Abstract
Pro-inflammatory cytokines and chemokines play critical roles in autoimmune diseases including rheumatoid arthritis (RA). Recently, it has been reported that β-arrestin 1 and 2 are involved in the regulation of inflammation. We hypothesized that β-arrestin 1 and 2 play critical roles in murine models of RA. Using a collagen-induced arthritis (CIA) and a human TNFα transgenic (TNFtg) mouse model, we demonstrated that β-arrestin 1 and 2 expression are significantly increased in joint tissue of CIA mice and TNFtg mice. In fibroblast-like synoviocytes (FLS) isolated from hind knee joint of CIA mice, we observed an increase of β-arrestin 1 and 2 protein and mRNA levels in the early stage of arthritis. In FLS, low molecular weight hyaluronan (HA)-induced TNFα and IL-6 production was increased by overexpression of β-arrestin 1 but decreased by overexpression of β-arrestin 2 demonstrating isoform specific regulation. TNFα and HA induced an increase of β-arrestin 1 and 2 expression in FLS, while high mobility group box (HMGB)-1 only stimulated β-arrestin 1 expression. TNFα- or HA- induced β-arrestin 2 expression was blocked by a p38 inhibitor. To examine the in vivo role of β-arrestin 2 in the pathogenesis of arthritis, WT and β-arrestin 2 KO mice were subjected to collagen antibody-induced arthritis (CAIA). β-arrestin 2 KO mice exhibited more severe arthritis in CAIA. Thus β-arrestin 2 is anti-inflammatory in CAIA. These composite observations suggest that β-arrestin 1 and 2 differentially regulate FLS inflammation and increased β-arrestin 2 may reduce experimental arthritis severity.
Keywords: CIA, β-arrestin, FLS, CAIA
1. Introduction
Chronic inflammation is a key component of autoimmune diseases such as rheumatoid arthritis (RA). RA affects approximately 1% of the global population and induces significant morbidity and associated economic costs (Firestein, 2003). RA is characterized by chronic inflammation of synovial joints leading to cartilage damage and ultimately total joint destruction. Pro-inflammatory cytokines and chemokines play critical roles in autoimmune diseases (Tremoulet and Albani, 2006). TNFα, IL-1β and IL-6 contribute to the development of RA (Houssiau et al., 1988; Saxne et al., 1988; Youn et al., 2002). A number of agents that block the TNFα, IL-1β and IL-6 have been introduced into clinical practice or are currently in clinical trials (Moreland, 2004). Understanding the signaling pathways regulating pro-inflammatory cytokine and chemokine production in RA may provide novel treatment strategies.
Recent studies have addressed the potential role of Toll-like receptors (TLR)s in arthritis. Involvement of various TLRs in the onset and perpetuation of experimental arthritis has been convincingly demonstrated (Joosten et al., 2003; Lee et al., 2005). TLRs and several of their endogenous ligands such as hyaluronan (HA) and high-mobility group box 1 (HMGB1) are highly expressed in synovial tissue from RA patients compared with that from healthy donors (Radstake et al., 2004; Roelofs et al., 2005; Seibl et al., 2003). TLR4-mediated activation of dendritic cells (DCs) from RA patients induced significantly higher levels of pro-inflammatory cytokines compared to DCs from healthy volunteers (Roelofs et al., 2005). Injection of the TLR4 ligand bacterial endotoxin LPS into murine synovial joints induced experimental arthritis that shares similar features with human RA (Kyo et al., 2005). Convincing evidence therefore suggests that the local activation of TLRs by either intrinsic or extrinsic TLR ligands are critical inflammatory events that initiate and/or exacerbate the development of RA.
Beta-arrestin 1 and 2 are adaptor proteins that lead to termination of G protein activation and desensitization of G protein-coupled receptor (GPCR)s (Luttrell and Lefkowitz, 2002; Pitcher et al., 1998). β-arrestins mediate GPCR desensitization by facilitating endocytosis by interaction with clathrin in clathrin-coated pits (Goodman et al., 1996; Krupnick et al., 1997; Miller and Lefkowitz, 2001). However, in addition to GPCR desensitization, it has been shown that β-arrestin 1 and 2 function as multifunctional scaffold/adaptor proteins for GPCR activation of MAP kinases including ERK1/2 (DeFea et al., 2000a; DeFea et al., 2000b; Luttrell et al., 2001), JNK (McDonald et al., 2000), p38 (Sun et al., 2002), and Src family kinases (Luttrell et al., 1999). The latter signaling events can occur independent of G protein signaling. β-arrestins also regulate cellular survival/apoptosis pathways such as PI3K (Povsic et al., 2003), ERK (DeFea et al., 2000a), JNK (McDonald et al., 2000; Miller et al., 2001) and p38 (Bruchas et al., 2006; Miller et al., 2003; Sun et al., 2002) mediated signaling. Recently, it has been reported that β-arrestin 1 and 2 are involved in regulation of inflammation. We have observed a differential effect of β-arrestin 2 on mediating TLR4-induced activation of ERK 1/2 but suppression of NFκB activation (Fan et al., 2007). NFκB is inhibited as a consequence of β-arrestins associated with TNF receptor-associated factor (TRAF) 6 and inhibiting TRAF6 ubiquitination (Wang et al., 2006). β-arrestin 1 and 2 also bind and prevent degradation of IκBα-inhibiting NFκB activation (Gao et al., 2004; Witherow et al., 2004). Our recent studies have demonstrated that β-arrestin 2 negatively regulates polymicrobial sepsis-induced inflammation (Fan et al., 2010).
We hypothesized that β-arrestin 1 and 2 play a critical role in arthritis. Expression of β-arrestin 1 and 2 were examined in joint of CIA mice and human TNFα transgenic (TNFtg) mice. Since fibroblast-like synoviocytes (FLS) in synovium are the primary effectors of cartilage destruction and inflammatory pathogenesis, ex vivo FLS were isolated from CIA mice and expression of β-arrestin 1 and 2 protein and mRNA, and inflammatory mediators were determined. The effects of β-arrestin 1 and 2 overexpression on HA induction of inflammatory mediators were determined and in subsequent studies signaling pathways inducing β-arrestin 1 and 2 were examined in FLS. In the in vivo studies, we examined the susceptibility of β-arrestin 2 KO mice in collagen antibody-induced arthritis (CAIA). Collectively these studies suggest that β-arrestin 1 and 2 differentially regulate FLS inflammatory mediator production and β-arrestin 2 is anti-inflammatory in experimental arthritis. The newly discovered isoform specific role of β-arrestins as regulators of inflammation may provide insights into molecular mechanisms of arthritis pathogenesis from which novel molecular specific therapeutic approaches may be derived.
2. Materials and Methods
2.1. Mice
β-arrestin 2(−/−) mice and littermate WT mice with C57BL/6 background were generated by breeding heterozygous animals. Studies employed 5 to 8 week old β-arrestin 2(−/−) and age matched WT mice for all the experiments. The original heterozygous mice were obtained from Dr. Robert J. Lefkowitz (Duke University Medical Center, Durham, NC). PCR was performed with genomic DNA from 4-week-old mice tails. The following primer pairs were used: forward, 5'-GATCAAAGCCCTCGATGATC-3'; reverse, 5'-ACAGGGTCCACTTTGTCCA-3' and 5'-GCTAAAGCGCATGCTCCAGA-3'. The reactions were run for 35 cycles. Western blot analysis of splenocytes from WT and β-arrestin 2(−/−) confirmed the absence of β-arrestin 2 with no effect on β-arrestin 1 expression (Fan et al., 2010).
