Abstract
Background
Low socioeconomic status (SES) is a strong predictor of many health problems, including asthma impairment; however, little is understood about why some individuals defy this trend by exhibiting good asthma control despite living in adverse environments.
Objective
This study sought to test whether a psychological characteristic – “shift-and-persist” (dealing with stressors by reframing them more positively, while at the same time, persisting in optimistic thoughts about the future) - protects low SES children with asthma.
Methods
121 children physician-diagnosed with asthma, ages 9-18, were recruited from medical practices and community advertisements (M age=12.6, 67% male, 61% Caucasian). Shift-and-persist and asthma inflammation (eosinophil counts, stimulated IL-4 cytokine production) were assessed at baseline, and asthma impairment (daily diary measures of rescue inhaler use and school absences), and daily peak flow were monitored at baseline and at a 6-month follow-up.
Results
Children who came from low SES backgrounds but who engaged in shift-and-persist strategies displayed less asthma inflammation at baseline (β=.19, p<.05), as well as less asthma impairment (reduced rescue inhaler use and fewer school absences; β=.32, p<.01) prospectively at a 6 month follow-up period. In contrast, shift-and-persist strategies were not beneficial among high SES children with asthma.
Conclusion
An approach that focuses on the psychological qualities that low SES children develop to adapt to stressors may represent one practical and effective starting point for reducing health disparities. Moreover, the approaches that are effective in low SES communities may be different from those that are optimal in a high SES context.
Keywords: socioeconomic status, asthma, children, psychological, stress
INTRODUCTION
Among social factors, low socioeconomic status (SES) exhibits one of the strongest and most consistent associations with morbidity and mortality across a wide range of diseases 1, 2, including childhood asthma. For example, low SES children with asthma are significantly more likely to be hospitalized or visit an emergency department for asthma. Compared to higher-SES children with asthma, they also have more symptoms and more severe exacerbations 3-9.
However, these observations have often left unanswered one important question: Why do some individuals not get sick despite facing persistent and severe adversity? While there are certainly numerous environmental and behavioral factors that explain why low SES is detrimental to health, including heightened exposure to neighborhood pollutants, a greater likelihood of engaging in risky behaviors such as smoking, and the experience of negative psychological states such as depression 10-12, these factors cannot explain why some individuals thrive, despite confronting adverse circumstances such as poverty. This type of thriving has been labeled by researchers as resilience 13, 14.
Contributors to resilience among children facing adversity have been discussed extensively within the mental health literature 15-17. However, rarely has the notion of psychological resilience been explored with respect to physical health outcomes. Moreover, the small existing literature with respect to physical health outcomes has typically relied on either self-report measures of health or nonspecific physiological indicators 18-21.
Stress is known to be one of the primary psychological pathways linking low SES to poor health 22, 23. Hence our group has hypothesized that to the extent that low SES individuals develop specific strategies that allow them to adapt well to the types of stressors that accompany low SES life, they may show reduced physiological responses to stressors, and over the long-term, lower risk for a number of diseases.
Specifically, we postulated that low SES children who are resilient will use a “shift-and-persist” approach to dealing with the stressors of low SES life 24. This approach entails both shifting (adjusting oneself to stressors through trying to find the positive in them) and persisting (staying optimistic about the future and pursuing future goals). In the context of some of the uncontrollable stressors that many low SES families face, this approach is hypothesized to be more adaptive than actively trying to confront stressors. As a result, this model hypothesizes that this subgroup of low SES children will be less likely to display negative psychological responses, and in turn reduced physiological activation (of the hypothalamic-pituitary-adrenal axis and of the sympathetic nervous system) acutely in response to stressors. Over time, with reduced exposure to the stress hormones secreted by these systems, pathogenic processes such as inflammation are diminished, ultimately reducing risk for diseases involving those processes 25-27.
