Abstract
Many of our everyday actions are only appropriate in certain situations and selecting the appropriate behavior requires that we use current context and previous experience to guide our decisions. The current study examined hippocampal functional connectivity with prefrontal and striatal regions during a task that required participants to make decisions based on the contextual retrieval of overlapping sequential representations. Participants learned four sequences comprised of six faces each. An overlapping condition was created by having two sequences with two identical faces as the middle images. A non-overlapping condition contained two sequences that did not share any faces between them. Hippocampal functional connectivity was assessed during the presentation period and at the critical choice, where participants had to make a contextually dependent decision. The left hippocampus showed significantly increased functional connectivity with dorsal and ventral striatum and anterior cingulate cortex during the presentation period of the overlapping compared to the non-overlapping condition after participants knew the sequences. At the critical choice point of the overlapping condition, the left hippocampus showed stronger functional connectivity with the orbitofrontal cortex. These functional connectivity results suggest the hippocampus may play a role in decision making by predicting the possibilities of what might come next, allowing orbitofrontal and striatal regions to evaluate the expected choice options in order to make the correct action at the choice point.
Keywords: Striatum, learning, retrieval, prefrontal cortex, valuation
1. Introduction
Decision making involves comparing the value of choice options and selecting a course of action based upon that valuation (Kable and Glimcher, 2009; Rangel et al., 2008). The medial prefrontal and orbitofrontal cortex, as well as both dorsal and ventral striatum, form part of the valuation circuitry important for determining the reward value of the outcome of a choice (Hare et al., 2008; Kable and Glimcher, 2007; Lau and Glimcher, 2008; Padoa-Schioppa and Assad, 2006; 2008; Plassmann et al., 2010). Critically, some choices can have multiple outcomes associated with them where an action taken in one context may be appropriate while that same action in a different context may not. The hippocampus is critical for forming and retrieving item-context associations (Cansino et al., 2002; Davachi et al., 2003; Eichenbaum et al., 2007; Giovanello et al., 2004; Komoroski et al., 2009; Manns and Eichenbaum, 2006; Ranganath et al., 2004; Rolls, 1996; Ross et al., 2008; Slotnick, 2010; Staresina and Davachi, 2008; Yonelinas et al., 2001) and has also been linked to the separation, or disambiguation, of overlapping sequences in both animals and humans (Agster et al., 2002; Brown et al., 2010; Hasselmo and Eichenbaum, 2005; Kumaran and Maguire, 2006; Lipton et al., 2007; Ross et al., 2009; Shohamy and Wagner, 2008; Zilli and Hasselmo, 2008). Importantly, the hippocampus shows stronger fMRI activity when context is used to guide choices (Brown et al., 2010), suggesting the hippocampus may work with prefrontal and striatal regions when making decisions at choice points where the options have been associated with different reward values and the current context can be used to implement the correct choice.
Within the prefrontal cortex, the orbitofrontal cortex may be particularly important to the selection of the appropriate response when confronted with choice options whose reward values change in different contexts. The orbitofrontal cortex signals the expected value of a choice (Plassmann et al., 2010; Schoenbaum et al., 1998; 2003; 2009; Takahashi et al., 2009) and is critical for reversal learning (Berlin et al., 2004; Chudasama and Robbins, 2003; Fellows and Farah, 2003; Hornak et al., 2004; McAlonan and Brown, 2003; Meunier et al., 1997; Rudebeck and Murray, 2008; Schoenbaum et al., 2003; Tsuchida et al., 2010). Additionally, the orbitofrontal cortex is activated at the choice point where two overlapping navigational routes diverge (Brown et al., 2010), suggesting the orbitofrontal cortex may be critical for the type of flexible behavior necessary to select appropriate responses in situations with changing contingencies. These previous studies suggest the hippocampus and orbitofrontal cortex may act as a functional network in order to separate the individual value of each choice option within the current context in order to guide the decision making process.
In the current study, we used a previously collected data set (Ross et al., 2009) examining sequence learning in humans to examine functional interactions between the hippocampus, prefrontal cortex, and striatum during a task that required participants to make decisions based on the contextual retrieval of overlapping sequential representations. Participants were asked to make choices in the context of a sequence learning task. Critically, an overlapping sequence condition was created where the correct choice required participants to use the context of the current sequence to make their decision (critical choice point). The overlapping condition was formed by creating two non-spatial sequences of six faces which shared the same middle two faces (i.e. the third and fourth faces of the sequence), while a non-overlapping (NOL) sequence condition was composed of two additional sequences of six unique faces always shown in the same order (Fig. 1). After viewing the first four faces in a sequence, participants were shown two faces simultaneously and were asked to choose which of the faces belonged in the current sequence (Fig. 2). Left and right hippocampal spherical seed regions with a radius of 5 mm centered on Montreal Neurological Institutes (MNI) coordinates ±30, −24, −15 were derived from a previous study illustrating the importance of the hippocampus to sequence learning (Schendan et al., 2003). Regions showing functional connectivity with these seed regions were assessed using a beta-series correlation functional connectivity method (Rissman et al., 2004). The fMRI data were split into a learning phase and experienced phase based on each individual’s behavioral performance. The data split allowed us to examine differences in functional connectivity when participants were learning the correct choices to make in the overlapping sequences as well as during contextually driven decision making when participants knew the sequences. Additionally, a random order condition (RAN) where two groups of six faces were always shown in a unique order was included. Inclusion of the random condition allowed us to examine functional connectivity during sequence learning and retrieval by comparing the non-overlapping condition to the random condition in the learning and retrieval phases of the experiment.
Figure 1.
Graphical representation of the overlapping and non-overlapping conditions. Each condition consisted of six faces represented by letters and numbers. The middle two faces of the two overlapping sequences were identical (X and Y). No faces were shared between the two non-overlapping sequences. Faces in the overlapping and non-overlapping sequences were always presented in the same order.
Figure 2.
Behavioral paradigm. During the presentation phase, the first four faces of a sequence were shown paired with a blurred face for 4 second each. The presentation phase was followed by an 8 sec delay period where participants focused on a fixation dot. During the choice period, participants selected between two faces in order to complete the fifth and sixth elements of the sequence. The choice for the fifth element was termed the “critical choice” and the choice for the sixth element was termed the “final choice”. Face pairs during the choice phase were presented for 4 sec and response feedback indicated to participants whether or not they were correct. The choice phase was followed by a 6 sec inter-trial-interval (ITI). A 2 sec cue was shown after the ITI to indicate to participants if the upcoming trial was going to be a sequence trial (either overlapping or non-overlapping) or a random trial. Finally, a 2 sec prompt for the participant to “Get Ready” for the next trial was shown after the cue.
