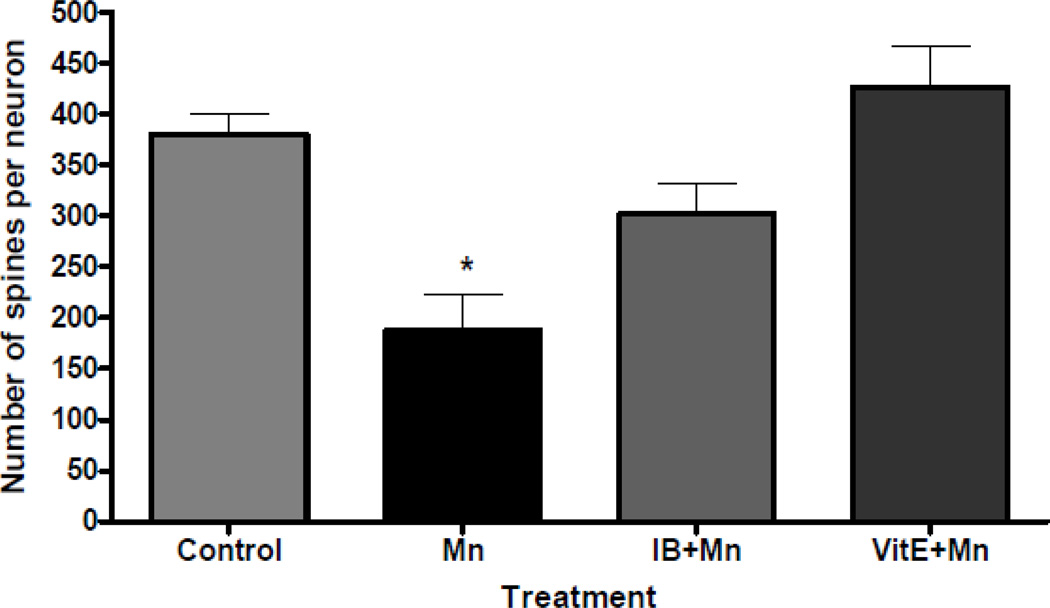
Figure 6.
Quantitative analyses of the total spine number of medium spiny neurons from the striatum of mice treated with saline (control) or Mn (100 mg/kg, s.c.) with or without pretreatment with ibuprofen (IB, 140 µg/ml in drinking water for 2 weeks) or vitamin E (Vit E, α-tocopherol, 100 mg/kg, i.p., for 3 days) and sacrificed 24 hours after the treatment. Tracing and counting of four to six Golgi-impregnated striatal MSNs were done using a Neurolucida system at 60× magnification (MicroBrightField, VT). *Significant difference between control and Mn-treated mice (P<0.05). Treatment with Mn induced the degeneration of the striatal dendritic system and a decrease in the total number of spines of MSNs.
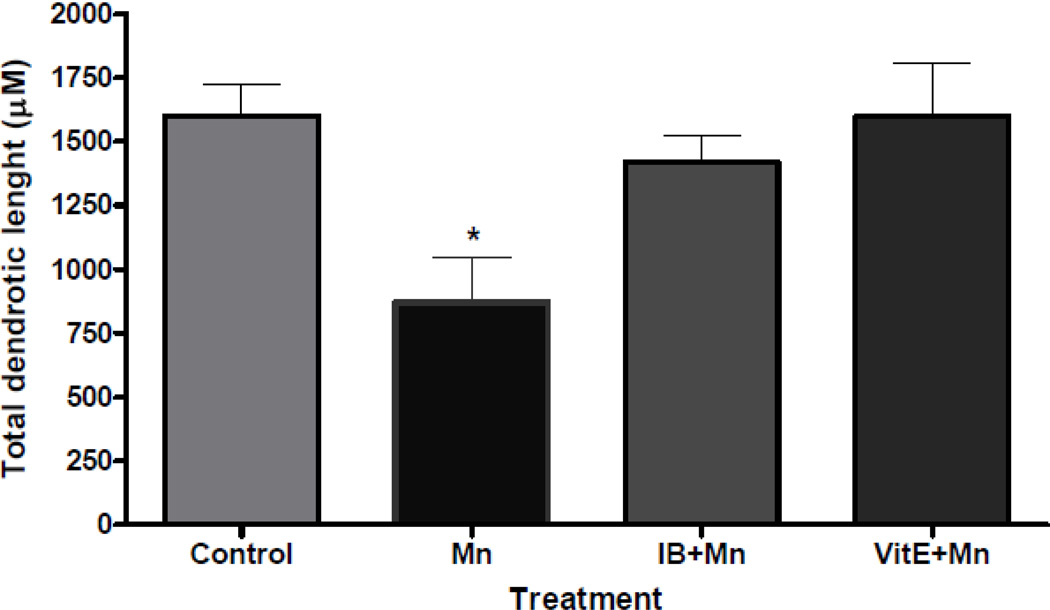
Figure 7.
