Abstract
Reports indicate that prostaglandin (PG)E2 markedly enhances antigen-mediated degranulation in mouse bone marrow-derived mast cells (BMMCs) but not in human mast cells (HuMCs). We have examined the underlying mechanism(s) for this disparity in HuMCs derived from the peripheral blood of multiple donors in addition to mouse BMMCs. HuMCs from half of these donors failed to respond to PGE2 and the PGE2 EP3 receptor agonist, sulprostone. However, HuMCs from the remaining donors and the LAD2 human MC line responded to PGE2 and sulprostone with marked enhancement of antigen-mediated degranulation and IL-8 production in a similar manner to that observed in mouse BMMCs. The EP2 agonist, butaprost, failed to modulate antigen-mediated responses in any type of MCs. These distinct phenotypes could not be explained by differences in EP2 or EP3 expression nor by differences in the ability of PGE2 to elevate levels of cAMP, a signal recognized to down-regulate mast cell activation. Moreover, both responder and non-responder HuMC populations exhibited similar activation of phosphatidylinositol 3-kinase, and MAP kinases. However, translocation of PLCγ1 to the cell membrane and the associated calcium signal were enhanced only in the responder HuMC population indicating that the link between EP3 and PLCγ is impaired in the non-responder HuMCs.
Conclusions
These data provide a cautionary note for the translating of observations in the mouse to human mast cell-dependent disorders, but may also provide a basis for examining the effects of co-activating receptors in patients susceptible to allergic conditions.
Keywords: Mast Cells, PGE2, degranulation, cytokine release, signalling
Introduction
Inflammatory mediators released from activated mast cells play a major role in the initiation of allergic reactions associated with anaphylaxis, atopy, rhinitis and allergic ocular disorders [1]. The manifestations of these reactions are the direct consequence of activation of a complex signaling cascade within mast cells upon antigen/IgE-dependent aggregation of cell surface high affinity receptors for IgE (FcεRI) [2]. Mast cells also express a variety of other classes of receptors that have the capacity to potentiate FcεRI-mediated mast cell responses [3–5]. Such receptors include the growth factor receptor KIT [6,7], Toll like receptors 2, and 4 [8], the IL-33 receptor [9], and specific G protein-coupled receptors (GPCRs) including the EP3 prostaglandin (PG)E2 receptor, the A2b and A3 adenosine receptors and the C3a complement receptor [10–12].
The ability of these co-activating receptors to enhance antigen-mediated mast cell activation has led to the suggestion that, under specific circumstances, these receptors have the capacity to contribute to allergic responses in vivo [13,14]. However, the extent to which they actually influence mast cell activation in a physiological setting has yet to be determined. Nevertheless, studies conducted in both mouse bone marrow-derived mast cells (BMMCs) and human mast cells derived from peripheral blood progenitor cells (HuMCs) have revealed that the KIT ligand, stem cell factor (SCF), markedly enhances antigen-mediated mast cell degranulation and cytokine production [6,7]. The synergistic interactions between KIT and the FcεRI are regulated by an amplification pathway involving the phosphoinositide 3 kinase (PI3K)/Btk axis leading to enhanced phospholipase (PL) Cγ activation and an elevated calcium signal [15,16]. At least in the case of degranulation, these events may be coordinated by the transmembrane adaptor molecule, linker for activation of T cells 2 (LAT2; also known as LAB or NTAL) [7,17]. As with SCF, both IL-33 [9] and specific TLR [8] agonists markedly enhance FcεRI-mediated cytokine generation in mouse BMMCs and/or the mouse MC9 mast cell line. However, in contrast to the SCF-mediated response, the consensus of reports [8,18], including our own unpublished observations, suggest that neither agonist enhances mast cell degranulation or the release of arachidonic acid which is necessary for production of eicosanoids. This may be explained by the fact that both of these agents signal through the adaptor molecule MyD88 [19] which appears to lack the ability to generate the required calcium signal [8].
Of the various G protein coupled receptor (GPCR) agonists known to potentiate the actions of antigen, we found that PGE2 was the most effective and that it acted primarily through the EP3 subset of PGE2 receptors [20]. Although the synergistic actions of PGE2 and SCF were remarkably similar, in that both degranulation and cytokine production were enhanced [6,15,20], the underlying signaling events regulating these responses were different. The synergistic actions of PGE2 on mediator release were not dependent on phosphatidylinositol 3-kinase (PI3K), as was the case of SCF [6], but rather on a cross-synergy in the membrane translocation and activation of PLCβ and PLCγ [20]. PGE2 acts through PLCβ via the EP3 receptor via Gαi in contrast to the activation of PLCγ by antigen via FcεRI [20].