The joint tissue of human TNFα transgenic mice (Taconic Farms, Germantown, NY) was provided by Dr. Gary Gilkeson (Medical University of South Carolina). TNFtg mice spontaneously develop severe chronic arthritis by 20 weeks of age (Baker et al., 2010). The joint tissue was collected from WT and human TNFα transgenic mice at 30 weeks of age. DBA/1J mice were purchased from Harlan laboratories. The investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and commenced with the approval of the institutional animal care and use committee.
2.2.Flag-β-arrestin 1 and 2 lentivirus construction
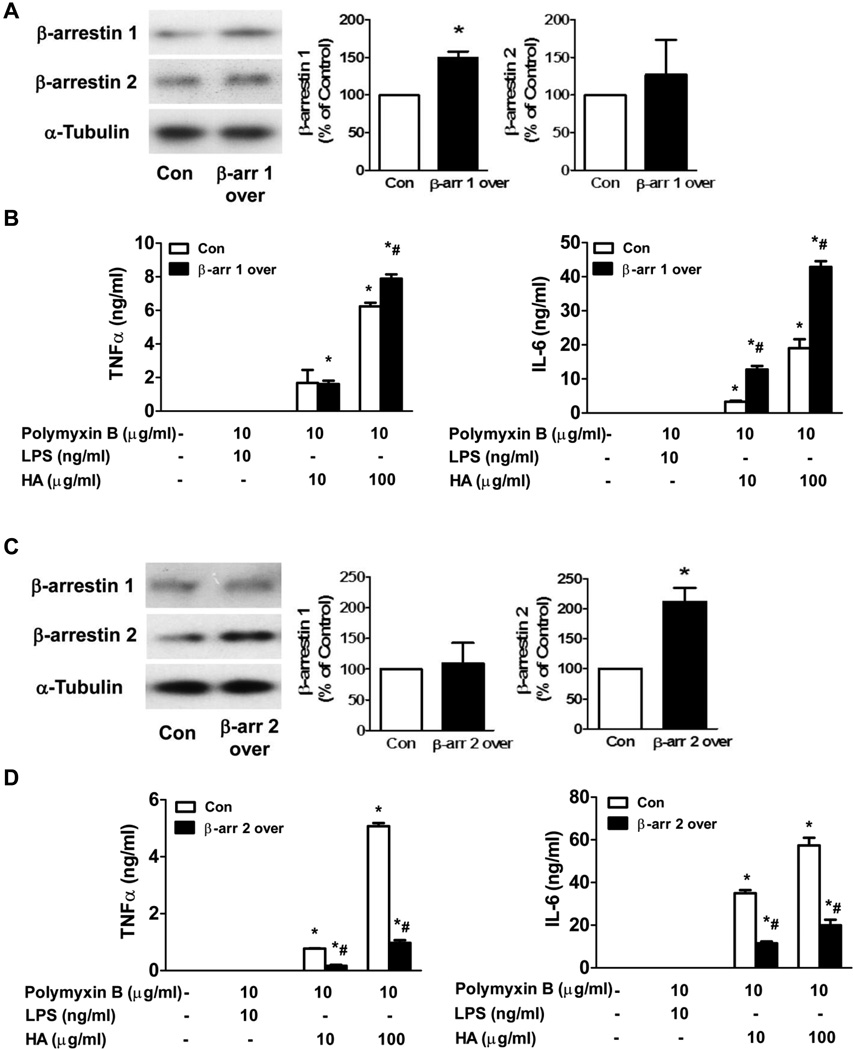
Flag-β-arrestin 1 and Flag-β-arrestin 2 were cloned into a ViraPower Lentiviral Expression System using pLenti6/V5 directional TOPO cloning kit. A pLenti6/V5-GW/lacZ plasmid was used as a control plasmid. Lentivirus containing β-arrestin 1, β-arrestin 2 and control vector were generated following manufacture’s instructions (Invitrogen). A representative Western blot shows the β-arrestin 1 and β-arrestin 2 lentivirus transduced cells overexpress β-arrestin 1 (Fig. 4A) and β-arrestin 2 (Fig. 4C).
Figure 4. The effects of β-arrestin 1 and 2 overexpression on HA-induced TNFα and IL-6 production in FLS.
FLS were isolated from DBA/1J mice and transfected with control, flag-β-arrestin 1 or flag-β-arrestin 2 lentivirus. Flag-β-arrestin 1 (A) and 2 (C) overexpression were determined by Western blot. Effects of flag-β-arrestin 1 (B) and 2 (D) overexpression on HA-induced TNFα and IL-6 production in FLS were determined by ELISA. Data represent means ± S.E from four independent experiments. *p<0.05 compared to unstimulated group. #p<0.05 compared to control group.
2.3.Induction of arthritis and scoring
CIA was studied in DBA/1J mice (7–8 weeks old). Mice were immunized at the base of the tail with 100 µg bovine type II collagen mixed with CFA containing 4 mg/ml M. tuberculosis (Chondrex). Twenty-one days after the first injection, the mice received a booster injection of bovine type II collagen (100 µg) mixed with IFA. The mice were monitored daily for swelling of paws as a sign of arthritis. The severity of the arthritis was scored from 0 to 4 as follows: grade 0, normal; grade 1, redness and mild swelling of the ankle or wrist; grade 2, moderate redness and swelling of the ankle or wrist; grade 3, severe swelling of the entire paw; and grade 4, deformity or ankylosis. Each limb was graded, giving a maximum possible score of 16 per animal. At 4, 6, 8 and 10 weeks after first collagen injection, mice were sacrificed and joint inflammation and erosions were examined with haematoxylin and eosin (H&E) stain of sections of the hind knee.
The CAIA was studied in WT and β-arrestin 2 KO mice in C57BL/6 background. The mice were injected intravenously with a cocktail of 4 monoclonal antibodies, clone A2-10 (IgG2a), F10-21 (IgG2a), D8-6 (IgG2a), and D1-2G(IgG2b), (4 mg/0.4 ml/mouse, Chondrex) through the tail vein (day 0). Three days later LPS (50 µg/0.2 ml/mouse) was injected intraperitoneally. The mice were monitored daily for swelling of paws as a sign of arthritis. The severity of the arthritis was graded as described previously and paw thickness was measured with 0- to 10-mm calipers. Fourteen days after antibodies injection, mice were sacrificed and joint inflammation and erosions were examined with H&E stain of sections of the hind knee.
2.4.Histological sectioning
Histopathology studies were performed on a hind knee to assess the disease state. The hind knee was removed and fixed in 10% formalin in phosphate-buffered saline, decalcified in 10% ethylenediaminetetra-acetic acid and embedded in paraffin. Sections of the hind knee were stained with H&E. Evaluation of the severity of arthritis was carried out by a pathologist who was blinded to the experimental groups.
