Abstract
Background
Much of the morbidity following trauma results from excessive activation of the innate immune system. This is manifested as a systemic inflammatory response and associated end-organ damage. Although mast cells are known to be important in many immune responses, their role in the systemic response to severe trauma is unknown.
Study Design
C57BL/6J-KitW-sh/BsmJ (mast cell deficient) and wild type mice were subjected to 1.5h of hemorrhagic shock plus bilateral femur fracture and soft tissue injury (HS/T), followed by resuscitation at 4.5h. Blood withdrawal volumes, mean arterial pressures; circulating cytokine, chemokine, High Mobility Group Box-1 (HMGB-1), double strain DNA (dsDNA), transaminase levels, and histology in liver and lung were compared between groups.
Results
Mast cell deficient mice exhibited greater hemodynamic stability than wild type mice. At baseline, the mast cell deficient mice exhibited no difference in any of the organ injury or inflammatory markers measured. As expected, wild type mice subjected to HS/T exhibited end organ damage manifested by marked increases in circulating Alanine transaminase (ALT), aspartate aminotransferase (AST), and double-strain DNA (dsDNA) levels, as well as histological evidence of tissue necrosis. In clear contrast, mast cell deficient mice exhibited almost no tissue damage. Similarly, the magnitude of increased circulating cytokine and chemokine induced by HS/T was much less in the mast cell deficient mice than in the wild type group.
Conclusions
Mast cell deficiency resulted in a damped systemic inflammatory response, greatly attenuated multiple organ injury, and more stable hemodynamics in HS/T. Thus, mast cells appear to be a critical component of the initial host response to severe injury.
Introduction
Trauma is among the leading causes of mortality and morbidity in the United States and worldwide (1). The survivors of severe trauma develop systemic inflammatory response syndrome (SIRS), which contributes to early organ injury and dysfunction (2, 3). SIRS is a result of activation of innate immune mechanisms, such as toll-like receptor signaling, and complement systems (4, 5). For example, we (5, 6, 7) and others have shown that toll-like receptor 4 and toll-like receptor 9 signaling, as well as complement activation are critical to the HS/trauma inflammatory response (3). However, other cellular components of the innate immune system may be important to the initial inflammatory response following HS/trauma. Mast cells (MC) are among cells known to be first line but unstudied responders in trauma.
MC were first described by Paul Ehrlich in 1878. They are widely distributed in most tissues and are well known for their role in allergy and anaphylaxis. MC granules are readily released upon stimulation and contain histamine, heparin, proteases, cytokines, chemokines, and lipid mediators. In recent years, MC have been found to be important in the immune response to sepsis (8, 9). Furthermore, IL-6 and TNFα have been shown to be vital for mast cell function (10, 11). Moreover, MC have been shown to be involved in wound healing, electro sensitivity, cerebral ischemia-reperfusion injury, and burns (12, 13, 14, 15, 16). MC stabilizer treatment reduced the induction of joint capsule fibrosis after traumatic injury (17). Previous work showed that HS could activate mast cells in the stomach (18, 19). However, the contribution of MC to trauma induced-inflammation is unknown. To address this question, we used MC deficient and wild type control mice in a model of combined hemorrhagic chock and trauma (HS/T).
Methods
Reagents
Mouse cytokine immunoassay Luminex kit was purchased from Millipore Corporation (Billerica, MA). Anti-HMGB1 antibody and anti-histamine antibody were purchased from Abcam Inc. (Cambridge, MA). Quant-iT™ PicoGreen dsDNA Kits were bought from Molecular Probes, Inc. (Eugene, OR). Lipopolysaccharide was bought from List Biological Laboratories (Campbell, CA).
Animal preparation
Male C57BL/6-Kit W-sh/W-sh mice (Mast cell deficient, M−/−) and their wild type counterparts (WT), C57/BL6 mice (Jackson Laboratories, Bar Harbor, Maine) were used in our study. All mice were 12 weeks old and weighed 25 to 30g. C57BL/6-Kit W-sh/W-sh mice have been found to be mast cell deficient at of 12weeks of age and afterwards. Animal handling and care complied with the National Institutes of Health’s regulations regarding the care and use of experimental animals and was approved by the University of Pittsburgh’s Institutional Animal Use and Care Committee. The animals were maintained in the animal facility with a 12 hour light-dark cycle and had free access to standard laboratory food and water.
Animal model of hemorrhagic shock + bilateral femur fracture + soft tissue injury (STI)
Animals were anesthetized with intra-peritoneal pentobarbital (70mg/kg) throughout the procedures. Hemorrhagic shock was performed as previously described by our group (7). Described briefly, bilateral groin dissections were performed. After identification of the femoral artery, a small arteriotomy was made, and the femoral arteries were cannulated with sterile PE-10 catheters. Both femurs were then fractured with the same hemostat 2–3mm above the knee joint. STI was induced by using the same hemostat to squeeze thigh muscles adjacent to the fracture to the second click for a period of 30 seconds. Animals were hemorrhaged to a mean arterial pressure (MAP) of 25 mmHg via one femoral artery cannula. The contralateral cannula was used to monitor mean arterial pressure and heart rate throughout the experiment. All surgical procedures were performed using aseptic techniques. Sham animals underwent bilateral femoral artery cannulation only. After 90 minutes of hemorrhagic shock and trauma, animals were resuscitated with three times the volume of shed blood with Ringer’s lactate. Throughout the procedure, body temperature was monitored via a rectal temperature probe and maintained at 37°C using a heating pad and a heating lamp.
