Abstract
Background
Interleukin (IL)-5 plays a central role in the development and maintenance of eosinophilia and eosinophil activation in a wide variety of eosinophilic disorders. Although IL-5, IL-3 and GM-CSF can modulate expression of IL-5 receptor α (IL-5Rα) on eosinophils in vitro, little is known about soluble and surface IL-5Rα levels in vivo.
Objective
To assess surface and soluble IL-5Rα levels in patients with eosinophilia and/or mastocytosis.
Methods
Surface IL-5Rα expression was assessed by flow cytometry in blood and/or bone marrow from subjects with eosinophilia (n=39), systemic mastocytosis (n=8) and normal volunteers (n=28). Soluble IL-5Rα (sIL-5Rα) was measured in a cohort of 177 untreated subjects and correlated with eosinophilia, eosinophil activation, serum tryptase and cytokine levels.
Results
Whereas IL-5Rα expression on eosinophils inversely correlated with eosinophilia (r=−0.48, p<0.0001), serum levels of sIL-5Rα increased with eosinophil count (r=0.56, p<0.0001), serum IL-5 (r=0.40, p<0.0001) and IL-13 levels (r=0.29, p=0.004). Of interest, sIL-5Rα was significantly elevated in patients with systemic mastocytosis without eosinophilia. Although sIL-5Rα levels correlated with serum tryptase levels in these patients, eosinophil activation, assessed by CD69 expression on eosinophils and serum eosinophil-derived neurotoxin levels, was increased compared to normal subjects.
Conclusion
These data are consistent with an in vivo IL-5Rα regulatory pathway in human eosinophils similar to that described in vitro and involving a balance between surface and soluble receptor levels. This may have implications with respect to the use of novel therapeutic agents targeting IL-5 and its receptor in patients with eosinophilia and/or mastocytosis.
Keywords: Hypereosinophilic syndrome, interleukin-5, mast cell, eosinophil, mastocytosis
Introduction
The IL-5 receptor (IL-5R) is a high affinity receptor expressed on eosinophils, basophils and mast cells and occurs as a heterodimer of IL-5Rα with the β subunit common to IL-5, IL-3 and GM-CSF receptors1. Data from numerous studies in animals and humans have demonstrated that IL-5 signaling through this receptor is essential for eosinophil development, survival and activation. Consequently, recent attention has focused on the development of therapeutic agents that target the IL-5/IL-5R axis for use in a wide variety of disorders characterized by eosinophilic inflammation. These include humanized monoclonal antibodies to IL-5 itself, already proven to be effective in the treatment of both hypereosinophilic syndrome (HES)2 and eosinophilic asthma3,4 , and a monoclonal antibody to IL-5Rα (benralizumab, MEDI-563) that results in enhanced susceptibility of eosinophils to antibody-dependent cell-mediated cytotoxicity5,6.
A soluble form of IL-5Rα (sIL-5Rα) can be generated by differential splicing of mRNA transcripts7 or by cleavage of surface receptors8. Increased serum levels of sIL-5Rα have been described in subjects with acute exacerbations of chronic obstructive pulmonary disease9 and in patients with nasal polyposis with asthma10 and have been suggested to play a role in the down-modulation of the eosinophilic inflammatory response. In fact, recombinant sIL-5Rα has been shown to bind IL-5 with high affinity and to prevent binding of IL-5, but not IL-3 or GM-CSF, to surface IL-5R, attenuating signal transduction, mediator release and survival of eosinophils in vitro11.
Decreased surface expression of IL-5Rα on eosinophils has been reported following in vitro incubation with IL-5, IL-3, GM-CSF or IL-9 and is accompanied by a reciprocal increase in sIL-5Rα in the culture supernatants8,12-14. A similar phenomenon has been observed in vivo in association with eosinophil recruitment to the airways following airway allergen challenge and resulted in a lack of eosinophil responsiveness to exogenous IL-515. Theoretically, such alterations in membrane and soluble IL-5Rα levels could interfere with the efficacy of monoclonal antibodies to bind IL-5Rα on eosinophils, as well as on basophils and mast cells. Despite these concerns, little is known about IL-5Rα regulation in patients with peripheral eosinophilia and/or mastocytosis.
Thus, the aim of the present study was to determine whether modulation of membrane and soluble IL-5Rα levels occurs in vivo in patients with marked eosinophilia and/or mastocytosis. Not only did surface and soluble IL-5Rα levels increase with eosinophil count and serum IL-5 level, as predicted from in vitro studies, but serum levels of sIL-5Rα were significantly increased in patients with systemic mastocytosis (SM) without eosinophilia. Consequently, the relative contributions of eosinophils and mast cells to sIL-5R levels were also explored.