Much of the experimental evidence for the enhancement of mast cell activation by co-activating receptors has been obtained from studies of mouse BMMCs [3] although the synergy between KIT and FcεRI has been replicated in HuMCs [6,7]. This is in contrast to the reports that PGE2 enhances antigen-mediated activation of BMMCs [21,22] but inhibits antigen-induced degranulation in HuMCs [23,24]. In preliminary studies we too were unable to detect any potentiation of degranulation by PGE2 in HuMCs derived from CD34+-peripheral blood progenitor cells. Thus, at least in terms of the PGE2 response, the mouse model may not reflect the situation in humans. We thus investigated this apparent dichotomy in HuMCs derived from multiple donors and in BMMCs.
As reported here, in contrast to previous observations, we find that as in BMMCs, PGE2 can synergistically enhance mast cell degranulation and cytokine production in HuMCs. However, this enhancement was donor dependent as mast cells from half the human donors failed to respond to PGE2, hereafter respectively referred to as the responder and non-responder HuMC populations. The differences between PGE2-responders and non-responders were unrelated to EP receptor expression, but rather reflected differences in downstream signaling. These data suggest caution must be applied when observations in the mouse are translated to human systems and reminds us of the heterogeneity of human immunologic responses. Regardless, as will be discussed, the data may also provide insight as to how co-activating receptors may impact mast cell driven disease in the human.
Materials and Methods
Human and mouse mast cell cultures
CD34+ peripheral blood progenitor cells for this study were selected at random from stored samples obtained from normal donors. These samples were obtained following informed consent under a protocol approved by the NIAID IRB. Primary human mast cells (HuMCs) were obtained from these progenitor cells by culturing in StemPro-34 culture media containing recombinant human IL-3 (30 ng/ml) (first week only), IL-6 (100 ng/ml), and SCF (100 ng/ml) (Peprotech) as described [25,26]. Experiments were conducted on these cells 7–10 weeks after the initiation of culture. The LAD2 human mast cells were cultured in StemPro-34 culture media containing SCF (100 ng/ml) (Peprotech) as described [27].
Mouse bone marrow-derived mast cells (BMMCs) were obtained by flushing bone marrow cells from the femurs of C57BL/6J mice (The Jackson Laboratory), then culturing the cells for 4–6 weeks in RPMI 1640 supplemented with 10% FBS, glutamine (4 mM), sodium pyruvate (1 mM), penicillin (100 units/ml), streptomycin (100 µg/ml), non-essential amino acids (Sigma), HEPES (25 mM), β-mercaptoethanol (50 µM) and mouse recombinant IL-3 (30 ng/ml) (Peprotech). Cultures were maintained at 37 °C in a humidified incubator of 95% air, 5% CO2. Mouse studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee at NIH.
Cell activation
For the initial activation experiments, HuMCs were grown from 8 individual donors (H1–H8). Subsequent signaling studies were conducted on a subset of cells obtained from selected donors displaying specific phenotypes, depending on availability. HuMCs or BMMCs were sensitized overnight with an optimal concentration (100 ng/ml) of biotinylated human IgE or mouse SPE-7 (IgE anti-DNP) (Sigma) respectively, in cytokine- and SCF-free medium. The following day, the cells were washed with cytokine-free medium or HEPES buffer (10 mM HEPES [pH 7.4], 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4·7H2O, 5.6 mM glucose, 1.8 mM CaCl2·2H2O, 1.3 mM MgSO4·7H2O) containing 0.04% BSA (Sigma) to remove excess IgE and then resuspended in medium or HEPES buffer at the required cell density for a specific assay. BMMCs or HuMCs were stimulated with DNP-HSA (10 ng/ml) or with streptavidin (SA, 10 ng/ml) at 37 °C respectively.
Degranulation and Cytokine Production
For degranulation, mast cells were sensitized overnight as described above and then rinsed with HEPES buffer containing 0.04% BSA. HuMCs and LAD2 cells (1×104 cells/well) and BMMCs (3–5×104 cells/well) were aliquoted into individual wells of a 96 well plate and triggered in the same buffer with antigen/SA (0–100 ng/ml) and/or indicated GPCR agonists (Sigma) for 30 min. Degranulation was monitored by the release of β-hexosaminidase into the supernatants [7,20] and calculated as a percentage of the total content (cells and media) after cell activation.