Experimental groups
Animals were divided into the following six groups:
Anesthesia in Wild-type mice (n=6)
Anesthesia in C57BL/6-Kit W-sh/W-sh mice (n=4)
Bilateral femoral artery cannulation in wild-type mice (n=6)
Bilateral femoral artery cannulation in C57BL/6-Kit W-sh/W-sh mice (n=4)
HS + BFF + STI in wild-type mice (n=7)
HS + BFF + STI in C57BL/6-Kit W-sh/W-sh mice (n=7)
Mean arterial blood pressure (MAP) measurement and blood withdrawal calculation
A catheter in one femoral artery was connected to a transducer and then to a BLOOD PRESSURE ANALYZER MODEL 400 (Micro-Med, Inc, KY) to monitor mean arterial blood and heart rate and the MAP reading was recorded every 15mins. The shed blood was collected and the total shed volumes were measured before starting resuscitation.
Sample collection
At the conclusion of the experiment, animals were sacrificed with overdose isoflurane, and blood was harvested by cardiac puncture. Serum was collected by centrifuging the blood at 5,000 revolutions per minute for 10min at 4°C and then aliquoted and stored at −80°C until further analysis. The same hepatic lobe in each mouse was harvested and snap-frozen in liquid nitrogen and then stored at −80 degrees. After perfusion with phosphate buffer saline and 2% paraformaldehyde, the remaining liver and lungs were removed and fixed in 2% paraformaldehyde for an additional 18 hours prior to paraffin embedding and Hematoxylin and Eosin (HE) staining. Tongues were harvested for immunofluorescence staining.
Lipopolysaccharide (LPS)-induced macrophage activation
Mice were injected intraperitoneally with 1ml 4% thioglycollate in advance. Four days later, after euthanasia with overdose Isoflurane inhalation, the mice were sterilized with 75% alcohol, and 3ml of cold RPMI serum free media were injected into the peritoneal cavities. The abdomen was massaged gently for 1 min and the media aspirated and filtered with a 70um cell strainer (BD Biosciences, MD). After centrifugation at 1,000rpm for 10min, red blood cell lysis buffer was added and centrifuged again for 15min. The cell pellet was reconstituted with RPMI with 10% FBS and 5×105 cells per well were layered on a 24-well plate. After incubation for 12h at 37°C 5% CO2, 5% O2, and 90% N2 incubator, non-adherent cells were washed off and 100 μl of low serum Opti-MEM media (Invitrogen) with or without 10 μg/ml LPS was added to each well. 6hr later, supernatant and cells were harvested and TNFα (eBioscience) together with IL-6 levels (R&D) were measured by ELISA.
Immunofluorescence staining and Confocal Microscopy
Tongues were fixed in 2% paraformaldehyde followed by performing the previously described standardized protocol for cryopreservation (7). Histamine content in MC was assayed in the tongue as follows: 5 micron tongue sections were incubated with 2% bovine serum albumin (BSA) in PBS for 1 hour, followed by 5 washes with PBS+0.5% BSA (PBB). The samples were then incubated with rat polyclonal anti-CD45 (1:200, BD Pharmigen) and rabbit polyclonal anti-histamine (1:1000, Abcam) primary antibodies in PBB for 1h at room temperature. Samples were washed 5 times with PBB followed by incubation in the appropriate AlexaFluor-488 (1:500, Invitrogen) and Cy3 (1:1000, Jackson ImmunoResearch Laboratories) secondary antibodies diluted in PBB. Samples were washed three times with PBB, followed by a single wash with PBS prior to 30-second incubation with Hoechst nuclear stain. The nuclear stain was removed and samples were washed with PBS prior to placing a coverslip using Gelvatol (23 g poly [vinyl alcohol 2000], 50 ml glycerol, 0.1% sodium azide to 100 ml PBS). Positively stained cells in 6 random fields were imaged on a Fluoview 1000 confocal scanning microscope (Olympus, Melville, NY). Imaging conditions were maintained at identical settings within each antibody-labeling experiment with original gating performed using the negative control.
Analyses of Lung Injury
To analyze lung injury, 8–10 randomly selected fields (magnification 20×) were assessed in each mouse by 2 investigators independently who were blinded to the identity of the slides. Quantitative assessment of injury was made by grading four histological findings: congestion, edema, inflammation, and hemorrhage. The lung injury degree was scored on a scale of 0 to 4 (0=normal, 1=mild, 2=moderate, 3=severe, and 4=very severe) for each feature with a cumulative maximum score of 16.
Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) measurement
Serum ALT and AST were measured with HESKA Dri-Chem 4000 (HESKA, Loveland, CO; slides from Fujifilm Japan).
Cytokine levels
Serum interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), interleukin-10 (IL-10), chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-X-C motif) ligand 9 (CXCL9), and chemokine (C-X-C motif) ligand 10 (CXCL10) levels were assessed by Luminex assay according to the manufacturer’s instructions.
Serum HMGB1 levels
Western blot analysis was used to assess serum HMGB1 levels. Serum (3 μl) was separated by SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was blocked for 1h in PBS-Tween (0.1%) with 5% milk, followed by immunostaining with optimized dilutions of a polyclonal rabbit anti-mouse HMGB1 antibody (1:1,000) in 1% milk in PBS-Tween overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies were added and membranes were developed with the Super Signal West Pico chemiluminescent kit (Thermo Fisher Scientific) and exposed to film. Adobe Photoshop CS5 (Adobe Systems) was used to quantify each band and their relative intensity were calculated and compared.
Serum dsDNA detection
Serum dsDNA levels were detected with Quant-iT™ PicoGreen dsDNA Kits according to the product instructions.
Statistical analysis
Results are expressed as the mean ± standard error of mean (SEM). Data were analyzed using SigmaPlot 11.0 (Systat Software, Inc, Point Richmond, CA). We used the student’s t-test or Mann-Whitney Rank Sum Test when appropriate for two group comparisons. Significance was established at the 95% confidence interval (P < 0.05).
