Abstract
A role for mitochondria in tumor formation is suggested by mutations in enzymes of the TCA cycle: isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH) and fumarate hydratase (FH). Although they are all components of the TCA cycle, the resulting clinical presentations do not overlap. Activation of the hypoxia pathway can explain SDH phenotypes, but recent data suggest that FH and IDH mutations lead to tumor formation by repressing cellular differentiation. Here we discuss recent findings in the context of both mitochondrial and cytoplasmic components of the TCA cycle, and we propose that extra-metabolic roles of TCA cycle metabolites result in the reduced cellular differentiation. Furthermore, the activation of the pseudo-hypoxia pathway likely promotes the growth of these neoplasias into tumors.
Fundamentals of the TCA cycle
The TCA cycle is a central pathway in the metabolism of sugars, lipids and amino acids [1]. The original investigations of the TCA cycle took place in the context of oxidative metabolism, and it is often presented in a simplistic perspective of a cyclic pathway constantly oxidizing the acetyl moiety of acetyl-CoA to CO2, generating NADH and FADH2, which feed electrons to the respiratory chain (Figure 1 and Box 1). The work that defined the TCA cycle was performed using whole tissue homogenates. When differential centrifugation techniques allowed the preparation of pure mitochondria, it became evident that although mitochondria were necessary and sufficient to perform the cycle, isoforms of some of the enzymes were also found in the cytoplasm not associated with mitochondria (Table 1).
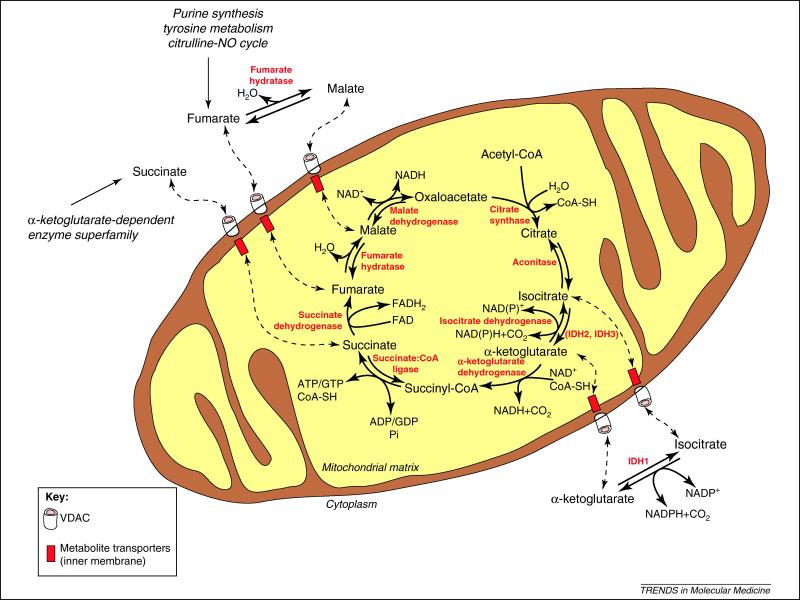
Figure 1. The TCA cycle.
Shown here are the core mitochondrial components and some of the cytoplasmic enzymes that catalyze steps in the TCA cycle. The cytopasmic and mitochondrial transamination reactions involving TCA cycle metabolites were omitted for simplicity. The transport of the metabolites across the inner membrane is catalyzed by a number of carriers and antiporters, and the metabolites cross the outer membrane by diffusing through channels such as VDAC. The metabolites are shown in black, the enzymes are shown in red, and the pathways in italics. Full arrows represent the direction of a reaction, intermittent arrows represent the translocation of metabolites between mitochondria and cytoplasm. Abbreviations: IDH, isocitrate dehydrogenase.
Box 1. Traditional formulation of the TCA cycle.
The TCA cycle [1] can be divided into two stages: decarboxylating, in which citrate (6 carbon atoms) is converted to succinyl-CoA (4 carbons) releasing two CO2 molecules; and reductive, the successive oxidations of succinate to fumarate, fumarate to malate, and malate to oxaloacetate (Figure 1).
The “first” reaction of the cycle is the condensation of acetyl-CoA with oxaloacetate to form citrate, catalyzed by citrate synthase. Citrate can be exported to the cytoplasm and serve as a precursor for lipid synthesis or remain in the mitochondria, where it is converted to isocitrate by aconitase. Aconitase contains a non-heme 4Fe-4S cluster and has one cytoplasmic isoform, which also functions as an iron regulatory protein.
The conversion of isocitrate in α-ketoglutarate is the first oxidative decarboxylation of the cycle. This reaction is catalyzed by isocitrate dehydrogenase, which occurs in three forms: NAD+-dependent and localized to mitochondria (IDH3), as well as NADP+-dependent and localized to either mitochondria (IDH2) or the cytoplasm (IDH1).
The α-ketoglutarate dehydrogenase complex (α-kgDHC) catalyses the conversion of α-ketoglutarate to succinyl-CoA and CO2. α-ketoglutarate is a substrate for the α-ketoglutarate–dependent dioxygenase superfamily, which includes the prolyl-hydroxylases regulating the α-subunits of hypoxia-inducible factors.