2. Results
2.1 Behavioral results
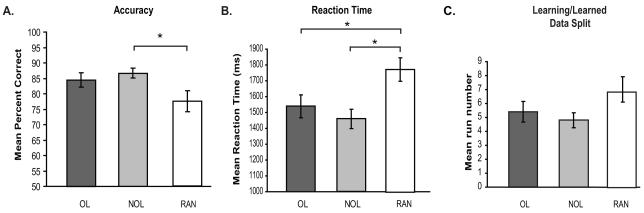
We examined both accuracy and reaction time at the critical choice point to determine whether there were any differences between the overlapping (OL), non-overlapping (NOL) and random conditions (RAN). A repeated measures ANOVA revealed a significant difference in percent accuracy (F(2,28) = 3.489, p < 0.05) and reaction time (F(2,28) = 9.352, p < 0.01) across the OL, NOL, and RAN conditions at the critical choice (Fig. 3a and 3b). The significant effect of accuracy at the critical choice was caused by a significant difference between the NOL and RAN condition (t(14) = 2.310, p < 0.05) while the significant effect found in reaction time was caused by a significant difference between the OL and RAN conditions (t(14) = 4.026, p < 0.01) and a significant difference between the NOL and RAN conditions (t(14) = 3.784, p < 0.01). Importantly, no significant differences in either percent accuracy or reaction time were found between the OL and NOL conditions at the critical choice (mean accuracy; OL 84.44 ± 2.35%, NOL 86.67 ± 1.64%; mean reaction time; OL 1539.78 ± 72.64 ms, NOL 1458.18 ± 60.81 ms) suggesting the two sequence conditions were of equal difficulty.
Figure 3.
Behavioral results. (A) Mean percent accuracy at the critical choice point for the overlapping (OL) sequences (dark grey bars), non-overlapping (NOL) sequences (light grey bars) and random (RAN) conditions (white bars). (B) Mean reaction time in milliseconds at the critical choice point. (C) Mean run number at which participants reached the behavioral criteria and the data split into the learning and experienced phase for each condition. Asterisks indicate significance at p < 0.05.
2.2 Behavioral results for learning phase and experienced phase
Each individual participant’s behavioral performance at the critical choice point was used to separate the data into a learning phase and an experienced phase separately for the OL, NOL, and RAN conditions. The data were split at the point where each individual participant’s performance reached a predetermined criterion of six in a row correct. The mean run where participants reached the behavioral criteria of six correct critical choices in a row was 5.4 ± 0.75 in the OL condition, 4.80 ± 1.44 in the NOL condition and 6.8667 ± 1.87 in the RAN condition (Fig. 3c). A one-way repeated measures ANOVA revealed no significant difference between conditions at the run where participants reached behavioral criteria (F(2,28) = 2.319, p = 0.117).
2.3 Functional connectivity results
We wanted to examine regions showing significantly stronger functional connectivity with the hippocampus when participants could use contextual retrieval in order to disambiguate two overlapping choice options. Additionally, we wanted to examine disambiguation related functional connectivity with the hippocampus when participants viewed the faces leading up to the choice point. Therefore, we examined where the functional connectivity was stronger with the hippocampus during the presentation phase and the critical choice phase of the overlapping condition compared to the non-overlapping condition during both the learning and experienced phase of the experiment (OL > NOL). We limited this analysis to those regions which showed significant functional connectivity with the hippocampus in the OL condition.
A secondary goal of the experiment was to examine which brain regions showed stronger functional connectivity with the hippocampus during sequence learning and retrieval. Therefore, we also contrasted hippocampal functional connectivity during the presentation phase and critical choice phase of the non-overlapping condition to the random condition (NOL > RAN) during both the learning and experienced phase. Here we limited the analysis to regions showing significant hippocampal functional connectivity during the NOL condition.
2.3.1 Disambiguation related functional connectivity at presentation period
We contrasted left and right hippocampal functional connectivity during the presentation period of the overlapping condition with the non-overlapping condition to isolate differences in hippocampal connectivity during the disambiguation of overlapping sequences at time points leading up to the critical choice. We did not see any significant differences in hippocampal functional connectivity between the OL and NOL conditions during the learning phase. In the experienced phase of the task (i.e. after participants knew the sequences), the left hippocampal seed region (−30,−24,−15) showed extensive disambiguation related functional connectivity during the presentation period (Table 1). Of particular interest was the greater connectivity between the left hippocampus and the dorsal and ventral striatum bilaterally, anterior cingulate cortex bilaterally (Brodmann area 24; BA 24), and posterior cingulate cortex bilaterally (BA 23) (Fig. 4). The increased hippocampal connectivity with the dorsal striatum in the presentation period of the experienced phase included both left and right caudate nucleus as well as right putamen. Disambiguation related functional connectivity with the hippocampus during the viewing of the first four faces of a sequence after participants were correctly identifying the critical choice consistently suggests that hippocampal function is more tightly correlated with striatal, anterior cingulate, and posterior cingulate, functioning when retrieving an overlapping sequence.
Table 1.
Presentation period of experienced phase disambiguation related connectivity
| kE | Left/Right | T | x,y,z | |
|---|---|---|---|---|
| Connectivity with left hippocampus (−30,−24,−15) | ||||
| Lingual gyrus (BA 18) | 1563 | R | 6.81 | 2,−58,0 |
| Posterior cingulate cortex (BA 23) | R | 5.46 | 8,−44,18 | |
| Posterior cingulate cortex (BA 23) | L | 4.38 | −4,−42,20 | |
| Posterior parahippocampal gyrus | R | 4.68 | 22,−30,−16 | |
| Lingual gyrus (BA 17) | R | 3.38 | 8,−66,14 | |
| Middle frontal gyrus (BA 6) | 1080 | L | 5.07 | −48,2,42 |
| Anterior cingulate cortex (BA 24) | R | 5.66 | 4,10,24 | |
| Posterior insula | L | 4.29 | −34,−12,18 | |
| Precentral gyrus (BA 6) | L | 3.89 | −48,−14,32 | |
| Thalamus | L | 3.46 | −12,−16,10 | |
| Middle frontal gyrus (BA 6) | 460 | L | 6.01 | −30,2,64 |
| Superior frontal gyrus (BA 6) | R | 4.71 | 6,6,62 | |
| Precentral gyrus (BA 4) | L | 4.36 | −34,−8,66 | |
| Cerebellum | 811 | R | 5.46 | 28,−70,−28 |
| Fusiform gyrus (BA 37) | R | 4.99 | 46,−52,−20 | |
| Cerebellum | L | 3.66 | −16,−86,−26 | |
| Dorsal striatum (caudate) | 282 | R | 4.02 | 16,16,14 |
| Dorsal striatum (putamen) | R | 3.03 | 22,8,6 | |
| Ventral striatum (nucleus accumbens) | R | 3.17 | 10,10,−6 | |
| Dorsal striatum (putamen) | R | 2.90 | 26,10,−8 | |
| Dorsal striatum (caudate) | 162 | L | 4.23 | −6,12,−2 |
| Ventral striatum (nucleus accumbens) | L | 3.51 | −18,12,−6 | |
| Precentral gyrus (BA 6) | 156 | R | 3.52 | 64,0,26 |
| Anterior cingulate cortex (BA 24) | 327 | L | 4.87 | −6,24,34 |
| Lateral occipital gyrus (BA 19) | 480 | R | 5.42 | 38,−88,−10 |
| Angular gyrus (BA 39) | 146 | L | 3.43 | −34,−72,22 |
| Thalamus | 128 | R | 3.63 | 14,−16,2 |
| Lateral occipital gyrus (BA 19) | 101 | L | 3.21 | −28,−90,−8 |
| Connectivity with right hippocampus (30,−24,−15) | ||||
| Lateral occipital gyrus (BA 18) | 131 | R | 4.26 | 22,−100,−8 |
kE = cluster size in voxels. Regions active within the same cluster are grouped together.