Quantitative analyses of the total dendritic length of medium spiny neurons from the striatum of mice treated with saline (control) or Mn (100 mg/kg, s.c.) with or without pretreatment with ibuprofen (IB, 140 µg/ml in drinking water for 2 weeks) or viatmin E (Vit E, α-tocopherol, 100 mg/kg, i.p., for 3 days) and sacrificed 24 hours after the treatment. Tracing and counting of four to six Golgi-impregnated striatal MSNs were done using a Neurolucida system at 60× magnification (MicroBrightField, VT). *Significant difference between control and Mn-treated mice (P<0.05). Treatment with Mn induced the degeneration of the striatal dendritic system and as well as a decrease in the length of the dendrites of MSNs.
DISCUSSION
Human exposure to Mn is of growing concern due to its prevalence not only in occupational settings, but also within the population at large. Although the cellular and molecular mechanisms of Mn neurotoxicity are not yet fully understood, our recent studies highlighted the link between oxidative stress, mitochondrial dysfunction, inflammation and neurodegeneration due to exposure to this metal (Milatovic et al., 2007, 2009). The present study further explores the mechanisms associated with Mn-induced neurotoxicity, probing the effects of antioxidants and non-steroidal anti-inflammatory drugs on oxidative injury and associated dendritic degeneration. Our findings have revealed that Mn-induced oxidative stress and high-energy phosphate depletion in primary neuronal cultures are completely protected by the soluble mimetic of vitamin E, trolox, and the NSAID, indomethacin. Importantly, our results also demonstrated that oxidative damage, neuroinflammation and the dendritic degeneration of striatal MSNs are completely protected by the antioxidant, vitamin E, and the NSAID, ibuprofen, in a mouse model of Mn neurotoxicity.
These latest results corroborate previous findings which demonstrated that the generation of ROS and altered mitochondrial activity play important roles in Mn neurotoxicity (Dobson et al., 2004; Milatovic et al., 2009). Once inside the neurons, Mn induces a transient increase in ROS generation and the simultaneous collapse of the mitochondrial membrane potential, thus exacerbating defects in electron transport chain to slow ATP production. The depletion of high-energy phosphates and the disruption of mitochondrial Ca2+ signaling is supported by previous data confirming that Mn inhibits Na+-dependent Ca2+ efflux (Gavin et al., 1990) and respiration in brain mitochondria (Zhang et al., 2004), processes that are critical for maintaining normal ATP levels and ensuring adequate inter-mitochondrial signaling. The release of ROS potentially damages mitochondria directly or indirectly through the effects of secondary oxidants such as superoxide, H2O2 or peroxynitrite (ONOO−) (Turrens and Boveris 1980). Superoxide produced in the mitochondrial electron transport chain may catalyze the transition shift of Mn2+ to Mn3+ through a set of reactions similar to those mediated by superoxide dismutase, thus leading to the increased oxidant capacity of this metal (Gunter et al., 2006).
Results from our previous in vitro studies suggested that increased lipid peroxidation and the depletion of high-energy phosphates represent early events and key mechanisms in Mn-induced astrocytic and neuronal dysfunction (Milatovic et al., 2007, 2009). Data from the present study confirm these findings and indicate that trolox and the NSAID, indomethacin, suppress Mn toxicity in cultured neurons. Trolox is a soluble mimetic of vitamin E and can act as a scavenger of radicals via the H-donating groups (Frankel, 2005). Several studies have demonstrated that trolox serves as a better antioxidant than vitamin E (Sagach et al., 2002; Raspor et al., 2005) due to its improved access to the hydrophilic compartments of the cells as well as its ability to trap two membrane lipid peroxyl radicals per molecule of trolox (Barclay and Vinqvist, 1994). Treatment with trolox has been shown to be protective in a variety of experimental studies including in human cells (Wu et al., 1990), in a model of neonatal rat cardiac myocyte cultures (Massey and Burton, 1990), in response to MeHg-induced neurotoxicity (Kaur et al., 2010), and decreases the enzymatic activities of the in mitochondrial electron transport system (Usuki et al., 2001). The recognition of the protective effects of trolox indicates that vitamin-dependent antioxidant defenses are important factors in attenuating the neurotoxic effects of Mn.