Succinyl-CoA is the precursor for heme synthesis in animals. If metabolized within the citrate cycle, succinyl-CoA generates succinate and GTP or ATP. The reaction is catalyzed by succinate:CoA ligase (SUCL), which is a dimer of the α-subunit (SUCLG1) and one of the β-subunits, either ATP-forming (SUCLA2) or GTP-forming (SUCLG2).
Succinate is oxidized to fumarate by succinate dehydrogenase (SDH), composed of four subunits termed A-D (SDHA-D). The SDH reaction is part of both the citrate cycle and the respiratory chain, where SDH is referred as complex II. All other oxidative steps of the cycle generate NADH to feed complex I of the respiratory chain, while the electrons removed from succinate are channeled through FAD to ubiquinone. Succinate is also product of the reactions catalyzed by the α-ketoglutarate–dependent dioxygenase superfamily, and can inhibit those reactions.
Fumarate hydratase (FH) catalyzes the hydration of the double bond in fumarate, generating malate. In most tissues, FH is found both in the cytoplasm and in mitochondria.
The “last” reaction of the cycle recycles oxaloacetate from malate. This reaction is catalyzed by malate dehydrogenase (MDH), and couples the oxidation of malate with the reduction of NAD+. MDH is localized to both mitochondria and the cytoplasm.
Table 1.
Compartmentalization of the TCA cycle enzymes and diseases arising from their malfunction.
| Activity | Enzymes | Localization | Mutation | Diseases | Refs. | |
|---|---|---|---|---|---|---|
| citrate synthase | CS | mitochondria | ||||
| citrate lyase | ACLY | cytoplasm | ||||
| aconitase | ACO1 | cytoplasm | ||||
| ACO2 | mitochondria | |||||
| isocitrate dehydrogenase | IDH1 (NADP+-dependent) |
cytoplasm | dominant (neomorphic activity) |
gliomas, acute myeloid leukemia |
[7, 9] | |
| IDH2 (NADP+-dependent) |
mitochondria | dominant (neomorphic activity) |
gliomas, acute myeloid leukemia |
[7, 9] | ||
| IDH3 α subunit (NAD+-dependent) |
mitochondria | |||||
| IDH3 β subunit (NAD+-dependent) |
mitochondria | |||||
| IDH3 γ subunit (NAD+-dependent) |
mitochondria | recessive | retinitis pigmentosa | [60] | ||
| α-ketoglutarate dehydrogenase |
α-ketoglutarate decarboxylase |
OGDH | mitochondria | |||
| dihydrolipoyl succinyltransferase |
DLST | mitochondria | ||||
| dihydrolipoyl dehydrogenase |
DLD | mitochondria | recessive | encephalopathy | [61] | |
| succinate:CoA ligase | SUCLG1 | mitochondria | recessive | hepatoencephalomyopathy | [62] | |
| SUCLA2 | mitochondria | recessive | hepatoencephalomyopathy | [62] | ||
| SUCLG2 | mitochondria | |||||
| succinate dehydrogenase | SDHA | mitochondria | recessive | encephalomyopathy | [63] | |
| dominant | gastrointestinal stromal tumors, pheochromocytomas, paragangliomas |
[5, 16, 64] |
||||
| SDHB | mitochondria | dominant | gastrointestinal stromal tumors, pheochromocytomas, paragangliomas |
[5, 16, 64] |
||
| SDHC | mitochondria | dominant | gastrointestinal stromal tumors, pheochromocytomas, paragangliomas |
[5, 16, 64] |
||
| SDHD | mitochondria | dominant | pheochromocytomas, paragangliomas |
[5, 16] | ||
| succinyl-CoA-3-oxoacid-CoA transferase |
SCOT | mitochondria | recessive | ketoacidosis | [65] | |
| fumarate hydratase | FH | mitochondria cytoplasm |
recessive | encephalopathy | [66] | |
| dominant | leiomyomas, renal cell cancer, Leydig cell tumors, ovary cystadenomas, cerebral cavernomas, uterine leiomyosarcomas and breast cancer |
[21] | ||||
| malate dehydrogenase | MDH1 (NAD+-dependent) |
cytoplasm | ||||
| MDH2 (NAD+-dependent) |
mitochondria | |||||
| malic enzyme | ME1 (NADP+-dependent) |
cytoplasm | ||||
| ME2 (NAD+-dependent) |
mitochondria | recessive | idiopathic generalized epilepsy |
[67] | ||
| ME3 (NADP+-dependent) |
mitochondria | |||||
| glutamate-oxaloacetate transaminase | GOT1 | cytoplasm | ||||
| GOT2 | mitochondria | |||||
| glutamate-pyruvate transaminase | GPT1 | cytoplasm | ||||
| GPT2 | mitochondria | |||||
The presence of these activities in the cytoplasm, as well as sophisticated shunts within the cycle, suggest another layer of complexity to this pathway. In addition, the cycle is not a closed pathway but rather integrates several metabolic pathways in the cell, such as the metabolism of amino acids, fatty acids and heme [1]. The cytoplasmic and mitochondrial pools of TCA cycle intermediates are in tight connection, and accumulation of a metabolite in one of these pools is reflected in the other. The metabolites can freely diffuse through channels in the outer mitochondrial membrane and can be transported across the inner mitochondrial membrane through active carriers (e.g., the tricarboxylate carrier, the dicarboxylate carrier and several antiporters) [1].