x,y,z coordinates in MNI space. L =left hemisphere, R = right hemisphere
Figure 4.
Disambiguation related functional connectivity (overlapping > non-overlapping) with the left hippocampal seed region during the presentation period after participants knew the sequences. (A) Left hippocampal functional connectivity at the presentation phase with regions of the dorsal and ventral striatum (top panels and bottom left panel), anterior cingulate cortex (top left panel), posterior cingulate cortex (top right panel and bottom left panel), angular gyrus (bottom right panel), insula (bottom right panel) and middle frontal gyrus (bottom right panel). All images displayed on an average structural image of all 15 participants. (B) Results rendered on a whole brain. Red areas indicate regions showing significantly stronger functional connectivity with the left hippocampus during the overlapping condition compared to the non-overlapping condition during the presentation period after participants knew the sequences. All images displayed using p < 0.01 with 96 contiguous voxels. L = left hemisphere, R = right hemisphere
2.3.2 Disambiguation related functional connectivity at the critical test
At the critical test, participants had to indicate which of the two faces belonged with the four faces shown during the presentation phase. In the overlapping condition, participants needed to use the context of the current sequence to guide their choice because the faces immediately preceding the choice point and the faces at the choice point were always the same. The left hippocampus showed significant disambiguation related functional connectivity between many cortical regions at the critical choice point of the experienced phase, including the left rostral prefrontal cortex (BA 10p), left superior frontal sulcus (BA 8), supramarginal gyrus bilaterally (BA 40), and left superior parietal lobule (BA 7) (Fig. 5, Table 2). Importantly, the left hippocampus showed significantly greater functional connectivity with the left orbitofrontal cortex (BA 12/47) during the critical choice of the overlapping condition compared to the non-overlapping condition in the experienced phase of the task (Fig 5). Consistent with our hypothesis, these results suggest that when making a choice after a period of overlap in a known sequence, the hippocampus works together with the orbitofrontal cortex in order to select the appropriate choice option for the current sequence.
Figure 5.
Disambiguation related functional connectivity with the left hippocampal seed region during the critical choice in the experienced phase of the task. (A) Left hippocampal functional connectivity with the orbitofrontal cortex (top left panel), rostral prefrontal cortex (top right panel), and supramarginal gyrus (bottom panels) when choosing between two overlapping choice options. (B) Results rendered on a whole brain. Red areas indicate regions showing significantly stronger functional connectivity with the left hippocampus during the overlapping condition compared to the non-overlapping condition at the critical choice point after participants knew the sequences. All images displayed using p < 0.01 with 96 contiguous voxels. L = left hemisphere, R = right hemisphere
Table 2.
Left hippocampal disambiguation related connectivity at critical choice of experienced phase
| kE | Left/Right | T | x,y,z | |
|---|---|---|---|---|
| Lateral orbitofrontal cortex (BA 12/47) | 157 | L | 4.93 | −36,54,−4 |
| Rostral prefrontal cortex (BA 10p) | L | 3.04 | −20,66,10 | |
| Precentral gyrus (BA 4) | 349 | L | 5.88 | −42,10,44 |
| Middle frontal gyrus (BA 6) | L | 4.66 | −40,18,46 | |
| Supramarginal gyrus (BA 40) | 314 | L | 5.28 | −50,−60,44 |
| Superior parietal lobule (BA 7) | L | 4.38 | −40,−68,44 | |
| Fusiform gyrus (BA 19) | 274 | R | 6.73 | 34,−84,−16 |
| Cerebellum | R | 3.57 | 28,−78,−30 | |
| Superior frontal gyrus (BA 8) | 179 | L | 4.74 | −6,32,54 |
| Supramarginal gyrus (BA 40) | 107 | R | 5.11 | 46,−56,46 |
| Lateral occipital gyrus (BA 18) | 224 | L | 3.95 | −18,−100,0 |
| Middle temporal gyrus (BA 21) | 112 | L | 6.06 | −46,−30,−10 |
kE = cluster size in voxels. Regions active within the same cluster are grouped together.
x,y,z coordinates in MNI space. L =left hemisphere, R = right hemisphere
2.3.3 Random compared to overlapping condition
One possible explanation for the differences in functional connectivity between the overlapping and non-overlapping conditions detailed above could be related to working memory load. It may be that participants are maintaining the first few faces seen in the overlapping condition in working memory whereas they only need to maintain the prior face in the non-overlapping condition to get the critical choice correct. If working memory load is causing the differences in hippocampal functional connectivity between the overlapping and non-overlapping conditions, then one would expect the random condition, which has the greatest working memory load, to show greater hippocampal functional connectivity than the overlapping condition. Therefore, we compared hippocampal functional connectivity during the random condition to the overlapping condition. We limited these comparisons to the presentation and critical choice period of the experienced phase in the left hippocampus because this is where we found disambiguation related functional connectivity differences. The random condition showed significantly greater left hippocampal functional connectivity with only the cerebellum (x,y,z mni coordinate 0, −38, −34) during the presentation period of the experienced phase. At the critical test of the experienced phase, there were no brain regions which showed significantly greater left hippocampal functional connectivity during the random condition than the overlapping condition. Importantly, these results rule out working memory load as an explanation for the connectivity differences seen between the overlapping and non-overlapping condition.