Subjects and Methods
Patient populations
Subjects with eosinophilia or mastocytosis underwent detailed clinical and laboratory evaluation as part of Institutional Review Board (IRB)-approved clinical protocols to study these disorders (NCT00001406, NCT00044122). Healthy volunteers were recruited under an IRB-approved clinical protocol designed to obtain controls for in vitro research (NCT00090662). All participants gave written informed consent. Subjects with eosinophilia were defined as subjects with a peak peripheral eosinophil count >1500/mm3 evaluated between January 1991 and November 2009 and were classified into subtypes as follows: chronic eosinophilic leukemia (CEL; presence of the FIP1L1/PDGFRA fusion gene), hypereosinophilic syndrome (HES; unexplained eosinophilia >1,500/mm3 with evidence of signs or symptoms attributable to the eosinophilia), parasitic (PARA; evidence of helminth infection and resolution of eosinophilia with treatment), benign eosinophilia (BE; persistent eosinophilia >1500/mm3 for more than 5 years without signs or symptoms), secondary eosinophilia (Other EO; eosinophilia >1,500/mm3 of known cause, such as due to a neoplasm or drug hypersensitivity). Subjects with mastocytosis were defined according to the 2008 WHO classification of myeloproliferative diseases16 as systemic mastocytosis (SM; eosinophilia <1000/mm3), systemic mastocytosis with eosinophilia (SM-eo; eosinophilia >1000/mm3) or cutaneous mastocytosis (CM; urticaria pigmentosa in the absence of bone marrow involvement). Demographic and laboratory characteristics of the different patient populations are presented in Table 1.
Table 1.
Demographic and laboratory characteristics of study population.
| Diagnosis* | Normal (n=38) |
CEL (n=6) |
HES (n=34) |
PARA (n=16) |
BE (n=10) |
Other EO (n=8) |
SM-eo (n=5) |
SM (n=54) |
CM (n=6) |
|---|---|---|---|---|---|---|---|---|---|
| Median Age (range) |
44 (22-79) |
35 (17-78) |
41 (1-82) |
27 (11-80) |
53 (28-74) |
52 (32-73) |
59 (54-74) |
49 (21-69) |
31 (20-54) |
| Gender (M/F) | 11/31 | 6/0 | 19/15 | 9/7 | 7/3 | 5/3 | 3/2 | 20/34 | 0/6 |
| Median eos/mm3 (range)** |
124 (30-480) |
7067 (3719-23074) |
2950 (471-45990) |
1908 (540-10496) |
2220 (1696-4270) |
1818 (340-8437) |
1999 (1186-2806) |
164 (18-952) |
106 (40-876) |
| Median serum tryptase ng/mL (range) |
ND | 14.3 (6.46-46.7) |
11.2 (2.23-28.1) |
7.85 (3.7-16.9) |
7.18 (3.31-12.7) |
8.78 (4.26-11.7) |
123 (34-567) |
42 (5-780) |
6 (4-13) |
| Median serum IgE IU/mL (range) |
ND | 14 (4-22) |
243 (4-13263) |
898 (15-6293) |
60 (4-628) |
273 (2-1422) |
4 (4-29) |
7 (0-1370) |
41 (6-173) |
CEL = FIP1L1/PDGFRA-positive chronic eosinophilic leukemia, HES = hypereosinophilic syndrome, including idiopathic and lymphocytic variants, PARA = helminth infection, BE = benign eosinophilia (eosinophilia >1500/mm3 without clinical manifestations), Other EO = secondary eosinophilic disorders (e.g. neoplasms, drug hypersensitivity, allergic disease)
Although subjects were classified on the basis of peak recorded eosinophil counts, laboratory results listed in the table are from the date of the sIL-5R assay. Consequently, the lower range of eosinophils/mm3 is <1500/mm3 in some eosinophilic groups. ND = not done
Assessment of surface receptor expression by flow cytometry
Surface expression of IL-5Rα and activation markers on peripheral blood eosinophils was assessed using whole blood from all subjects evaluated for eosinophilia or mastocytosis between March and November 2009. A normal volunteer was included as a control with each assay. Whole blood was stained with CDw125-PE (clone A14; BD Biosciences, San Jose, CA), CD25-FITC, CD40-FITC, CD9-FITC, CD69-FITC, and CD16-PE (BD Biosciences, San Diego, CA). Irrelevant, directly conjugated, murine IgG1 was used to ascertain background staining. CD9 was used as a positive control for eosinophils. Samples were analyzed on a FACSCanto II flow cytometer using Cellquest software (BD Biosciences). Eosinophils were separated from granulocytes by their characteristic high side scatter and dim staining for CD16. Percent positive for each surface molecule was ascertained using a FITC conjugated subclass control and setting a marker so that >98% of the control was defined as negative. The normal ranges for surface receptor expression represent the 95% confidence interval for % expression on eosinophils from 40 normal volunteers. Quantibrite PE (BD Biosciences) was used to estimate the antibodies bound per cell (ABC) for CDw125-PE according to the manufacturer’s instructions.