We tested the idea that a shift-and-persist approach to dealing with stress could protect low SES children from detrimental asthma outcomes. We recruited a sample of children with asthma across the SES spectrum, and measured pulmonary and immune outcomes, as well as asthma impairment, with daily monitoring of pulmonary and impairment outcomes repeated 6 months later. We hypothesized that to the extent they used shift-and-persist strategies, low SES children would be buffered from poor asthma outcomes over time, relative to low SES children who did not make use of these strategies. As a result, low SES children who used shift-and-persist would have asthma outcomes similar to their high SES counterparts.
METHOD
Participants
121 youth physician-diagnosed with asthma (83% with allergic asthma) were recruited from Vancouver, B.C from asthma clinics, newspaper advertisements, and school and community center postings. Youth ranged in age from 9-18, were fluent in English, free of acute respiratory illness at the time of their visit, had not had a prednisone course for at least 2 weeks, and had no chronic illnesses other than asthma.
Participants came for a laboratory visit and then completed home daily diaries (see below for a description of the questions) and peak flow monitoring for two weeks (time 1). Six months later, participants repeated daily diary and peak flow monitoring (time 2). The protocol was approved by the University of British Columbia Research Ethics Board, and informed consent was obtained from parents and assent from children.
Measures
SES
Socioeconomic resources were measured by asking parents about the amount of assets that their family could easily convert to liquid cash in an emergency (family savings). This measure is recommended by the MacArthur Research Network on Socioeconomic Status and Health (www.macses.ucsf.edu), and widely used in SES research 28. Resource-based measures of SES like this have more robust associations with health-related outcomes in childhood than prestige-based measures (e.g., education) 29, 30.
Measure of shift-and-persist
The tendency to shift oneself in response to stressors was measured using the Cognitive Restructuring scale of the Responses to Stress questionnaire 31. Three items (e.g., “I thought about the things I was learning from the situation, or about something good that would come from it”) were queried on a 4 point scale (ranging from “not at all” to “a lot”). These items tap the extent to which individuals try to deal with stressful situations by thinking about them in more positive ways. Items were coded such that higher scores indicated a higher tendency to positively reappraise stressful situations. This measure has been validated in children, and linked to mental health outcomes such as depression 31, 32.
As an indicator of future persistence, a measure of positive thinking about the future was included. The Life Orientation Test taps the extent to which individuals have positive expectations for their future (e.g., “I always feel good about my future”) 33. This measure consists of six items rated on a 3 point scale. Items were coded such that higher scores indicated higher optimism. This measure has been used in children 34, and has established links with disease outcomes in adults 35, 36.
To create a total shift-and-persist score, responses to the shift and persist measures were first standardized (because they are on different scales), and then summed. Thus higher scores indicate using a higher combination of both shift and persist strategies.
Asthma measures
(1) Inflammation
At baseline, a complete blood count with five-part differential (Bayer ADVIA 70 hematology system, Holiston, Massachusetts) was performed to obtain eosinophil counts. In addition, white blood cells’ secretion of the Th-2 cytokine IL-4 in response to mitogen stimulation was measured. At baseline peripheral blood was drawn into BD Vacutainer Cell Preparation Tubes containing Sodium Heparin, and 3 × 106 peripheral blood mononuclear cells (PBMC’s) were isolated through density-gradient centrifugation. PBMC’s were resuspended in culture medium consisting of RPMI plus 10% fetal calf serum, and incubated with phorbol myristate acetate (PMA 25ng/ml) and ionomycin (INO 1 ug/ml) for a period of 48 hours at 37°C in 5% CO2. This PMA/INO combination has been successful in stimulating asthma-relevant cytokines in other studies 37, 38. Supernatants were frozen until the end of the study, and then assayed to determine levels of IL-4 using enzyme-linked immunosorbent assays (ELISA). Intra-assay CVs ranged from 3.68 – 4.76%. To create a composite measure, eosinophil and IL-4 values were first standardized (z scores were calculated, given the different ranges for these two measures), and the two scores were then summed.