RESULTS
Mast cells degranulate following HS/T
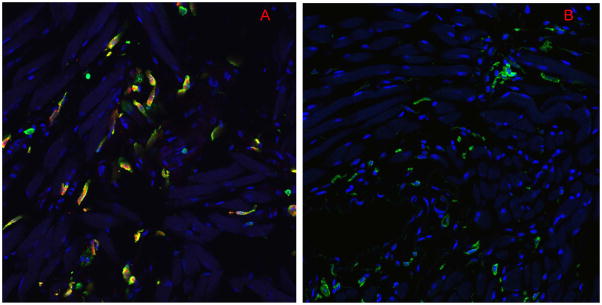
To determine if MC degranulation occurs following HS/T, we assessed CD45, a MC surface marker and histamine, a constituent of MC granules by immunofluorescence staining in C57BL/6 mice. As shown in Figure 1A, CD45 and histamine co-localization were easily detected in the tongues of sham mice. However, 6h after HS/T, there was almost no detectable histamine in the tongues (Fig. 1B). A pilot time-course study showed most of the histamine was released prior to resuscitation, with only small amounts still detectable at the 4.5h time point following HS/T (data not shown). Thus MC degranulation is an early event following HS/T.
Figure 1.
Mast cell degranulation in tongues of mice subjected to HS/T. Immunofluorescent staining showe d CD45 and histamine double positive cells in (A) the sham tongues, while (B) histamine staining was lost in HS/T tongues at the 6 h time point. (× 60) (Red=histamine, Green=CD45, Blue=nucleus).
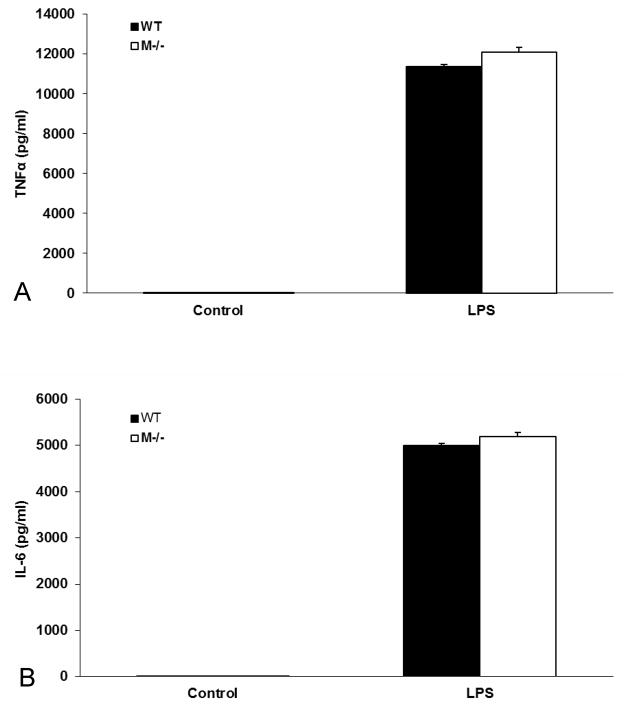
To confirm that defect in C57BL/6-Kit W-sh/W-sh mice is selective to MC and does not influence the response of macrophages, we harvested peritoneal macrophages from both WT and MC deficient mice and assessed their responses to LPS. The results showed that macrophages isolated from MC deficient mice were comparable to cells from WT mice (Fig. 2A and B).
Figure 2.
LPS-induced Macrophage activation comparison between WT and M−/− mice. Values were expressed as mean ± SEM. There was no difference between control WT and M−/− macrophages. After 6 h of LPS treatment, macrophages from M−/− and WT mice released comparable (A) TNFα and (B) IL-6 (n=3/group, p>0.05).
Mast cell deficiency leads to better hemodynamics in the trauma setting
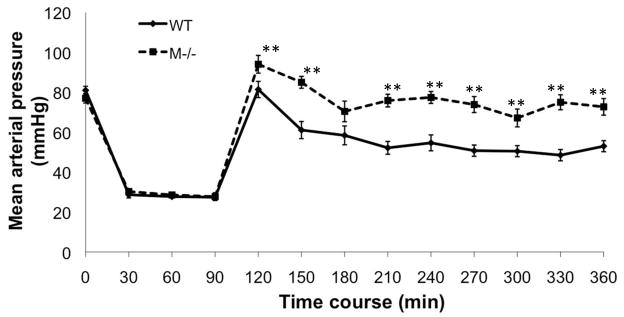
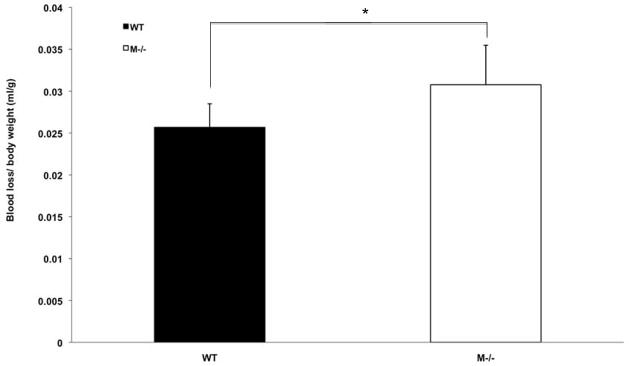
Severe trauma induces vascular dysfunction and is characterized by fluid leak and loss of vascular tone. Experimentally this contributes to blood pressure (BP) lability following resuscitation. Interestingly, MC deficient mice exhibited higher BPs following resuscitation (Fig. 3). Furthermore, the quantity of shed blood required to induce a sustained mean BP of 25mmHg was significantly higher in the MC deficient mice compared to the WT mice (Fig. 4). Thus, early MC activation may contribute to vascular dysfunction following HS/T.
Figure 3.
Time course of hemodynamic response of WT and mast cell deficient mice after HS/T. There was no mean arterial pressure difference between WT and mast cell deficient mice at baseline. After 90 min of HS/T with a MAP 25–30 mmHg, all the mice were resuscitated with Ringer’s lactate. MAPs were then recorded every 30 min. At most time points mast cell deficient mice had significantly higher MAPs than WT mice. Values are expressed as mean ± SEM. N=7/group, ** p<0.01.
Figure 4.