Bone marrow aspirates from subjects with SM (n=17) or HES (n=6) were stained using CD45-PerCP (clone 2D1), CD117-APC (clone 104D2), CD34-FITC (clone 8G12), CD49d-PE (clone 9F10), CD16-FITC (clone 3G8), CD9-PerCP-Cy5.5 (M-L13), CD3- FITC (clone SK7), CD19-APC (clone SJ25C1), CDw125-PE (clone A14) (BD Biosciences, San Jose, CA) and CD14-APC (TuK4; Invitrogen, Carlsbad, CA). In each sample, a minimum of 300,000 events was acquired. Median number of events for eosinophils was 6,262 (range 2,692-122,789), mast cells 103 (range 11-549) and lymphocytes 38,635 (range 8,002-66,597). Mast cells were identified as CD117 bright positive, CD34 negative, CD45 positive cells with characteristic forward and side scatter properties. Eosinophils were identified as CD45 positive, CD49d positive, CD16 dim positive, CD9 positive, CD14 negative, CD3 negative, CD19 negative cells cells with characteristic high side scatter properties. FlowJo (Version 8.8.2, Tree Star, Ashland, OR) software was used for data analysis. Flow sorting of bone marrow mast cells and cDNA preparation were performed as previously described17. Sort purity routinely exceeded 98%.
Measurement of serum levels of sIL-5Rα
Soluble IL-5Rα levels in serum were assessed using a chemiluminescence capture ELISA. Briefly, FluoroNunc Maxi-sorp plates were coated with monoclonal anti-IL-5Rα antibody (KM 1257) at 1.0 μg/ml in PBS overnight at 4°C. The plates were blocked for 1 hour with PBS/Tween/20% casein prior to incubation with sera for 2 hours at room temperature. sIL-5Rα was detected by sequential incubation with biotinylated goat anti-human IL-5Rα (R&D) and streptavidin-horseradish peroxidase (GE Healthcare). After washing with 0.2M phosphate buffer, plates were developed with FemtoGlow (Michigan Diagnostics) and read on a luminometer. All assays were performed in duplicate, and values were calculated based on a standard curve. The sensitivity of the assay was 7.8 pg/ml.
Cytokine analysis
Serum cytokine and eotaxin levels were measured by suspension array technology in multiplex using a Milliplex kit for human IL-2, IL-3, IL-5, IL-13, IL-17, GM-CSF and eotaxin (Millipore Corp, St. Charles, MO) according to the manufacturer’s instructions18. The minimal detectable concentrations were as follows: IL-2 (0.4 pg/ml), IL-3 (9.8 pg/ml), IL-5 (0.1 pg/ml), IL-13 (0.3 pg/ml), IL-17 (0.4 pg/ml), GM-CSF (2.3 pg/ml) and eotaxin (2.1 pg/ml).
Serum EDN assay
Serum levels of EDN were measured by ELISA as described previously19. Briefly, Immulon 4 flat bottom plates were coated with monoclonal antibody (6D1.5/A5 anti-EDN) at 2.5 μg/ml in PBS overnight at 4°C. The plates were blocked for 1 hour with PBS/Tween/0.1% bovine serum albumin prior to overnight incubation of sera. Granule proteins were detected by sequential incubation with rabbit polyclonal anti-EDN followed by alkaline phosphatase-labeled goat anti-rabbit IgGFc and alkaline phosphatase substrate (Sigma). All assays were performed in duplicate, and values were calculated based on a standard curve. The limit of detection for the assay was approximately 2 ng/ml.
Quantification of isoforms IL-5Rα mRNA transcripts for soluble and membrane forms
Eosinophils were purified from peripheral blood by negative selection as described previously19 and frozen in TRIzol (Life Technologies) to be processed at a later date. Total RNA was quantified using a NanoDrop, ND-1000 (NanoDrop Technologies) and 1.0 μg of RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Approximately 50 ng of RNA equivalent cDNA template was used per 1X SYBR Green (SaBiosciences) in a final 10 μl reaction volume. Real-time amplification was performed in a 384 well plate using a GeneAmp 7900HT Sequence Detection System (Applied Biosytem). Transmembrane (TM) IL-5Rα and soluble (SOL) IL-5Rα isoform variants were amplified using a common forward primer annealing to exon 10 (5′-GCAGCAGTGAGCTCCATGTG-3′) and isoform-specific reverse primers (TM: 5′-AGGGCTTGTGTTCATCATTTCC-3′, SOL: 5′-TGGATGTTATCTCCTTTATCTTGAGAA-3′), generating PCR products of 89 bp and 95 bp, respectively20. Each sample was run in duplicate, and cycle threshold (CT) values were normalized using β-actin CT values from corresponding samples. Real-time PCR efficiencies were compared and were equivalent between IL-5Rα transcript variants using a standard curve consisting of 10-fold serial dilutions of plasmids containing their respective target sequences.
Statistical analyses
Both medians and geometric means (GM) were used as measurements for central tendency. The Mann-Whitney-U or Fisher’s exact test was used for comparisons between groups and Spearman correlations were used with approximate confidence intervals based on Fisher’s Z transformation. Responses below the level of detection were given the lowest rank. P values were corrected for multiple comparisons where stated using the Holm’s correction21. All analyses were performed using Prism V5.0 (GraphPad Software) or R version 2.11.1.