Our data also showed that the non-specific cyclooxygenase (COX) inhibitor, indomethacin, attenuates the neurotoxic effects of Mn (Figures 1 and 2). COX is an important source of ROS in the pathologic brain (Marnett et al., 1999) and may function as a cellular factor to induce superoxide-mediated cell death in primary cortical neurons (Im et al., 2006). Enhanced COX-2 activity may deplete the levels of the reduced state of electron donors (i.e., possible antioxidants, such as GSH and NADH) thus increasing the potential for vulnerability to cytotoxic insult. It has been suggested that, due to generated superoxide anions, increased COX activity in primary cortical neurons greatly increases the neurotoxic effects of low doses of Fe2+, H2O2, Zn2+ and sodioum nitroprusside (Im et al., 2006). Exposure to indomethacin has also been shown to cause a decrease in ROS levels, an increase in cell viability in NMDA-treated rat cerebellar granule cells (Boldirev et al., 1999), suggesting that COX contributes to neuronal damage given its propensity to generate ROS.
An additional goal of this study was to determine whether antioxidants and NSAIDs suppress lipid peroxidation and the resultant neurodegeneration of MSNs in the striatal area in a mouse model of Mn-induced neurotoxicity. Antioxidants play an important role in preventing many human diseases, including, but not limited to cancer, atherosclerosis, stroke, rheumatoid arthritis and neurodegeneration (Fang et al., 2002). Vitamin E has been recognized as one of the most potent and important antioxidants. It acts as a chain-breaking antioxidant and radical scavenger, thus protecting cells from the peroxidation of polyunsaturated fatty acids in phospholipids and the consequent membrane degeneration (VanAcker et al., 1993). Our previous studies showed that vitamin E pretreatment protected oxidative injury in models of activated innate immunity and excitotoxicity (Milatovic et al., 2003, 2004, 2005; Zaja-Milatovic et al., 2008, 2009). Vitamin E pretreatment was also effective in suppressing organophosphate diisopropylphosphorofluoridate-induced increases in the cerebral and neuronal biomarkers of oxidative damage, F2-IsoPs and F4-neuroPs, the production of citrulline and the depletion of ATP and phosphocreatine (Milatovic et al., 2009). A decreased level of vitamin E in response to hyperoxia or treatment with a convulsant (Onodera et al., 2003; Rauca et al., 2004) suggested that this vitamin is consumed to prevent oxidative damage. In addition, vitamin E maintains oxidative phosphorylation in mitochondria and accelerates the restitution of high-energy metabolites (Punz et al., 1998; Kotegawa et al., 1993). Results of the present study confirm the protective effect of antioxidants and show that vitamin E pretreatment fully protects the Mn-induced increase in cerebral markers of oxidative damage, F2-IsoPs (Figure 3).
Importantly, the results from this study also showed that the protection of lipid peroxidation prevents the Mn-induced neurodegeneration of striatal MSNs. Images of Neurolucida-traced MSNs with Golgi-impregnated striatal sections from control and Mn-exposed animals (Figure 5) confirmed our previous findings which demonstrated that Mn exposure alters the integrity of the dendritic system with the profound dendrite regression of striatal MSNs. Nigrostriatal dopamine axons synapse onto striatal MSNs, which appear to be particularly sensitive to Mn-induced toxicity (Defazio et al., 1996; Sloot and Gramsbergen, 1994). It has been reported that Mn exposure causes long-term reductions in striatal DA levels and induces a loss of autoreceptor control over DA release (Autissier et al., 1982; Komura and Sakamoto, 1992). Results from the current study, in which dendritic morphology was assessed in randomly selected striatal MSNs, revealed that vitamin E pretreatment protects the Mn-induced decrease in the spine number (Figure 6) and total dendritic length (Figure 7) of MSNs. We observed a close concordance between these results, indicating that protecting the cerebrum from neuronal oxidative damage also protects striatal MSNs from dendritic degeneration.