Blood withdrawal volume comparison between WT and M−/− mice subjected to HS/T. Values were expressed as mean ± SEM. M−/− mice required significantly more blood to be withdrawn than WT mice basing on body weight (0.0308±0.0047mL/g vs 0.0257 ±0.0028mL/g, **p<0.01).
Mast cells contribute to end organ damage after trauma
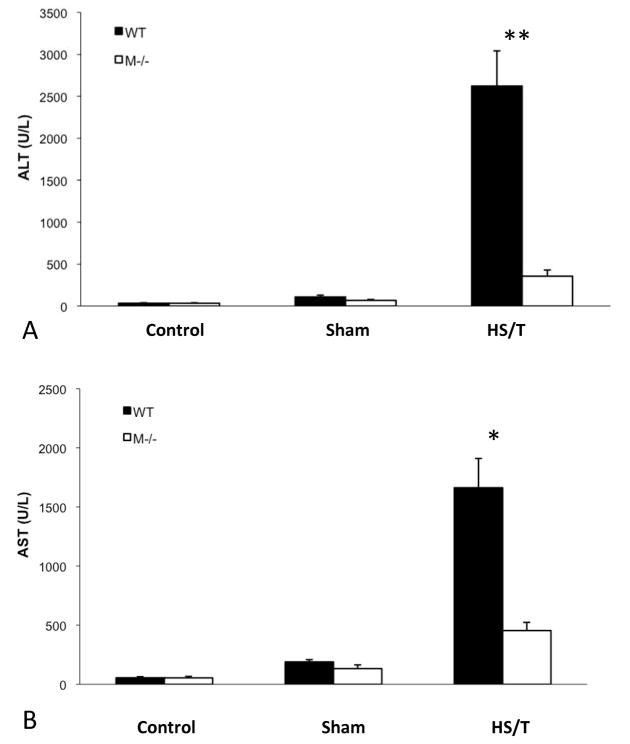
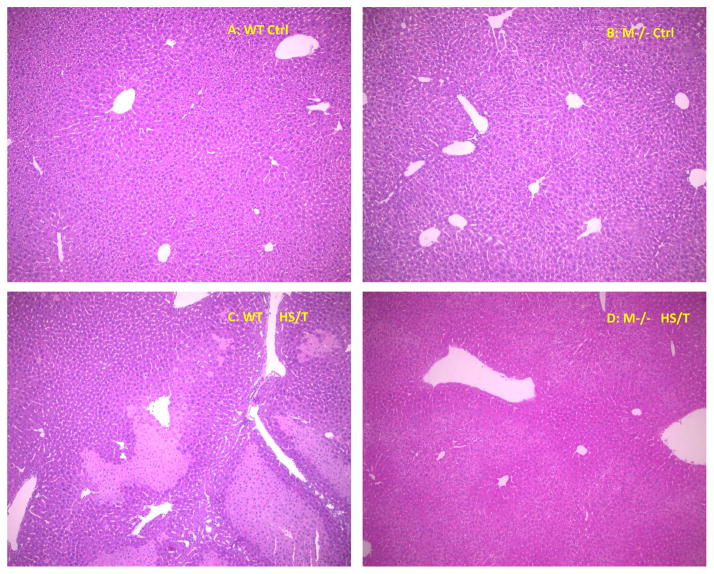
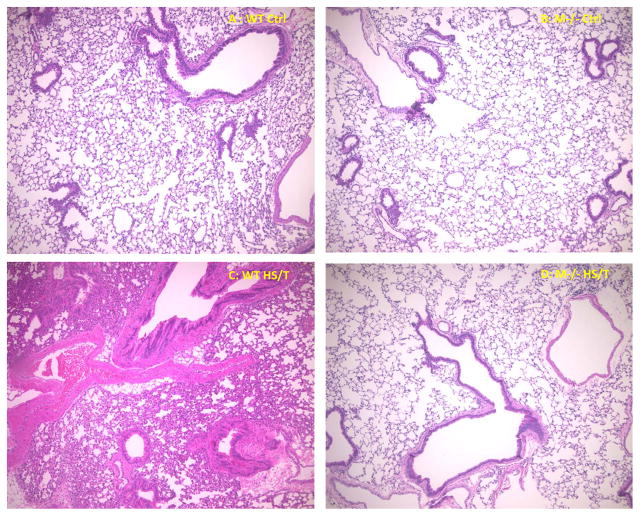
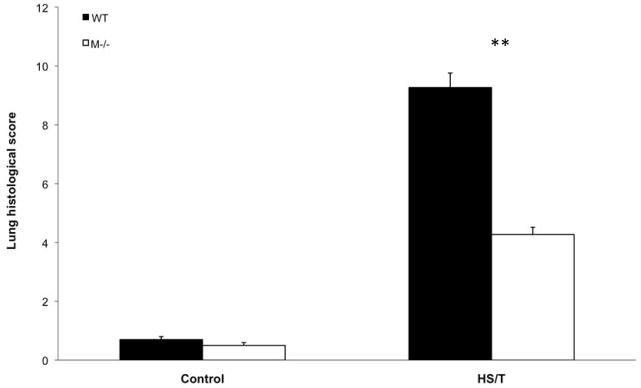
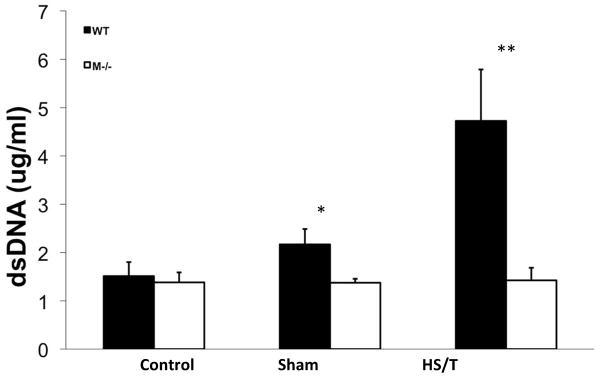
Major trauma with or without HS induces remote organ injury, such as liver damage and acute lung injury. To assess the importance of MC in the end organ damage seen in our model, C57BL/6-Kit W-sh/W-sh mice were again compared with WT. For liver injury, we assessed both hepatic histology and circulating ALT and AST levels. As shown in Fig. 5A and 5B, MC deficiency resulted in significantly less liver injury following HS/T as measured by circulating transaminases. By histology WT mice exhibited obvious hepatic necrosis after HS/T at the 6h time point (Fig. 6), while MC deficient mice maintained normal liver structure. HS/T also caused acute lung injury in wild type mice at the 6h time-point manifested by pulmonary edema, capillary leakage, and leukocyte infiltration (Fig. 7), together with higher histological lung injury scores (Fig. 8); however, MC deficient mouse lungs basically did not exhibit these changes and had much lower lung injury scores. This supports MC’s role in acute liver and lung injury. Moreover, as recent evidence indicates that dsDNA can be released from tissues after trauma and the level of free DNA correlates to injury severity (20, 21, 22), we measured plasma dsDNA levels. As shown in Fig. 9, wild type mice subjected to HS/T exhibited marked elevation in dsDNA, which was not seen in the absence of MC.