2.3.4 Sequence related functional connectivity in learning phase
We also examined brain regions showing stronger functional connectivity with the hippocampus during sequence learning by contrasting the non-overlapping condition to the random condition (NOL > RAN) and masking the results with one-sample t-tests examining hippocampal functional connectivity in the NOL condition. The complete list of regions showing significant sequence related functional connectivity with the hippocampus during sequence learning and retrieval can be found in Table 3.
Table 3.
Sequence related connectivity (NOL>RAN) with the hippocampus
| kE | Left/Right | T | x,y,z | |
|---|---|---|---|---|
| Presentation period during learning phase | ||||
| Left hippocampus (−30,−24,−15) | ||||
| Posterior orbitofrontal cortex (BA 13L) | 214 | R | 4.78 | 26,28,−12 |
| Rostral prefrontal cortex (BA 10) | R | 3.51 | 6,40,−18 | |
| Ventral striatum (nucleus accumbens) | R | 3.19 | 8,16,−8 | |
| Right hippocampus (30,−24,−15) | ||||
| Ventral striatum (nucleus accumbens) | 134 | R | 4.07 | 10,16,−8 |
| Rostral prefrontal cortex (BA 10) | 98 | R | 4.29 | 20,56,10 |
| Critical test during learning phase | ||||
| Right hippocampus (30,−24,−15) | ||||
| Rostral prefrontal cortex (BA 10) | 148 | R | 5.83 | 4,60,8 |
| Middle frontal gyrus (BA 8) | 109 | L | 4.21 | −30,24,50 |
| Critical test during experienced phase | ||||
| Right hippocampus (30,−24,−15) | ||||
| Precuneus (BA 7m) | 800 | L | 6.85 | −2,−74,34 |
| Precuneus (BA 7p) | R | 5.73 | 12,−70,54 | |
| Posterior cingulate cortex (BA 31) | R | 4.42 | 12,−58,36 | |
| Lingual gyrus (BA 18) | 751 | L | 4.75 | −8,−84,−16 |
| Cuneus (BA 17) | L | 3.41 | −6,−88,4 | |
| Cuneus (BA 17) | R | 3.31 | 12,−80,6 | |
| Dorsal lateral prefrontal cortex (BA 9) | 236 | R | 5.14 | 30,40,40 |
| Superior frontal gyrus (BA 8) | R | 3.66 | 22,28,50 | |
| Precuneus (BA 7p) | 130 | L | 4.03 | −10,−72,56 |
| Posterior cingulate cortex (BA 31) | 289 | L | 4.50 | −4,−32,50 |
| Cuneus (BA 17) | 161 | R | 4.67 | 14,−100,−6 |
| Medial rostral prefrontal cortex (BA 10) | 130 | R | 5.51 | 12,44,−6 |
| Superior parietal lobule (BA 7) | 139 | L | 3.96 | −34,−40,54 |
kE = cluster size in voxels. Regions active within the same cluster are grouped together.
x,y,z coordinates in MNI space. L =left hemisphere, R = right hemisphere
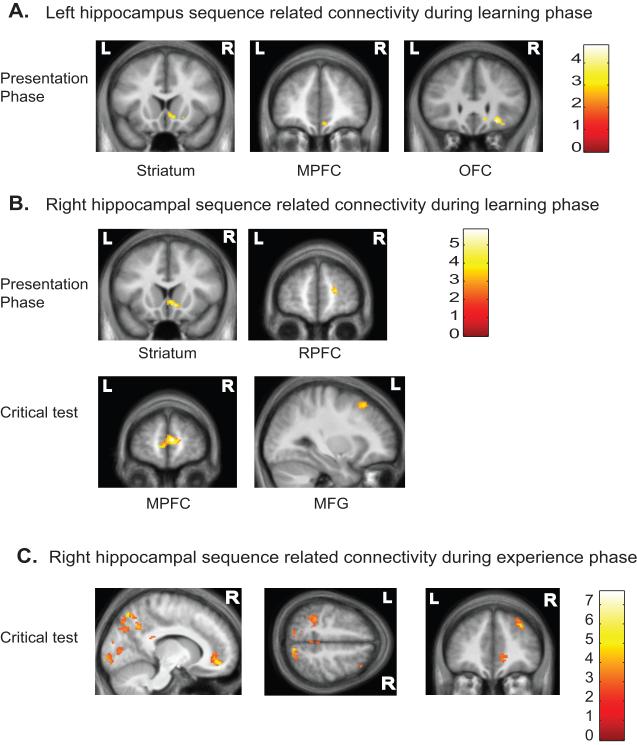
During the presentation phase of the task, the left hippocampal seed region showed significantly stronger functional connectivity with right posterior orbitofrontal cortex (BA 13l), right medial prefrontal cortex (BA 10r), and right ventral striatum (Fig. 6a) when viewing faces from the non-overlapping sequence condition compared to the random condition. The right hippocampal seed region showed significant sequence related connectivity with right lateral rostral prefrontal cortex (BA 10p) and right ventral striatum during sequence learning in the presentation phase (Fig. 6b top panel).
Figure 6.
Regions showing significant sequence related functional connectivity (non-overlapping > random) with the hippocampus. (A) Left hippocampus sequence related functional connectivity during the presentation phase while participants were learning the sequences with the ventral striatum, medial prefrontal cortex (MPFC) and the orbitofrontal cortex (OFC). (B) The top panels illustrate significant sequence related functional connectivity between the right hippocampus and the ventral striatum and rostral prefrontal cortex (RPFC) in the presentation phase while participants were learning the sequences. The bottom panels show medial prefrontal cortex (MPFC) and middle frontal gyrus (MFG) functional connectivity to the right hippocampus at the critical choice while participants were learning the sequences. (C) Regions showing significant functional connectivity with the right hippocampus at the critical choice after participants knew the sequences. All images displayed using p < 0.01 with 96 contiguous voxels. L = left hemisphere, R = right hemisphere
At the critical test of the learning phase, the right hippocampal seed region showed significant sequence related functional connectivity with the medial rostral prefrontal cortex (BA 10) and left middle frontal gyrus (BA 8) (Fig. 6b bottom panel). These results suggest that during sequence learning, the hippocampus works in conjunction with ventral striatum, orbitofrontal cortex, medial prefrontal cortex, and lateral rostral prefrontal cortex.
2.3.5 Sequence related functional connectivity in experienced phase
We examined brain regions showing significantly increased functional connectivity with the hippocampus during sequence retrieval by contrasting the presentation period and critical choice of the non-overlapping condition to the random condition in the experienced phase of the task. There was extensive sequence related functional connectivity with the right hippocampal seed region during the critical choice of the experienced phase (Fig. 6c). The right hippocampus showed significantly stronger functional connectivity during the critical choice of non-overlapping sequences compared to the random condition with the precuneus bilaterally (BA 7p and 7m), posterior cingulate cortex bilaterally (BA 31), cuneus bilaterally (BA 17), right dorsal lateral prefrontal cortex (BA 9), right medial rostral prefrontal cortex (BA 10r) and left superior parietal lobule (BA 7). These results suggest the hippocampus works with occipital, parietal, and prefrontal cortical regions when correctly identifying the next face in a sequence.