(2) Pulmonary function
At baseline and 6 months later, children monitored peak expiratory flow (PEF) at home using an electronic monitor (Quadromed, Hoechberg, Germany). 3 PEF readings were taken upon awakening and again at bedtime each day for two weeks, and the highest value at each time point was used. Daily PEF variability was calculated, (|morning PEF − evening PEF|/average of morning + evening PEF)*100, and averaged across the two week monitoring period.
(3) Impairment
At baseline and 6 months later, children recorded daily rescue inhaler use (use of a short-acting beta agonist for symptom relief during the day) and school absences (missing school because of asthma) at the end of every day for two weeks. A composite measure was created reflecting the percentage of days inhaler use or absences were reported. 95% of children completed daily diaries and peak flow measures at baseline, and 86% completed them at the 6 month follow-up.
Potential confounders
Variables included as covariates in statistical analyses included asthma severity, determined from the NAEPP/EPR2 Guidelines based on the higher of symptom frequency and medication use, paralleling the approach of previous researchers 39. Children also brought in their asthma medications to the research center, and inhaled corticosteroid use was coded (yes/no in the past two weeks, as well as the number of times used in the past two weeks), as was beta agonist use (yes/no in the past two weeks). In addition, we included the demographic characteristics of child age, gender, and ethnicity as covariates in statistical analyses.
Statistical analyses
Descriptive information about the sample is provided in Table 1. Multiple regression analyses were conducted in which asthma variables were predicted from (1) time 1 asthma variable (for follow-up analyses) + covariates described above; (2) main effect of SES (family savings) and main effect of shift-and-persist; and (3) the interaction between SES and shift-and-persist. For inflammation, measures at baseline formed the dependent variable. For asthma impairment and pulmonary function, measures at the 6-month follow-up formed the dependent variables. These prospective analyses allowed us to test directionality – that is, the notion that shift-and-persist at baseline would forecast asthma impairment or pulmonary function at a future time point (controlling for baseline values). Including the interaction term allowed us to test the hypothesis that shift-and-persist strategies would benefit low SES children differently from high SES children. Tests of interactions were conducted according to the recommendations of Aiken and West 40, whereby variables are first centered, and then the interaction is calculated as the product of the two centered variables.
Table 1.
Descriptive sample information (N=121)
| % | M | SD | |
|---|---|---|---|
| Age | 12.61 | 2.63 | |
| Gender (% male) | 67 | ||
| Ethnicity | |||
| Caucasian | 61 | ||
| Asian | 26 | ||
| Other | 13 | ||
| Family savings | 4.76 | 2.50 | |
| Asthma control | |||
| Well-controlled | 45 | ||
| Not well-controlled | 41 | ||
| Very poorly controlled | 14 | ||
| Currently taking ICS | 70 | ||
| Shift strategies | 7.14 | 2.26 | |
| Persist strategies | 13.90 | 2.08 | |
| Eosinophils (×109 cells/L, time 1) | 0.37 | 0.28 | |
| IL-4 production (pg/ml, time 1) | 9.28 | 10.34 | |
| Peak flow variability (%, time 1) | 10.84 | 8.98 | |
| Peak flow variability (%, time 2) | 9.22 | 6.04 | |
| School absences (time 1) | 0.63 | 3.61 | |
| School absences (time 2) | 0.58 | 3.00 | |
| Rescue inhaler use (time 1) | 17.14 | 28.27 | |
| Rescue inhaler use (time 2) | 8.93 | 18.26 |
Note: Family savings is on a 1-9 scale. Asthma control was determined based on impairment and risk scores, according to the NHLBI NAEPP/EPR-3 Guidelines 46. ICS = inhaled corticosteroids. Shift strategy scores ranged from 3-12. Persist strategy scores ranged from 3-18. School absences and rescue inhaler use refer to the percentage of days these were endorsed during the 2 week monitoring period.