Results
Serum sIL-5Rα levels are elevated in patients with eosinophilia and/or mastocytosis
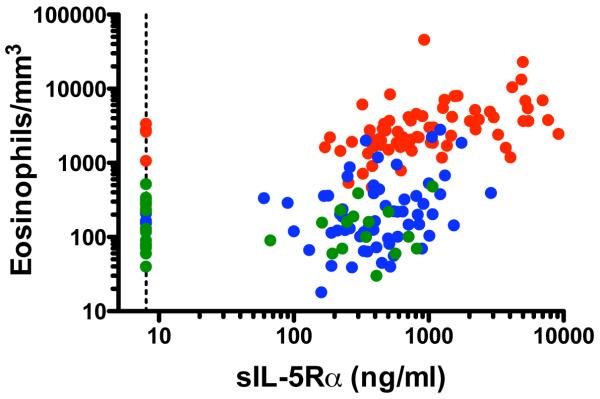
Levels of sIL-5Rα were measured in serum from a cohort of patients with untreated eosinophilia >1500/mm3 (n=74), SM (n=59) or CM (n=6) and in normal controls (n=38) (see Table 1). Serum sIL-5Rα levels were positively correlated with eosinophilia in the group as a whole (r=0.56, p<0.0001, Spearman rank correlation), in subjects with eosinophilia (r=0.52, p<0.0001) and in subjects with SM (r=0.31, p=0.02), but not in normal subjects (r=−0.04, p=0.8) (Figure 1).
Figure 1.
Serum levels of sIL-5Rα are positively correlated with peripheral blood eosinophilia. Each circle represents an individual data point. Subjects with eosinophilic disease are shown in red, those with mastocytosis in blue and normals in green. The dotted line represents the limit of detection of the assay. P<0.0001, r = 0.54, Spearman rank correlation
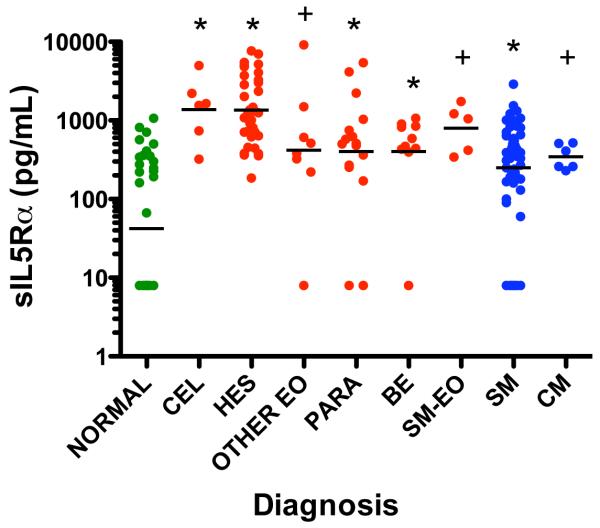
When serum levels of sIL-5Rα were compared among the different patient groups, as expected, sIL-5Rα levels were significantly elevated in patients with a wide variety of eosinophilic disorders as compared to normal subjects (Figure 2). However, serum levels of sIL-5Rα were also increased in patients with SM without eosinophilia (geometric mean [GM] 251 pg/ml, 95% CI 164.9, 381.9) as compared to normals (GM 42.3 pg/ml, 95% CI 22.4, 79.7). In fact, sIL-5Rα was detectable in serum from 70/75 patients with eosinophilia and 47/54 patients with SM without concomitant eosinophilia compared to 17/38 normal controls (P<0.0001, Fisher’s exact tests comparing each group to normal). When the ratio of sIL-5Rα to absolute eosinophil count (AEC) was examined comparing the 8 patient groups to the normal group, only the SM group had a significantly higher ratio (sIL-5Rα/AEC) than the normal group (P=0.007 Holm’s adjusted for multiple comparisons).
Figure 2.
Serum IL-5Rα levels are increased in patients with eosinophilia and mastocytosis without eosinophilia. Each symbol represents an individual data point. Subjects with eosinophilia are indicated in red, with mastocytosis in blue and normal controls in green. Group geometric means are indicated by horizontal lines. * p<0.001 or + p<0.05 compared to normal group (Holm’s adjusted p-value using the Mann-Whitney-U test)
Paired serum samples and eosinophil counts were available from two time points for 22 subjects with eosinophilia, 2 subjects with SM and 2 normal subjects with measurable sIL5Rα levels. As expected, the change in sIL5Rα level was strongly correlated with the change in eosinophil count (r=0.66, p<0.001, Spearman rank correlation).
Elevated sIL-5Rα is associated with increased serum IL-5 levels and eosinophil activation
Since in vitro studies had demonstrated an increase in sIL-5Rα levels in response to stimulation with IL-5, GM-CSF and IL-3, serum levels of these, as well as other cytokines important in eosinophilia and eosinophil activation, were measured in 142 of the subjects for whom sufficient serum was available. After correction for multiple comparisons, sIL-5Rα levels were correlated only with serum IL-5 and IL-13 levels (P <0.001 and P=0.004, respectively, Spearman rank correlation; Table 2). Interestingly, serum levels of IL-13 were significantly increased in eosinophilic patients (median 2.18 pg/ml, range 0-557 pg/ml) as compared to normal controls (median 0 pg/ml, range 0-326 pg/ml; P=0.04) and correlated with eosinophil count in the group as a whole (r=0.27, p=0.0012).. Serum levels of sIL-5Rα were also correlated with serum levels of the eosinophil granule protein, eosinophil-derived neurotoxin (EDN) (r=0.55, P<0.0001, Spearman rank correlation) and with serum IgE levels (r=0.26, P=0.006, Spearman rank correlation).