Next, we tested the efficacy of ibuprofen, a NSAID with some COX-independent actions (Asanuma et al., 2001; Weggen et al., 2001), in attenuating the Mn-induced effects. Our previous studies have confirmed that, in parallel with increased levels of biomarkers of oxidative damage, Mn exposure induces increased inflammation, which is characterized by elevated PGE2 levels both in vitro and in vivo (Milatovic et al., 2009). Recent studies have also shown an inflammatory response in glial cells following Mn exposure (Zhang et al., 2009; Zhao et al., 2009). Mn potentiates lipopolysaccharide (LPS)-induced increases in proinflammatory cytokines and nitric oxide production in glial cultures (Filipov et al., 2005). We have previously shown that ibuprofen protected oxidative damage and neurodegeneration in pyramidal neurons from the CA1 hippocampal area following kainic acid-induced excitotoxicity and activation of innate immunity (Milatovic et al., 2003; Zaja-Milatovic et al., 2008). This protection was at least an order of magnitude more potent than that resulting from naproxen or acetylsalicylic acid (Milatovic et al., 2004). Analogous to the tested antioxidant, ibuprofen completely protected the increased lipid peroxidation (Figure 3) and generation of PGE2 (Figure 4) and reversed the Mn-induced changes in dendrite length and spines (Figures 6 and 7). While NSAID acts predominantly through the non-selective inhibition of COX, it is also possible that its neuroprotective effect is COX-independent, residing in its antioxidant activity (Asanuma et al., 2001). DAergic neurons possess reduced antioxidant capacity, as evidenced by low intracellular GSH, which renders these neurons more vulnerable to oxidative stress and glial activation relative to other cell types (Sloot et al., 1994; Greenamyre et al., 1999). ROS may act in concert with reactive nitrogen species (RNS) derived from glia to facilitate the Mn-induced degeneration of DAergic neurons. Therefore, inflammation in conjunction with the over-activation of glia and the release of additional neurotoxic factors may represent a crucial component associated with the degenerative process of DAergic neurons in response to Mn.
In summary, our data revealed that lipid peroxidation and mitochondrial dysfunction are closely associated with dendritic degeneration following Mn-induced neurotoxicity. Furthermore, this study investigated different pathways to attenuate biomarkers of oxidative damage associated with Mn neurotoxicity and the extent to which such attenuation is accompanied by rescue from striatal neurodegeneration. Specifically, vitamin E and ibuprofen were shown to efficiently protect oxidative injury and to prevent the Mn-induced synaptodendritic degeneration of MSNs. Our findings suggest that the mediation of oxidative stress/mitochondrial dysfunction and the effective control of alterations in biomarkers of oxidative injury, neuroinflammation and synaptodendritic degeneration may provide an effective, multi-pronged therapeutic strategy for preventing dysfunctional dopaminergic transmission and slowing the progression of Mn-induced neurodegenerative processes.
Research Highlights.
Mn exposure leads to neurotoxicity in vitro and in vivo.
Antioxidants and anti-inflammatory agents attenuate Mn-induced oxidative injury.
These agents also protect the striatal neurons from dendritic atrophy and spine loss.
These prophylactic strategies may be effective against Mn neurotoxicity.