2.5. FLS isolation, culture and stimulation
FLS were isolated from control and arthritic DBA/1J mice. Briefly, hind knee joint tissue was minced with scalpels and incubated with 1 mg/ml of collagenase type II (Invitrogen, Carlsbad, CA) for 2 hours at 37°C. Cells were passed through a 40µm nylon mesh to obtain single cell suspensions and washed with culture medium. FLS in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with heat inactivated 10% fetal calf serum, 2% penicillin/streptomycin and 0.25 µg/ml fungizone (Invitrogen, Carlsbad, CA) were grown in 150cm2 tissue culture flasks and maintained at 37°C in 5% CO2, 95% air. Non-adherent cells were removed during cell culture, and cells between passages 3 to 9 were used.
FLS were transduced with lentivirus (MOI=1) containing β-arrestin 1, β-arrestin 2 or control vector and stimulated with HA (10 µg/ml and 100 µg/ml, Calbiochem) in the presence of polymyxin B (10 µg/ml) for 24 h. Polymyxin B, which binds to and inactivates LPS (100 ng/ml, ultra pure LPS from E.coli O111:B4), was added to rule out any potential LPS contamination in HA. The supernatants were collected to determine the TNFα and IL-6 production by ELISA.
FLS from DBA/1J mice were stimulated with TNFα (10 ng/ml, Sigma), HA (100 µg/ml) and HMGB1 (1 µg/ml, Prospec) for 6 h, 12 h, 24 h and 48 h. After stimulation, the protein was collected to determine β-arrestin 1 and 2 expression in FLS. To determine the effects of PI3K, ERK 1/2, JNK and p38 kinases on TNFα- and HA- induced increases of β-arrestin 1 and 2 expression in FLS, cells were pretreated with PI3K inhibitor Wortmannin (10 nM, Calbiochem), MEK inhibitor PD98059 (10 µM, Calbiochem), JNK inhibitor SP600125 (10 µM, Calbiochem) or p38 inhibitor SB203580 (10 µM, Calbiochem) for 1 hour followed by stimulation with TNFα (10 ng/ml) or HA (100 µg/ml) for 24 h. β-arrestin 1 and 2 expression were examined by Western blot analysis.
2.6. Western blot
The joint tissues were minced and lysed with ice-cold RIPA lysis buffer (10 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin, 1 µg/ml leupeptin, and 1 µg/ml pepstatin A). Cells were also washed and lysed with ice-cold RIPA lysis buffer. All lysed samples were kept on ice for 30 min, and centrifuged for 10 min at 4°C at 10,000g. The supernatant was collected and stored at −20°C until Western blot analysis.
For Western blotting, lysates were added to Laemmli sample buffer and boiled for 4 min. Subsequently, protein from each sample was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membranes were washed with Tris-buffered saline-Tween 20 (TBST; 20 mM Tris, 500 mM NaCl, and 0.15% Tween 20) and blocked with 5% milk in TBST (20 mM Tris, 500 mM NaCl, and 0.1% Tween 20) for 1 h. After washing with TBST (20 mM Tris, 500 mM NaCl, and 0.1% Tween 20) twice, membranes were incubated with primary antibody overnight at 4°C. The following antibodies were used: the monoclonal anti-β-arrestin 1 antibody was purchased from BD Transduction Laboratories; a polyclonal anti-β-arrestin2 antibody was from Abcam and a monoclonal anti-α-tublin antibody was from Cell Signaling. The membranes were washed twice with TBST (20 mM Tris, 500 mM NaCl, and 0.1% Tween 20) and incubated with HRP conjugated secondary antibody in blocking buffer for 1 h. After washing three times with TBST (TBST; 20 mM Tris, 500 mM NaCl, and 0.15% Tween 20), immunoreactive bands were visualized by incubation with ECL plus detection reagents (GE Healthcare) for 5 min and exposure to ECL Hyperfilm (GE Healthcare). The densitometry of bands was quantified with NIH image 1.63 software. We found α-Tubulin levels are consistent in the control and RA groups (Data not shown). Therefore, α-Tubulin was used as the loading control.
2.7. Real-time RT-PCR
Total RNA was extracted from FLS using RNeasy mini kit (QIAGEN). The purity and concentration of RNA is determined by plate reader with Gen5 1.09 software (BioTek). cDNA was synthesized with superscript II Reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR was performed by prism 7000 Real Time PCR System (ABI) using SYBR Green PCR kit (QIAGEN) in a final reaction volume of 10 µl with 2 ρM of each primer (primers for α-tubulin, β-arrestin 1, β-arrestin 2 were from IDT, sequences are available upon request). A negative control without cDNA did not produce any amplicons. Melting curve analysis was performed to verify that only one product was amplified. The size of products was verified by agarose gel electrophoresis. Data were analyzed with 2−ΔΔCt value calculation using α-Tubulin for normalization.