RESULTS
Preliminary analyses
At baseline lower SES was associated with greater asthma-related inflammation (r=−.26, p=.007), as well as greater asthma impairment (r=−.34, p=.001), although not with peak flow variability (p=.39) or inhaled corticosteroid use (p=.85). There were no significant differences by SES (p=.64), ethnicity (p=.73) or sex (p=.09) in use of shift-and-persist strategies; however, older children were more likely to engage in shift-and-persist strategies (r=.18, p=.049).
SES, shift-and-persist, and inflammation
Table 2 presents regression coefficients for SES and shift-and-persist variables for all asthma measures. After controlling for demographic variables, asthma severity, and asthma medication use, there was a significant main effect of SES (p<.001), such that lower SES was associated with greater asthma-related inflammation. There was no main effect of shift-and-persist (p=.40). There was, however, a significant interaction of SES with shift-and-persist (p=.047). Results remained the same if number of days of ICS use was included as a covariate instead of yes/no usage (interaction p=.047).
Table 2.
Multiple regression analyses of socioeconomic status and shift-and-persist strategies predicting asthma measures
| Outcome | β | p |
|---|---|---|
| Asthma inflammation composite (baseline) | ||
| SES | −.355 | <.001 |
| Shift-and-persist | −.083 | .403 |
| SES × shift-and-persist | .187 | .047 |
| Asthma impairment (at 6 month follow up) | ||
| SES | −.273 | .006 |
| Shift-and-persist | −.160 | .109 |
| SES × shift-and-persist | .315 | .001 |
| Peak flow variability (at 6 month follow up) | ||
| SES | −.014 | .899 |
| Shift-and-persist | −.017 | .881 |
| SES × shift-and-persist | .186 | .084 |
Note: All analyses control for child age, gender, ethnicity, asthma severity, inhaled corticosteroid use, beta agonist use. In addition, analyses that predicted outcomes at 6 month follow up controlled for baseline values. The asthma inflammation composite is a standardized score reflecting eosinophil counts and stimulated IL-4 production. The asthma impairment measure is a composite reflecting the percentage of days of school absences and rescue inhaler use during the 2 week daily diary assessment. Peak flow variability was calculated based on 2 weeks of morning and evening peak flow readings.
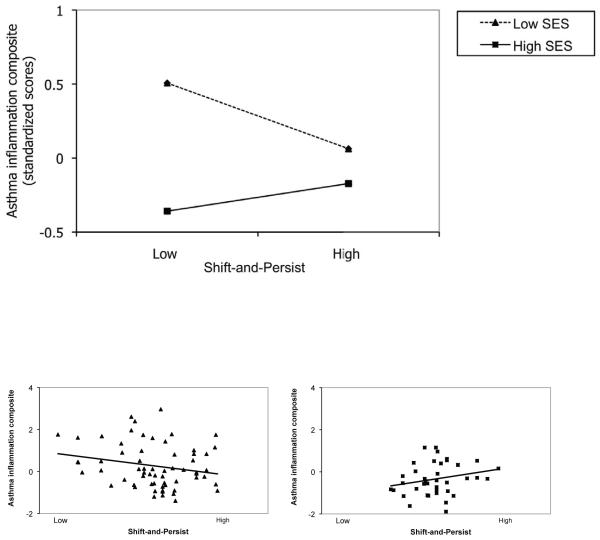
Figure 1 depicts this interaction graphically. Note that although SES was modeled as a continuous variable in regression analyses, the nature of an interaction between two continuous variables can be difficult to visualize, and hence significant interactions were plotted by graphing the relationship between shift-and-persist and asthma outcomes at low (−1 SD) and high (+1 SD) levels of SES. This creates an artificial distinction within one of the continuous variables, but allows one to more easily see how the relationship between shift-and-persist and asthma outcomes varies at different levels of SES. Thus the top graph in Figure 1 depicts estimated regression lines when asthma inflammation is regressed onto shift-and-persist scores (controlling for covariates) at 2 different levels of SES. As can be seen in the figure, children low in SES who were also low in shift-and-persist displayed the greatest scores on the inflammation composite. As shift-and-persist scores increased, low SES children with asthma displayed inflammatory profiles more similar to high SES children with asthma.
