Abstract
Based on reactions with five flavonoids, the regioselectivities of twelve human UDP-glucuronosyltransferase (UGT) isozymes were elucidated. The various flavonoid glucuronides were differentiated based on LC-MS/MS fragmentation patterns of [Co(II)(flavonoid – H)(4,7-diphenyl-1,10-phenanthroline)2]+ complexes generated upon post-column complexation. Glucuronide distributions were evaluated to allow a systematic assessment of the regioselectivity of each isozyme. The various UGT enzymes, including eight UGT1A and four UGT2B, displayed a remarkable range of selectivities, both in terms of the positions of glucuronidation and relative reactivity with flavanones versus flavonols.
Keywords: Human UDP-Glucuronosyltransferase, Flavonoid, Regioselectivity, Mass Spectrometry, Metal Complexation, Glucuronidation
1. Introduction
Flavonoids, a class of polyphenols found in fruits and vegetables, have been shown not only to have anti-inflammatory properties but also to exhibit promising chemopreventive properties against cancer and cardiovascular disease [1,2]. The positive bioactivities of flavonoids have been demonstrated in a variety of in vitro, in vivo, and case control studies [2-4]. In recent years the presumed chemopreventive properties have been under closer scrutiny due to the poor bioavailability of most unmodified flavonoid aglycones in the body [5], coupled with the growing documentation that flavonoids undergo extensive biotransformation [6-8]. The metabolism of flavonoids has a great impact on their absorption and distribution, and importantly biotransformation can substantially alter the chemical properties of the flavonoids, such as altering the bioactivities [9].
Most flavonoids are found naturally as glycosylated forms in fruits and vegetables. When ingested the flavonoid glycosides undergo deglycosylation by β-glucosidase or lactose phloridzin hydrolase enzymes primarily found in the small intestine [10]. After loss of their sugar side-chains, flavonoids are rapidly metabolized by mainly Phase II enzymes found in small intestine, kidneys, and most importantly the liver [10]. This process results in glucuronidation, sulfation, methylation, or hydroxylation depending on the nature of the interacting enzyme [11]. Any flavonoid compound metabolized or unmodified that is not absorbed prior to reaching the large intestine may be absorbed by microflora, a process leading to decomposition of the flavonoid by ring fission and causing the release of small phenolic acids that are excreted in the urine [10]. Since most of the flavonoids ingested are conjugated and consequently absorbed as conjugates, there has been increasing interest in understanding the formation, uptake, distribution, and chemopreventive properties of the conjugates. To facilitate such investigations, the development of sensitive analytical methods to characterize, identify and track the flavonoid conjugates is paramount.
Glucuronidation of flavonoids is carried out in the body by the UDP-glucuronosyltransferase (UGT) family of enzymes. These enzymes have been found in every major organ involved in digestion, as well as the kidneys and liver [11]. To date, nineteen different isomers of the UGT enzyme have been identified [12], which are categorized into three different subgroups (UGT1As, UGT2As and UG2Bs). There are nine isoforms of the UGT1A group and seven in the UGT2B group, and together they play a major role in Phase II metabolism. The role of UGT2A isoforms remains unknown [12]. UGT enzymes catalyze the addition of glucuronic acid at a hydroxyl group, carboxylic acid, sulfide group, amine, or in rare cases a methyl group [11]. Flavonoids possessing one to multiple hydroxyl groups may undergo O-glucuronidation at various positions when metabolized by UGT enzymes. However, the specific positions which are glucuronidated by each enzyme are still not fully established.
The structural characterization of flavonoids and their metabolites has proven to be a challenging task. All flavonoids share the same basic three-ring structure and may differ by the position of a single functional group, making their positive identification difficult by many analytical methods. Mass spectrometry has proven to be one of the most effective tools for identification of flavonoids, in large part due to the informative fragmentation patterns generated by collision induced dissociation (CID) upon application of MS/MS strategies [13], especially when coupled with HPLC to allow separation of complex mixtures of flavonoids [14-20]. We have extended the capabilities of MS/MS methods for differentiation of flavonoids by formation of complexes containing a flavonoid, a metal, and an auxiliary organic ligand [21]. These complexes, upon CID, give unique fragmentation patterns that allow confident identification and differentiation of flavonoids, even for isomers. We have evaluated a number of metal complexation approaches and shown their versatility [22-29], including the adaptation of the methods for identification of metabolites in urine and plasma [30-33]. More recently, we applied the metal complexation/MS/MS methodology to gain insight into the regioselectivity of the UGT1A1 enzyme with various flavonoids [34]. In this prior study, the products were confidently identified and the distributions of various glucuronidated products were quantified.
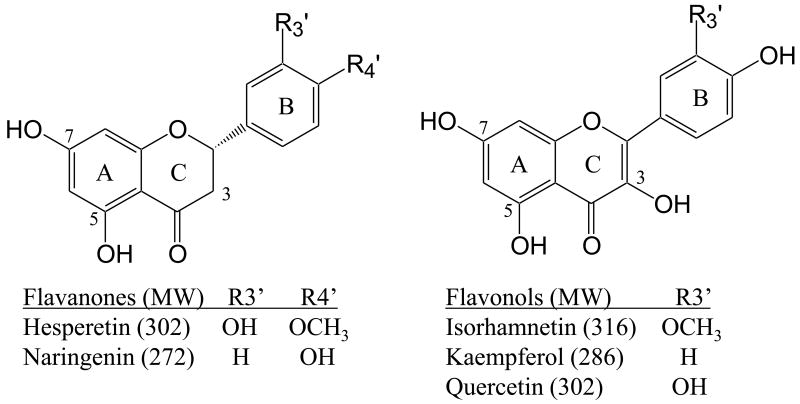
In this present study, we have expanded our investigation of the selectivity of glucuronidation of the twelve most common UGT enzymes (1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, 2B17) for five of the most commonly consumed flavonoids (hesperetin, isorhamnetin, kaempferol, naringenin, quercetin). (Figure 1) While biotransformation of flavonoids has been an area of much interest [12,35-40], this is the first time the isomeric flavonoid glucuronide products of such a large array of enzymatic syntheses have been determined, thus providing detailed insight into the selectivities of the UGT isoenzymes. This systematic study provides benchmark data for assessment of UGT enzymatic regioselectivity and establishes predictive correlations of biotransformation upon consumption of flavonoids.