For cytokine release studies, cells were sensitized as above, washed with cytokine-free medium. HuMCs (1×106 cells/ 1ml/ well) were triggered in this media for 6–8 hours with SA (10 ng/ml), PGE2 (100 nM), and/or SCF (100 ng/ml). Cytokines were measured in the cell culture supernatant by human IL-8 Quantikine ELISA kits (R&D Systems).
Fractionation of Cells and Immunoblotting
Membrane fractions were prepared as described [20]. Briefly, HuMCs (1×106 cells/sample) were sensitized as described above and washed with HEPES (0.04% BSA) buffer, then stimulated with SA (10 ng/ml) and/or PGE2 (100 nM) at 37 °C for 2 min. After lysis of the cells and sonication, the resultant preparations were centrifuged at 20,000×g for 30 min. Proteins in the pellet fractions were then solubilized with 1% Triton X-100, 1% NP40, and 0.1% SDS in lysis buffer followed by centrifugation at 15,000×g for 15 min to collect the solubilized membrane fraction.
For immunoblot analyses, HuMCs (0.5×106 cells/sample) were sensitized with IgE and washed with HEPES (0.04% BSA) buffer, then stimulated with SA (10 ng/ml) and/or PGE2 (100 nM) at 37 °C for 2 and 10 min. HuMC lysates were prepared as described [20,28]. Total cell lystates or membrane fractions were subjected to electrophoresis on 4–12% NuPAGE BisTris gels (Invitrogen). Following membrane transfer, proteins were probed utilizing the following antibodies: anti-phospho-AKT (Ser(P)-473) pAb, anti-phospho-ERK1/2 (Thr(P)-202, Tyr(P)-204) pAb, anti-phospho-JNK (Thr(P)-183, Tyr(P)-185) pAb, anti-phospho-p38 (Thr(P)-180, Tyr(P)-182) pAb (Cell Signaling, Beverly, MA); anti-phospho-PLCγ1 (Tyr(P)-783) pAb (BIOSOURCE, Invitrogen), anti-phospho-Btk (Tyr(P)-551) mAb (BD bioscience); anti-PLCγ1 (1249) (Santa Cruz Biotechnology). To normalize protein loading, membranes were stripped and probed for Syk (total lysates) or KIT (membrane fraction) (Santa Cruz Biotechmology) or, alternatively, identically loaded samples were probed for Syk or KIT. Changes in protein phosphorylation or translocation were determined by scanning the ECL films using a Quantity One scanner (Bio-Rad)
Real-time RT-PCR
Total RNA was isolated from each HuMC (1 × 106/sample) population or BMMCs (1 × 106/sample) using the RNeasy Mini Kit (Qiagen). One microgram of total cellular RNA was treated to remove genomic DNA contamination and reverse transcribed using SA Biosciences Reverse Transcription reagents and oligo(dT) (SA Biosciences). PGE2 EP receptor expression was analyzed using real-time PCR on an ABI7500 SDS system (Applied Biosystems). All EP probes and GAPDH were purchased from Applied Biosystems. All reactions were performed in triplicate for 40 cycles. The relative expression levels of EP receptors were calculated as follows: for each sample the threshold cycle (Ct) was determined and normalized to the average value for GAPDH housekeeping gene (ΔCt). The value for ΔCt was then subtracted from EP3 (ΔΔCt) and the relative fold expression was calculated using the equation 2ΔΔCt × 100.
Measurement of cAMP accumulation
Cyclic AMP accumulation was measured using a kit, following the manufacturer’s instructions (Cayman chemical). Briefly, HuMCs (1 × 106/sample), LAD cells (1 × 106/sample) and BMMCs (2 × 106/sample) were incubated in cytokine-free medium for 16–20h. Cells were pre-incubated with the cAMP phosphodiesterase inhibitor Isobutyl methylxanthine (IBMX) (100 µM) for 10 min at 37 °C and then the cells were stimulated with PGE2 (100 nM) or forskolin (10 µM) for 20 min at 37 °C. After spinning, the levels of cAMP were determined in the supernatants. For comparison, the results are normalized to pmol in 1 × 106 cells/50 µl.
Intracellular Ca2+ Determination
Ca2+ flux was measured in the HuMCs following loading of the cells with Fura-2 AM ester (Molecular Probes) as described [25]. Cells were loaded with Fura-2 AM (2 µM) for 30 min at 37 °C, rinsed, resuspended in HEPES buffer containing 0.04% BSA and sulfinpyrazone (0.3 mM) (Sigma), and then placed in a 96-well black culture plate (2×104 cells/well) (CulturPlat-96 F, PerkinElmer Life Sciences). Fluorescence was measured at two excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm. The ratio of the fluorescence readings was calculated following subtraction of the fluorescence of the cells that had not been loaded with Fura-2 AM.