The extraordinary complexity in terms of shunts, anaplerotic reactions (reactions that replenish the TCA cycle metabolite pools, such as generation of oxaloacetate from pyruvate) and the dual compartmentalization of reactions and metabolites suggests that the TCA cycle may operate in a cell-specific manner and respond to environmental and developmental cues within the same cell type, depending, for example, on the expression levels of the different enzyme isoforms and whether circumstances require anabolism or catabolism. The TCA cycle occurs in multiple flavors beyond the formulation by Hans Krebs; there are many different “TCA cycles” ongoing in the human body at any given moment.
In this review, the molecular mechanisms linking TCA cycle defects and tumor formation will be discussed. In particular, the dual-compartmentalization of the TCA cycle between mitochondria and the cytoplasm, how mutations in TCA cycle enzymes have implications for tumor promotion by activating the hypoxia response pathway, and the role of TCA cycle metabolites in the regulation of cellular differentiation will be reviewed in detail.
Genetic defects in the TCA cycle
Genetic defects affecting TCA cycle proteins have been known for more than twenty years. Until recently, only recessive defects were known, and their clinical outcomes were generically similar to defects in the respiratory chain and oxidative phosphorylation, consisting of severe multi-system disorders but no tumor predisposition [2]. Mutations in mtDNA cause defects in the respiratory chain and oxidative phosphorylation, but not in the TCA cycle. Several mtDNA mutations have been observed in association with both primary tumors and metastasis. However, it remains unclear if those mtDNA mutations were the primary cause of the tumors or if they conferred growth advantage to tumors already formed. Importantly, over 250 known pathological germline mtDNA mutations in patients with metabolic diseases do not appear to predispose to tumor formation. The only respiratory chain defect that is known to lead to the formation of tumors is MERRF (myoclonic epilepsy and ragged-red fibers); patients with MERRF have presented with lipomas [3]. In contrast to the germline mtDNA mutations, a vast literature supports the role of somatic mtDNA mutations in increasing the metastatic potential and aggressiveness of tumors [4], highlighting that defects in mitochondrial metabolism are likely lethal in early stages of development while developed tissues are able to cope and adapt.
Recently, dominant defects associated with tumor formation were described in three enzymes involved in the TCA cycle: isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH) and fumarate hydratase (FH) [5-7]. This review will focus on the mechanisms that link these defects with tumor formation.
Isocitrate Dehydrogenase
NADP+-IDH is monomeric in the cytoplasm and dimeric in mitochondria, while NAD+-IDH occurs in a complex of subunits of three different types, α, β and γ, in the stoichiometry 2α:1β:1γ [1]. Mutations in the NADP+-dependent IDH subunits IDH1 and IDH2 have been found in benign as well as advanced-stage astrocytomas and oligodendromas [7]. These mutations were also present in a small fraction of acute myeloid leukemia patients [8]. In contrast to the heritable tumor suppressor nature of the dominant SDH and FH defects, the IDH somatic mutations do not involve a loss of heterozygosity and behave like oncogenic gain-of-function mutations [9, 10]. Interestingly, identical germline mutations in IDH have been found in patients with d-2-hydroxyglutaric aciduria, for which no evidence of tumor formation has been reported [11]. This suggests that the IDH mutations confer a growth advantage not in the initial stages of tumorigenesis but later during tumor progression.
The neomorphic activity of mutant IDH1 and IDH2 converts α-ketoglutarate to 2-hydroxyglutarate, thus depleting α-ketoglutarate. The decrease in α-ketoglutarate depletes the rest of the cycle (succinate, fumarate, malate), regardless of whether the mutation is in the cytoplasmic or the mitochondrial enzyme [12]. The perturbation of TCA cycle function by mutant IDH1/2 also underlies major increases in amino acid levels, enabling anabolism [12]. Interestingly, recessive mutations in the β subunit of IDH3, the mitochondrial NAD+-dependent enzyme that functions in the traditional formulation of the TCA cycle, are loss-of-function mutations that but do not predispose to tumor formation (Table 1).
Succinate Dehydrogenase
The SDH complex is a heterotetramer of the SDHA, SDHB, SDHC and SDHD subunits. The recently discovered SDH5 is a factor required for the flavination of SDHA and assembly of the tetramer [13]. Dominant mutations in the SDH subunits SDHB, SDHC and SDHD predispose carriers to carotid body paragangliomas and adrenal gland pheochromocytomas [5, 14-16]. More recently, dominant defects in SDH5 were also found to predispose individuals to paragangliomas [13]. A germline SDHA mutation has also been found in a patient presenting with paraganglioma [17]. Finally, mutations in SDHB are associated with renal cell carcinoma and T-cell acute leukemia [18, 19], and mutations in SDHB, SDHC and SDHD are associated with gastrointestinal stromal tumors [20]. It is presently unclear why certain individuals with SDH mutations develop these secondary tumors.