Acknowledgements
The authors gratefully acknowledge support by grant from Department of Defense W81XWH-05-1-0239 (MA, DM) and the National Institute of Environmental Health Science (NIEHS) grant R01 10563.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asanuma M, Nishibayashi-Asanuma S, Miyazaki I, Kohno M. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J. Neurochem. 2001;76:1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese in health and disease: from transport to neurotoxicity. In: Massaro E, editor. Handbook of neurotoxicology. Totowa, NJ: Humana Press; 2000. pp. 195–209. [Google Scholar]
- Autissier N, Rochette L, Dumas P, Beley A, Loireau A, Bralet J. Dopamine and norepinephrine turnover in various regions of the rat brain after chronic manganese chloride administration. Toxicology. 1982;24:175–182. doi: 10.1016/0300-483x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB, Soares FA. A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci. 2010;115:194–201. doi: 10.1093/toxsci/kfq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awas JA, Soteriou MC, Drougas JG, Stokes KA, Roberts LJ, 2nd, Pinson CW. Plasma prostaglandin E1 concentrations and hemodynamics during intravenous infusions of prostaglandin E1 in humans and swine. Transplantation. 1996;61:1624–1629. doi: 10.1097/00007890-199606150-00013. [DOI] [PubMed] [Google Scholar]
- Barclay LR, Vinqvist MR. Membrane peroxidation: inhibiting effects of water-soluble antioxidants on phospholipids of different charge types. Free Radic. Biol. Med. 1994;16:779–788. doi: 10.1016/0891-5849(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Carpenter DO, Huentelman MJ, Peters CM, Johnson P. Sources of reactive oxygen species production in excitotoxin- stimulated cerebellar granule cells. Biochem. Biophys. Res. Commun. 1999;256:320–324. doi: 10.1006/bbrc.1999.0325. [DOI] [PubMed] [Google Scholar]
- Bolte S, Normandin L, Kennedy G, Zayed J. Human exposure to respirable manganese in outdoor and indoor air in urban and rural areas. J. Toxicol. Environ Health A. 2004;67:459–467. doi: 10.1080/15287390490276485. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese induced parkinsonism. Neurotoxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Defazio G, Soleo L, Zefferino R, Livrea P. Manganese toxicity in serumless dissociated mesencephalic and striatal primary culture. Brain Res. Bull. 1996;40:257–262. doi: 10.1016/0361-9230(96)00041-x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat. Disord. 2007;13 Suppl 3:S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann. N.Y. Acad. Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal ganglia accumulation and motor assessment following manganese chloride exposure in the C57Cl/6 mouse. Int. J. Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Clewell HJ, 3rd, Andersen ME. Application of pharmacokinetic data to the risk assessment of inhaled manganese. Neurotoxicology. 2006;27:752–764. doi: 10.1016/j.neuro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol. Sci. 2007;97:459–466. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Guoyao W. Free radicals, antioxidant, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Frankel EN. Antioxidants. In: Frankel EN, editor. Lipid oxidation. Bridgwater, United Kingdom: The Oily Press; 2005. pp. 209–258. [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem. J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol. Appl. Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. 1992. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson's disease. Biochem. Soc. Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK. Manganese inhibits NMDA receptor channel function: implications to psychiatric and cognitive effects. Neurotoxicology. 2007;28:1147–1152. doi: 10.1016/j.neuro.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Normandin L, Norenberg MD, Kennedy G, Yi JH. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosci. Lett. 2006;396:167–171. doi: 10.1016/j.neulet.2005.11.064. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Kubota M, Uchiyama M, Yagi M, Kanada S, Yamazaki S, Murata H, Okamoto K, Suzuki M, Nitta K. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Pediatr. Surg. Int. 2003;19:268–272. doi: 10.1007/s00383-002-0929-6. [DOI] [PubMed] [Google Scholar]
- Im JY, Kim D, Paik SG, Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic. Biol. Med. 2006;41:960–972. doi: 10.1016/j.freeradbiomed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. The in vitro effects of Trolox on methylmercury-induced neurotoxicity. Toxicology. 2010;276:73–78. doi: 10.1016/j.tox.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Yang Y, Hirata Y, Kanthasamy A, Kanthasamy AG. Activation of protein kinase Cδ by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem. Pharmacol. 2005;69:133–146. doi: 10.1016/j.bcp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Komura J, Sakamoto M. Effects of manganese forms on biogenic amines in the brain and behavioral alterations in the mouse: long-term oral administration of several manganese compounds. Environ. Res. 1992;57:34–44. doi: 10.1016/s0013-9351(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Kotegawa M, Sugiyama M, Shoji T, Haramaki N, Orgura R. Effect of α-tocopherol on high energy phosphate metabolite levels in rat heart by 31 P-NMR using a Langendorff perfusion technique. J. Mol. Cell Cardiol. 1993;25:1067–1074. doi: 10.1006/jmcc.1993.1119. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 2005;1(313):46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors T. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ. Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism:conceptual model based on the evolution of exposure. Neuromol. Med. 2009;11:311–321. doi: 10.1007/s12017-009-8108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- Massey KD, Burton KP. Free radical damage in neonatal rat cardiac myocyte cultures: effects of alpha-tocopherol, Trolox and phytol. Free Radic. Biol. Med. 1990;8:449–458. doi: 10.1016/0891-5849(90)90058-q. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, et al. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J. Neurochem. 2003;87:1518–1526. doi: 10.1046/j.1471-4159.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Milatovic S, Montine K, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immunity response in vivo. J. Neuroinflammation. 2004;1(20(1–7)) doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827:88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sydoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Science. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Aschner M. Measurement of isoprostanes as markers of oxidative stress in neuronal tissue. Current Protocols in Toxicology unit. 2009:1–12. doi: 10.1002/0471140856.tx1214s39. 12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, 2nd, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- Onodera K, Omoi NO, Fukui K, Hayasaka T, Shinkai T, Suzuki S, Abe K, Urano S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Radic. Res. 2003;37:367–372. doi: 10.1080/1071576031000090019. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Punz A, Nanobashvili N, Feugl A, et al. Effect of α-tocopherol pretreatment on high energy metabolites in rabbit skeletal muscle after ischemia-reperfusion. Clin. Nutr. 1998;17:85–87. doi: 10.1016/s0261-5614(98)80311-7. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: Clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Raspor P, Pleniscar S, Gazdag Z, Pesti M, Miklavcic M, Lah B, Logar-Marinsek R, Poljsak B. Prevention of intracellular oxidation in yeast: the role of vitamin E analogue, Trolox (6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxyl acid) Cell Biol. Int. 2005;29:57–63. doi: 10.1016/j.cellbi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Rauca C, Wiswedel I, Zerbe R, Keilhoff G, Krug M. The role of superoxide dismutase and alpha-tocopherol in the development of seizures and kindling induced by pentylenetetrazol—influence of the radical scavenger alpha-phenyl-N-tert-butylnitrone. Brain Res. 2004;1009:203–212. doi: 10.1016/j.brainres.2004.01.082. [DOI] [PubMed] [Google Scholar]
- Sagach VF, Scrosati M, Fielding J, Rossoni G, Galli C, Visioli F. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol. Res. 2002;45:435–439. doi: 10.1006/phrs.2002.0993. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB. Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication: a positron emission tomography study. Neurology. 1997;48:1053–1056. doi: 10.1212/wnl.48.4.1053. [DOI] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JBP. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J. Neurochem. 1994;62:205–216. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Suzuki Y, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J. Exp. Med. 1975;22:5–10. [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res. Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;1(208):1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki F, Yasutake A, Umehara F, Tokunaga H, Matsumoto M, Eto K, Ishiura S, Higuchi I. In vivo protection of a water-soluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci. Lett. 2001;304:199–203. doi: 10.1016/s0304-3940(01)01764-5. [DOI] [PubMed] [Google Scholar]
- VanAcker SABE, Koymans LMH, Bast A. Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radic. Biol. Med. 1993;15:311–328. doi: 10.1016/0891-5849(93)90078-9. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietzik CU, Findlay KA, Smith TE, Murphy MP, Butler T, Kang DE, Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic A[beta]42 independently of cyclo-oxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Wilson P, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J. Comp. Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wu TW, Hashimoto N, Wu J, Carey D, Li RK, Mickle DA, Weisel RD. The cytoprotective effect of Trolox demonstrated with three types of human cells. Biochem. Cell Biol. 1990;68:1189–1194. doi: 10.1139/o90-176. [DOI] [PubMed] [Google Scholar]
- Yang MS, Yu LC, Gupta RC. Analysis of changes in energy and redox states in HepG2 hepatoma and C6 glioma cells upon exposure to cadmium. Toxicology. 2004;201:105–113. doi: 10.1016/j.tox.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz A, Zhang J, Montine K, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in late-stage Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Gupta RC, Aschner M, Montine TJ, Milatovic D. Pharmacologic suppression of oxidative damage and dendritic degeneration following kainic acid-induced excitotoxicity in mouse cerebrum. Neurotoxicology. 2008;29(4):621–627. doi: 10.1016/j.neuro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Gupta RC, Aschner M, Milatovic D. Protection of DFP-Induced Oxidative Damage and Neurodegeneration by Antioxidants and NMDA Receptor Antagonist. Toxicol. Appl. Pharmacol. 2009;240:124–131. doi: 10.1016/j.taap.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z. In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol. In Vitro. 2004;18:71–77. doi: 10.1016/j.tiv.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Lokuta KM, Turner DE, Liu B. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia. J. Neurochem. 2010;112:434–443. doi: 10.1111/j.1471-4159.2009.06477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong TA, Lokuta KM, Turner DE, Vujisic K, Liu B. Microglia enhance manganese chloride-induced dopaminergic neurodegeneration: role of free radical generation. Exp. Neurol. 2009;217:219–230. doi: 10.1016/j.expneurol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol. Sci. 2009;107:156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]