Figure 5.
(A) ALT levels comparison in mast cell deficient and wild type mice subjected to HS/T. Values are expressed as mean ± SEM. There is no statistical difference between anesthesia or sham groups. However, the mast cell deficient group had a much lower ALT level after HS/T (356.4±73.5 vs. 2622.1±420.2U/L, n=7/ group, **p<0.01). (B) AST level comparison in mast cell deficient and wild type mice subjected to HS/T There was no statistical difference between mast cell deficient and wild type anesthesia or sham mice. However, the mast cell deficient group had a much lower AST level than wild type controls after HS/T (454.7±68.9 vs. 1662.6±247.8U/L, n=7/ group, *p<0.05).
Figure 6.
Hepatic histology comparison between WT and M−/− mice. Representative histopathological liver images of WT and M−/− mice. (A) liver structure in WT control mice (× 100). (B) liver structure in M−/− control mice (× 100). (C) WT liver after 6h HS/T (× 100). Shows massive hepatic necrosis. (D) M−/− mice after 6h HS/T (× 100) Shows well-kept hepatic structure.
Figure 7.
Lung histology comparison between WT and M−/− mice. Representative histopathological lung images of WT and M−/− mice. (A) lung structure in WT control mice (× 100). (B) lung structure in M−/− control mice (× 100). (C) WT lung after 6 h HS/T (× 100). Shows pulmonary edema, capillary leakage, and leukocyte infiltration in WT lungs. (D) M−/− mice lung after 6 h HS/T (× 100). Shows basically normal lung structure in M−/− mice.
Figure 8.
Comparison of lung injury scores between WT and mast cell deficient groups. Values are expressed as mean ± SD. There were no difference in lung injury score between WT and mast cell-deficient control group mice. However, compared to WT HS/T lungs, mast cell deficient mice had statistically lower lung injury (9.27±0.49 vs. 4.27±0.25, **p<0.001).
Figure 9.
Circulating dsDNA level comparison between WT and mast cell deficient groups. Circulating dsDNA levels were measured by Quant-iT™ PicoGreen dsDNA kit. Values are expressed as mean ± SEM. * p<0.05, WT vs M−/− sham, n=6 and 4 respectively. **p<0.01, WT vs. M−/− after HS/T, n=7/group.
Circulating IL-1β, TNFα, IL-6, and IL-10 levels in HS/T are mast cell dependent
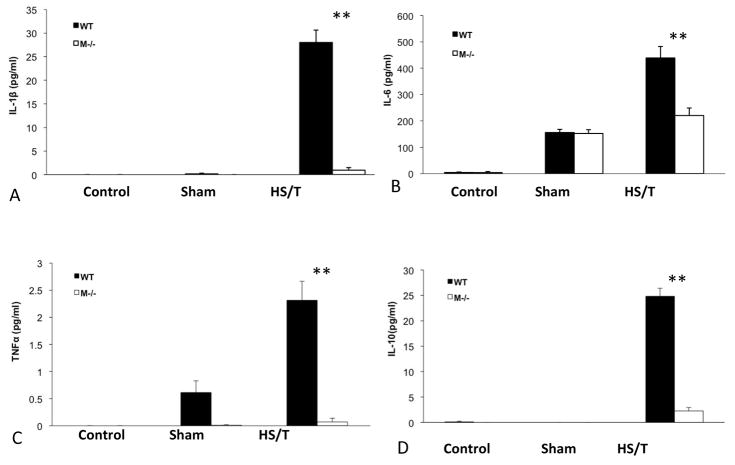
Trauma results in the induction of a systemic inflammatory response manifested by significant increases in circulating cytokines. This cytokine storm has been implicated in multiple organ dysfunction syndrome and mortality. To assess the impact of MC on the initial inflammatory response after trauma, circulating IL-1β, TNFα, IL-6 and IL-10 levels were measured. MC deficiency resulted in significantly lower IL-1β, TNFα, IL-6 and IL-10 levels following HS/T at 6h compared to the control group (Fig. 10). Importantly, the baseline levels between groups were comparable. These observations indicate that MC are essential to the inflammatory response initiated by HS/T.
Figure 10.
Cytokine level comparison between WT and mast cell deficient mice. Serum cytokine levels were measured by luminex assay at 6h following HS/T. (A) IL-1β level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group). (B) IL-6 level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group). (C) TNFα level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group). (D) IL-10 level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group).