Table 2.
Serum levels of IL-5Ra are correlated with serum levels of IL-5, IL-13, IgE and EDN, but not with serum levels of eotaxin, IL-2, IL-3, GM-CSF or IL-17
| Analyte* | Spearman R | P value** |
|---|---|---|
| IL-2 | −0.004 | NS |
| IL-3 | −0.06 | NS |
| IL-5 | 0.40 | <0.0001 |
| IL-13 | 0.29 | 0.004 |
| IL-17 | −0.03 | NS |
| eotaxin | 0.05 | NS |
| GM-CSF | 0.08 | NS |
| EDN | 0.55 | <0.0001 |
| IgE | 0.26 | 0.038 |
n=142 for all cytokines, n=129 for IgE and n=151 for EDN
Holm’s adjusted for multiple comparisons
Although subjects with SM and AEC <1000/mm3 had eosinophil counts that were comparable to those in the normal control group, serum levels of EDN were increased in these subjects compared to normal controls (GM 66.15 ng/ml, 95% CI 54.40, 80.43 vs. GM 26.84 ng/ml, 95% CI 17.21, 41.88; p=0.001, Mann-Whitney-U test), consistent with increased eosinophil activation in the patients with SM. As expected, EDN levels in the patients with eosinophilic disorders were significantly elevated (GM 296.3 ng/ml, CI 218.6, 401.6) compared to patients with SM and AEC <1000/mm3 or to normal controls (P<0.0001). The surface phenotype was assessed on peripheral blood eosinophils in 9 consecutive subjects with SM without eosinophilia. Expression of the eosinophila ctivation marker, CD69, ranged from 0.9% to 14.8% (normal range ≤2.2%) and was elevated in 6 of the 9 subjects. Surface expression of CD40 and CD25 were within normal limits.
IL-5Rα expression on peripheral blood eosinophils is negatively correlated with eosinophilia
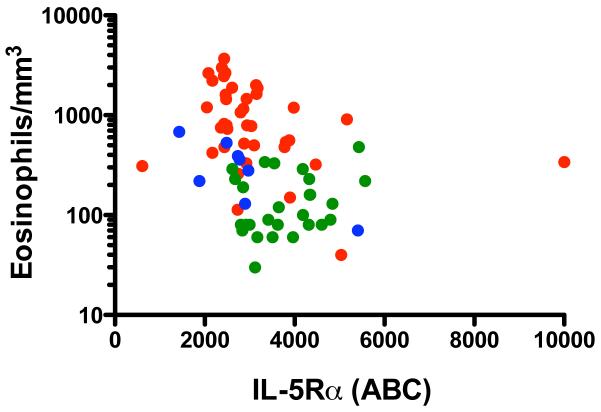
After preliminary, semi-quantitative studies showed a decrease in membrane expression of IL-5Rα on eosinophils from eosinophilic patients as compared to normals (data not shown), surface IL-5Rα expression was quantified on peripheral blood eosinophils from patients with eosinophilic disorders (n=39), KIT D816V-positive systemic mastocytosis (n=8) and normal volunteers (n=28). As shown in Figure 3, an inverse correlation was observed between surface expression of IL-5Rα and absolute eosinophil count (r = −0.48, p<0.0001, Spearman rank correlation).
Figure 3.
Surface expression of IL-5Rα on eosinophils is inversely correlated with peripheral blood eosinophilia. Each circle represents an individual data point. Subjects with eosinophilic disease are shown in red, those with mastocytosis in blue and normals in green. ABC = antibodies bound per cell. P<0.0001, r = −0.48, Spearman rank correlation
To examine the role of mRNA expression in the downregulation of surface IL-5Rα on peripheral eosinophils in vivo, transmembrane (TM) and soluble (SOL) IL-5Rα mRNA was quantified in eosinophils from 21 untreated subjects with varied eosinophilic disorders and in 3 normal controls. The level of TM IL-5Rα mRNA was negatively correlated with eosinophilia (Spearman rank correlation: r=−0.52 (−0.781, −0.096), p=0.016). Although TM IL-5Rα mRNA appeared to increase with increasing surface receptor expression (r=0.56), paired data were available for only 10 subjects, and the correlation was not statistically significant. SOL IL-5Rα mRNA levels were not correlated with eosinophil count (n=21) or soluble receptor levels (n=18).