3. Discussion
3.1 Disambiguation related functional connectivity
The hippocampus has been shown to be critical when learning and retrieving both non-spatial and spatial sequences which have overlapping elements (Agster et al., 2002; Brown et al., 2010; Kumaran and Maguire, 2006; Ross et al., 2009; Shohamy and Wagner, 2008). Here, direct comparison of hippocampal functional connectivity during the learning and retrieval of overlapping versus non-overlapping sequences of faces allowed for the isolation of disambiguation related functional connectivity. The most extensive disambiguation related functional connectivity was seen in the experienced phase of the task, after participants knew the sequences. The left hippocampus showed disambiguation related functional connectivity with both dorsal and ventral striatum, anterior cingulate, and posterior cingulate during the presentation period of the experienced phase of the task. At the critical choice point, where the context of the current sequence could be used to make a choice, the left hippocampus showed stronger functional connectivity with orbitofrontal cortex, rostral prefrontal cortex, and supramarginal gyrus during the overlapping compared to the non-overlapping condition. These results suggest the hippocampus works in conjunction with a large network of regions, including prefrontal and striatal regions when there is a need to disambiguate overlapping non-spatial sequential representations to guide decision making.
3.1.1 Orbitofrontal-hippocampal connectivity during decision making
The orbitofrontal cortex may be critical to the selection of the correct stimulus under changing contextual situations. At the critical choice point, we found significant disambiguation related functional connectivity between the hippocampus and orbitofrontal cortex in the experienced phase of the task. The orbitofrontal cortex has been shown to be critically important for promoting flexible behavior (Arana et al., 2003; Chudasama and Robbins, 2003; Elliott et al., 2000; McAlonon and Brown, 2003; Meunier et al., 1997; Murray and Izquierdo, 2007; O’Doherty et al., 2003; Schoenbaum et al., 1998; 2003; Takahashi et al., 2009). In a recent fMRI study examining disambiguation during spatial navigation, the orbitofrontal cortex was found to be active along with the hippocampus and parahippocampal cortex at the point where two overlapping mazes diverged (Brown et al. 2010). Based on theoretical work (Hasselmo and Eichenbaum, 2005), Brown and colleagues (2010) hypothesized that the orbitofrontal cortex may help in the flexible selection of the correct response at the critical choice point by working with contextual information retrieved by the hippocampus. A recent electrophysiological study demonstrated that over the course of learning, neurons in the hippocampus develop item-in-context representations (Komorowski et al., 2009). These item-in-context representations may help signal the importance of an item in one contextual situation over another. We suggest the observed increase in connectivity between the left hippocampus and orbitofrontal cortex at the critical choice point may be a reflection of an item-in-context representation being used by the orbitofrontal cortex to help guide the selection of the appropriate face in the current context.
3.1.2 Hippocampal contributions to decision making
The current task requires an individual to make a choice between two equally rewarded stimuli at the critical choice point. At the critical choice of the overlapping condition, earlier elements of the sequence need to be retrieved to guide the decision. The dorsal and ventral striatum, orbitofrontal cortex, anterior cingulate, posterior cingulate, and ventral lateral prefrontal cortex have all been implicated in the decision making process (Breiter et al., 2001; den Ouden et al., 2010; Haber and Knutson, 2010; Hare et al., 2008; 2009; Kable and Glimcher, 2007; 2009; Lau and Glimcher, 2008; Padoa-Schioppa and Assad, 2006; 2008; Plassmann et al., 2010; Redish et al., 2008; Schoenbaum et al., 1998; 2003; 2009; Takahashi et al., 2009; van der Meer et al., 2010). Our results showed significant disambiguation related functional connectivity between the hippocampus and these decision making regions, providing an important link between hippocampal function and decision making.
One possible way the hippocampus might help in the decision process is to predict what is to come after the currently presented stimulus. Electrophysiological studies in rodents have shown that the hippocampus may look ahead during decision making (Ferbinteanu and Shapiro, 2003; Johnson and Redish, 2007; Johnson et al., 2007), and studies in humans have shown the hippocampus is active when thinking of the future (Addis et al., 2007; 2009; Botzung et al., 2008; Okuda et al., 2003). This hippocampal look ahead might allow for evaluation of an upcoming choice by decision making circuitry to commence before the actual choice is encountered. In the current task, the look ahead may be accomplished by the hippocampus reading out the sequence of faces. In the overlapping condition, the sequence read out would introduce ambiguity at the overlap point since the overlapping stimuli are followed by two different faces. The greater connectivity seen between the hippocampus and regions involved in decision making after participants know the overlapping sequences may be due to the uncertainty caused by the overlapping sequences. The ventral striatum has long been associated with reward value (Breiter et al., 2001; Carlezon and Wise, 1996; Haber and Knutson, 2010; Phillips and Fibiger, 1978; van der Meer et al., 2010). We suggest the increased hippocampal connectivity with the ventral striatum during the presentation phase of the overlapping sequences is a reflection of hippocampal retrieval of the upcoming choice options which then allows the ventral striatum to retrieve both the positive and negative reward values previously associated with each of the critical choice stimuli. The hippocampus may then signal that in this particular sequence, one of the stimuli has been associated with the positive reward value while the other stimulus has been associated with the negative reward value in preparation for the upcoming choice.
The hippocampal look ahead may also prepare the participants to take action when the choice stimuli appear. The current results showed significant disambiguation related functional connectivity with the dorsal striatum during the presentation phase of the task. The dorsal striatum has been shown to associate action with the current situation (Balleine et al., 2007; Poldrack et al., 2001; Poldrack and Packard, 2003; Redish et al., 2008; van der Meer et al., 2010). The hippocampal connectivity with the dorsal striatum at the presentation phase may suggest a preparedness to act when the choice stimuli appear. In this way, the dorsal striatum may act as a filtering mechanism where only the relevant choice stimulus is allowed to enter working memory, allowing for the selection of the correct action (Baier et al., 2010; McNab and Klingberg, 2008). In the presentation period of the experienced phase of the overlapping compared to the non-overlapping condition, the hippocampus also demonstrated significantly stronger functional connectivity with the anterior cingulate cortex, which has been associated with goal based action selection (Shima and Tanji, 1998; Matsumoto et al., 2003; 2004; Ridderinkhof et al., 2004; Hadland et al., 2003; Bush et al., 2002). When combined with these prior studies, our results suggest the hippocampus works closely with the dorsal striatum and anterior cingulate cortex in situations where the current context and prior experience can be used to prepare a response.