The majority of SDHD defects are nonsense mutations, while in SDHB there is a similar proportion of missense and nonsense mutations [21]. Interestingly, SDH mutations often cause carotid body paraganglioma, which has increased incidence in high-altitude dwellers, suggesting that SDH mutations mimic the biological pathways triggered by environmental hypoxia. Although SDHB, SDHC and SDHD are physically interacting structural subunits of SDH, there are certain differences among the phenotypes arising from germ line subunit mutations. While germ line mutations in SDHB associate with extra-adrenal and malignant paragangliomas, those in SDHD cause mostly benign head and neck paragangliomas [21]. Notably, SDHD and SDH5 (SDHAF2) mutations show a puzzling pattern of predisposition: maternally-inherited mutations are at no risk of developing tumors, while the paternally-inherited ones carry an age-dependent risk [5, 13]. The mechanism underlying this parent-of-origin effect remains unclear, but probably involves epigenetic regulation (i.e. imprinting) that may be tissue-specific.
Fumarate Hydratase
Active FH is a homotetramer [1]. Recessive mutations in FH cause severe encephalopathies and early death, while dominant FH mutations predispose to tumor formation [6]. The recessive and dominant mutations are equally distributed along the FH gene [22]. FH behaves as a tumor suppressor, with the normal allele being lost in the tumors due to loss of heterozygosity [23]: the parents of FH recessive patients (defect in both alleles, either homozygous or compound heterozygous) have a dominant defect (heterozygous mutation; tumors develop when the normal FH allele is lost) and often present with tumors [22]. Dominant FH defects predispose to multiple cutaneous and uterine leiomyomas (MCUL), as well as to hereditary leiomyomatosis and renal cell cancer (HLRCC) [6]. While the skin and uterine leiomyomas are benign, the kidney tumors in HLRCC are particularly aggressive [24]. The morphologic spectrum of kidney tumors in HLRCC patients is broad, including papillary type II, tubulo-papilar, tubular, collecting duct and clear cell carcinoma [25-28]. Clinical presentations with minor penetrance in patients with dominant FH mutations include Leydig cell tumors, ovary cystadenomas, cerebral cavernomas, uterine leiomyosarcomas and breast cancer [29].
The majority of defects in FH are missense mutations, with next most frequent being frameshift and nonsense mutations [22]. Large scale FH deletions also occur [6].
The cytoplasmic and mitochondrial FH isoforms are encoded by the same gene [30]. Cytoplasmic FH exists in all tissues except the brain [30]. In cells from patients with FH mutations, mitochondrial FH is present at normal levels, albeit with markedly reduced activity, while no FH is detected in the cytoplasm [30]. FH mutant cells are thus null for cytoplasmic FH activity, implying that the tumor suppressor role is associated with the cytoplasmic FH isoform. Accordingly, overexpression of cytoplasmic FH in FH-/- MEFs rescued most of the fumarate accumulation [31]. Because fumarate is generated in the cytoplasm by several pathways, it is likely that the primary function of cytoplasmic FH is to maintain low fumarate levels in the cytoplasm by conversion to malate, thus providing increased substrate for the generation of cytoplasmic NADPH through the malic enzyme reaction. This suggests that cytoplasmic FH is important for antioxidant defense and anabolism. Along these lines, a role for cytoplasmic FH in the DNA damage response was reported [32], indicating that the absence of cytoplasmic FH would result in increased genomic instability.
The TCA cycle in control of the hypoxia response
Cells and tissues react to lower O2 availability by triggering a stress pathway designated as the hypoxia response. The term pseudo-hypoxia is often used to refer to the activation of this pathway under non-hypoxic conditions. The hypoxia response is a common feature of solid tumors and is characterized by increased glycolytic metabolism and promotion of angiogenesis [33]. The induction of the hypoxia pathway in tumors therefore underlies the so-called glycolytic shift, which is a typical feature of tumors [33]. This glycolytic shift was first observed in the 1920s by Otto Warburg, who noted that tumor cells rely on anaerobic ATP production through glycolysis, even in the presence of oxygen (i.e., aerobic glycolysis).
The major regulator of the hypoxia response is the transcription factor hypoxia inducible factor (HIF) [33], whose activity is regulated by TCA cycle metabolites. HIF is a heterodimer, with one α subunit and one β subunit. There are three known α subunits (HIF-1α, HIF-2α and HIF-3α) and two β subunits, ARNT1 (aryl hydrocarbon receptor nuclear translocator 1, often designated HIF-1β) and ARNT2. HIF-3α functions as a dominant negative regulator of HIF-1α and HIF-2α by binding to the β subunits but not to DNA [33]. While the β subunits are constitutively expressed, the α subunits are normally degraded in the presence of O2. This process involves the hydroxylation of proline residues in the α subunits, which is catalyzed by prolyl hydroxylases (PHD) (Figure 2). The PHDs are members of the superfamily of α-ketoglutarate-dependent dehydrogenases, which couple the hydroxylation of the substrates with the oxidation of α-ketoglutarate to succinate in reactions that are dependent on O2 and Fe2+ [33]. Importantly, the PHDs can be inhibited by several TCA cycle metabolites, as discussed below. The hydroxylated proline residues are recognized by an E3-ubiquitin ligase, pVHL (the product of the von Hippel Lindau gene), and targeted to the proteasome. When the levels of O2, Fe2+ or α-ketoglutarate are low, the PHD reaction stops or slows, allowing the α subunits to accumulate. These translocate to the nucleus, dimerize with the β subunits, and drive the hypoxia response transcriptional program (Box 2). In addition to the proline residues, asparagine residues of HIF α-subunits are also subject to hydroxylation, catalyzed by Factor Inhibiting HIF (FIH), inhibiting the binding of HIF α-subunits to transcriptional coactivators [33].