Bone marrow mast cells may contribute to the increased levels of soluble IL-5Rα in patients with SM
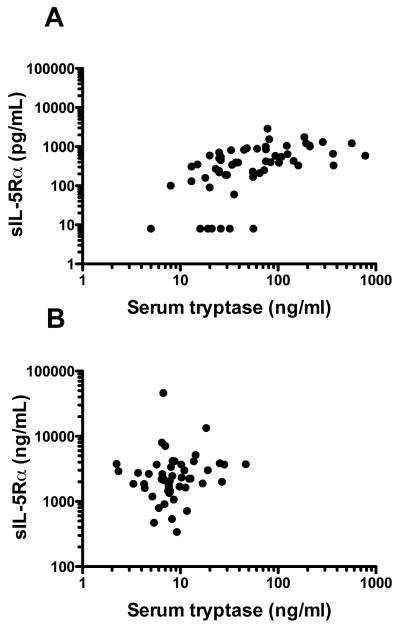
In order to confirm that mast cells express IL-5Rα, bone marrow aspirates were obtained from 17 patients with KIT D816V-positive SM and 6 patients with FIP1L1/PDGFRA-negative HES. As expected, bone marrow eosinophilia was significantly increased in HES patients, whereas mast cells were markedly increased in patients with SM (See Table E1 in the Online Repository). IL-5Rα expression was detected on mast cells from all patients tested and was comparable to that seen on bone marrow eosinophils (Figure E1 in the Online Repository). To determine if mast cells are capable of producing transcript for the soluble form of the receptor, bone marrow mast cells were flow sorted from a patient with KIT D816V-positive SM. Soluble transcript was detected by RT-PCR (Figure E2). Serum levels of sIL-5Rα were positively correlated with serum tryptase, a surrogate for total mast cell burden22, in patients with SM (r=0.62, P<0.0001, Spearman rank correlation; Figure 4a), but not in patients with eosinophilia in the absence of SM (r=0.19, P=0.2, Spearman rank correlation; Figure 4b).
Figure 4.
Serum levels of sIL-5Rα are correlated with serum tryptase levels in patients with SM, but not HES without SM. Serum tryptase and sIL-5Rα levels are shown for subjects with SM (r=0.62, P<0.0001, Spearman rank correlation, panel A) and eosinophilia without SM (r=0.19, P=0.2, panel B). Each circle represents an individual data point.
Discussion
IL-5 and its receptor play a central role in eosinophilia and eosinophil activation in eosinophilic disorders and have been exploited successfully as therapeutic targets in hypereosinophilic syndrome2, Churg-Strauss syndrome23 and eosinophilic asthma3,4,6. In the current study, we examined a large cohort of patients with a broad range of peripheral eosinophil counts and quantified expression of both membrane and soluble IL-5Rα protein in vivo. Not only did we observe an increase in soluble receptor levels and a decrease in membrane receptor expression on eosinophils with increasing peripheral blood eosinophilia, but these changes were also correlated with serum levels of IL-5 and IL-13. In contrast to in vitro studies, no correlation was observed with serum levels of IL-3 or GM-CSF. Although eosinophil production of IL-13 cannot be entirely excluded as the reason for the observed correlation with serum IL-13 levels, recent data demonstrating that T cells are the primary source of IL-13 in patients with eosinophilic lung disease24 suggest that the relationship between IL-13 and sIL-5Rα is more likely due to a generalized skewing of the immune response towards a type 2 phenotype. This is supported by the fact that sIL-5Rα levels were also correlated with serum IgE levels. In contrast to a recent study describing the association of a polymorphism in the promoter for IL-13 with SM25, serum IL-13 levels were not increased in our patients with SM as compared to normal controls (data not shown).
Of note, levels of sIL-5Rα were significantly increased in subjects with SM without eosinophilia. Although the expression of functional IL-5Rα on mast cells cultured in vitro from human cord blood derived CD133+ progenitors has been previously reported26-28, information on IL-5Rα expression on bone marrow mast cells ex vivo is lacking. In the current study, we demonstrate that bone marrow mast cells from patients with either KIT D816V-positive SM or FIP1L1/PDGFRA-negative HES express IL-5Rα at levels comparable to bone marrow eosinophils. Moreover, sIL-5Rα mRNA was detected in the bone marrow mast cells of a patient with KIT D816V-positive SM, suggesting that mast cells are capable of producing the soluble form of the receptor. Serum levels of sIL-5Rα did correlate with serum tryptase levels in patients with SM, consistent with mast cells as a potential source of the increased soluble receptor levels in these patients. However, the increased surface expression of CD69 on eosinophils and elevated serum EDN levels in patients with SM without eosinophilia suggest that activated eosinophils may also play a role. Although eosinophilia has been reported in a subset of patients with SM, the mechanism of eosinophil activation in SM without peripheral eosinophilia has not been studied and may be due to mast cell production of mediators known to activate eosinophils, including IL-33 and IL-5 or direct effects of the KITD816V mutation, which has been shown to be present in eosinophils from patients with SM29.