Our functional connectivity results closely mirror known patterns of anatomical connections. The hippocampus sends projections to the orbitofrontal cortex (Barbas and Blatt, 1995; Cavada et al., 2000; Roberts et al., 2007), the anterior cingulate cortex (Barbas and Blatt, 1995) and the ventral striatum (Groenewegen et al., 1987; 1999). The orbitofrontal cortex forms a fronto-striatal loop with the dorsal striatum while the anterior cingulate cortex forms a separate fronto-striatal loop with the ventral striatum (Alexander et al., 1986). The pattern of these anatomical connections coupled with the current functional connectivity results suggest the hippocampus may play a role in decision making by predicting the possibilities of what might come next, allowing reward valuation regions to evaluate the expected choice options in order to make the correct action at the appropriate choice point.
In our previous study examining hippocampal contributions to sequence learning using a univariate analysis (Ross et al., 2009), we showed that hippocampal activity was not significantly different between the overlapping and non-overlapping conditions, even in the experienced phase of the task. Interestingly, by looking at the network activity, the current functional connectivity analysis identifies differences in hippocampal functional connectivity in the experienced phase using the same data set. In the Ross et al. 2009 study, we speculated that we did not see differences between the overlapping and non-overlapping conditions in the univariate analysis because the sequences were newly learned. We went on to suggest that well learned sequences might show a difference between overlapping and non-overlapping conditions. The connectivity results support this idea. In a subsequent study using a spatial sequencing task, we demonstrated that well learned overlapping sequences more strongly activate the hippocampus compared to well learned non-overlapping sequences (Brown et al., 2010). We suggest that the differences in hippocampal functional connectivity we see for newly learned overlapping sequences in the current analysis may precede activation differences seen in well learned sequences.
3.2 Sequence related functional connectivity with the hippocampus
A second goal of the present study was to examine hippocampal functional connectivity during sequence learning and retrieval. The hippocampus has been shown to be critically important to learning the order of events (Downes et al., 2002; Fortin et al., 2002; Kesner et al., 2002). Our results showed significant sequence learning related connectivity in the presentation phase between the hippocampus and orbitofrontal cortex, rostral prefrontal cortex, and ventral striatum.
After participants knew the sequences, there was significant sequence related functional connectivity between the hippocampus and the medial rostral prefrontal cortex, precuneus/posterior cingulate cortex, and dorsal lateral prefrontal cortex at the critical choice point. The precuneus and posterior cingulate cortex have been linked to memory retrieval (Bledowski et al., 2009; Buckner et al., 2008; Cavanna and Trimble, 2006; Uncapher and Wagner, 2009; Wagner et al., 2005) while the dorsolateral prefrontal cortex has been shown to be important for maintaining representations in working memory (Kirchhoff et al., 2000; Miller and Cohen, 2001; Owen et al., 1996; Petrides, 1994; 2005). The dorsal lateral prefrontal cortex may be maintaining representations of the two critical choice stimuli as the hippocampus, precuneus, and posterior cingulate cortex retrieve the correct stimulus from long-term memory.
3.3 Conclusion
Making the correct contextually dependent choice at a divergent point in two overlapping sequences increases the connectivity between the hippocampus, a region known to be important for disambiguating overlapping representations, and regions important for coding the value of choice options. Contextual retrieval of earlier elements of the sequence may play an important role in deciding between two overlapping representations at a choice point. We suggest the connectivity between the hippocampus and prefrontal cortical areas (orbitofrontal cortex and medial prefrontal cortex) may allow item-in-context representations coded in the hippocampus to influence the value of stimuli associated with both positive and negative outcomes, allowing for a contextually dependent choice to be made. In this way, the hippocampus may be assisting in the decision making process. In conclusion, we suggest the hippocampus works directly with the reward valuation and action selection systems when a choice must be made between two overlapping representations.
4. Experimental Procedure
4.1 Participants
The data used in the current study are the same as previously used for an experiment examining the role of the human hippocampus during learning and retrieval of sequences (Ross et al., 2009). The prior experiment used univariate statistical methods to examine the role of the human hippocampus during learning and retrieval of sequences (Ross et al., 2009) whereas the focus of the current analysis was to examine hippocampal functional connectivity during context dependent decision making. Twenty-one participants between the ages of 18-35 (mean age 20.7 ± 0.69 years; eight males) with normal or corrected-to-normal vision were recruited for this study from the Boston University community. A total of six participants were eliminated from the analysis, two due to excess motion during fMRI scanning and four because of poor behavioral performance, leaving fifteen participants for analysis. Each participant gave informed consent before participation in accordance with the experimental protocol approved by both the Massachusetts General Hospital Internal Review Board and the Institutional Review Board of Boston University.
4.2 Experimental Protocol
4.2.1 Behavioral paradigm
Thirty-six pictures of different faces (18 male; 18 female) served as stimuli in the task. Each stimulus was pseudo-randomly assigned to create six groups of six faces. Each group contained three male and three female faces with each face appearing in only one group. Randomly paired groups were assigned to the overlapping sequence, non-overlapping sequence, or random conditions.
In the overlapping sequence condition, six faces were always shown in the same order and the third and fourth faces of each sequence were identical, thereby creating two overlapping sequences. The non-overlapping condition contained two groups of six faces that were shown in the same order but did not share faces between them (Fig. 1). The random condition contained two groups of six faces that always stayed the same but were shown in a random order each trial.
A trial consisted of Presentation, Delay, and Choice phases followed by an intertrial interval (ITI) (Fig. 2). In the Presentation phase, a serial presentation of four faces was paired with a blurred face for 4 seconds. In the Delay period, a fixation cross was shown for 8 seconds. In the choice phase, participants were shown two faces and asked to indicate which face belonged in the group. The choice for the fifth face in the group was specified as the “critical choice”, while the choice for the sixth face in the group was termed the “final choice”. The fifth face in the group was determined to be the critical choice because it was the choice right after the period of overlap in the two sequences. Feedback was immediately given to the participants whether their response was correct or incorrect.
Participants were encouraged to learn the order of the faces in the overlapping and non-overlapping sequence conditions in order to make the correct decision at the critical choice point. Participants were told that they would be tested on their knowledge after fMRI scanning. Participants viewed all six groupings of faces (two overlapping, two non-overlapping, and two random) in a run. The order of presentation of the two groupings within a condition was split across trials. The groupings were counterbalanced so that each pair of six faces served as stimuli in all three conditions across participants.