Figure. 1. Structures of flavonoids.
2. Methods and Materials
2.1 Reagents
All UDP-glucuronosyltransferase isozymes were purchased from BD Biosciences (Woburn, MA, USA). UDP-Glucuronic acid (UDPGA) trisodium salt, 4,7-diphenyl-1,10-phenanthroline (4,7-dpphen), cobalt(II) bromide, hesperetin, naringenin, isorhamnetin, kaempferol, and quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade acetonitrile, HPLC grade water, potassium phosphate, and methanol were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
2.2 Synthesis of Flavonoid Glucuronides by UGT Enzymes
The procedure for the glucuronidation reactions was modified from the protocol reported in Davis et al. [34]. Each enzyme was divided into 25 μL aliquots and stored at -80 °C until use. The following reaction was set up for each combination of UGT enzyme (UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, 2B17) and flavonoid (hesperetin, isorhamnetin, kaempferol, naringenin, quercetin). All volumes were delivered using appropriate micropipettes. The synthesis was carried out by adding 2 mM aqueous UDPGA (65 μL), 20 mM potassium phosphate buffer pH 7.0 (378.75 μL), and 10 mM methanolic solution of flavonoid (6.25 μL) to a microcentrifuge tube. The reaction was started by addition of 25 μL of a UGT enzyme (5 mg/mL). This concentration of enzyme was used based on experiments reported by Plumb et al.[41]. The mixture was incubated at 37 °C overnight. To stop the reaction, 1.5 mL of acetone was added. The tubes were centrifuged for 10 min at 16,000 g. The supernatant was removed and the acetone was evaporated by placing the tubes in a Savant DNA120 SpeedVac Concentrator (Thermo Electron, Waltham, MA, USA) on low heat for 1 hour and 40 min. The remaining mixture was refrigerated until analysis. In a study by Easterbrook et al. [42], the activities of UGT enzymes were evaluated in the presences of organic solvents at various concentrations, and it was reported that there were no apparent effects on enzyme activities for solutions containing up to 2% methanol content, so a significant effect on enzymatic activity was not anticipated for the present study. A low concentration of methanol was used in the present study to enhance solubility of the flavonoids and assure more accurate concentrations in solution.
2.3 LC-MS/MS Analysis
LCMS analysis of the flavonoid glucuronides was undertaken using a Waters Alliance 2695 HPLC system (Milford, MA, USA) or a Hitachi L-7000 HPLC system (Hitachi High Technologies America, Pleasanton, CA, USA) and LCQ Duo quadrupole ion trap mass spectrometer (Thermo Electron, Waltham, MA, USA) with electrospray ionization (ESI). The column was a Waters Symmetry C18 column, 2.1 × 50 mm, 3.5 μm particle size, with a guard column. The injection volume was 30 μL. The mobile phase was 0.33% formic acid in water (A) and 0.33% formic acid in acetonitrile (B). The gradient used began at 15 % B and increased to 40 % over 30 min.
Samples were first analyzed in the negative ESI mode in order to search for flavonoid glucuronides. The spray voltage was set at 4.5 kV, the heated capillary temperature was 200 °C, and the automatic gain control was set to 5 × 107 ions with a maximum injection time of 500 ms and 5 microscans averaging. All other parameters were set to obtain optimal signal. The positive ESI mode was used for MS/MS analysis of the flavonoid/metal complexes. The metal complexes were formed by post-column addition of a methanolic solution of 5 μM CoBr2 and 4,7-dpphen, which was infused at a rate of 20 μL/min controlled by a syringe pump. The spray voltage for the positive ion mode was set to 5 kV, and the heated capillary temperature was 200 °C. The automatic gain control for MS/MS was set to 2 × 107 ions with a maximum injection time of 500 ms and 5 microscan averaging, the isolation width was set to 4 Da, and a collision energy of 35% normalized collision energy was used for collision induced dissociation.
2.4 LC-UV Monitoring of Reaction Products
In order to estimate the relative product distributions of different flavonoid glucuronides for each enzymatic reaction leading to multiple products, the peak area for each resulting product was integrated based on its LC-UV chromatographic profile at 360 nm. The area of each product peak was divided by the total area of all product peaks in order to calculate the product distributions as percentages. This percentage was rounded to the nearest 5%.
3. Results
Our objective was to map the formation of various flavonoid glucuronides for each glucuronosyltransferase enzyme. In order to differentiate the flavonoid glucuronide isomers, MS/MS spectra of the metal complexes [Co(II) (FG – H) (4,7-dpphen)2]+ were analyzed along with HPLC retention times (where FG represents a flavonoid glucuronide). These metal complexes were produced via post-column complexation in LCMS runs of the product mixtures obtained for each flavonoid/glucuronosyltransferase combination. Whereas the fragmentation patterns of deprotonated flavonoid glucuronides are typically not distinctive for isomers, the MS/MS spectra of the metal complexes allows differentiation and confident elucidation of the isomers. As described previously [30,34], the metal complexes containing 7-O-glucuronides show losses of the auxiliary ligand (-Aux) and the glucuronic acid moiety (-GlcA), both individually or together (−(GlcA+Aux)), upon CID. The loss of the flavonoid aglycon (-Agl) is also a characteristic fragment of 7-O-glucuronides. The metal complexes of the 5-O-glucuronides and 3-O-glucuronides both show a prominent loss of the glucuronic acid moiety in conjunction with the auxiliary ligand (−(GlcA+Aux)); moreover, the 5-O product has been shown to elute prior to the 7-O product [34] whereas the 3-O-glucuronide elutes after. For B-ring-glucuronides, the characteristic fragments include the loss of the auxiliary ligand (−Aux) and the losses of both the auxiliary ligand and glucuronide moiety together (−(GlcA+Aux)) [30]. 4′-O-glucuronides elute before 3′-O glucuronides and after the 3-O-glucuronides. These systematic MS/MS and elution patterns were utilized for assignment of the glucuronide products in the present study. A table listing all the fragments found for the products is shown in Supplement Table 1.