Modulation of IL-5Rα expression on eosinophils in vitro has been described in response to IL-5, IL-3, GM-CSF and IL-98,12-14, although the mechanism of this modulation remains unclear. Although internalization of the common β-chain of the IL-5R has been described in response to IL-5 binding and is essential for signaling30, the mechanism of IL-5Rα modulation has been less well-studied. Some in vitro studies have demonstrated changes in isoform splicing in response to cytokine stimulation12,13; whereas others have supported cleavage of surface receptors as the primary mechanism8. In a mouse model of schistosomiasis, serum IL-5 levels had no effect on differential splicing of the isoforms in Siglec-F+ bone marrow cells31. In contrast, in a study of 34 patients with nasal polyposis (NP), the relative amount of soluble to membrane IL-5Rα mRNA expression was increased in nasal polyp tissue from asthmatic patients with NP as compared to non-asthmatic patients with NP and normal controls32. Furthermore, mRNA levels for the soluble and membrane isoforms were correlated (positively and negatively, respectively) with tissue eosinophilia. Serum levels of sIL-5Rα (and peripheral blood eosinophilia) were also increased in the patients with NP and asthma, although no correlation with eosinophilia was observed. In the present study, we found evidence to support modulation of membrane, but not soluble, IL-5Rα levels at the mRNA level in peripheral eosinophils in vivo. Whether the discordance between our results and those reported in the nasal polyp study is due to differences between blood and tissue eosinophils, technical issues or other factors remains to be elucidated.
Modulation of IL-5Rα on the cell surface, as well as increased serum levels of the soluble receptor, in subjects with eosinophilia and mastocytosis raise the possibility that agents targeting the IL-5R may be less effective in these patient groups. Although serum levels of soluble receptor exceeded 5 ng/ml in many subjects in the current study, this is unlikely to be sufficient to interfere with agents currently in development. In fact, in a recent study of the IL-5Rα monoclonal antibody, benralizumab (MEDI-563) therapy in asthma, mean maximum antibody concentrations after a single dose of 0.03 to 3 mg/kg ranged from 1 μg/ml to 82 μg/ml and were dose-dependent6. The effect of downmodulation of surface receptor on dose response is less clear and will require additional studies. In summary, surface expression of IL-5Rα on eosinophils is decreased and serum levels of sIL-5Rα are increased in patients with eosinophilia and/or mastocytosis. These data are consistent with an in vivo IL-5R regulatory pathway in human eosinophils (and possibly mast cells) similar to that described in vitro and involving a balance between surface and soluble receptor levels. The observed increase in serum sIL-5Rα is correlated with serum levels of IL-5 and IL-13, as well as with serum EDN levels. Serum sIL-5Rα levels are also correlated with serum tryptase levels in patients with SM. Whether mast cells are directly responsible for the increase in sIL-5Rα observed in SM patients without eosinophilia and/or act indirectly by stimulating eosinophils remains to be elucidated. Increases in serum levels of soluble IL-5Rα and downmodulation of IL-5Rα on the cell surface in patients with eosinophilic and mast cell disorders may have important implications with respect to the efficacy of novel therapeutic agents targeting IL-5 and its receptor in these patient groups.
Supplementary Material
Table E1. Bone marrow features of subjects with SM or HES.
Key Messages.
Surface expression of IL-5Rα on eosinophils is decreased and serum levels of sIL-5Rα are increased in patients with eosinophilia and/or systemic mastocytosis.
Soluble IL-5Rα levels are positively correlated with eosinophilia, serum levels of IL-5 and IL-13 and markers of eosinophil activation in all subjects and with serum tryptase levels in subjects with systemic mastocytosis.
These findings are consistent with an in vivo IL-5R regulatory pathway in human eosinophils (and possibly mast cells) similar to that described in vitro and involving a balance between surface and soluble receptor levels and may have therapeutic implications.
Acknowledgments
We thank all the subjects as well as the many clinical research staff, including Cheryl Talar-Williams, Melissa Law, Kathryn Spates, Kristin Young, Amara Pabon, Eunice Fox, Linda Scott, Laura Wisch, Daly Cantave, Nina Jones and Yun Bai, for their contributions. T.M.W. and I.M. helped design and perform the research, analyze the data and write the paper; J.S., M.B., O.S., C.S. and P.K. helped perform the research and analyze the data; M.P.F. analyzed the data and performed the statistical analyses, A.K. and R.K. contributed vital new reagents and protocols and participated in the writing of the paper; D.D.M. helped design the research and write the paper; and A.D.K. directed the research and participated in the design and performance of the research, analysis of the data, and writing of the paper.
Funding: This study was supported in part by the Division of Intramural Research of the NIAID, NIH. MedImmune LLC provided the protocol and reagents for the sIL-5Rα ELISA.