Figure 1.
Top figure: Interaction of socioeconomic status (SES: family savings) by shift-and-persist strategies predicting asthma inflammation composite at baseline. The inflammation composite reflects eosinophil counts and stimulated IL-4 production. Estimated values are plotted at ±1 SD of SES. Bottom panels: individual data points for low and high SES patients by median split. To obtain adjusted values for each participant, asthma inflammation was regressed onto covariates, and residualized scores were plotted by shift-and-persist values.
SES, shift-and-persist, and asthma impairment
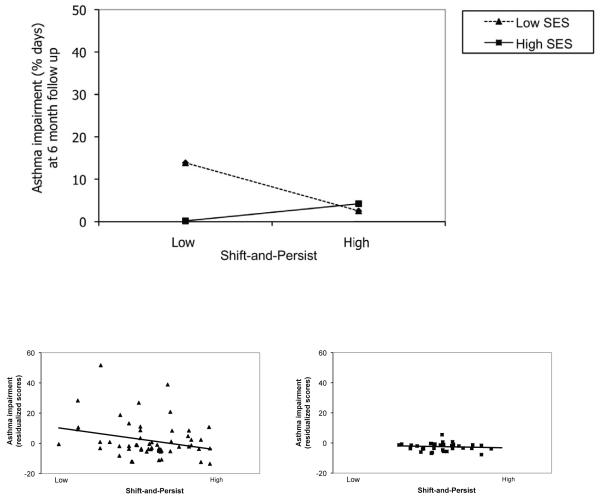
With respect to functional impairment, there was a main effect of SES (p=.006), such that lower SES at baseline was associated prospectively with greater asthma impairment at Time 2 (greater rescue inhaler use & school absences). There was no main effect of shift-and-persist (p=.11). There was, however, a significant interaction of SES with shift-and-persist (p=.001). This pattern of results remained the same if number of days of ICS use was included as a covariate instead of yes/no usage (interaction p=.001). As depicted in Figure 2, children low in SES who were also low in shift-and-persist had the greatest asthma impairment at time 2 (adjusting for time 1 values plus other covariates). As shift-and-persist scores increased, low SES children showed less asthma impairment at Time 2, and began to resemble high SES children with asthma.
Figure 2.
Top figure: Interaction of socioeconomic status (SES: family savings) by shift-and-persist strategies predicting asthma impairment at 6 month follow-up, controlling for time 1 values. Asthma impairment reflects school absences and rescue inhaler use. Estimated asthma impairment values are plotted at ±1 SD of SES. Bottom panels: individual data points for low and high SES patients by median split. To obtain adjusted values for each participant, asthma impairment was regressed onto covariates, and residualized scores were plotted by shift-and-persist values. Because these are standardized residuals, values can be less than 0.
SES, shift-and-persist, and pulmonary function
With respect to daily peak flow, there were no main effects of either SES (p=.90) or shift-and-persist (p=.88). There was, however, a marginal interaction of SES with shift-and-persist (p=.084). This interaction was such that as SES declined, greater shift-and-persist scores at baseline were associated with less peak flow variability at time 2.
Inflammation links to asthma over time
Given that similar patterns emerged for inflammation measures at baseline and asthma impairment prospectively, we tested the connection between these findings by examining whether our measures of inflammation at baseline predicted asthma impairment or pulmonary function at Time 2. After controlling for baseline impairment and other covariates described above, greater inflammation at baseline predicted greater asthma impairment at the 6 month follow up (β = .47, p < .001). No significant associations were found for pulmonary function.
Given these links, we tested whether shift-and-persist’s prospective association with asthma impairment was due to its concurrent relationship with inflammation. In further analyses where baseline inflammation was controlled, the interaction between SES and shift-and-persist continued to forecast asthma impairment at time 2 (β = .26, p=.009). These findings indicate that shift-and-persist was protective to low SES children above and beyond their baseline inflammation levels.