Statistical Analysis
Data are represented as the mean ± S.E. The n values represent the number of experiments from multiple preparations from the indicated donors.
Results
Potentiation of antigen-mediated mast cell degranulation by PGE2
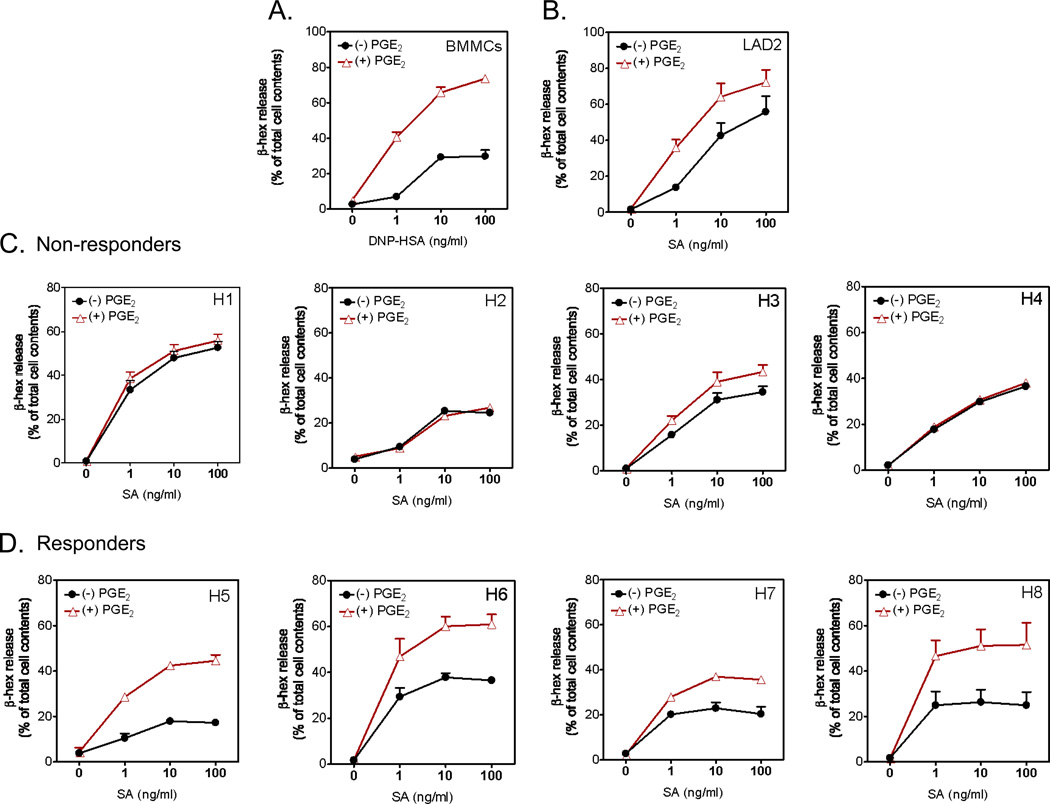
In contrast to our previous observations in mouse BMMCs [20], we did not detect synergistic enhancement of antigen-dependent degranulation and cytokine production by PGE2 in HuMCs in preliminary experiments (data not shown). In investigating the underlying cause of this disparity, we collected CD34+ progenitor cells for culture from additional donors to obtain mature mast cells as described in “Materials and Methods”. These cells were sensitized with biotinylated IgE and then activated through FcεRI aggregation with streptavidin in the absence or presence of an optimal concentration of PGE2 at 100 nM [20,29]. Degranulation was assessed by the release of the granule marker, β-hexosaminidase, and compared to that determined in the LAD2 human mast cell line and, as a positive control, mouse BMMCs.
As before [20], PGE2 markedly enhanced antigen-mediated degranulation in the C57BL/6J BMMCs without producing significant degranulation in the absence of antigen (Figure 1A). PGE2 similarly potentiated antigen-mediated degranulation in 129s/v and Balb/c BMMCs (data not shown). PGE2 also enhanced antigen-mediated degranulation in the LAD2 human mast cell line, although to a lesser extent than in BMMCs (Figure 1B). However, in mast cell cultures derived from peripheral blood progenitors from 4 of the 8 donors tested, PGE2 neither enhanced nor reduced antigen-mediated degranulation (Figure 1C). In contrast, mast cells from the remaining 4 donors showed a similar degree of enhancement to that observed in the mouse BMMCs (Figure 1D). These differences were conserved in replicate cultures from each donor (data not shown) and thus reflected consistent differences rather than variable responses due to culture conditions. Although we observed some variability in the degree of antigen-mediated degranulation between the individual donor mast cells, there were no marked differences between the PGE2-responsder and non-responder groups in the range of degranulation observed in response to antigen in the absence of PGE2. Therefore, the ineffectiveness of PGE2 in enhancing release in the non-responders did not relate to the maximal possible degranulation levels achieved by antigen in the absence of PGE2.