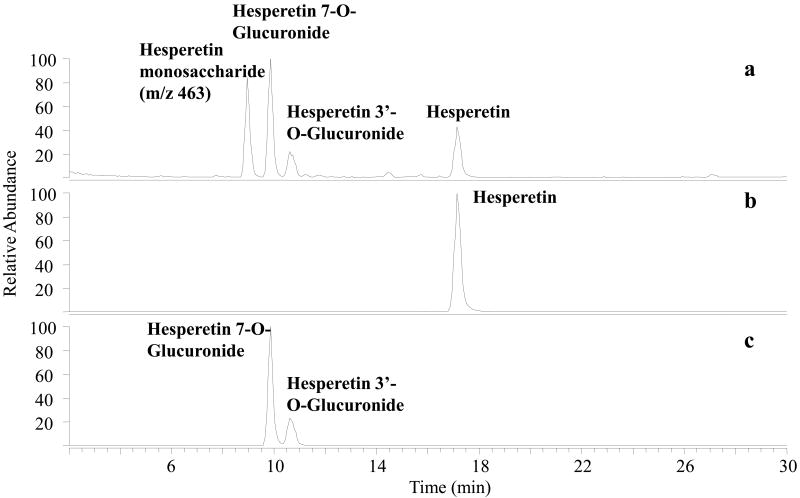
Once the enzymatic reactions were quenched, each of the incubates was centrifuged and the supernatant was screened by LCMS in the negative ESI mode. The resulting total ion chromatograms were searched both for the unreacted flavonoid aglycon, monoglucuronidated flavonoid products (aglycon + 176), and diglucuronidated products (aglycon +176 +176) based on the m/z values of the anticipated products. A typical total ion chromatogram obtained for the hesperetin product mixture is shown in Figure 2a with extracted ion chromatograms shown in Figures 2b and 2c for deprotonated hesperetin (m/z 301) and the monoglucuronidated products (m/z 477). No diglucuronidated products were detected. After screening the enzymatic incubates, then the metal complexes were generated upon LCMS by post-column complexation and subjected to CID for structural characterization. Examples of the resulting MS/MS spectra are shown in Figure 3 for hesperetin glucuronides produced from UGT1A6 and in Figure 4 for kaempferol glucuronides produced from UGT1A1. All products identified are summarized for the two classes of flavonoids (flavanones and flavanols) in Table 1 along with the distribution of products based on integration of the chromatographic peak areas of each product and unreacted flavonoid. Inclusion of the unreacted flavonoids gives an indication of the relative reactivity promoted by each enzyme for a particular flavonoid.
Figure. 2.
a) Total ion chromatogram for UGT1A10 reaction with hesperetin, including background subtraction. b) Selected ion chromatogram for deprotonated hesperetin (m/z 301). c) Selected ion chromatogram for deprotonated hesperetin monoglucuronide (m/z 477).
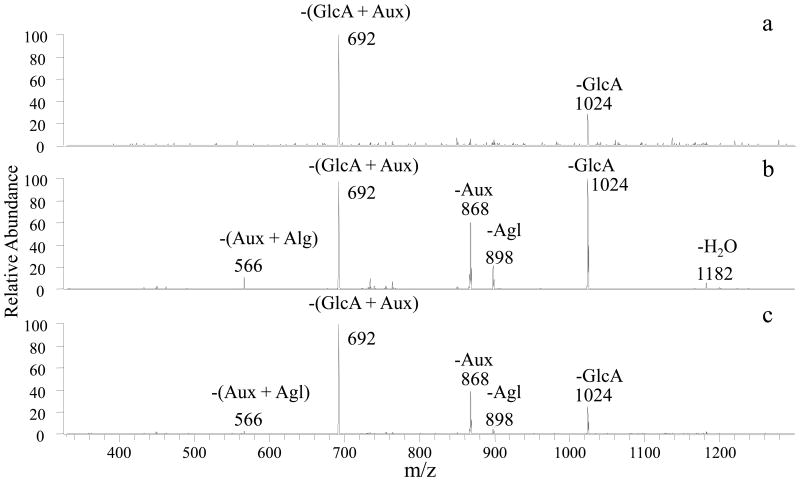
Figure 3.
CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all UGT1A6/hesperetin products: a) 5-O glucuronide, m/z 1200 b) 7-O glucuronide, m/z 1200 c) 3′O- glucuronide, m/z 1200. –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
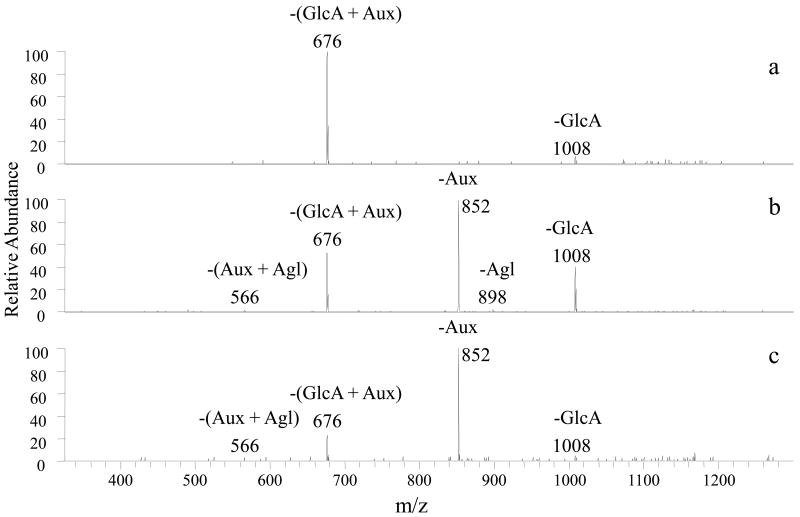
Figure 4.
CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all UGT1A1 kaempferol products: a) 5-O-Glucuronide, (m/z 1184) b) 7-O-Glucuronide, (m/z 1184) c) 4′-O-Glucuronide, (m/z 1184). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Table 1. Glucuronide product distributions relative to unreacted flavonoid.