Mast cell deficiency is associated with reduced chemokine release in HS/T
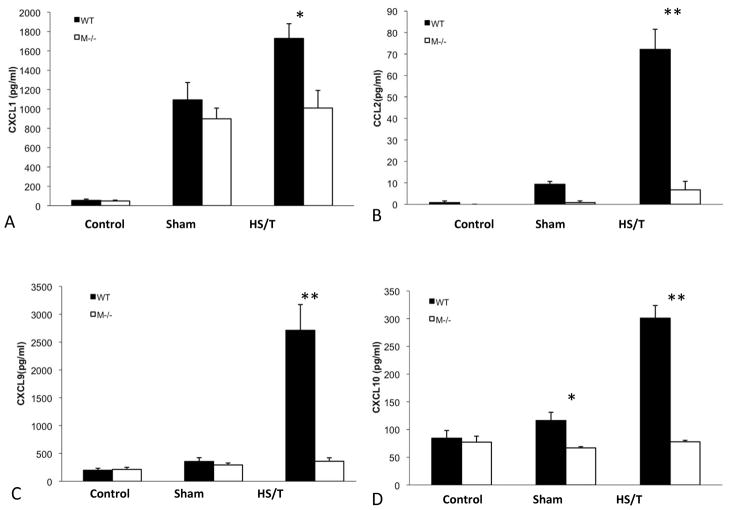
Increased chemokine synthesis leads to leukocyte mobilization and accumulation in tissues following injury (23). Therefore, we postulated that MC deficiency would be associated with reduced chemokine levels. As shown in Fig. 11, trauma induced increased CCL2, CXCL1, CXCL9, and CXCL10 serum levels in wild type mice subjected to HS/T. However, MC deficient mice subjected to HS/T exhibited a much lower chemokine release at 6h than seen in the wild type mice. Thus, MC activation appears to be vital for chemokine induction following injury.
Figure 11.
Circulating chemokine level comparison between M−/− and WT mice. Serum cytokine levels were measured by luminex assay at 6h following HS/T. Values are expressed as mean ± SEM. (A) CXCL1 level comparison between groups. *p<0.05 (WT vs M−/− HS/T, n=7/group). (B) CCL2 level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group). (C) CXCL9 level comparison between groups. **p<0.01 (WT vs M−/− HS/T, n=7/group). (D) CXCL10 level comparison between groups. *p<0.05, (WT vs M−/− sham, n=6 and 4 respectively) **p<0.01 (WT vs M−/− HS/T, n=7/group).
Mast cells are required for increases in circulating HMGB1 in trauma
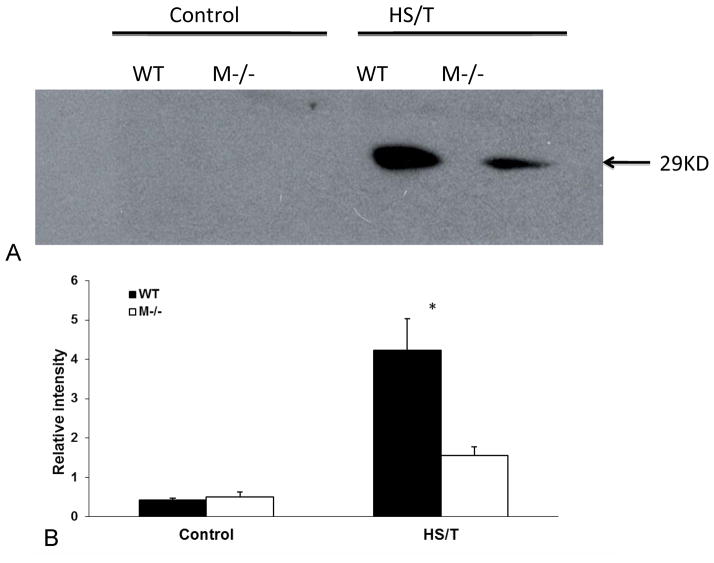
We (24, 25) and others (26, 27) have previously found that HMGB1 is an important mediator of the inflammatory response and end organ damage in HS and tissue trauma. To assess the importance of MC in the release of HMGB1 in this model, circulating HMGB1 levels were measured by Western Blot analysis. As shown in Fig. 12, HS/T in wild type animals resulted in an increase in detectable HMGB1 in the circulation. However, in the absence of MC, HMGB1 levels were markedly lower. These observations indicate that MC activation contributes to the release of HMGB1 into the circulation following injury.
Figure 12.
Western blot of HMGB1. (A) The bands were around 29KD. There was no detectable HMGB-1 in either sham or control groups. A strong HMGB1 band (29KD) was detected in the WT HS/T serum, which was not present in the M−/− HS/T group. Western blot represents an experiment that was repeated 3 times. (B) Relative intensity of paired bands was compared. There was no statistical difference between 2 control groups. In HS/T groups, WT mice had higher intensity than M−/− ones (*p<0.05, n=3/group).
Discussion
The role of MC in either the initiation of the inflammatory response or end-organ damage in trauma has not been established. Here, using a strain of MC-deficient mice and wild-type mice in a model closely mimicking severe clinical trauma, we found that MC deficiency led to a markedly diminished systemic inflammatory response following HS/T as measured by circulating cytokines, chemokines, and HMGB1 levels. This was associated with a reduction in tissue injury measured by circulating ALT, AST, dsDNA levels, and liver and lung histology. MC deficiency also resulted in improved vascular compensation capacity and better hemodynamic stability after HS/T. These findings point to the central role of MC in the early innate immune systemic responses following trauma.
The innate immune system consists of immune cells such as macrophages, NK cells, NKT cells, and MC (28, 29, 30, 31). These cells are likely contributors to the initial inflammatory response following HS and tissue injury. MC are widely distributed sentinels in the body, especially at sites that have contact with the outside environment. MC have characteristic granules and can react very quickly when activated. We now know MC are much more than just effector cells to allergenic responses, but much of our knowledge about MC in the systemic inflammatory response syndrome comes from sepsis studies. It has been shown that IL-6 and TNFα release by MC plays an important role in eliminating bacteria in sepsis (10, 11). However, the activators of immune responses in sepsis and trauma are distinct. We utilized the MC deficient C57BL/6-KitW-sh/W-sh mouse strain, a robust and specific strain for studies of MC function in vivo. These mice have an inversion mutation in the transcriptional regulatory element upstream of the c-kit transcription start site on mouse chromosome 5. The reduction in c-kit expression on stem cells in the bone marrow prevents the myeloid progenitor from differentiating into MC precursors, which results in MC deficiency. No other known disruptions in the development of hematopoietic stem cell derivatives have been shown. In addition, c-kit receptor mRNA and protein expression are normal in other tissues (32). We confirmed that macrophage responses to LPS are intact in the MC deficient strain.