Figure 1.
Comparative effects of PGE2 on antigen-induced degranulation in BMMCs (A), LAD2 cells (B) or HuMCs (C and D). BMMCs, LAD2 cells or HuMCs from eight different donors were sensitized overnight and then challenged with PGE2 (100 nM) or antigen (DNP-HSA for BMMCs, streptavidin (SA) for HuMCs and LAD2) or antigen with PGE2 added concurrently for 30 min and β-hexosaminidase (β-hex) release then determined. Data are represented as the mean ±S.E and representative of n=2–3 experiments conducted in duplicate. H1–H8 refers to HuMC1–HuMC8 from eight different donors. This designation and order were made following the identification of the two phenotypes.
Relative expression of EP2 and EP3 receptors in responder and non-responder HuMC populations
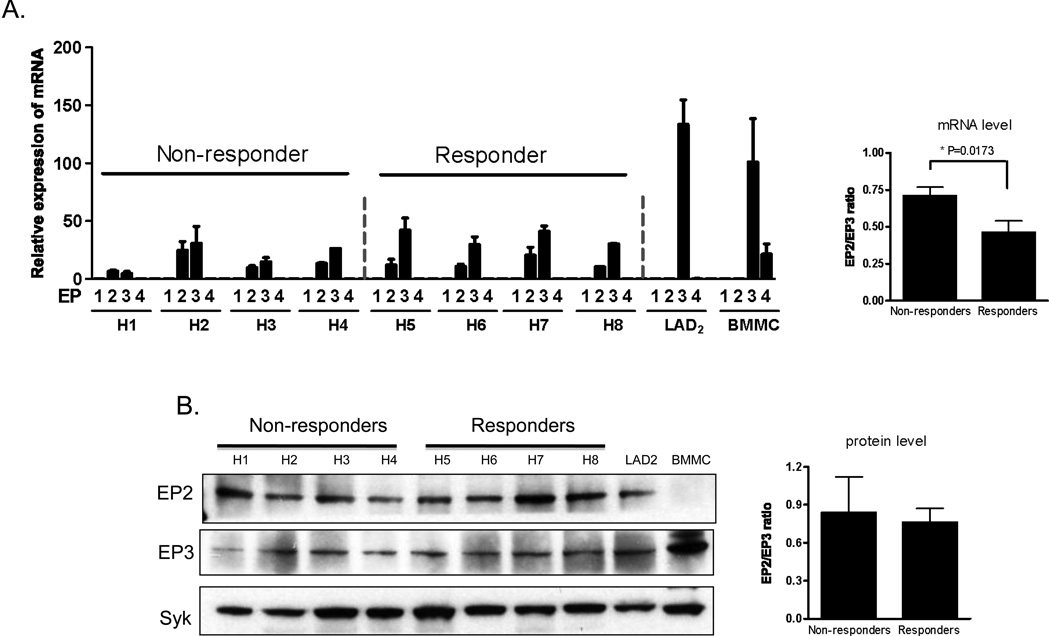
Mast cells express PGE2 Gαs-coupled EP2 receptors that regulate intracellular cAMP production and Gαi-coupled EP3 receptors that are linked to PLCβ and PI3Kγ activation [20,30]. EP2 receptors are reported to down-regulate mast cell responses to antigen via cAMP production [23,24] whereas evidence obtained from knockout mice reveal that EP3 receptors up-regulate these responses [21]. In determining whether the responder and non-responder HuMC phenotypes could be attributed to differential expression of these two receptors, we found by real time PCR that both BMMCs and LAD2 cells expressed message for EP3 and, to a minor extent, EP4 (Figure 2A). EP2 message was not detected in either cell type. Both the responders and non-responders appeared to express similar levels of EP2 and EP3 message although the ratio between the expression of EP3 compared to EP2 was approximately 30% higher in the responders (Figure 3A). Nevertheless, at the protein level, EP2 and EP3 receptors were detected at comparable levels in both HuMC phenotypes and LAD2 cells, although EP2 protein was not detected in the BMMCs (Figure 2B) and the levels of message for LAD2 cells was appreciably lower than that observed for BMMCs. This may be due to differences in the half life for EP2 mRNA and protein in these cells. Regardless, the data as a whole suggest that it is likely that the phenotypic differences between responder and non-responder HuMC populations were not a result of differential expression of these receptors. In a similar manner, we observed no differences in FcεRI expression between responder and non-responder populations (data not shown).