All values are percentages of total product and unreacted flavonoid distribution. Values in parenthesis indicate products with unconfirmed structures, meaning that two alternatives are possible. A dash is used to indicate the absence of a product. All values rounded to the nearest 5%. The average standard deviation is ±5%.
| Hesperetin | 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B4 | 2B7 | 2B15 | 2B17 |
| Hesperetin | 60 | 15 | 100 | 10 | 45 | 70 | 30 | 75 | 80 | 60 | 100 | 100 |
| 5-O-Glucuronide | - | - | - | Trace | - | - | - | - | - | - | - | - |
| 7-O-Glucuronide | 30 | 10 | - | 90 | 5 | 10 | 30 | 15 | 20 | 30 | Trace | Trace |
| 3′-O-Glucuronide | 10 | 75 | - | Trace | 50 | 20 | 40 | 10 | Trace | 10 | Trace | Trace |
| Naringenin | 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B4 | 2B7 | 2B15 | 2B17 |
| Naringenin | 40 | 50 | 100 | 30 | 80 | 65 | 20 | 90 | 70 | 80 | 95 | 95 |
| 5-O-Glucuronide | - | - | - | Trace | - | - | - | - | - | - | - | - |
| 7-O-Glucuronide | 50 | 50 | - | 70 | 20 | 35 | 80 | 10 | 30 | 20 | 5 | 5 |
| 4′-O-Glucuronide | - | - | - | - | - | - | - | - | - | - | - | - |
| Isorhamnetin | 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B4 | 2B7 | 2B15 | 2B17 |
| Isorhamnetin | 80 | 35 | 100 | 100 | 90 | 100 | 95 | 95 | 100 | 100 | 100 | 100 |
| 5-O-Glucuronide | - | - | - | - | 10 | - | 5 | Trace | - | - | - | - |
| 7-O-Glucuronide | - | 50 | - | - | Trace | - | - | Trace | - | - | - | - |
| 3-O-Glucuronide | (20) | (15) | - | - | - | - | - | (5) | - | - | - | - |
| 4′-O-Glucuronide | (20) | (15) | - | - | - | - | - | (5) | - | - | - | - |
| Kaempferol | 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B4 | 2B7 | 2B15 | 2B17 |
| Kaempferol | 40 | 90 | 100 | 60 | 75 | 90 | 75 | 100 | 95 | 100 | 100 | 100 |
| 5-O-Glucuronide | 5 | Trace | - | 30 | 10 | Trace | 10 | Trace | - | Trace | - | - |
| 7-O-Glucuronide | 50 | 10 | - | 10 | 15 | 5 | 15 | Trace | 5 | Trace | - | - |
| 3-O-Glucuronide | - | - | - | - | - | - | - | - | - | - | - | - |
| 4′-O-Glucuronide | 5 | Trace | - | Trace | - | 5 | Trace | Trace | - | Trace | - | - |
| Quercetin | 1A1 | 1A3 | 1A4 | 1A6 | 1A7 | 1A8 | 1A9 | 1A10 | 2B4 | 2B7 | 2B15 | 2B17 |
| Quercetin | 25 | 55 | 100 | 95 | 100 | 100 | 70 | 95 | 100 | 90 | 100 | 100 |
| 5-O-Glucuronide | - | - | - | Trace | - | - | - | - | - | Trace | - | - |
| 7-O-Glucuronide | 5 | 25 | - | 5 | - | Trace | 30 | 5 | - | - | - | - |
| 3-O-Glucuronide | - | - | - | - | - | - | - | - | - | - | - | - |
| 4′-O-Glucuronide | 10 | 5 | - | - | - | Trace | - | Trace | - | - | - | - |
| 3′-O-Glucuronide | 60 | 15 | - | - | - | Trace | - | Trace | - | 10 | - | - |
3.1 Flavanone Glucuronides
Hesperetin formed monoglucuronidated products upon exposure to all the glucuronosyltransferases except for UGT1A4 (which in fact produced no glucuronides for any of the flavonoids). These products were analyzed by the metal complexation/MS/MS method described in the Experimental section and assigned based on their characteristic fragmentation patterns and retention times. For UGT1A1, 1A3, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17, two products were found (Table 1). The MS/MS spectrum of the first glucuronide product showed losses of GlcA, Agl, and Aux, which is characteristic of 7-O-glucuronide products. The second eluting product yielded the prominent loss of (GlcA+Aux) as well as the loss of Aux, which as described above is characteristic of 3′-O-glucuronidation. For UGT1A6, hesperetin showed a total of three products (see corresponding MS/MS patterns in Figure 3). Two of the products are identical to those described above for the other glucuronosyltransferase reactions. The third product eluted prior to the 7-O-glucuronide, and the MS/MS spectrum shows one prominent loss of (GlcA+Aux), thus confirming this product as a 5-O-glucuronide.
For naringenin all of the glucuronosyltransferases resulted in only a single product except for UGT1A4, which promoted no products at all and UGT1A6 which led to the formation of two products. The single dominant product dissociated by pathways characteristic of a 7-O-glucuronide (losses of GlcA, Agl, and Aux). For the UGT1A6 reaction the second product showed a prominent loss of (GlcA +Aux) which is assigned as the 5-O-glucuronide owing to its elution prior to the 7-O-glucuronide product. (See CID mass spectra in Supplementary Figure 1.)
3.2 Flavonol Glucuronides
Upon reactions with most of the glucuronosyltransferases, isorhamnetin displayed no reactivity in the presence of the UGT1A4, 1A6, 1A8, 2B4, 2B7, 2B15, and 2B17 enzymes. Reaction with the UGT1A3 enzymes led to the formation of two products. The first product showed losses of GlucA, Agl and Aux upon CID, allowing ready assignment as the 7-O-glucuronide. The second product demonstrated loss of (GlcA+Aux) as the major product, and also showed a loss of Aux though this fragment occurred in very low abundance. This product could either be a 3-O glucuronide or a 4′-O glucuronide because its elution after the 7-O glucuronide ruled it out as a 5-O glucuronide. Moreover the 3′ position has a methoxy group, and the absence of a methyl group loss allowed a 3′-glucuronide structure to be ruled out. The minor loss of Aux in the MS/MS spectrum suggested that this was the 4′-O glucuronide, but the evidence was somewhat inconclusive due to the lack of other diagnostic ions.