A total of 12 runs were shown to each participant using E-Prime 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA). Each group of six faces was shown 12 times over all the runs for a total of 24 overlapping condition trials; 24 non-overlapping condition trials and 24 random condition trials. The order of presentation for each condition within a run was counterbalanced across all 12 runs.
4.2.2 Postscan test
After scanning, participants were administered a postscan test assessing their knowledge of the overlapping and non-overlapping sequences. All six faces in a sequence were presented to the participants in a scrambled order on a piece of paper. Participants were asked to label the faces in order of 1 through 6. Any participant who made more than two sequence errors on the postscan test was excluded from the functional connectivity analysis.
4.3 Imaging Acquisition
Imaging was conducted using a 3 Tesla Siemens MAGNETOM TrioTim scanner (Siemens AG, Medical Solutions, Erlangen, Germany) with a 12-channel Tim® Matrix head coil. Two high-resolution T1-weighted multiplanar rapidly acquired gradient echo (MP-RAGE) structural scans were acquired using generalized autocalibrating partially parallel acquisitions (GRAPPA) (TR = 2,530 ms; TE = 3.44 ms; flip angle = 7°; slices = 176, field of view = 256; resolution = 1 mm × 1 mm × 1 mm). Functional T2*-weighted BOLD images were acquired using an echo planar imaging (EPI) sequence (TR = 2000 ms; TE = 30 ms; flip angle = 90°; acquisition matrix = 64 × 64, field of view = 256; slices = 32; resolution = 4.0 mm isotropic). Slices were aligned along the anterior commissure/ posterior commissure line.
4.4 fMRI Pre-processing
Functional imaging data were preprocessed using the SPM8 software package (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). All BOLD images were first reoriented so the origin (i.e. coordinate xyz = [0 0 0]) was at the anterior commissure. Then the images were corrected for differences in slice timing and realigned to the first image collected within a series. Motion correction was conducted next and included realigning and unwarping the BOLD images to the first image in the series in order to correct for image distortions caused by susceptibility-by-movement interactions. Realignment was estimated using 2nd degree B-spline interpolation with no wrapping while unwarp reslicing was done using 4th degree B-spline interpolation with no wrapping. The high-resolution structural images were then coregistered to the mean BOLD image created during motion correction and segmented into white and gray matter images. The bias-corrected structural images and the coregistered BOLD images were then spatially normalized into standard MNI (Montreal Neurological Institute) stereotactic space using the parameters derived during segmentation with resampling of the BOLD images to 2 × 2 × 2 mm isotropic voxels. The normalized structural images of all sixteen participants were averaged after normalization for overlay of group Statistical Parametric Maps (SPMs). Finally, BOLD images were spatially smoothed using a 6 mm full-width at half-maximum Gaussian filter to reduce noise.
4.5 Data Analysis
4.5.1 Behavioral analysis
Percent accuracy and reaction times were recorded using E-prime 2.0. Paired samples t-tests were used to assess whether differences existed between the overlapping and non-overlapping conditions in percent accuracy and reaction time at the critical choice point. If participants scored less than 75% correct on the critical choice for either the overlapping or non-overlapping condition, they were excluded from the study. Behavioral analysis was done using SPSS 16 (SPSS, Inc., Chicago, IL).
4.5.2 Learning phase versus experience phase behavioral analysis
We used each individually participant’s behavioral performance at the critical choice point to split the fMRI data into a learning phase and experienced phase. All trials beginning with and following the trial in which participants correctly identified the critical choice stimulus six times in a row comprised the experienced phase while all trials before the experienced phase constituted the learning phase. For example, if a participant correctly identified the critical choice stimulus six times in a row beginning with the fifth run, then all trials in the fifth through twelfth run were included as part of the experienced phase whereas all trials in the first four runs were included as part of the learning phase. Six correct critical choices was chosen as a criteria because it required the participants to correctly identify the critical choice of both sets of faces in a condition three times in a row.
4.6 fMRI Analysis
4.6.1 Region-of-interest (“seed”) selection
The hippocampus served as our region of interest (ROI) in this study based on its known involvement in sequence learning and retrieval and our interest in characterizing this region’s functional connectivity during the disambiguation of overlapping sequences. The left and right hippocampal coordinates (±30,−24,−15) were derived from Schendan et al., 2003. The seed regions were created as 5 mm spherical ROIs centered on the coordinate using the MarsBar region of interest toolbox for SPM8 (Brett et al., 2002).
4.6.2 Beta series correlation analysis
Functional connectivity analyses were conducted using the beta series correlation analysis method (Rissman et al., 2004). The first step of the beta series correlation method was to adapt the univariate fMRI data analysis so that the magnitude of the task-related BOLD response was estimated separately for each experimental trial. Therefore, a design matrix with 290 regressors was convolved with the canonical hemodynamic response function in SPM8. During model generation, the data was filtered with a 0.008 Hz high-pass filter. The regressors were created for each participant as a function of condition (overlapping, non-overlapping, random), trial phase (presentation, delay, critical choice, and final choice) and individual trial. Additional regressors included as part of the design matrix were a nuisance regressor comprised of the inter trial intervals, the feedback, cue period, and get ready signal as well as 6 motion regressors. Due to the short feedback time in our study design, there is a small amount of collinearity between the critical choice and the feedback period which may have influenced the results at the critical choice. Regressors were modeled as square waves, or boxcars, starting at the onset of the event with length equal to the corresponding trial phase (e.g. presentation regressors were modeled as 16 s square waves). Critical choice and final choice regressors were modeled as square waves equal to the time it took for the participant to respond on that particular trial.
Parameter estimates, or beta values, were computed for each regressor using the least squares solution of the GLM in SPM8. We then sorted these beta values into the individual trials of the presentation and critical choice phase of the overlapping, non-overlapping, and random conditions which were subsequently divided into learning and experienced trials yielding a set of 144 beta values for every voxel in the brain for the beta series correlation analysis. Beta series were then formed by stringing together the beta values for each individual trial in the appropriate condition. This method assumes the extent to which two brain voxels interact during a given condition can be quantified by the extent to which their respective condition-specific beta series are correlated (Rissman et al., 2004). We used the left and right seed regions of the hippocampus to construct correlation maps specific to each condition by determining the correlation of that specific seed region’s beta series with the beta series of all other voxels in the brain using a custom MATLAB (MathWorks, Natick, MA) script generously provided by Dr. Jesse Rissman.