2.8. Assay for TNFα and IL-6 production
TNFα and IL-6 production were measured using an ELISA with mouse TNFα and IL-6 ELISA kits (eBioscience, San Diego, CA).
2.9. Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by analysis of variance (ANOVA) with Fisher’s probable least-squares difference test or Student’s t-test using GraphPad Prism software. P< 0.05 value was considered statistically significant.
3. Results
3.1.β-arrestin 1 and 2 protein levels are increased in joint tissue in CIA mice
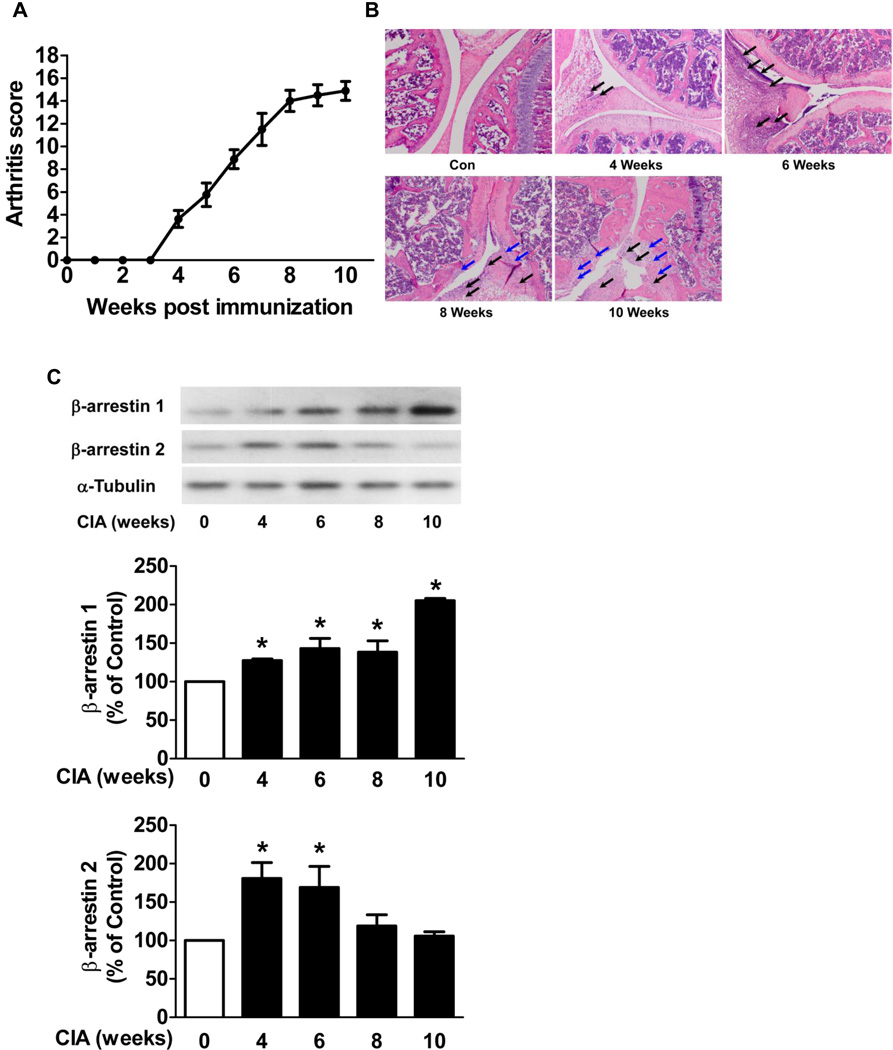
To determine the β-arrestin 1 and 2 protein levels in the murine model of RA, CIA was induced in DBA/1J mice. In DBA/1J mice, arthritis was apparent at 4 weeks post collagen injection and reached a mean arthritis score of 15 at 10 weeks (Fig. 1A). The H&E stained sections of the hind knee joint revealed the severity of disease development. Synovial inflammation was observed at 4 and 6 weeks and bone erosion was evident at 8 and 10 weeks after first collagen injection (Fig. 1B). The hind knee joint tissue were collected from CIA mice and control mice at 4, 6, 8 and 10 weeks after the first collagen injection. In the hind knee joint tissue from CIA mice, β-arrestin 1 expression was significantly increased at 4, 6, 8 and 10 weeks (1.3 ± 0.02 fold, n=5, 1.4 ± 0.1 fold, n=6, 1.4 ± 0.2 fold, n=6, and 2.1 ± 0.03 fold, n=5, respectively, p < 0.05) and β-arrestin 2 expression was significantly increased at 4 and 6 weeks (1.8 ± 0.2 fold, n=5, and 1.7 ± 0.3 fold, n=6, respectively, p < 0.05) compared to the control mice (Fig. 1C). These observations demonstrate that increased β-arrestin 2 expression in joint tissue is transient whereas increased β-arrestin 1 expression persisted during disease severity.
Figure 1. β-arrestin 1 and 2 expression in joint tissue in CIA mice.
CIA was studied in DBA/1J mice. The mean arthritis score reveals the disease severity (A). The hind knee joint tissue was collected from CIA mice and control mice at 4, 6, 8 and 10 weeks after the first collagen injection. Sections of joint were subjected to H&E stain (B). Black arrows indicate the PMNs and macrophages that infiltrated into synovial tissue and cavity and blue arrows indicate the bone erosion. The hind knee joints (C) were homogenized and subjected to Western blot. The densitometric levels of the scanned gels were normalized to control levels. α-Tubulin was used as an internal control. Data represent means ± S.E of five to nine mice per group from three independent experiments. *p<0.05 compared to the control mice.
3.2. β-arrestin 1 and 2 protein levels are increased in joint tissue from human TNFα transgenic mice
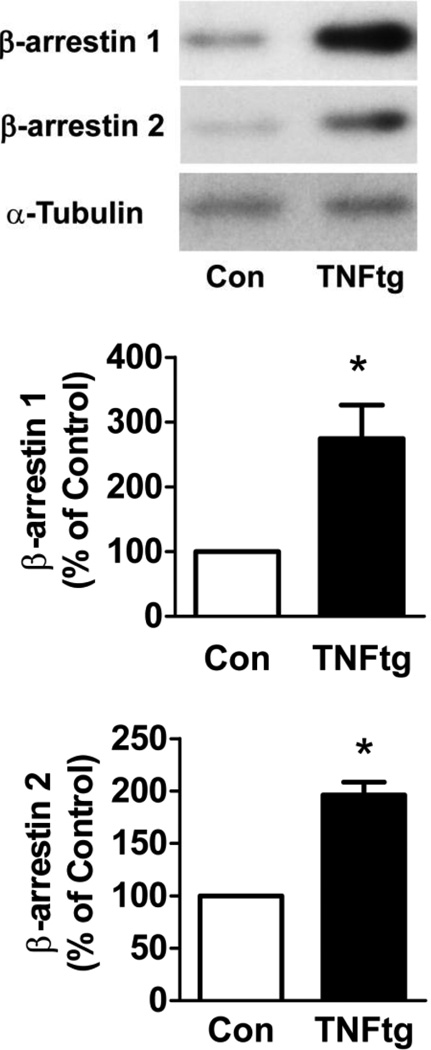
Since TNFα is overexpressed in RA, we sought to determine what effects TNFα may have on β-arrestin levels. Thus, we used a model of RA that is an overexpression of human TNFα. The hind knee joint tissue was collected from the TNFtg mice and WT mice at 30 weeks of age. Our results demonstrated that β-arrestin 1 and 2 expression were significantly increased (2.7 ± 0.5 fold and 2.0 ± 0.1 fold, respectively, n=5, p < 0.05) in the hind knee joint tissue from the TNFtg mice compared to the control mice (Fig. 2). Thus β-arrestins expression is also increased in the arthritic joint induced by excess expression of TNFα in addition to immune complex formation.
Figure 2. β-arrestin 1 and 2 expression in joint tissue in TNFα transgenic mice.
The TNFα transgenic mice were employed as a murine model of RA. The hind knee joint tissue was collected from the TNFtg mice and control mice at 30 weeks of age. The hind knee joints were subjected to Western blot. α-Tubulin was used as an internal control. Data represent means ± S.E from five mice per group from two independent experiments. *p<0.05 compared to the control mice.