Figure 2. Role of TCA cycle metabolites in the regulation of HIF α-subunits in hypoxic, pseudo-hypoxic and non-hypoxic conditions.
The regulation of the three α-subunits (HIF-1α, HIF-2α and HIF-3α) by hydroxylation is similar, but for simplicity only HIF-1α is represented in this figure. The asparaginyl hydroxylase reaction, the phosphorylation-dependent nuclear import, and the coactivators and polymerase complex were omitted for simplicity. The HIF-1β subunit is expressed constitutively in the nucleus, and is available for dimerization with α subunits that reach the nucleus. Abbreviations: Ub, ubiquitin; HRE, hypoxia response element; TSS, transcription start site.
Box 2. The hypoxia response, the TCA cycle and tumor formation.
The relationships between hypoxia, the TCA cycle and tumor formation are complex. While the general notion is that tumors are inherently hypoxic and non-respiring, multiple evidence shows that only the core of solid tumors is hypoxic [33]. A hallmark of hypoxia is the induction of pyruvate dehydrogenase kinase (PDK), which inactivates pyruvate dehydrogenase (PDH) by phosphorylation [33]. PDH converts pyruvate to acetyl-CoA, and its inactivation results in an inability to feed pyruvate into the TCA cycle. While this results in lower TCA cycle flow, lower electron supply to the respiratory chain and therefore less aerobic ATP production, this effect can be counteracted by the hypoxia-driven increase in glycolysis [42]. However, tumors have heightened anabolic needs, to meet their growth rate, particularly building blocks used in the synthesis of proteins, lipids and nucleic acids. The hypoxia response addresses these anabolic needs of tumors by up-regulating the oncogenic transcription factor Myc, which induces the enzyme machinery allowing mitochondrial import of glutamine, its conversion to glutamate, and deamination of glutamate to α-ketoglutarate, thus replenishing the TCA cycle [68]. The α-ketoglutarate can be reduced to isocitrate, through isocitrate dehydrogenase, and isocitrate is then converted to citrate by aconitase. Citrate can be exported from mitochondria to cytoplasm, where it is cleaved by citrate lyase to generate oxaloacetate and acetyl-CoA, which is the source for lipid synthesis [42]. Hypoxia also induces the expression of transketolase, an enzyme of the pentose phosphates pathway, resulting in increased production of ribose-5-phosphate, the building block for nucleotide synthesis [69]. Therefore, the hypoxia response addresses the fundamental adaptive steps that the cells need to take in order to gain growth advantage.
Mutations in the TCA cycle cause perturbations of the metabolite pools which can result in pseudo-hypoxia. While recessive germline mutations causing loss-of-function of the enzymes lead to serious neuromuscular disorders and infantile death, dominant mutations in the same nucleotides with loss of heterozygosity predispose to tumor formation [30]. One possible explanation for this discrepancy is that tissues that are still developing, in particular the brain, are not able to cope with the perturbation in the TCA cycle in the long term and thus fail to perform and eventually degenerate. A fully developed tissue, however, may adapt to the TCA cycle defect, and that adaptation may result so “successful” that it results in overgrowth and tumor formation.
Because α-ketoglutarate and succinate are directly involved in the PHD reaction that stabilizes HIF α-subunits, the TCA cycle stands to have a major role in the regulation of the hypoxia response. While α-ketoglutarate is necessary for the reaction to occur, succinate, fumarate and oxaloacetate inhibit the prolyl hydroxylases, leading to pseudo-hypoxia in tumor and embryonic cells [31, 32, 34-37] but not in non-transformed cells [30, 36, 38]. Pyruvate, citrate, isocitrate, fumarate and succinate inhibit PHDs in tumor cells, with fumarate showing the strongest effect [36, 39-41]. Citrate inhibits FIH, while neither succinate nor fumarate are FIH inhibitors [31, 36]. The decrease in α-ketoglutarate levels resulting from the neomorphic activities of mutated IDH1 and IDH2 also has the potential to affect the PHD reaction and thus the accumulation of HIF α subunits. While these observations clearly place the TCA cycle upstream of the hypoxia response, it is worth highlighting that one of the consequences of the hypoxia/pseudo-hypoxia response is a decreased supply of pyruvate flow to the TCA cycle and an increase in glutamine catabolism that feeds the α-ketoglutarate pool [42]. The TCA cycle and the hypoxia response therefore form a complex regulatory network in which each of the pathways reciprocally affects the other (see Box 2).
While it is clear that the pseudo-hypoxia response confers a growth advantage to the cells with IDH, FH and SDH defects, it remains to be determined if it is sufficient to initiate a tumor, or if the pseudo-hypoxia response confers an advantage in a later stage of tumor progression. The following section will address this question.