In our study, the rapid release of histamine in tongue MC is consistent with the previous finding that stomach MC degranulate during HS (18, 19). Histamine can increase capillary permeability and low dose of histamine injection in humans induces a rapid decrease in mean arterial blood pressure (33, 34). Thus the requirement for greater volumes of blood withdrawal to achieve the target BP in MC deficient mice may be due to the absence of MC vasoactive substances. The absence of MC products may also be responsible for the improved post-resuscitation hemodynamics.
Severe trauma initiates inflammatory cascades and excessive cytokine production, which is one of the bases of systemic inflammatory response syndrome and multiple organ failure. Serum IL-1β, TNFα, IL-6 and IL-10 levels are key indicators of the magnitude of the systemic inflammatory response (35, 36, 37, 38). We found MC deficiency remarkably attenuated these cytokine responses, implicating MC as one of the drivers of the initial systemic inflammatory response following HS/T. Chemokines, which are important for leukocyte recruitment into tissues are classified by the cysteine-containing amino acid sequence at the amino terminus of each molecule. Chemokine nomenclature includes C, CC, CXC, and CX3C designations. Chemokine (C-C motif) ligand 2 (CCL2) can recruit monocytes, memory T cells, and dendritic cells to sites of tissue injury and inflammation (23, 39). CXCL1 exhibits neutrophil chemoattractant activity (40, 41). CXCL9 is a T-cell chemoattractant, while CXCL10 exhibits chemoattraction for monocytes, T cells, NK cells, and dendritic cells (42, 43, 44). The attenuated chemokine levels in MC deficient mice imply MC either directly or indirectly promote the influx of inflammatory cells into tissues and organs to further amplify inflammatory responses. Since MC are activated at sites remote from the site of tissue injury, MC are likely to contribute to leukocyte recruitment into organs in the systemic response to injury.
HMGB1 is a nuclear protein that can be actively released by cells in response to stresses such as hypoxia or passively released by cells undergoing necrosis (24, 27). Therefore, it is a marker of both tissue stress responses and tissue damage. Once released, HMGB1 plays a critical role in the initiation and propagation of the inflammatory response following trauma, and has been implicated in the pathogenesis of trauma and sepsis both experimentally and clinically (24, 27, 45, 46, 47). Our group previously (24, 48, 49) showed that neutralizing HMGB1 antibodies reduces cytokine release in models of HS, ischemia/reperfusion, and femur fracture. HMGB1 neutralizing antibodies also attenuated hemorrhage-induced acute lung injury as well as gut barrier dysfunction and ultimately led to improved survival (25, 26). Here, we found that MC deficiency was associated with lower circulating HMGB1 levels. Whether this is due to a reduction in tissue damage or a role of MC in regulating HMGB1 release is uncertain. However, some of the impact of MC on the systemic inflammatory response could be mediated through HMGB1.
Experimental and clinical studies have shown that trauma can result in cell-free DNA release and its level is related to severity (20, 21). Both nuclear and mitochondrial DNA (mtDNA) can be released in trauma (20, 22). The MC-dependent increase in circulating dsDNA seen in our study could be the result of tissue damage. Whether DNA sensing drives this MC-dependent inflammatory response is unknown. Our lab previously (6) found marked suppression of HS/T-induced inflammation in TLR9 mutant mice, suggesting that DNA can serve as an endogenous trigger of inflammation in our rodent model. In the MC deficient group, tissue damage was greatly blunted but not completely prevented, which suggests that MC activity is only one of the effectors leading to organ damage. We would expect that immune mediators and immune cells other than MC also play roles in inducing inflammation after injury.
MC are sentinel cells of the innate immune system and as such are widely distributed in tissues. Their ability to store and quickly release numerous mediators required for induction of acute inflammatory response and activation of leukocytes could account for the attenuated response seen in MC-deficient mice in our model. In anaphylaxis MC are activated by antigen- and IgE-dependent aggregation of IgE receptor FcεRI (8). In the setting of bacterial infection, TLR4 products of complement activation, or endothelin-1 have been shown to contribute to MC activation (50, 51, 52). Our previous work (5, 7) has shown TLR4 and complement factor C3 are vital to inflammatory response and organ damage in HS/T. Therefore, we would speculate that TLR4 signaling and complement activation could contribute to MC activation in HS/T. Ongoing work is underway to address this question.
In conclusion, our study provides evidence that MC are a key cellular component contributing to the pathobiology of severe injury manifested by an exaggerated inflammatory response and end-organ damage. Although further work is still needed to clarify the mechanism of MC activation and action, our results raise the possibility that suppressing MC activation could be beneficial in the setting of severe injury.
Acknowledgments
The authors thank Lauryn Tait, John M Brumfield, Frank Alicia, and Derek Barkay for their technical assistance, and Heiner Christine for her review of this paper.