Figure 2.
Expression of EP receptors in BMMCs, LAD2 cells or HuMCs. A. RNA and cDNA were prepared from the cells as described in ‘Material and Methods’, and real time PCR was performed using commercial Taq-Man EP probes. The expression was normalized to the GAPDH probe and then normalized to BMMCs EP3 expression. The relative ratios between the EP2 and EP3 message in non-responders and responders are presented in the bar graph. B. Total cell lysates were prepare from HuMCs, LAD2 or BMMCs and performed gel electrophoresis, the proteins were probed with antibodies recognizing EP2 or EP3. Protein loading of the samples was normalized by stripping and then probing for Syk. Data are represented as the mean ±S.E. The relative ratios between the EP2 and EP3 protein in non-responders and responders are presented in the bar graph following scanning of the gel. The relative expression of EPs are representative of n=2–3 experiments conducted in triplicate. Blots are representative of three independent experiments.
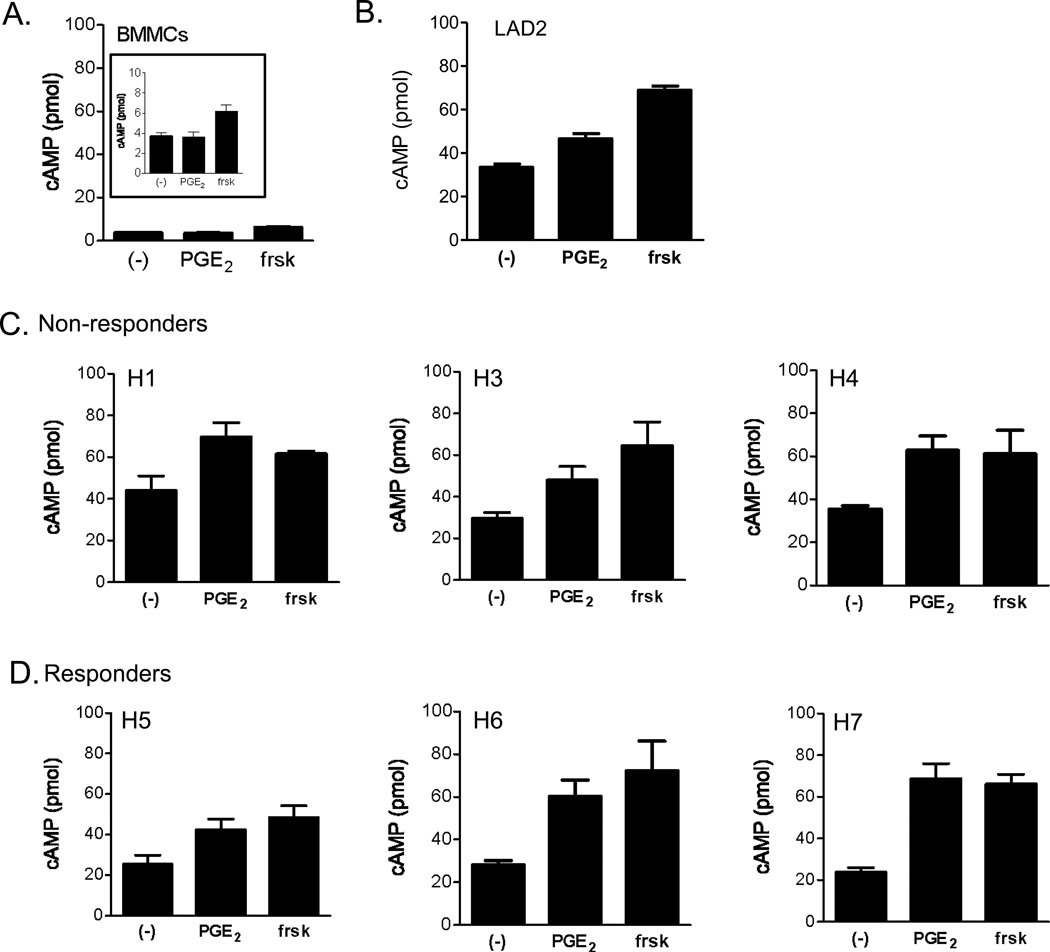
Figure 3.