Mechanisms beyond hypoxia
The molecular mechanisms linking TCA cycle defects to tumor formation remain elusive. The pseudo-hypoxia response has been a major focus of research in the field, and several lines of evidence have accumulated supporting a role for the hypoxia response in tumors of patients with FH or SDH defects [21]. The changed levels of α-ketoglutarate, succinate and fumarate in cells with IDH, SDH or FH mutations support the idea of perturbed PHD function. Therefore, defects in IDH, SDH and FH seem to decrease hydroxylation of HIF α-subunits, thus triggering the pseudo-hypoxia response. However, the PHDs are part of a superfamily of proteins, α-ketoglutarate-dependent dioxygenases, converting α-ketoglutarate to succinate, while hydroxylating a wide range of substrates much broader than the HIF α-subunits [43]. Furthermore, while the clinical presentations for SDH dominant defects are similar to other hypoxic syndromes, such is not the case for the IDH and FH phenotypes. Hypoxia has been presented as the driving mechanism of the formation of the tumors in TCA cycle defects, but it is fundamental to distinguish if hypoxia is indeed the initiator mechanism or of it is instead a growth-promotion mechanism that confers advantage to these tumors.
Uterine myomata are benign uterine smooth muscle tumors, and constitute the major clinical presentation in patients with dominant FH mutations [6]. These myomata accumulate fumarate as well as succinate [44]. The uterine myomata in patients with FH mutations have increased HIF-1α protein levels, HIF-target expression, and vascular density [44, 45]. Myomata from HLRCC patients were shown by transcriptional profiling to have increased glycolytic potential compared to sporadic myomas, albeit no evidence for increased hypoxia response under the same conditions [46]. Both fumarate and succinate inhibit the PHDs, particularly PHD2, and trigger the pseudo-hypoxia response in tumor cells in vitro [35-37, 39]. Silencing of FH in cultured tumor cells also lead to increased glycolytic metabolism and accumulation of HIF α-subunits [35]. However, no accumulation of HIF-1α was detected in primary fibroblasts with recessive FH defects and fumarate accumulation [30, 38]. It seems thus that intracellular fumarate accumulation triggers the pseudo-hypoxia response in tumor or embryonic cells, but not in primary fibroblasts. Mice with a kidney-specific FH knock-out have renal cysts which display hypoxia response [47]. It should however be noted that the kidney FH knock-out is not a model for FH defects arising in normal adult kidney, but for FH defects occurring in the kidney since the embryonic stage until adulthood [48]. In patients, the kidney tumors arise in normal kidney having one mutated FH allele, upon loss of the normal allele, but the conditional knock-out mouse lost both FH alleles in embryonic kidney [6]. Therefore, the kidney-specific FH knock-out is not a good model to study the role of FH in kidney tumors. Furthermore, the penetrance of FH mutations in kidney tumors is very low, suggesting that other factors may be involved [49].
The paradigm for the hypoxia pathway is the von Hippel-Lindau (VHL) disease, a dominant familial cancer syndrome caused by mutations in the VHL gene [33]. pVHL targets the HIF α-subunits to the proteasome, resulting in accumulation of HIF-1α and HIF-2α in VHL disease [33]. The clinical presentations of the VHL syndrome are clear cell renal carcinoma, pheochromocytoma, pancreatic tumors and hemangioblastomas [50]. There is no overlap between the clinical presentations of VHL disease and of IDH mutations. VHL and SDH syndromes both have high penetrance in pheochromocytomas. The only phenotype shared in VHL syndrome and dominantly inherited FH defects is the kidney cancer, but while VHL leads to clear cell renal carcinoma, FH- leads to papillary type II, collecting duct morphology, or mixed [24, 25, 28].
The absence of overlap between the clinical presentations for VHL and IDH or FH suggests that the pseudo-hypoxia response is not an initiator event, but rather a growth-promoting mechanism. In the case of SDH, which leads to phenotypes similar to VHL disease and to clinical presentations of patients living in chronic hypoxia due to high altitude, it remains possible that the pseudo-hypoxia response may contribute to the growth of the tumors from an earlier stage. The events that initiate the tumors in IDH and FH mutations remain unclear, and are discussed in the next section.
Regulation of cellular differentiation by TCA cycle metabolites
A comparison of the expression profiles of FH defects in diploid primary fibroblasts and in uterine myomata revealed that the transcriptional activity of Serum Response Factor (SRF), a major regulator of smooth muscle differentiation, was down-regulated in both samples [51]. This implies a loss of SRF signaling before the tumors form, raising the possibility that SRF may have a role in the initial events of tumor formation. SRF signaling is essential for smooth muscle differentiation [52]. The homeostasis of smooth muscle is maintained by progenitor cells, which have stem cell-like characteristics, and SRF triggers the differentiation of these cells to terminally differentiated myocytes [52]. A down-regulation of SRF signaling in FH deficiency would in principle result in inhibition of smooth muscle progenitor cell differentiation, and consequently in the accumulation of cells with stem cell-like behavior. This scenario could explain the formation of myomata, benign smooth muscle tumors which are the major clinical presentation in patients with dominant FH defects. The proliferation, differentiation and death of stem cell-like populations are regulated by the redox environment [53]. The redox environment in primary cells with FH mutations is highly reduced, a condition which favors proliferation of stem cell-like populations, while inhibiting their differentiation or death [30]. Therefore, SRF down-regulation and a reduced redox environment would contribute to the same effect: accumulation of smooth muscle progenitor cells culminating in the formation of myomata. Interestingly, SRF signaling is also down-regulated in sporadic myomata, without FH mutations, implying that perturbation of SRF function is necessary for the formation of myomata [51]. While it is tempting to speculate that FH defects “hijack” this myomata-causing perturbation of SRF signaling, the question of how mutations in FH result in the down-regulation of SRF signaling remains open. Given that SRF is regulated by phosphorylation [52], it is likely that some of the signaling cascades upstream of SRF are affected by fumarate intracellular accumulation. Fumarate can modulate the activity of several enzymes (e.g., dopamine β-mono-oxygenase, malic enzyme, prolyl hydroxylases) [33, 54]. In addition, fumarate can react with exposed protein thiols and lysines, forming adducts, a process designated succinylation (because the adduct formed resembles succinate) [55]. Succinylation is likely to affect the activity of the modified proteins, analogous to other post-translational modifications such as phosphorylation and acetylation. Interestingly, acetylation of cytoplasmic proteins is regulated by acetyl-CoA generated by the cleavage of citrate in cytoplasm [56]. Perturbations of the TCA cycle metabolite pools caused by IDH, SDH and FH mutations affect the cytoplasmic level of acetyl-CoA [12] and may therefore regulate by acetylation the activity of key proteins involved in tumorigenesis.