Discussion
Our results demonstrated that among low SES children, those who engaged in a specific psychological strategy for dealing with daily life stress, labeled shift-and-persist, showed better asthma profiles prospectively. That is, lower SES children with asthma who worked to reinterpret stressors in a more positive light (shifting) while remaining optimistic about their futures (persisting) had less asthma inflammation at baseline, as well as less asthma impairment (less rescue inhaler use and school absences) at a 6 month follow-up assessment. In fact, low SES children with asthma with high levels of shift-and-persist resembled high SES children with asthma on a number of these dimensions. This study is the first that we are aware of to demonstrate the health-protective effects – both biologically and clinically – of a psychological characteristic for coping with stress that uniquely protects low SES children confronting a chronic disease.
There were no main effects of shift-and-persist, only an interaction with SES, indicating that shift-and-persist strategies are not uniformly beneficial, but rather specifically helpful to those who come from low SES backgrounds. Because low SES individuals on average live under circumstances consisting of more frequent stressors that are more uncontrollable 41, an approach that emphasizes shifting oneself (reframing stressors more positively) may be beneficial for slowing down the pathophysiologic processes that contribute to diseases such as asthma. In addition, maintaining optimism about the future may provide meaning in life, and foster striving towards long-term goals, processes which in turn mitigate asthma-relevant physiologic processes over time. Thus there may be psychological qualities that are uniquely beneficial to low SES children’s asthma, and that are different from those that are beneficial to high SES children.
Our findings are consistent with several adult studies that have examined individual psychological characteristics that moderate the effects of SES on health outcomes. For example, perceived control buffered a community sample of adults who were low in SES from poor self-reported health, acute health symptoms, and functional limitations 18. Similarly, high purpose in life buffered a community sample of adults who were low in SES from high levels of the inflammatory cytokine IL-6 19. Our study is novel in documenting specific protective factors that emerge in childhood, and in documenting their relevance to clinically relevant outcomes in a chronic disease such as asthma.
In addition, our findings are also consistent with other studies that have examined the benefits of positive childhood social relationships for those low in SES. These studies have documented that factors such as maternal warmth can buffer low SES individuals from adverse physiological and inflammatory risk profiles in both childhood and adulthood 21, 42, 43. In the present study, rather than focusing on broader family contexts, we focused on children themselves, and the characteristics that they can acquire to protect themselves from adverse health outcomes.
This type of work has important implications for efforts to reduce the increased burden of asthma among those lower in SES 44, 45. Our findings are important in documenting that it is possible for some children, despite being dealt a life of adversity, to show good asthma control. Furthermore, this study identifies a set of psychological factors that contributes to this resilience. Because we pinpointed qualities that naturally occur in some low SES children, this will hopefully allow researchers and clinicians to identify realistic targets for future interventions. That is, if we can identify characteristics that some low SES children already possess that promote good asthma profiles, these may be ones that may be most possible to alter through intervention in other low SES individuals. In order to meaningfully reduce health disparities, we may need to tailor interventions to the realities of low SES life, and to acknowledge that approaches that work in higher SES communities may not be similarly effective in a low SES context.
Strengths of the present study include the longitudinal design, the multiple measures of inflammatory, pulmonary, and impairment outcomes, and the novel approach of focusing on strengths (rather than detrimental factors) within low SES communities. Limitations include not having objective records of physician visits and hospitalizations, and not having longer monitoring periods for asthma outcomes and for examining variations by seasonality in asthma. In addition, as this study was observational we cannot draw firm conclusions about causality. Future studies could undertake experimental manipulations of shift-and-persist strategies and test the effects on asthma impairment in low SES children. Finally, future studies should explore other factors associated with shift-and-persist, such as temperament or family relationships, that may help explain the use of shift-and-persist strategies and their association with asthma outcomes.