3.3 β-arrestin 1 and 2 protein expression and mRNA levels are increased in FLS of CIA mice
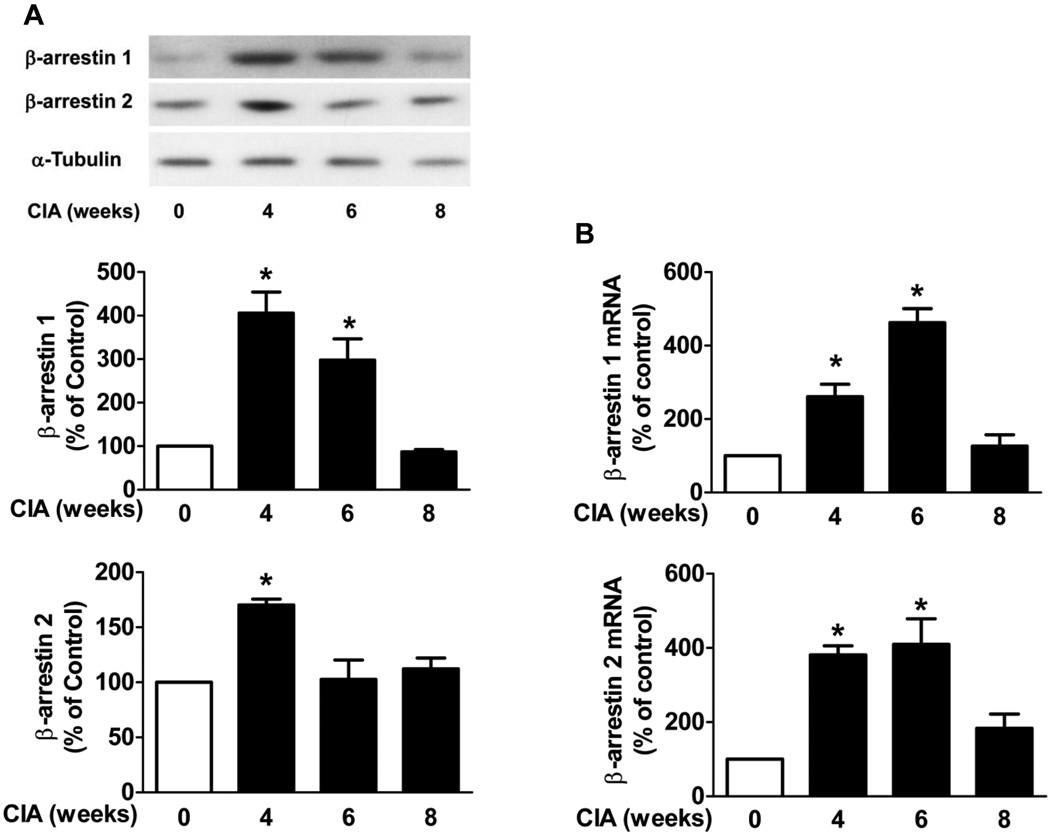
To investigate if specific cells exhibit increased β-arrestin expression in the joint tissue, FLS were isolated from the hind knee joint tissue from the CIA mice and control mice at 4, 6 and 8 weeks. Cells were cultured and used at the third passage. In FLS from arthritic mice, β-arrestin 1 protein expression was significantly increased at 4 and 6 weeks (4.1 ± 0.5 fold and 3.0 ± 0.5 fold, respectively, n=4, p < 0.05) and β-arrestin 2 expression was significantly increased at 4 weeks (1.7 ± 0.1 fold, n=4, p < 0.05) compared to the control mice (Fig. 3A). In the FLS from arthritic mice, mRNA levels of β-arrestin 1 (2.6 ± 0.3 fold and 4.6 ± 0.4 fold, respectively, n=3, p < 0.05) and β-arrestin 2 (3.8 ± 0.3 fold and 4.1 ± 0.7 fold, respectively, n=3, p < 0.05) were both significantly increased at 4 and 6 weeks compared to the control mice (Fig. 3B). These studies suggested that FLS in part contribute to the increased β-arrestin isoform expression in arthritic joint.
Figure 3. β-arrestin 1 and 2 protein expression and mRNA levels in FLS.
FLS were isolated from the hind knee joint tissue from the CIA mice at 4, 6 and 8 weeks and control mice. FLS were cultured for three passages. (A) Cell lysates were subjected to Western blot. The densitometric levels of the scanned gels were normalized to control levels. (B) Total RNA was extracted and mRNA levels of β-arrestin 1 and β-arrestin 2 were determined by quantitative real-time PCR. α-Tubulin was used as an internal control for both protein and mRNA levels. Data represent means ± S.E from three to four mice from three independent experiments. *p<0.05 compared to the control FLS.
3.4 Overexpression of β-arrestin 1 and 2 differentially regulates HA-induced TNFα and IL-6 production in FLS
Since the increases in β-arrestin 1 and 2 occurred coincidentally with the increases in mediators, β-arrestin 1 and 2 could induce the mediators or negatively regulate the mediators. Therefore HA induced TNFα and IL-6 production were determined in FLS transduced with control, β-arrestin 1 or β-arrestin 2 lentivirus. β-arrestin 1 or 2 lentivirus transduction increased β-arrestin 1 or 2 levels by 1.5±0.08 fold (Fig. 4A) and 2.1±0.23 fold (Fig. 4C) respectively. In FLS transduced with control lentivirus, HA induced a significant increase of TNFα and IL-6 production. In FLS transduced with β-arrestin 1 lentivirus, HA-induced TNFα and IL-6 production were significantly increased (1.3 ± 0.04 fold for TNFα and 3.9 ± 0.3 and 2.3 ± 0.1 fold for IL-6 respectively, n=4, p < 0.05) compared to control lentivirus-transduced FLS (Fig. 4B). In FLS transduced with β-arrestin 2 lentivirus, HA-induced TNFα and IL-6 production was significantly decreased (78 ± 4.4% and 81 ± 1.8% for TNFα and 67 ± 2.4% and 65 ± 4.6% for IL-6 respectively, n=4, p< 0.05) compared to control lentivirus transduced FLS (Fig. 4D). These data suggest that β-arrestin 2 is anti-inflammatory whereas β-arrestin 1 is pro-inflammatory in experimental arthritis.
3.5 TNFα, HA and HMGB1 induce increases of β-arrestin 1 and/or 2 expression in FLS
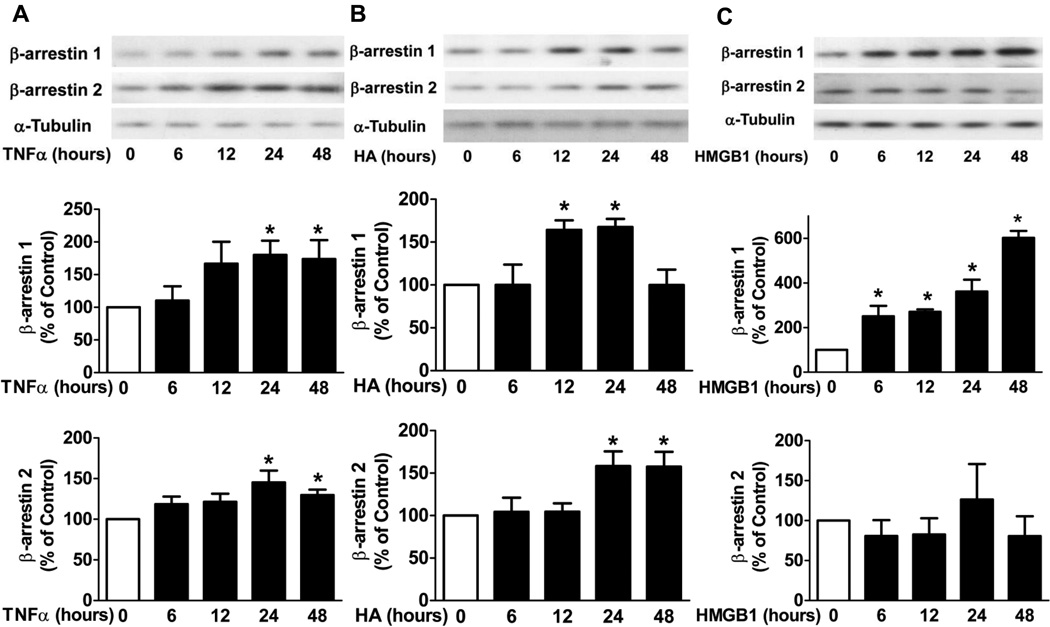
The potential roles of TLR ligands and TNFα in RA have been reported. To investigate which agent may contribute to the increased β-arrestin 1 and 2 expression in FLS in CIA mice, we tested the effects of TNFα, HA which is TLR2/4 endogenous ligand, and HMGB1 a TLR4 endogenous ligand. FLS from DBA/1J mice were stimulated with TNFα (10 ng/ml), HA (100 µg/ml) and HMGB1 (1 µg/ml) for 6 h, 12 h, 24 h and 48 h. After stimulation, protein was collected to determine β-arrestin 1 and 2 expression. β-arrestin 1 (1.8 ± 0.2 fold and 1.7 ± 0.3 fold respectively, n=4, p < 0.05) and β-arrestin 2 (1.5 ± 0.2 fold and 1.3 ± 0.1 fold respectively, n=4, p < 0.05) expression were both significantly increased in FLS stimulated with TNFα for 24 h and 48 h (Fig. 5A). β-arrestin 1 expression was increased in FLS stimulated with HA for 12 h and 24 h (1.6 ± 0.1 fold and 1.7 ± 0.1 fold respectively, n=3, p < 0.05) and β-arrestin 2 expression was increased in FLS stimulated with HA for 24 h and 48 h (1.6 ± 0.2 fold and 1.6 ± 0.2 fold respectively, n=4, p < 0.05) (Fig. 5B). β-arrestin 1 expression was significantly increased (2.5 ± 0.5 fold, 2.7 ± 0.1 fold, 3.6 ± 0.5 fold, and 6.0 ± 0.3 fold, respectively, n=3, p < 0.05) in FLS stimulated with HMGB1. Interestingly, HMGB1 did not induce β-arrestin 2 expression (Fig. 5C). Taken together, HMGB1 induced only β-arrestin 1 expression, while TNFα and HA induced both β-arrestin 1 and 2 expression in FLS.