The effect of fumarate in cellular differentiation described above may represent a novel paradigm for the extra-metabolic roles of TCA cycle metabolites. Another example of a metabolite regulating cellular differentiation is 2-hydroxyglutarate, generated from α-ketoglutarate by the neomorphic activities of mutated IDH1 and IDH2. The metabolite 2-hydroxyglutarate is structurally similar to α-ketoglutarate, and inhibits α-ketoglutarate-dependent enzymes, particularly telomerase and histone demethylases [57-59]. Interestingly, the effect of 2-hydroxyglutarate on telomerase prevents differentiation of stem cells, elucidating the leukemogenic effect of IDH mutations [57]. Furthermore, a reductive shift in the redox environment is associated with IDH defects, similar to what is observed in FH defects and consistent with undifferentiated stem cells [12, 30].
Therefore, we propose a model in which mutations in FH and IDH cause perturbations in the cytoplasmic TCA cycle metabolite pools resulting in the inhibition of proteins (e.g., SRF, telomerase) necessary for cellular differentiation, leading to the accumulation of undifferentiated cells. The activation of the pseudo-hypoxia response at a later stage likely provides these cells with necessary adaptations to proliferate efficiently under varying conditions, explaining also the association of this response with TCA-related mutations that cause cancer.
Concluding remarks
The direct involvement of TCA cycle enzymes and metabolites in tumor formation brought a pathway long resigned to dusty biochemistry text books back to the limelight. The extra-metabolic functions of the TCA cycle metabolites are now a major focus of research. While some issues have been resolved, many questions remain. First and foremost, how the “TCA cycles” operate in different cells, different tissues, and even under different conditions in the same cell (Box 3). The heroic efforts of the chemists and biochemists that originally defined this key metabolic pathway, relying on a vast and solid knowledge of chemistry, can now be joined by researchers with contemporary genomic and metabolomic approaches to bring about a systematic understanding of TCA cycle metabolism and the vast implications this holds for biology and medicine.
Box 3. Outstanding questions.
The role of TCA cycle metabolites in tumor formation is only beginning to be unraveled. A fundamental question that remains unanswered is the tissue-specificity of the phenotypes. Are certain tissues affected because their “normal” TCA cycle is less able to buffer fluctuations in specific metabolites? The answer is likely to bring us a new level of understanding of how the TCA cycle integrates with other pathways in different tissues and different conditions. The current state of metabolomic methodologies makes accessible the study of the “TCA cycles” occurring in the human body, both physiological and pathological.
What are the extra-metabolic roles for the TCA cycle metabolites? While some of these roles have been identified, it is certainly possible that the TCA cycle metabolites have the ability to modulate the activity of signaling cascades or transcription factors, thus effecting profound regulatory effects on several cellular decisions.
How are α-ketoglutarate-dependent dioxygenases inhibited by TCA cycle metabolites? While the prolyl hydroxylases and telomerase are already known to be inhibited by TCA cycle metabolites, it is likely that other proteins of the same family also have this regulatory potential. Identification of the tissue distribution and functions of proteins in that family would certainly contribute to the understanding of the role of TCA cycle metabolites in tumor formation.
Acknowledgements
This work was supported by NIH grants CA112364 and ES011163 to Gerald S. Shadel.