Abbreviations
- ABC
antibodies bound per cell
- AEC
absolute eosinophil count
- BE
benign eosinophilia
- CEL
chronic eosinophilic leukemia
- CM
cutaneous mastocytosis
- CT
cycle threshold
- EDN
eosinophil-derived neurotoxin
- EO
eosinophilia
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HES
hypereosinophilic syndrome
- IL
interleukin
- IL-5R
IL-5 receptor
- sIL-5R
soluble IL-5 receptor
- PARA
helminth infection
- PBS
phosphate-buffered saline
- SOL
soluble
- SM
systemic mastocytosis
- TM
transmembrane
- SM-eo
systemic mastocytosis with eosinophilia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tavernier J, Devos R, Cornelis S, et al. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 3.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 5.Kolbeck R, Kozhich AA, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–1353. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor α antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Tavernier J, Tuypens T, Plaetinck G, Verhee A, Fiers W, Devos R. Molecular basis of the membrane-anchored and two soluble isoforms of the human interleukin 5 receptor α subunit. Proc Natl Acad Sci. 1992;89:7041–7045. doi: 10.1073/pnas.89.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL-5 receptor α on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 9.Rohde G, Gevaert P, Holtappels G, et al. Soluble interleukin-5 receptor alpha is increased in acute exacerbations of chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2004;135:54–61. doi: 10.1159/000080043. [DOI] [PubMed] [Google Scholar]
- 10.Gevaert P, Bachert C, Holtappels G, et al. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003;58:371–379. doi: 10.1034/j.1398-9995.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 11.Monahan J, Siegel N, Keith R, et al. Attenuation of IL-5-mediated signal transduction, eosinophil survival, and inflammatory mediator release by a soluble human IL-5 receptor. J Immunol. 1997;159:4024–4034. [PubMed] [Google Scholar]
- 12.Tavernier J, Van der Heyden J, Verhee A, et al. Interleukin 5 regulates the isoform of its own receptor α -subunit. Blood. 2000;95:1600–1607. [PubMed] [Google Scholar]
- 13.Gregory B, Kirchem A, Phipps S, et al. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor α -chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor α expression with loss of IL-5 responsiveness, but upregulate IL-3 receptor α expression. J Immunol. 2003;170:5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 14.Gounni AS, Gregory B, Nutku E, et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2007;96:2163–2171. [PubMed] [Google Scholar]
- 15.Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL-5 receptor α on human eosinophils: I. Loss of membrane IL-5 receptor α on airway eosinophils and increased soluble IL-5 receptor α in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . In: Classification of Tumors: Tumours of the Haematopoietic and Lymphoid Tissues. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. International Agency for Research on Cancer (IARC) Press; Lyon, France: 2008. [Google Scholar]
- 17.Wilson TM, Maric I, Simakova O, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hildesheim A, Ryan RL, Rinehart E, et al. Simultaneous measurement of several cytokines using small volumes of biospecimens. Cancer Epidemiol Biomarkers Prev. 2002;11:1477–1484. [PubMed] [Google Scholar]
- 19.Klion AD, Law MA, Riemenschneider W, et al. Familial eosinophilia: a benign disorder? Blood. 2004;103:4050–4055. doi: 10.1182/blood-2003-11-3850. [DOI] [PubMed] [Google Scholar]
- 20.Perez C, Vandesompele J, Vandenbroucke I, et al. Quantitative real time polymerase chain reaction for measurement of human interleukin-5 receptor alpha spliced isoforms mRNA. BMC Biotechnol. 2003;3:17. doi: 10.1186/1472-6750-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright SP. Adjusted p-values for simultaneous inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
- 22.Sperr WR, Jordan JH, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Marogowda G, Oren E, et al. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–43. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui S, Cruse G, McKenna S, et al. IL-13 expression by blood T cells and not eosinophils is increased in asthma compared to non-asthmatic eosinophilic bronchitis. BMC Pulm Med. 2009;9:34. doi: 10.1186/1471-2466-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedoszytko B, Niedoszytko M, Lange M, et al. Interleukin-13 promoter gene polymorphism -1112C/T is associated with the systemic form of mastocytosis. Allergy. 2009;64:287–294. doi: 10.1111/j.1398-9995.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 26.Dahl C, Hoffmann HJ, Saito H, Schiotz PO. Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy. 2004;59:1087–1096. doi: 10.1111/j.1398-9995.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 27.Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and IL-5 prime human mast cells for different profiles of IgE dependent cytokine production. Proc Natl Acad Sci USA. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulka M, Metcalfe D. High-resolution tracking of cell division demonstrates differential effects of TH1 and TH2 cytokines on SCF-dependent human mast cell production in vitro: correlation with apoptosis and Kit expression. Blood. 2005;105:592–599. doi: 10.1182/blood-2004-07-2838. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–72. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 30.Lei JT, Martinez-Moczygemba M. Separate endocytic pathways regulate IL-5 receptor internalization and signaling. J Leuk Biol. 2008;84:499–506. doi: 10.1189/jlb.1207828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bystrom J, Dyer KD, Ting-De RSS, et al. Interleukin-5 does not influence differential transcription of transmembrane and soluble isoforms of IL-5R α in vivo. Eur J Haematol. 2006;77:181–190. doi: 10.1111/j.1600-0609.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 32.Gevaert P, Hellman C, Lundblad L, et al. Differential expression of the interleukin 5 α receptor isoforms in blood and tissue eosinophils of nasal polyp patients. Allergy. 2009;64:725–732. doi: 10.1111/j.1398-9995.2008.01885.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Bone marrow features of subjects with SM or HES.