cAMP production in response to PGE2 in mouse BMMCs (A) (insert magnifies cAMP scale from 0 to 10 pmoles), LAD2 cells (B) and HuMCs (C,D). Briefly, the cells were preincubated with the cAMP phosphodiesterase inhibitor, IBMX (100 µM) for 10 min at 37 °C then stimulated with PGE2 (100 nM) or forskolin (frsk: 10 µM) for 20 min at 37 °C. The reaction was stopped by the addition of HCl followed by centrifugation. The cAMP content of the supernatants was determined as described in “Material and Methods”. For comparison, the results are proportionally represented to pmol in 1 × 106 cells/50 µl. Data are presented as the mean ±S.E. from n=2–3 experiments conducted in duplicate.
PGE2-induced Cyclic-AMP production in human mast cells
Despite the similar expression of EP2 receptors in both sets of HuMC populations, differences in efficacies in coupling of EP2 to adenylate cyclase, and hence cAMP production might result in more pronounced suppression of mast cell activation in the non-responder but not the responder HuMC phenotype. This possibility was examined in BMMCs, LAD2 cells and the HuMC populations using the adenylate cyclase stimulant, forskolin, as a positive control. Mouse BMMCs had low basal levels of cAMP and failed to respond to PGE2 but responded to forskolin with an increase in levels of cAMP (Figure 3A) which likely reflects the low expression of EP2 receptors in these cells. In the LAD2 cells, PGE2 induced only a slight elevation in cAMP levels (Figure 3B) as compared to forskolin. In HuMCs, PGE2 elevated cAMP to levels approaching those induced by forskolin (Figure 3C and 3D). However, there were no marked differences in cAMP expression between the non responder (Figure 4C) and responder (Figure 4D) populations. These data support the conclusion that the efficacy in coupling of the EP2 receptor to cAMP production did not account for the phenotypic differences between HuMC populations. Furthermore, these data provide further support for our conclusion that differences in the ability of PGE2 to enhance antigen-mediated degranulation in human mast cells are not due to variations in EP2 receptor expression.
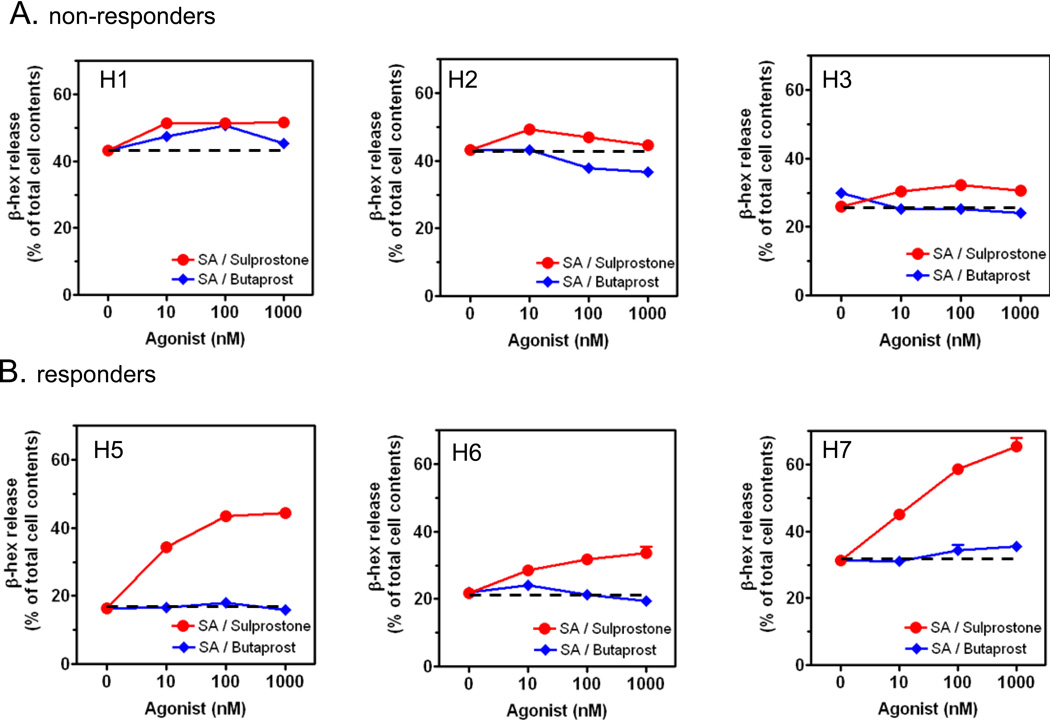
Figure 4.