In sum, children who came from low SES backgrounds and who engaged in shift-and-persist strategies (dealing with stressors by reframing them more positively, while at the same time, persisting in optimistic thoughts about the future) showed better asthma profiles, both in terms of reduced inflammation at baseline, as well as less asthma impairment at a 6-month follow-up. In contrast, shift-and-persist strategies were not beneficial to high SES children with asthma. Future studies should test whether these effects extend to other chronic illnesses as well. Given that broader social policies (e.g., anti-poverty programs) and environments (e.g., neighborhoods) can be difficult to change, an approach that focuses on low SES children themselves and the psychological qualities they could develop to adapt to the stressors they are forced to confront on a daily basis may be both a practical and effective starting point in efforts toward the long-term goal of eventually eliminating health disparities by social class.
Key Messages.
Low socioeconomic status (SES) is one of the most well-established social predictors of poor health, including asthma impairment; however, little is understood about why some low SES individuals are able to exhibit good asthma control despite living under adverse conditions.
This study identifies a psychological characteristic (shifting-and-persisting in response to stress) that protects children living in low SES homes from detrimental asthma outcomes.
An approach that focuses on the psychological qualities that children can possess for dealing with the stress of low SES life may represent one practical and effective starting point for efforts to reduce health disparities across society.
Capsule Summary.
This study identifies a psychological characteristic that protects children living in low SES homes from detrimental asthma outcomes. Such an approach may represent one practical and effective starting point for efforts to reduce asthma disparities.
Acknowledgments
This study was supported by NIH grant HL073975.
Glossary
Abbreviations
- SES
socioeconomic status
- PEF
peak expiratory flow
- IL
interleukin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 2.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 3.Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. American Journal of Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood PR, Smith LA, Romero D, Bradshaw P, Wise PH, Chavkin W. Relationships between welfare status, health insurance status, and health and medical care among children with asthma. American Journal of Public Health. 2002;92:1446–1452. doi: 10.2105/ajph.92.9.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon PA, Zeng ZW, Wold CM, Haddock W, Fielding JE. Prevalence of childhood asthma and associated morbidity in Los Angeles County: Impacts of race/ethnicity and income. J Asthma. 2003;40:535–543. doi: 10.1081/jas-120018788. [DOI] [PubMed] [Google Scholar]
- 6.Dales RE, Choi B, Chen Y, Tang M. Influence of family income on hospital visits for asthma among Canadian school children. Thorax. 2002;57:513–517. doi: 10.1136/thorax.57.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maziak W, von M E, Keil U, Hirsch T, Leupold W, Rzehak P, et al. Predictors of health care utilization of children with asthma in the community. Pediatr Allergy Immunol. 2004;15:166–171. doi: 10.1046/j.1399-3038.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 8.Cesaroni G, Farchi S, Davoli M, Forastiere F, Perucci CA. Individual and area-based indicators of socioeconomic status and childhood asthma. European Respiratory Journal. 2003;22:619–624. doi: 10.1183/09031936.03.00091202. [DOI] [PubMed] [Google Scholar]
- 9.Persky VW, Slezak J, Contreras A, Becker L, Hernandez E, Ramakrishnan V, et al. Relationships of race and socioeconomic status with prevalence, severity, and symptoms of asthma in Chicago school children. Annals of Allergy Asthma & Immunology. 1998;81:266–271. doi: 10.1016/S1081-1206(10)62824-4. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Y Acad Sci. 2010;1186:146–173. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 11.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviors and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science and Medicine. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 12.Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 13.Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: The moderating effects of individual and family qualities. Journal of Child Psychiatry. 1988;29:745–764. doi: 10.1111/j.1469-7610.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 14.Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 15.Garmezy N. Stress-resistant children: The search for protective factors. In: Stevenson JE, editor. Recent research in developmental psychopathology. Pergamon; Oxford: 1985. pp. 213–233. [Google Scholar]