The UGT1A1 enzyme promoted the formation of one product for isorhamnetin that dissociated by a major loss of (GlcA+Aux) and a minor loss of Aux which had a very low abundance. Since there are no other products in this reaction, confident identification of this product was not possible; however comparing the elution time of this product with confirmed products of other isorhamnetin/glucuronidation reactions shows that this product has a retention time matching that of the second product of the UGT1A3, which suggest that this product also having the minor loss of Aux in the CID spectrum appears to be a 4′-O-glucuronide. However this product could not be confidently distinguished between the 3-O-glucuronide and 4′-O-glucuronide structures. The UGT1A7 enzyme resulted in formation of two products upon reaction with isorhamnetin. The first product exhibited the dominant loss of (GlucA+Aux), and the second product displayed all the characteristic losses of a 7-O-glucuronide species (-GlucA, -Agl, -Aux). The first product corresponded to the 5-O-glucuronide due to its elution prior to the 7-O product. The UGT1A9 enzyme promoted formation of one product that dissociated by loss of (GlcA+Aux) upon CID, indicating that this could be a 3-O or 5-O glucuronide. With only a single product eluting, the only way this product can be identified is based on comparison of retention times to products from those of the 1A7 reaction. The elution time of the UGT1A9 product matched that of the 5-O-glucuronide identified from the 1A7 reaction mixture, thus confirming the UGT1A9 product as 5-O-glucuronide. (See CID mass spectra in Supplemental Figure 2).
The reaction of isorhamnetin with UGT1A10 yielded three products. The first two eluting products were identified as the 5-O glucuronide and 7-O glucuronide respectively; this is because the second product showed losses of GlucA, Agl, and Aux upon CID, allowing it to be identified as the 7-O glucuronide. The first eluting product showed a dominant loss of (GlucA+Aux), which because it eluted before the 7-O glucuronide can be readily assigned as the 5-O glucuronide. The third product showed a loss of (GlcA+Aux) as the major product, and also exhibited a loss of Aux although this latter fragment occurred in very low abundance similar to that of the second product of the UGT1A3 reaction. This product could not be confidently distinguished between the 3-O glucuronide and 4′-O glucuronide structures. NMR spectroscopy would arguably allow more confident identification and differentiation of these products, albeit requiring far greater quantities of products as well as isolation of individual products or use of LC-NMR.
Kaempferol formed three glucuronides (5-O, 7-O, and 4′-O) with the exception of reactions promoted by UGT1A4, UGT2B15, and UGT2B17 which resulted in no products, UGT1A7 which yielded only two products, and UGT2B4 which led to only one glucuronide. As described above, the identities of the products were assigned based on their MS/MS patterns, as illustrated in Figure 4, and their relative retention times. Upon analysis of the UGT1A1 glucuronides, the first eluting product dissociated by losses of GlcA and (GlucA+Aux), the second product demonstrated losses of GlcA, Agl, and Aux, some occurring together, and the third product showed prominent losses of Aux and (GlcA+Aux). The first product was readily identified as kaempferol-5-O-glucuronide because of the characteristic sole major loss of (GlucA+Aux), as well as the fact that this product eluted prior to the second product, the latter which is confidently assigned as the 7-O-glucuronide based on its characteristic fragmentation pattern. The third product could conceivably be a 3-O or 4′-O glucuronide, both which should elute after the 7-O-glucuronide. However, the fact that the loss of Aux was observed along with the combined loss of (GlcA+Aux) ruled out the 3-O-glucuronide. For the reactions with the UGT1A7 enzyme, the two products formed included the 5-O-glucuronide and 7-O-glucuronide but not the 4′-O-glucuronide. The one product formed for the UGT2B4 reactions was identified as the 7-O- glucuronide product, while neither the 5-O- glucuronide nor the 4′-O- glucuronide were produced.
Quercetin has five possible glucuronidation sites, and it generated three different products upon reaction with the UGT1A1, 1A3, 1A8, and 1A10 glucuronosyltransferases. Upon CID, the first eluting product showed fragment pathways characteristic of 7-O-glucuronide, including losses of GlcA, Agl, and Aux. The next two products both showed losses of Aux and Aux+GlcA. The first of these two products is assigned as the 4′-O-glucuronide based on its elution prior to the other 3′-O-glucuronide. Reaction with the UGT1A9 enzyme resulted in only one product, the 7-O-glucuronide. The UGT1A6 enzyme predominantly led to formation of the 7-O-glucuronide as well as the 5-O product which was not observed for any of the enzymatic reactions of quercetin. This 5-O product was confirmed because it showed only one characteristic loss (-(GlcA+Aux)) and eluted prior to the 7-O-glucuronide. The UGT2B7 enzyme led to formation of two products, the first of which showed the loss of (GlcA+Aux) as the only major fragmentation pathway while the second product showed losses of Aux and (GlcA+Aux). The fragmentation pattern of this second product was consistent with either a 3′-O glucuronide or a 4′-O glucuronide. The similar MS/MS patterns and elution times of 3′-O and 4′-O glucuronides did not allow conclusive assignment of this particular quercetin product. However, upon LCMS separation and analysis of a mixture containing the products from the UGT1A3 reaction and the products from this UGT2B7 reaction, the formation of the 4′-O glucuronide product was ruled out, and the product was identified as a 3′-O modification. The first product was identified as a 5-O glucuronide because of its similar retention time to the 5-O product confirmed from the UGT1A6 reactions. (See CID mass spectra in Supplemental Figure 3).