References
- 1.Scheffler IE. Mitochondria. 2nd ed John Wiley & Sons, Inc.; Hoboken, New Jersey , U.S.A.: 2008. [Google Scholar]
- 2.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holme E, et al. Multiple symmetric lipomas with high levels of mtDNA with the tRNA(Lys) A-->G(8344) mutation as the only manifestation of disease in a carrier of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet. 1993;52(3):551–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa K, Hayashi J. A novel function of mtDNA: its involvement in metastasis. Ann N Y Acad Sci. 2010;1201:40–3. doi: 10.1111/j.1749-6632.2010.05616.x. [DOI] [PubMed] [Google Scholar]
- 5.Baysal BE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287(5454):848–51. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson IP, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16(9):387–97. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–34. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranendijk M, et al. IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science. 2010;330(6002):336. doi: 10.1126/science.1192632. [DOI] [PubMed] [Google Scholar]
- 12.Reitman ZJ, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A. 2011;108(8):3270–5. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao HX, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325(5944):1139–42. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26(3):268–70. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 15.Astuti D, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69(1):49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baysal BE, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39(3):178–83. doi: 10.1136/jmg.39.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnichon N, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19(15):3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanharanta S, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74(1):153–9. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baysal BE. A recurrent stop-codon mutation in succinate dehydrogenase subunit B gene in normal peripheral blood and childhood T-cell acute leukemia. PLoS ONE. 2007;2(5):e436. doi: 10.1371/journal.pone.0000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med. 2009;266(1):43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5(11):857–66. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 22.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9(1):20. doi: 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam NA, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12(11):1241–52. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 24.Refae MA, et al. Hereditary leiomyomatosis and renal cell cancer: an unusual and aggressive form of hereditary renal carcinoma. Nat Clin Pract Oncol. 2007;4(4):256–61. doi: 10.1038/ncponc0773. [DOI] [PubMed] [Google Scholar]
- 25.Merino MJ, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31(10):1578–85. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 26.Lehtonen HJ, et al. Conventional renal cancer in a patient with fumarate hydratase mutation. Hum Pathol. 2007;38(5):793–6. doi: 10.1016/j.humpath.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Toro JR, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73(1):95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiuru M, Launonen V. Hereditary leiomyomatosis and renal cell cancer (HLRCC) Curr Mol Med. 2004;4(8):869–75. doi: 10.2174/1566524043359638. [DOI] [PubMed] [Google Scholar]
- 29.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011 doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 30.Raimundo N, et al. Differential metabolic consequences of fumarate hydratase and respiratory chain defects. Biochim Biophys Acta. 2008;1782(5):287–94. doi: 10.1016/j.bbadis.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 31.O’Flaherty L, et al. Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yogev O, et al. Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol. 2010;8(3):e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalgard CL, et al. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J. 2004;380(Pt 2):419–24. doi: 10.1042/BJ20031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacs JS, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Koivunen P, et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282(7):4524–32. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 37.Selak MA, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Ottolenghi C, et al. Clinical and biochemical heterogeneity associated with fumarase deficiency. Hum Mutat. 2011 doi: 10.1002/humu.21534. [DOI] [PubMed] [Google Scholar]
- 39.Hewitson KS, et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007;282(5):3293–301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 40.Lu H, et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51):41928–39. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 41.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 42.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozer A, Bruick RK. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat Chem Biol. 2007;3(3):144–53. doi: 10.1038/nchembio863. [DOI] [PubMed] [Google Scholar]
- 44.Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 45.Pollard P, et al. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2005;205(1):41–9. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 46.Vanharanta S, et al. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol Genet. 2006;15(1):97–103. doi: 10.1093/hmg/ddi431. [DOI] [PubMed] [Google Scholar]
- 47.Pollard PJ, et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell. 2007;11(4):311–9. doi: 10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Igarashi P, et al. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol. 1999;277(4 Pt 2):F599–610. doi: 10.1152/ajprenal.1999.277.4.F599. [DOI] [PubMed] [Google Scholar]
- 49.Lehtonen R, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164(1):17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13(2 Pt 2):680s–684s. doi: 10.1158/1078-0432.CCR-06-1865. [DOI] [PubMed] [Google Scholar]
- 51.Raimundo N, et al. Downregulation of SRF-FOS-JUNB pathway in fumarate hydratase deficiency and in uterine leiomyomas. Oncogene. 2009;28(9):1261–73. doi: 10.1038/onc.2008.472. [DOI] [PubMed] [Google Scholar]
- 52.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011;12(6):349–61. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 53.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh JY, et al. Long-range interaction between the enzyme active site and a distant allosteric site in the human mitochondrial NAD(P)+-dependent malic enzyme. Arch Biochem Biophys. 2009;487(1):19–27. doi: 10.1016/j.abb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, et al. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu W, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowdhury R, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12(5):463–9. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartong DT, et al. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat Genet. 2008;40(10):1230–4. doi: 10.1038/ng.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Odievre MH, et al. A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of alpha-ketoglutarate dehydrogenase deficiency. Hum Mutat. 2005;25(3):323–4. doi: 10.1002/humu.9319. [DOI] [PubMed] [Google Scholar]
- 62.Rouzier C, et al. The severity of phenotype linked to SUCLG1 mutations could be correlated with residual amount of SUCLG1 protein. J Med Genet. 2010;47(10):670–6. doi: 10.1136/jmg.2009.073445. [DOI] [PubMed] [Google Scholar]
- 63.Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10(5):289–91. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- 64.Pantaleo MA, et al. SDHA Loss-of-Function Mutations in KIT-PDGFRA Wild-Type Gastrointestinal Stromal Tumors Identified by Massively Parallel Sequencing. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- 65.Fukao T, et al. Clinical and molecular characterization of five patients with succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. Biochim Biophys Acta. 2011;1812(5):619–24. doi: 10.1016/j.bbadis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Bourgeron T, et al. Mutation of the fumarase gene in two siblings with progressive encephalopathy and fumarase deficiency. J Clin Invest. 1994;93(6):2514–8. doi: 10.1172/JCI117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg DA, et al. Malic enzyme 2 may underlie susceptibility to adolescent-onset idiopathic generalized epilepsy. Am J Hum Genet. 2005;76(1):139–46. doi: 10.1086/426735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao F, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene. 2010;29(20):2962–72. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]