Abbreviations
- HS/T
hemorrhagic shock/trauma
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- dsDNA
double-strain DNA
- BFF
bilateral femur fracture
- STI
soft tissue injury
- MC
mast cells
- M−/−
mast cell deficiency
- IL-1β
interleukin-1 beta
- TNFα
tumor necrosis factor alpha
- IL-6
interleukin- 6
- IL-10
interleukin-10
- CCL2
chemokine (C-C motif) ligand 2
- CXCL1
chemokine (C-X-C motif) ligand 1
- CXCL9
chemokine (C-X-C motif) ligand 9
- CXCL10
chemokine (C-X-C motif) ligand 10
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 2.Faist E, Baue AE, Dittmer H, Heberer G. Multiple organ failure in trauma patients. J Trauma. 1983;23:775–787. doi: 10.1097/00005373-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Napolitano LM, Ferrer T, McCarter RJ, Jr, Scalea TM. Systemic inflammatory response syndrome (SIRS) score on admission independently predicts mortality and length of stay in trauma patients. J Trauma. 2000;49:647–652. doi: 10.1097/00005373-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Barsness KA, Arcaroli J, Harken AH, et al. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 5.Prince JM, Levy RM, Yang R, et al. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Gill R, Ruan X, Menzel CL, et al. Systemic inflammation and liver injury following hemorrhagic shock and peripheral tissue trauma involve functional TLR9 signaling on bone marrow-derived cells and parenchymal cells. Shock. 2011;35:164–170. doi: 10.1097/SHK.0b013e3181eddcab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai C, Gill R, Eum HA, et al. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1175–R1182. doi: 10.1152/ajpregu.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland RE, Olsen JS, McKinstry A, et al. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiota N, Nishikori Y, Kakizoe E, et al. Pathophysiological role of skin mast cells in wound healing after scald injury: study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol. 2010;151:80–88. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- 13.Weller K, Foitzik K, Paus R, et al. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 14.Hart PH, Grimbaldeston MA, Swift GJ, et al. Dermal mast cells determine susceptibility to UVB-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strbian D, Kovanen PT, Karjalainen-Lindsberg ML, et al. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 2009;41:438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- 16.Younan G, Suber F, Xing W, et al. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J Immunol. 2010;185:7681–7690. doi: 10.4049/jimmunol.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monument MJ, Hart DA, Befus AD, et al. The mast cell stabilizer ketotifen fumarate lessens contracture severity and myofibroblast hyperplasia: a study of a rabbit model of posttraumatic joint contractures. J Bone Joint Surg Am. 2010;92:1468–1477. doi: 10.2106/JBJS.I.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debek W, Barczyk M, Chyczewski L, Roszkowska-Jakimiec W. Hemorrhagic shock activates mast cells in the rat stomach wall. Eur J Pediatr Surg. 2000;10:155–161. doi: 10.1055/s-2008-1072348. [DOI] [PubMed] [Google Scholar]
- 19.Kokoschka R, Göber I, Gebhart W. Gastric blood flow, mast cell degranulation and micromorphology of gastric mucosa following experimental haemorrhagic shock in dogs. Br J Surg. 1982;69:328–332. doi: 10.1002/bjs.1800690613. [DOI] [PubMed] [Google Scholar]
- 20.Chou CC, Fang HY, Chen YL, et al. Plasma nuclear DNA and mitochondrial DNA as prognostic markers in corrosive injury patients. Dig Surg. 2008;25:300–304. doi: 10.1159/000152846. [DOI] [PubMed] [Google Scholar]
- 21.Lam NY, Rainer TH, Chan LY, et al. Time Course of Early and Late Changes in Plasma DNA in Trauma Patients. Clin Chem. 2003;49:1286–1291. doi: 10.1373/49.8.1286. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is Released by Shock and Activates Neutrophils via P38 Map-Kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 23.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 24.Levy RM, Mollen KP, Prince JM, et al. Systemic inflammation and remote organ injury following trauma require HMGB-1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 25.Yang R, Harada T, Mollen KP, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Park JS, Strassheim D, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ombrellino M, Wang H, Ajemian MS, et al. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446–1447. doi: 10.1016/S0140-6736(99)02658-6. [DOI] [PubMed] [Google Scholar]
- 28.Barkhausen T, Frerker C, Pütz C, et al. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30:401–410. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]
- 29.Côte CH, Tremblay MH, Duchesne E, Lapoite BM. Inflammation-induced leukocyte accumulation in injured skeletal muscle: role of mast cells. Muscle Nerve. 2008;37:754–63. doi: 10.1002/mus.20998. [DOI] [PubMed] [Google Scholar]
- 30.Faunce DE, Gamelli RL, Choudhry MA, Kovacs EJ. A role for CD1d-restricted NKT cells in injury-associated T cell suppression. J Leukoc Biol. 2003;73:747–755. doi: 10.1189/jlb.1102540. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, Fujimi S, Lederer JA, et al. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–4520. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- 32.Grimbaldeston MA, Chen CC, Piliponsky AM, et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basheer M, Schwalb H, Nesher M, et al. Mast cell activation by fibrinogen-related homologous c-terminal peptides (haptides) modulates systemic blood pressure. J Allergy Clin Immunol. 2010;126:1041–1048. doi: 10.1016/j.jaci.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Vigorito C, Giordano A, De Caprio L, et al. Effects of histamine on coronary hemodynamics in humans: role of H1 and H2 receptors. J Am Coll Cardiol. 1987;10:1207–1213. doi: 10.1016/s0735-1097(87)80120-1. [DOI] [PubMed] [Google Scholar]
- 35.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135:291–295. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 36.Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42:863–870. doi: 10.1097/00005373-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Stensballe J, Christiansen M, Tønnesen E, et al. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53:515–521. doi: 10.1111/j.1399-6576.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin C, Boisson C, Haccoun M, et al. Patterns of cytokine evolution (tumor necrosis-α and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Semple BD, Bye N, Rancan M, et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritzman AM, Hughes-Hanks JM, Blaho VA, et al. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect Immun. 2010;78:4593–4600. doi: 10.1128/IAI.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu LL, Warren MK, Rose WL, et al. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 42.Carr MW, Roth SJ, Luther E, et al. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufour JH, Dziejman M, Liu MT, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 44.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 45.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Ochani M, Li J, et al. Reversussing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaczorowski DJ, Nakao A, Vallabhaneni R, et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;27:1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prodeus AP, Zhou X, Maurer M, et al. Impaired mast cell-dependent natural immunity in complement C3- deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 52.Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]