4.Discussion
4.1 Selectivity Trends
Each UGT enzyme exhibited selectivity with respect to the sites of glucuronidation of flavonoids. To establish a benchmark for evaluating the array of UGT glucuronosyltransferases, the glucuronidation trends for 1A1 in the present study were first compared to a previous limited set of results obtained using the same enzyme and LCMS/MS analysis [34]. It was previously reported that UGT1A1 selectively modifies only the hydroxyl group at the 7 position, unless there is a hydroxyl at the 3′ position in which case UGT1A1 also modifies hydroxyl groups on the B ring [34]. The relative quantities of the hesperetin and quercetin glucuronidation products in this current study were in good agreement with those found in the previous study [34]. The sole glucuronidation of naringenin at the 7-O position noted in the past study also matches the same finding in the present study. The glucuronidation of kaempferol, however, shows a discrepancy based on our present results relative to past results [34] and another recent study [40]. In one study, only the 7-O-glucuronide product was reported [34], unlike the three distinctive products (5-O, 7-O, and 4′-O) found in the present study. In a more recent study by Singh et al., the interaction of kaempferol with the UGT1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10, and 2B7 enzymes was evaluated based on a UV shift method for glucuronide differentiation [40]. Singh et al. reported modification of kaempferol at the 3-O position for several of the UGT enzymes, as well as a low likelihood of 5-O product formation, in contrast to our findings in which glucuronidation was found to occur at the 5-O position but not at the 3-O position based on the MS/MS patterns of the metal complexes. This difference may require the synthetic production of individual flavonoid glucuronides and NMR spectral analysis for confident resolution. Isorhamnetin is the only flavonoid whose glucuronidation in the presence of UGT1A1 has not been reported previously, and it in fact yields just one product.
The results for the UTG1A3 enzyme show that its selectivity differs slightly from that of UGT1A1, with greater preference for glucuronidation of the 7-O position of the flavonols. The best example of this is reflected by the quercetin and isorhamnetin results, in which the production of 7-O glucuronides is enhanced for the UGT1A3 reactions. This is particularly evident in the reactions of isorhamnetin with UGT1A3 for which the 7-O-glucuronide product was by far the most abundant, a product not even detected upon reaction in the presence of UGT1A1. The selectivity of the UGT1A3 enzyme for hesperetin is likewise very different from that of the UGT1A1 reaction, in that the 3′-O glucuronide is the most abundant product, not the 7-O product. A previous recent study reported the glucuronidation of isorhamnetin, kaempferol, and quercetin with UGT1A3 and UGT1A9 [35], but specific product identities were not assigned. In this past study three products were found for isorhamnetin, two for kaempferol, and four for quercetin [35]. For each of isorhamnetin and quercetin, this represents one additional product then reported in the present study but for kaempferol it is one less for the UGT1A3 reactions. Due to the lack of product identities [35], further comparisons to the present results were not possible.
Several other notable features emerged upon inspection of the glucuronidation results for the other enzymes. UGT1A6 proved to be unusual in that it is the only glucuronidase that is able to promote 5-O-glucuronidation of the two flavanones, hesperetin and naringenin, and also the flavonol quercetin. UGT1A7 and UGT1A9 exhibited a preference for modifying the 3′ position of the hesperetin, but favored the 7-O position of naringenin and the flavonols. In particular, the 3′-O glucuronides were most abundant for hesperetin, whereas kaempferol and quercetin showed no or low abundance B-ring products. Instead the most abundant products for kaempferol were the 7-O and 5-O (A-ring) glucuronides. The UGT1A8 enzyme displayed a high preference for modification of flavonoids on the B-ring. For all the flavonoids that yielded multiple products, the most abundant glucuronide involved the B-ring. UGT1A10 preferentially glucuronidated the 7-O position, except for isorhamnetin which showed the 3-O or 4′-O position as the more highly favored sites for glucuronidation
The UGT2B enzymes (UGT2B4, UGT2B7, UGT2B15, and UGT2B17) all shared one similar characteristic: they all showed poor to no reactivity with the flavonols, suggesting that the general flavanol structure restricted interaction with the UGT2B enzymes or otherwise deactivated the glucuronidation reactions.
There are also notable differences in the glucuronidation selectivities based on subtle structural differences of the flavonoids. For example, naringenin and kaempferol are analogs, with the former being a flavanone and the latter being a flavanol with its characteristic double bond on the C ring and hydroxyl at the 3-O position. The flavanol structure enhances the ability of the UGT enzymes to modify the 4′-O position. In no cases does naringenin undergo glucuronidation at the 4′-O position, whereas 4′-O glucuronide products are produced in nearly every reaction for kaempferol.
Kaempferol and quercetin also share similar structures except for the additional hydroxyl group at the 3′ position of quercetin. The 3′-OH group proved to be a very reactive site for glucuronidation for quercetin (as well as the flavanone hesperetin). Another point of interest when comparing kaempferol and quercetin is that the presence of the hydroxyl at the 3′position seems to render the 5-O position almost completely inactive, except for in the cases of the UGT1A6 and the UGT2B7 enzyme, which both lead to 5-O glucuronide as a minor product.
Quercetin also is similar in structure to isorhamnetin with the only difference being that the 3′-O position is methylated for isorhamnetin. The methoxy at the 3′ position appears to deactivate glucuronidation in general by the UGT enzymes since products are only detected for five of the UGT enzymes (UGT1A1, UGT1A3, UGT1A7, UGT1A9, and UGT1A10). Interestingly, isorhamnetin exhibited greater reactivity with UGT1A7 than was observed for quercetin (no products).
4.2 Conclusion
The regioselectivity of the reactions of twelve human UDP-glucuronosyl-transferase (UGT) isozymes with five common flavonoids were evaluated by LC-MS/MS with post-column metal complexation. Metal complexation results in the formation of [Co(II) (FG-H) (4,7-dpphen)2]+ ions which are key for confident identification of the modification site promoted by a given UGT isozyme due to the more diagnostic fragmentation patterns than produced by conventional deprotonated flavonoid glucuronides. The UGT1A enzyme selectivities are affected by the presence of a hydroxyl group at the 3, 4′, or 3′ positions as well as by the presence of a methoxy at the 3′ position. The UGT2B enzymes all share one similar trend: they all show poor to no reactivity with the flavonols. This result implies that the greater planarity of the flavonols compared to the non-planar structures of flavanones or the additional hydroxyl group at the 3 position of the flavonols inhibits interaction with the UGT2 enzymes. This study also shows the effectiveness of metal complexation/tandem mass spectrometry in conjunction with HPLC retention times for identification of flavonoid monoglucuonides. Of the 60 reactions reported in this study only three resulted in a product that could not be differentiated.