For specific details and validation of the beta-series correlation method, see Rissman et al., 2004. Briefly, condition-specific whole brain correlation maps were obtained by calculating the correlation of the specific seed region’s beta series with that of all other brain voxels. An arc-hyperbolic tangent transform was then applied. The arc- hyperbolic transformed correlation coefficients were then divided by the standard deviation to produce a map of z-scores. Group level random-effects statistical parametric maps (SPMs) for all 12 conditions of interest (presentation and critical choice periods of the overlapping, non-overlapping, and random conditions in the learning and experienced phases) were constructed using the z-transformed correlation maps of each individual participant in SPM8. Functional connectivity specifically related to the disambiguation of sequences was assessed in the presentation and critical choice periods of the learning and experienced phases by comparing the overlapping to the non-overlapping z-transformed correlation maps using paired t-tests in SPM8. This comparison was masked with the results of second level random effects analyses using one-sample t-tests examining hippocampal functional connectivity specific to the overlapping condition. For example, the comparison examining regions showing stronger hippocampal functional connectivity during the overlapping compared to the non-overlapping critical choice was masked with those regions showing significant hippocampal functional connectivity in the overlapping critical choice period. This masking was done to ensure that regions showing stronger disambiguation related functional connectivity (OL>NOL) was limited to those regions showing significant functional connectivity in the overlapping condition.
Sequence related functional connectivity was assessed in the presentation and critical choice periods of the learning and experienced phase by conducting paired t-tests on the z-transformed correlation maps of the non-overlapping sequence condition with the random condition in SPM8. These contrasts were masked with the results of results of second level random effects analyses using one-sample t-tests examining regions showing significant hippocampal functional connectivity in the non-overlapping condition.
Finally, we also compared left hippocampal functional connectivity during presentation and critical choice of the experienced phase in the random condition to the overlapping condition. These comparison analyses were not masked with any other results and were run to assess the impact of working memory load on hippocampal functional connectivity.
A cluster extent threshold was enforced in order to correct for multiple comparisons. Specifically, an individual voxel statistical threshold of p < 0.01 (T = 2.62) was enforced with a minimum cluster extent threshold of 96 resampled voxels (768 mm3) was used for the results of the OL>NOL and NOL>RAN functional connectivity comparisons. The SPMs resulting from the one-sample t-tests which were used to mask these comparisons used an individual voxel statistical threshold of p < 0.001 (T = 3.79 ) with a minimum cluster extent of 48 resampled voxels (384 mm3). A slightly stricter individual voxel threshold was used in the one-sample t-tests to ensure that the results of our contrasts were limited to those regions showing significant hippocampal functional connectivity in the individual conditions. Monte Carlo simulations with 10,000 iterations were run to determine the cluster extent necessary to correct for multiple comparisons. The Monte Carlo simulations modeled activity in each voxel using a normally distributed random number (mean of zero and unit variance) and type I error was assumed to be equal to the individual voxel threshold p value in a volume defined by the functional acquisition dimensions. Autocorrelation in the functional data was modeled in the Monte Carlo simulations with 6 mm full-width, half-maximum Gaussian kernels. The simulations then provided the number of voxels where the probability of obtaining that size cluster or larger was less than p < 0.01 when the individual voxel threshold was 0.01 and 0.001. Peak activations within each cluster of activation were identified in SPM8. Peaks of activation were reported if they were more than 4 mm apart and represented a different region of activity. If a specific region of activity had multiple peaks within a cluster, the peak with the highest t-value was reported. Brodmann areas were identified using a variety of reference materials (Damasio, 2005; Ongur et al., 2003; Petrides, 2005).
Research highlights.
Illustrates hippocampal functional connectivity during decision making
Results link hippocampus to striatum and prefrontal cortex function
Discusses how context may guide decision making
Acknowledgments
This work was conducted with the support of the Cognitive Neuroimaging Laboratory, Silvio O. Conte Center for Memory and Brain (NIH P50 MH071702), Boston University (Boston, MA) and the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. This work also involved the use of instrumentation supported by the NCRR Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number S10RR021110. We would like to thank Dr. Jesse Rissman for providing his MATLAB code for functional connectivity analysis and Thackery Brown for assisting in fMRI data collection.
Abbreviations
- OL
overlapping
- NOL
non-overlapping
- BA
Brodmann area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47(11):2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22(13):5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23(29):9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Muller NG. Keeping memory clear and stable--the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci. 2010;30(29):9788–9792. doi: 10.1523/JNEUROSCI.1513-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5(6):511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(Pt 5):1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Rahm B, Rowe JB. What “works” in working memory? Separate systems for selection and updating of critical information. J Neurosci. 2009;29(43):13735–13741. doi: 10.1523/JNEUROSCI.2547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn. 2008;66(2):202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci. 2010;30(21):7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12(10):1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16(9):3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23(25):8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. 2 ed Oxford University Press; New York: 2005. [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden HE, Daunizeau J, Roiser J, Friston KJ, Stephan KE. Striatal prediction error modulates cortical coupling. J Neurosci. 2010;30(9):3210–3219. doi: 10.1523/JNEUROSCI.4458-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40(7):853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40(6):1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14(1):5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The anterior cingulate and reward-guided selection of actions. J Neurophysiol. 2003;89(2):1161–1164. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18(9):1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 2007;27(45):12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17(6):692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63(6):733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20(16):6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29(31):9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49(4):617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58(3):451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton PA, White JA, Eichenbaum H. Disambiguation of overlapping experiences by neurons in the medial entorhinal cortex. J Neurosci. 2007;27(21):5787–5795. doi: 10.1523/JNEUROSCI.1063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301(5630):229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol. 2004;14(2):178–185. doi: 10.1016/j.conb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146(1-2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35(7):999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996;6(1):31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11(1):95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4(2):207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. The role of dopamine in maintaining intracranial self-stimulation in the ventral tegmentum, nucleus accumbens, and medial prefrontal cortex. Can J Psychol. 1978;32(2):58–66. doi: 10.1037/h0081676. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Moyano J. Creso, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414(6863):546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D’Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 2004;24(16):3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31(4):415–437. doi: 10.1017/S0140525X0800472X. discussion 437-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502(1):86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6(6):601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19(9):790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci. 2008;20(3):432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28(33):8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37(6):1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10(12):885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39(5):855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282(5392):1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD. Does the hippocampus mediate objective binding or subjective remembering? Neuroimage. 2010;49(2):1769–1776. doi: 10.1016/j.neuroimage.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 2008;20(8):1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ, Taylor AR, Burke KA, Schoenbaum G. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62(2):269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30(50):16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91(2):139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MA, Johnson A, Schmitzer-Torbert NC, Redish AD. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67(1):25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport. 2001;12(2):359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Zilli EA, Hasselmo ME. Analyses of Markov Decision Process Structure Regarding the Possible Strategic use of Interacting Memory Systems. Front Comput Neurosci. 2008;2:6. doi: 10.3389/neuro.10.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]