Figure 5. The effects of TNFα, HA and HMGB1 on β-arrestin 1 and 2 expression in FLS.
FLS from DBA/1J mice were stimulated with TNFα (10 ng/ml), HA (100 µg/ml) or HMGB1 (1 µg/ml) for 6 h, 12 h, 24 h and 48 h. After stimulation, protein was collected and subjected to Western blot analysis. TNFα- (A), HA- (B) and HMGB1- (C) induced β-arrestin 1 and 2 expression were determined. α-Tubulin was used as an internal control. Data represent means ± S.E from three-four independent experiments.*p<0.05 compared to the control FLS.
3.6 p38 MAPK mediates TNFα and HA-induced increases of β-arrestin 2
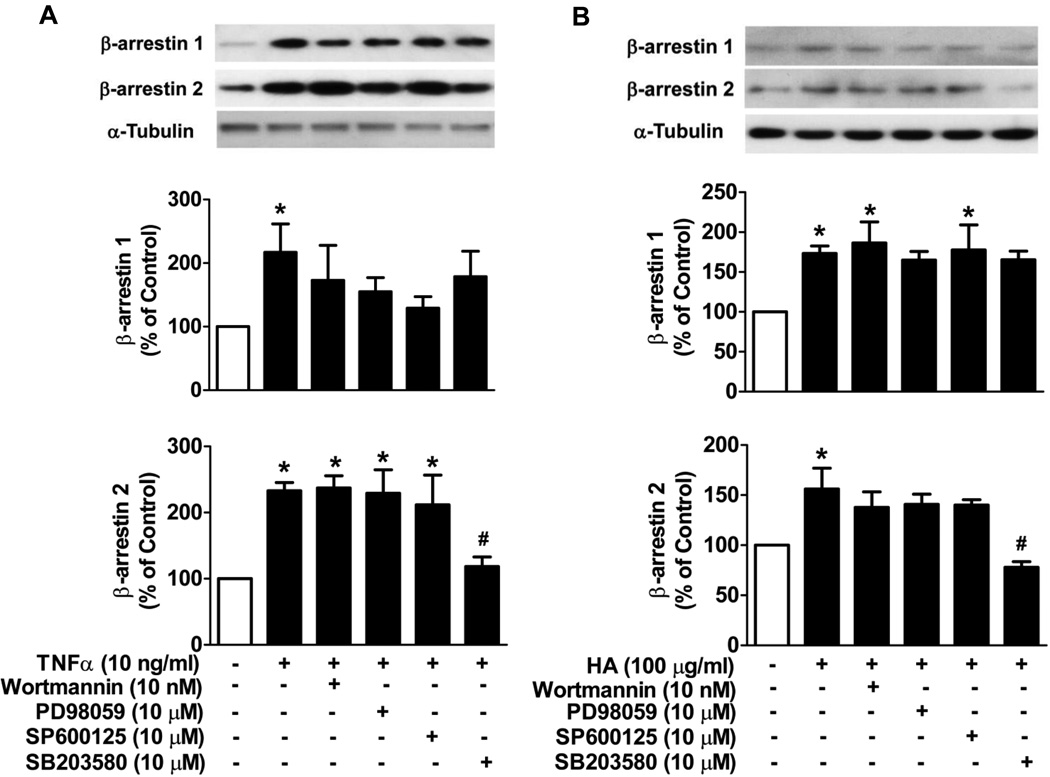
To further elucidate the signaling pathway which mediates TNFα and HA induced increases of β-arrestin 1 and 2 expression in FLS, we used PI3K, MEK, JNK and p38 kinases inhibitors. The concentrations of inhibitors we employed have been reported in several types of cells to selectively block the respective kinases (Kesherwani and Sodhi, 2007; Senokuchi et al., 2004; Zhang et al., 2011). When incubated with inhibitors alone, none of the inhibitors exhibited any effect on β-arrestin 1 and 2 levels in FLS (data not shown). Both TNFα- and HA-induced increases of β-arrestin 2 expression were blocked by SB203580 (p38 MAPK inhibitor) but not by the other inhibitors (Fig. 6A, B). On the other hand, TNFα- or HA-induced increases of β-arrestin 1 expression were unaltered by any of the inhibitors used (Fig. 6A, B). These observations suggest that distinct signaling pathways regulate β-arrestin 1 and 2 expression.
Figure 6. The effects of PI3K, MEK, JNK and p38 inhibitors on TNFα- or HA-mediated β-arrestin 1 and 2 expression in FLS.
FLS were isolated and cultured from DBA/1J mice. Cells were pretreated with or without the various inhibitors for 1 h followed by stimulation with TNFα or HA for 24 h. TNFα- (A) or HA- (B) mediated increases of β-arrestin 1 and 2 expression in FLS were determined. α-Tubulin was used as an internal control. Data represent means ± S.E from three independent experiments. *p<0.05 compared to unstimulated cells. #p<0.05 compared to TNFα or HA stimulation group.