Supplementary Material
Supplemental Table 1
–Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Fragments that were above 50% abundance are listed as major fragments.
Fragments that were below 50% abundance are listed as minor fragments.
Supplementary Figure 1: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all naringenin products: a) 5-O-Glucuronide, (m/z 1170) b) 7-O-Glucuronide, (m/z 1170). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Supplemental Figure 2: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all isohamnetin products: a) 5-O-Glucuronide, (m/z 1214) b) 7-O-Glucuronide, (m/z 1214) c) 4′-O-Glucuronide or 3-O-Glucuronide, (m/z 1214). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Supplemental Figure 3: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all quercetin products: a) 5-O-Glucuronide, (m/z 1200) b) 7-O-Glucuronide, (m/z 1200) c) 4′-O-Glucuronide, (m/z 1200), d) 3′-O-Glucuronide (m/z 1200). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Acknowledgments
Funding from the NIH (R03 CA133924-02) and the Welch Foundation (1155) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross JA, Kasum CM. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annual Review of Nutrition. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 2.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables –the millennium's health. International Journal of Food Science & Technology. 2001;36:703–725. [Google Scholar]
- 3.Kupeli E, Sahin FP, Yesilada E, Calis I, Ezer N. In vivo anti-inflammatory and antinociceptive activity evaluation of phenolic compounds from Sideritis stricta. Journal of Biosciences A. 2007;62:519–525. doi: 10.1515/znc-2007-7-810. [DOI] [PubMed] [Google Scholar]
- 4.Terao J. Dietary Flavonoids as Antioxidants. Forum of Nutrition. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- 5.Kroon PA, Clifford MN, Crozier A, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 7.Rice-Evans C. Flavonoid Antioxidants. Current Medicinal Chemistry. 2001;8:797. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 8.Atmani D, Chaher N, Atmani D, Berboucha M, Debbache N, Boudaoud H. Flavonoids in Human Health: From Structure to Biological Activity. Current Nutrition and Food Science. 2009;5:225–237. [Google Scholar]
- 9.Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radical Research. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 10.Williamson G, Day AJ, Plumb GW, Couteau D. Human metabolic pathways of dietary flavonoids and cinnamates. Biochem Soc Trans. 2000;28:16–22. doi: 10.1042/bst0280016. [DOI] [PubMed] [Google Scholar]
- 11.King C, Rios G, Green M, Tephly T. UDP-Glucuronosyltransferases. Current Drug Metabolism. 2000;1:143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 12.Wong YC, Zhang L, Lin G, Zuo Z. Structure-activity relationships of the glucuronidation of flavonoids by human glucuronosyltransferases. Exper Opin Drug Metab Toxicol. 2009;5m:1399–1419. doi: 10.1517/17425250903179300. [DOI] [PubMed] [Google Scholar]
- 13.Stobiecki M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry. 2000;54:237–256. doi: 10.1016/s0031-9422(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 14.Gutzeit D, Winterhalter P, Jerz G. Application of preparative high-speed counter-current chromatography/electrospray ionization mass spectrometry for a fast screening and fractionation of polyphenols. Journal of Chromatography A. 2007;1172:40–46. doi: 10.1016/j.chroma.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 15.Zettersten C, Co M, Wende S, Turner C, Nyholm L, Sjöberg PJR. Identification and Characterization of Polyphenolic Antioxidants Using On-Line Liquid Chromatography, Electrochemistry, and Electrospray Ionization Tandem Mass Spectrometry. Analytical Chemistry. 2009;81:8968–8977. doi: 10.1021/ac901397c. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt S, Zietz M, Schreiner M, Rohn S, Kroth LW, Krumbein A. Identification of complex, naturally occurring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode-array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:2009–2022. doi: 10.1002/rcm.4605. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Wu S, Li X, Li P. LC-ESI-MS-MS Determination of Rat Plasma Protein Binding of Major Flavonoids of Flos Lonicerae Japonicae by Centrifugal Ultrafiltration. Chromatographia. 2010;72:71–77. [Google Scholar]
- 18.Stobiecki M, Staszków A, Piaskecka A, Garcia-Lopez PM, Zamora-Natera F, Kachlicki P. LC-MSMS Profiling of Flavonoid Conjugates in Wild Mexican Lupine, Lupinus reflexus. Journal of Natural Products. 2010;73:1254–1260. doi: 10.1021/np100139d. [DOI] [PubMed] [Google Scholar]
- 19.Dehkharghanian M, Adenier H, Vijayalakshmi MA. Study of flavonoids in aqueous spinach extract using positive electrospray ionisation tandem quadrupole mass spectrometry. Food Chemistry. 2010;121:863–870. [Google Scholar]
- 20.Rak G, Fodor P, Abrank L. Three-step HPLC-ESI-MS/MS procedure for screening and identifying non-target flavonoid derivatives. International Journal of Mass Spectrometry. 2010;290:32–38. [Google Scholar]
- 21.Satterfield M, Brodbelt JS. Enhanced Detection of Flavonoids by Metal Complexation and Electrospray Ionization Mass Spectrometry. Analytical Chemistry. 2000;72:5898–5906. doi: 10.1021/ac0007985. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wang J, Brodbelt JS. Characterization of flavonoids by aluminum complexation and collisionally activated dissociation. J Mass Spectrom. 2005;40:350–363. doi: 10.1002/jms.793. [DOI] [PubMed] [Google Scholar]
- 23.Pikulski M, Brodbelt JS. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. Journal of the American Society for Mass Spectrometry. 2003;14:1437–1453. doi: 10.1016/j.jasms.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Brodbelt JS. Gas-Phase Hydrogen/Deuterium Exchange and Conformations of Deprotonated Flavonoids and Gas-Phase Acidities of Flavonoids. Journal of the American Chemical Society. 2004;126:5906–5919. doi: 10.1021/ja031655d. [DOI] [PubMed] [Google Scholar]