- 16.Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57:316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 17.Werner EE. Resilience in development. Current Directions in Psychological Science. 1995;4:81–85. [Google Scholar]
- 18.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology. 1998;74:763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 19.Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Ann N Y Acad Sci. 1999;896:96–115. doi: 10.1111/j.1749-6632.1999.tb08108.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Chen E. Why socioeconomic status affects the health of children: A psychosocial perspective. Current Directions in Psychological Science. 2004;13:112–115. [Google Scholar]
- 24.Chen E, Miller GE. “Shift-and-persist” strategies: Why being low in socioeconomic status isn’t always bad for your health. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 26.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 28.Juster FT, Smith JP, Stafford F. The measurement and structure of household wealth. Labour Economics. 1999;6:253–275. [Google Scholar]
- 29.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 31.Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: measurement of coping and involuntary stress responses. J Consult Clin Psychol. 2000;68:976–992. [PubMed] [Google Scholar]
- 32.Wadsworth ME, Rieckmann T, Benson MA, Compas BE. Coping and responses to stress in Navajo adolescents: Psychometric properties of the Responses to Stress Questionnaire. Journal of Community Psychology. 2004;32:391–411. [Google Scholar]
- 33.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 34.Chang EC, Sanna LJ. Experience of life hassles and psychological adjustment among adolescents: Does it make a difference if one is optimistic or pessimistic? Personality and Individual Differences. 2003;34:867–879. [Google Scholar]
- 35.Scheier MF, Matthews KA, Owens J, Magovern GJ, Lefebvre RC, Abbott RA, et al. Dispositional optimism and recovery from coronary artery bypass surgery: The beneficial effects on physical and psychological well-being. Journal of Personality and Social Psychology. 1989;57:1024–1040. doi: 10.1037//0022-3514.57.6.1024. [DOI] [PubMed] [Google Scholar]
- 36.Scheier MF, Matthews KA, Owens JF, Schulz R, Bridges MW, Magovern GJ, et al. Optimism and rehospitalization following coronary artery bypass graft surgery. Archives of Internal Medicine. 1999;159:829–835. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- 37.Magnan AO, Mely LG, Camilla CA, Badier MM, Montero-Julian FA, Guillot CM, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma: Increased IFN-_g_-producing CD8_+_ T cells in asthma. Am J Respir Crit Care Med. 2000;161:1790–1796. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 38.Schuerwegh AJ, De C LS, De S L, Bridts CH, Verbruggen A, Stevens WJ. Flow cytometric detection of type 1 (IL-2, IFN-_g_) and type 2 (IL-4, IL-5) cytokines in T-Helper and T-Suppressor/cytotoxic cells in rheumatoid arthritis, allergic asthma and atopic dermatitis. Cytokine. 1999;11:783–788. doi: 10.1006/cyto.1998.0483. [DOI] [PubMed] [Google Scholar]
- 39.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Sorkness CA. Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. American Journal of Respiratory and Critical Care Medicine. 2004;170:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 40.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; London: 1991. [Google Scholar]
- 41.Brady SS, Matthews KA. The effect of socioeconomic status and ethnicity on adolescents’ exposure to stressful life events. Journal of Pediatric Psychology. 2002;27:575–583. doi: 10.1093/jpepsy/27.7.575. [DOI] [PubMed] [Google Scholar]
- 42.Miller GE, Lachman ME, Chen E, Gruenewald TL, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against childhood poverty’s effects on metabolic syndrome at midlife. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. doi: 10.1038/mp.2010.53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busse WW. The National Institutes of Allergy and Infectious Diseases networks on asthma in inner-city children: an approach to improved care. J Allergy Clin Immunol. 2010;125:529–537. doi: 10.1016/j.jaci.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szefler SJ, Gergen PJ, Mitchell H, Morgan W. Achieving asthma control in the inner city: do the National Institutes of Health Asthma Guidelines really work? J Allergy Clin Immunol. 2010;125:521–526. doi: 10.1016/j.jaci.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Institute NHLaB . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health; Bethesda, MD: 2007. [Google Scholar]