3.7 β-arrestin 2 KO mice exhibit more severe arthritis in CAIA
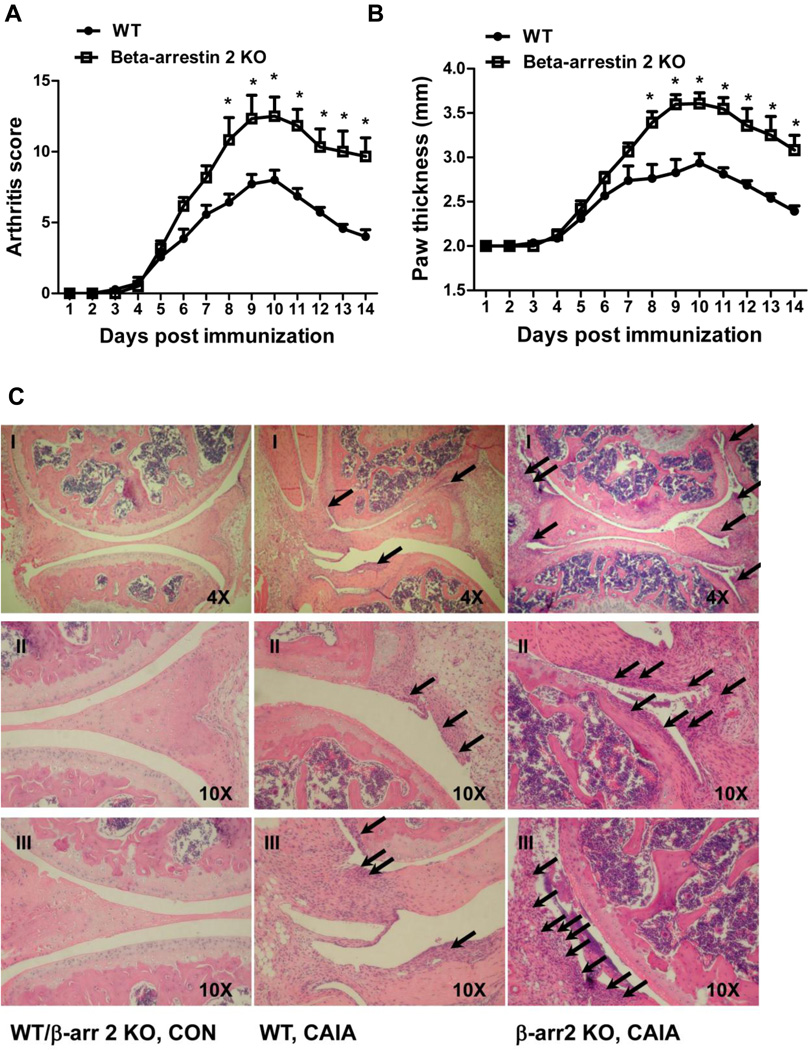
β-arrestin 2 KO mice are in a C57BL/6 background, which is not susceptible to CIA. To determine the effect of β-arrestin 2 gene deletion on arthritis development, collagen antibody induced arthritis (CAIA) was employed. β-arrestin 2 KO mice exhibited an increased mean arthritis score (Fig. 7A) and paw thickness (Fig. 7B) compared to the WT mice in CAIA. The H&E stain of sections of hind knee joint revealed the severity of disease development. More synovial inflammation was observed in the joint tissue from β-arrestin 2 KO mice compared to WT mice as indicated by increased PMNs and macrophages infiltrating into the synovial tissue and cavity. No pannus formation or joint erosion was observed (Fig. 7C). These observations support our hypothesis that β-arrestin 2 is anti-inflammatory in experimental arthritis.
Figure 7. Effect of β-arrestin 2 gene deletion on the collagen antibody-induced arthritis (CAIA).
CAIA was studied in WT and β-arrestin 2 KO mice. The mean arthritis score (A) and paw thickness (B) were determined as indicators of arthritis severity. Sections of the hind knee from WT/β-arrestin 2 KO control, WT CIA and β-arrestin 2 KO CIA were stained with haematoxylin and eosin (C). Picture II and III are enlarged sites of interest from picture I. Arrows indicate the PMNs and macrophages that infiltrated into synovial tissue and cavity. Data represent means ± SE from seven WT and six β-arrestin 2 KO mice from three independent experiments. *p<0.05 compared to the WT group.
4. Discussion
Our studies demonstrate that β-arrestin 1 and 2 expression are significantly increased in the joint tissue in CIA and TNFtg mice. In ex vivo FLS derived from joint tissue, β-arrestin 1 and 2 protein and mRNA levels were increased early in the course of CIA. Our studies demonstrated mRNA levels of TNFα and MMP3 were also increased in FLS in CIA (data not shown) suggesting β-arrestins may regulate inflammatory mediator expression. To determine if β-arrestins regulation of inflammation is isoform specific, we examined the effect of lentiviral overexpression of β-arrestin 1 or 2 in FLS. These studies demonstrated that HA-induced inflammatory mediators were increased by β-arrestin 1 overexpression but decreased by β-arrestin 2 overexpression. In subsequent studies, in vitro stimulation of FLS with TNFα or HA significantly increased β-arrestin 1 and 2 expression. Interestingly, HMGB1 stimulation only increased β-arrestin 1 but not β-arrestin 2 expression in FLS. By employing MAP kinase inhibitors, we found that TNFα- or HA- induced β-arrestin 2 expression appears to be regulated via p38 pathway. To examine role of β-arrestin 2 in the pathogenesis of arthritis, WT and β-arrestin 2 KO mice were subjected to the CAIA. β-arrestin 2 KO mice exhibited greatly exacerbated arthritis in CAIA suggesting that β-arrestin 2 ameliorates the inflammatory and degenerative sequelae of experimental arthritis. These observations demonstrate that both β-arrestin isoforms are increased in arthritis. However β-arrestin 1 and 2 appear to paly differential roles in regulating arthritis development. β-arrestin 2 may negatively regulate experimental arthritis whereas β-arrestin 1 may contribute to the development of arthritis.
Activated FLS in RA play a major role in joint tissue destruction. FLS become activated through growth factors, chemokines, cytokines and tissue degradation products such as endogenous TLR ligand HA (Noss and Brenner, 2008). Quantitative Real-time PCR and Western blot analysis confirmed the increased β-arrestin 1 and 2 expression on both mRNA and protein levels in FLS in hind knee joint tissue in the CIA mice. These results demonstrate that β-arrestin 1 and 2 in FLS may, in part, contribute to the observed increase of β-arrestins in arthritic joint tissue. It is interesting that increased β-arrestin 1 expression in knee joint is sustained during arthritis progression whereas increased β-arrestin 1 expression in FLS subsides at 8 weeks. These data suggest other immune cells in addition to FLS also contribute to β-arrestin 1 expression in arthritic joint. Indeed, we have observed that in splenic CD4+ T cells and dendritic cells β-arrestin 1 expression are increased during arthritis development (data not shown). These cells may be recruited into arthritic joints.
Activated FLS secrete numerous pro-inflammatory mediators and degradative enzymes which contribute to the inflammation and joint destruction in RA (Pap et al., 2005; Sweeney and Firestein, 2004). We observed that both β-arrestin isoforms protein and mRNA levels increased early in FLS in CIA mice at 4 and 6 weeks and subsequently returned to the baseline level at 8 weeks. Thus increased β-arrestin 1 and 2 expression occurs early in RA pathogenesis. Parallel to β-arrestin mRNA levels, we found TNFα and MMP3 mRNA levels were also increased in CIA mice (data not shown). Since lentiviral overexpression of β-arrestin 1 augmented inflammatory cytokines and overexpression of β-arrestin 2 suppressed inflammatory cytokines, the endogenous increase of β-arrestin isoforms may differentially regulate the inflammatory response. Our previous studies have demonstrated that β-arrestin 1 and β-arrestin 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression (Fan et al., 2007). Therefore β-arrestin 1 and β-arrestin 2 may induce or inhibit respectively, inflammatory events at different stages in arthritis progression.