- 25.Davis BD, Brodbelt JS. LC–MSn Methods for Saccharide Characterization of Monoglycosyl Flavonoids Using Postcolumn Manganese Complexation. Analytical Chemistry. 2005;77:1883–1890. doi: 10.1021/ac048374o. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Brodbelt JS. Silver Complexation and Tandem Mass Spectrometry for Differentiation of Isomeric Flavonoid Diglycosides. Analytical Chemistry. 2005;77:1761–1770. doi: 10.1021/ac048818g. [DOI] [PubMed] [Google Scholar]
- 27.Satterfield M, Brodbelt JS. Structural characterization of flavonoid glycosides by collisionally activated dissociation of metal complexes. Journal of the American Society for Mass Spectrometry. 2001;12:537–549. doi: 10.1016/S1044-0305(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Brodbelt JS, Wang J. Threshold dissociation and molecular modeling of transition metal complexes of flavonoids. Journal of the American Society for Mass Spectrometry. 2005;16:139–151. doi: 10.1016/j.jasms.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Pikulski M, Aguilar A, Brodbelt JS. Tunable Transition Metal-Ligand Complexation for Enhanced Elucidation of Flavonoid Diglycosides by Electrospray Ionization Mass Spectrometry. Journal of the American Society for Mass Spectrometry. 2007;18:422–431. doi: 10.1016/j.jasms.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Davis BD, Needs PW, Kroon PA, Brodbelt JS. Identification of isomeric flavonoid glucuronides in urine and plasma by metal complexation and LC-ESI-MS/MS. J Mass Spectrom. 2006;41:911–920. doi: 10.1002/jms.1050. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Satterfield MB, Brodbelt JS, Britz SJ, Clevidence B, Novotny JA. Structural Characterization and Detection of Kale Flavonoids by Electrospray Ionization Mass Spectrometry. Analytical Chemistry. 2003;75:6401–6407. doi: 10.1021/ac034795e. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Brodbelt JS. Screening flavonoid metabolites of naringin and narirutin in urine after human consumption of grapefruit juice by LC-MS and LC-MS/MS - Analyst (RSC Publishing) Analyst. 2004:1227–1233. doi: 10.1039/b412577k. [DOI] [PubMed] [Google Scholar]
- 33.Brett GM, Hollands W, Needs PW, et al. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Brithish Journal of Nutrition. 2009:664–675. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis BD, Brodbelt JS. Regioselectivity of Human UDP-Glucuronosyl-transferase 1A1 in the Synthesis of Flavonoid Glucuronides Determined by Metal Complexation and Tandem Mass Spectrometry. Journal of the American Society for Mass Spectrometry. 2008;19:246–256. doi: 10.1016/j.jasms.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Xie S, Chen S, Zeng S. Glucuronidation of flavonoids by recombinant UGT1A3 and UGT1A9. Biochemical Pharmacology. 2008;76:416–425. doi: 10.1016/j.bcp.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Xie S, You L, Zeng S. Studies on the flavonoid substrates of human UDP-glucuronosyl transferase (UGT) 2B7. Pharmazie. 2007;62:625–629. [PubMed] [Google Scholar]
- 37.Chen YK, Chen SQ, Li X, Zeng S. Quantitative regioselectivity of glucuronidation of quercetin by recombinant UDP-glucuronosyltransferases 1A9 and 1A3 using enzymatic kinetic parameters. Xenobiotica. 2005;35:943–954. doi: 10.1080/00498250500372172. [DOI] [PubMed] [Google Scholar]
- 38.Xie S, Chen Y, Chen S, Zeng S. Structure-metabolism relationships for the glucuronidation of flavonoids by UGT1A3 and UGT1A9. Journal of Pharmacy and Pharmacology. 2011;63:297–304. doi: 10.1111/j.2042-7158.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 39.Xie SG, You LY, Zeng S. Phase II metabolism of flavonoids mediated by human glucuronosyltransferase: an advanced research. Zhongguo Yaolixue Yu Dulixue Zazhi. 2007;21:438–443. [Google Scholar]
- 40.Singh R, Wu B, Tang L, Hu M. Uridine Diphosphate Glucuronosyltransferase Isoform-Dependent Regiospecificity of Glucuronidation of Flavonoids. Journal of Agricultural and Food Chemistry. 2011;59:7452–7464. doi: 10.1021/jf1041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plumb GW, O'Leary K, Day AJ, Williamson G. Enzymatic Synthesis of Quercetin Glucosides and Glucuronides. In: Santos-Buelga C, Williamson G, editors. Methods in Polyphenol Analysis. The Royal Society of Chemistry; Cambridge: 2003. pp. 177–186. [Google Scholar]
- 42.Easterbrook J, Lu C, Sakai Y, Li AP. Effects of Organic Solvents on the Activities of Cytochrome P450 Isoforms, UDP-Dependent Glucuronyl Transferase, and Phenol Sulfotransferase in Human Hepatocytes. Drug Metabolism and Disposition. 2001;29:141–144. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1
–Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Fragments that were above 50% abundance are listed as major fragments.
Fragments that were below 50% abundance are listed as minor fragments.
Supplementary Figure 1: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all naringenin products: a) 5-O-Glucuronide, (m/z 1170) b) 7-O-Glucuronide, (m/z 1170). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Supplemental Figure 2: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all isohamnetin products: a) 5-O-Glucuronide, (m/z 1214) b) 7-O-Glucuronide, (m/z 1214) c) 4′-O-Glucuronide or 3-O-Glucuronide, (m/z 1214). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)
Supplemental Figure 3: CID mass spectra of [Co(II) (FG-H) (4,7-dpphen)2]+ for all quercetin products: a) 5-O-Glucuronide, (m/z 1200) b) 7-O-Glucuronide, (m/z 1200) c) 4′-O-Glucuronide, (m/z 1200), d) 3′-O-Glucuronide (m/z 1200). –Aux (loss of auxiliary ligand); -GlcA (loss of glucuronic acid moiety); -Agl (loss of flavonoid aglycon)