To determine which inflammatory mediators induced β-arrestins, FLS were exposed to TNFα, HA (TLR2/4 endogenous ligand) and HMGB1 (TLR4 endogenous ligand). Consistent with our results in vivo in CIA and TNFtg mice, β-arrestin 1 and 2 protein levels were increased in vitro in FLS stimulated with TNFα and HA, while HMGB1 only stimulated β-arrestin 1 expression. Since HMGB1 may activate TLR4 and HA may stimulate TLR2 in addition to TLR4, it is possible that induction of β-arrestin 1 by HMGB1 may be TLR4 dependent whereas induction of β-arrestin 2 may be through TLR2. However HA also activates CD44 and HMGB1 additionally activates RAGE receptor (Ponta et al., 2003; Sims et al., 2010). These latter receptors may be also involved in induction of β-arrestin isoforms. To further study the signaling pathway contributing to the increases of β-arrestin 1 and 2 protein levels, PI3K, MEK, JNK and p38 kinases inhibitors were used in FLS. Our results demonstrate for the first time that the TNFα- and HA-induced increase of β-arrestin 2 protein levels appears to be regulated by p38 pathway.
Our studies demonstrating that β-arrestin 2 negatively regulates the inflammatory response in FLS and inhibits CAIA pathogenesis agree with our previous study that β-arrestin 2 negatively regulates sepsis-induced inflammation (Fan et al., 2010). The molecular pathways remain to be defined but our previous studies demonstrated that β-arrestins negatively regulate LPS-induced NFκB activation (Fan et al., 2007). Thus the protective effect of β-arrestin 2 in FLS may be through inhibiting NFκB signaling. The role of β-arrestin 1 in the inflammatory response remains to be further investigated. However, previous studies demonstrated that β-arrestin 1 and 2 reciprocally regulate GPCR mediated ERK 1/2 activation suggesting β-arrestin 1 and 2 may have distinct or even opposite functions (Ahn et al., 2004).
In conclusion, increased β-arrestin 1 and 2 expression are found in vivo and in vitro in joint and FLS in arthritis models. Moreover, the increased β-arrestin 2 expression seems to be mediated via p38 pathway and β-arrestin 2 negatively regulates the inflammatory response in FLS. Our findings that β-arrestin 2 prevents progress of CAIA supports this conclusion. Although our studies suggest β-arrestin 1 may enhance inflammation in FLS, the role of β-arrestin 1 in the in vivo experimental arthritis model remains to be further investigated. Our data provide a novel insight into the molecular mechanisms of regulating the pro-inflammatory response in RA pathogenesis suggesting that β-arrestins may become potential therapeutic targets for RA.
Highlight.
β-arrestin 1 and 2 expression are increased in experimental arthritis.
TNFα-, HA- and HMGB1-induced increases of β-arrestins.
β-arrestin 1 and 2 differentially regulate HA-induced pro-inflammatory cytokines.
β-arrestin 2 KO mice exhibited more severe arthritis in CAIA.
Acknowledgments
This work was supported by NIH AI079248 (HF) and NIH GM27673 (JAC).
Abbreviations
- RA
rheumatoid arthritis
- CIA
collagen-induced arthritis
- TNFtg
human TNFα transgenic
- FLS
fibroblast-like synoviocytes
- HA
low molecular weight hyaluronan
- HMGB-1
high mobility group box
- CAIA
collagen antibody induced arthritis
- DC
dendritic cells
- GPCR
G protein coupled receptor
- WT
wild type
- KO
knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pengfei Li, Email: lippe@musc.edu.
James A. Cook, Email: cookja@musc.edu.
Gary S. Gilkeson, Email: gilkeson@musc.edu.
Louis M. Luttrell, Email: luttrell@musc.edu.
Liping Wang, Email: wanglp@jlu.edu.cn.
Keith T. Borg, Email: borgk@musc.edu.
Perry V. Halushka, Email: halushpv@musc.edu.
Hongkuan Fan, Email: fanhong@musc.edu.
References
- Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000a;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000b;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Bitto A, Zingarelli B, Luttrell LM, Borg K, Halushka PV, Cook JA. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol. 2007;44:3092–3099. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Koenders MI, Smeets RL, Heuvelmans-Jacobs M, Helsen MM, Takeda K, Akira S, Lubberts E, van de Loo FA, van den Berg WB. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- Kesherwani V, Sodhi A. Quantitative role of p42/44 and p38 in the production and regulation of cytokines TNF-alpha, IL-1beta and IL-12 by murine peritoneal macrophages in vitro by concanavalin A. Cytokine. 2007;37:62–70. doi: 10.1016/j.cyto.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- Kyo F, Futani H, Matsui K, Terada M, Adachi K, Nagata K, Sano H, Tateishi H, Tsutsui H, Nakanishi K. Endogenous interleukin-6, but not tumor necrosis factor alpha, contributes to the development of toll-like receptor 4/myeloid differentiation factor 88-mediated acute arthritis in mice. Arthritis Rheum. 2005;52:2530–2540. doi: 10.1002/art.21213. [DOI] [PubMed] [Google Scholar]
- Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005;17:325–333. doi: 10.1093/intimm/dxh212. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- Miller WE, McDonald PH, Cai SF, Field ME, Davis RJ, Lefkowitz RJ. Identification of a motif in the carboxyl terminus of beta -arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–27777. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- Moreland LW. Biologic therapies on the horizon for rheumatoid arthritis. J Clin Rheumatol. 2004;10:S32–S39. doi: 10.1097/01.rhu.0000130688.68036.ef. [DOI] [PubMed] [Google Scholar]
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Pap T, Meinecke I, Muller-Ladner U, Gay S. Are fibroblasts involved in joint destruction? Ann Rheum Dis. 2005;64 Suppl 4:iv52–iv54. doi: 10.1136/ard.2005.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, Joosten LA, van den Berg WB. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, van den Berg WB, Radstake TR. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- Saxne T, Palladino MA, Jr, Heinegard D, Talal N, Wollheim FA. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31:1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senokuchi T, Matsumura T, Sakai M, Matsuo T, Yano M, Kiritoshi S, Sonoda K, Kukidome D, Nishikawa T, Araki E. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase mediate macrophage proliferation induced by oxidized low-density lipoprotein. Atherosclerosis. 2004;176:233–245. doi: 10.1016/j.atherosclerosis.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- Tremoulet AH, Albani S. Novel therapies for rheumatoid arthritis. Expert Opin Investig Drugs. 2006;15:1427–1441. doi: 10.1517/13543784.15.11.1427. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Kim HY, Park JH, Hwang SH, Lee SY, Cho CS, Lee SK. Regulation of TNF-alpha-mediated hyperplasia through TNF receptors, TRAFs, and NF-kappaB in synoviocytes obtained from patients with rheumatoid arthritis. Immunol Lett. 2002;83:85–93. doi: 10.1016/s0165-2478(02)00079-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shen M, Ding M, Shen D, Ding F. The neuroprotective action of pyrroloquinoline quinone against glutamate-induced apoptosis in hippocampal neurons is mediated through the activation of PI3K/Akt pathway. Toxicol Appl Pharmacol. 2011;252:62–72. doi: 10.1016/j.taap.2011.02.006. [DOI] [PubMed] [Google Scholar]