The effect of EP agonists on antigen-induced degranulation in responder (A) and non-responder (B) populations of HuMCs. Cells were sensitized overnight and then challenged with the indicated concentration of sulprostone or butaprost, in the presence of streptavidin (SA); (10 ng/ml) for 30 min and β-hexosaminidase (β-hex) release then determined. The dotted line represents the level of release obtained with antigen in the absence of EP receptor agonists. Data are represented as the mean ± S.E. The degranulation data are n=2–3 experiments conducted in duplicate.
Comparison of the effects of the EP-selective agonists butaprost and sulprostone on degranulation and cytokine production in responder and non-responder HuMC populations
Experiments were conducted with the selective EP2 and EP3 agonists, butaprost and sulprostone respectively, to verify that PGE2 enhanced degranulation acted through EP3 receptors; and that non-responder HuMC were refractory to either agonist as well as PGE2. As shown in Figure 4A, the populations that were determined to be non- responsive to PGE2 (Figure 1C) also failed to respond to both agonists (Figure 4A). In contrast, the previously identified responders (Figure 1D) displayed an enhancement of antigen-induced degranulation in response to the sulprostone but not butaprost (Figure 4B). These data indicated that, as in BMMCs [20,21], the potentiating effects of PGE2 were mediated through EP3.
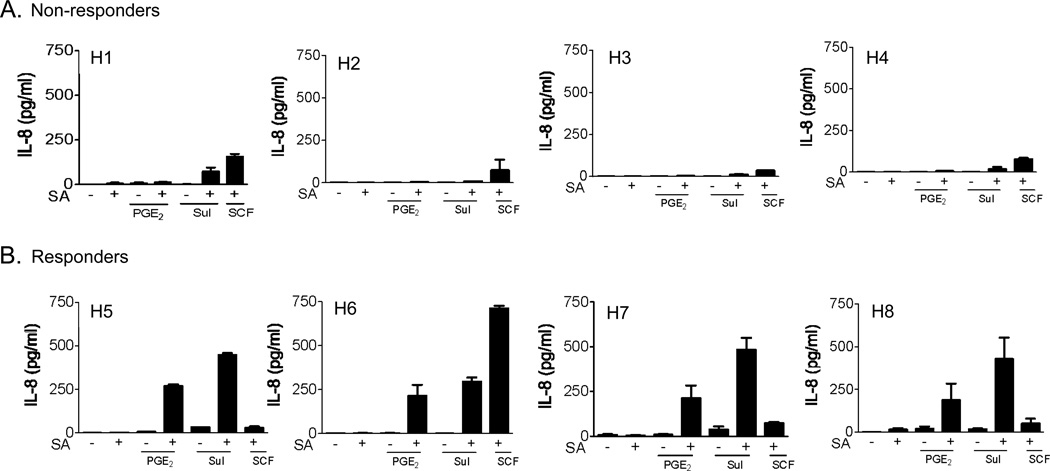
In addition to degranulation, PGE2 enhances antigen-induced cytokine production in mouse BMMCs [20,22]. We therefore determined whether production of cytokines, specifically IL-8, was resistant to the enhancing effects of PGE2 in the previously identified non-responding populations of HuMCs. In these studies, SCF was used as a control co-stimulant. As shown in Figure 5A, the non-responder HuMC populations exhibited no, or minimal, enhancement of antigen-mediated IL-8 release by PGE2 or sulprostone. This is in contrast to the responder populations which showed marked enhancement of IL-8 generation in response to these agents (Figure 5B). There were no overall differences between the populations in their ability to respond in a synergistic manner to SCF and antigen except for one (H6) from the responder population which exhibited an unusually high response. These data are consistent with the conclusion that the differential effect of PGE2 on mast cell mediator release is not due to inherent differences in the ability of the EP2 receptor to down-regulate mast cell activation but that that EP3-generated signals specifically fail to interact synergistically with FcεRI-mediated signals.
Figure 5.
The effect of PGE2 on IL-8 generation in non-responder (A) and responder (B) HuMC populations. Sensitized cells were washed with cytokine- and SCF-free medium. HuMCs (1×106 cells/ 1ml/ well) were triggered in this media for 6–8 hours with SA (10 ng/ml), PGE2 (100 nM), sulprostone (Sul, 100 nM), or SCF (100 ng/ml). Cytokine released into the cell culture supernatant was measured by a human IL-8 Quantikine ELISA kit. The cytokine data are represented as the mean ±S.E. from n=2–3 experiments conducted in duplicate.
Effect of the PGE2 on phosphorylation of key signaling events in responder and non-responder populations
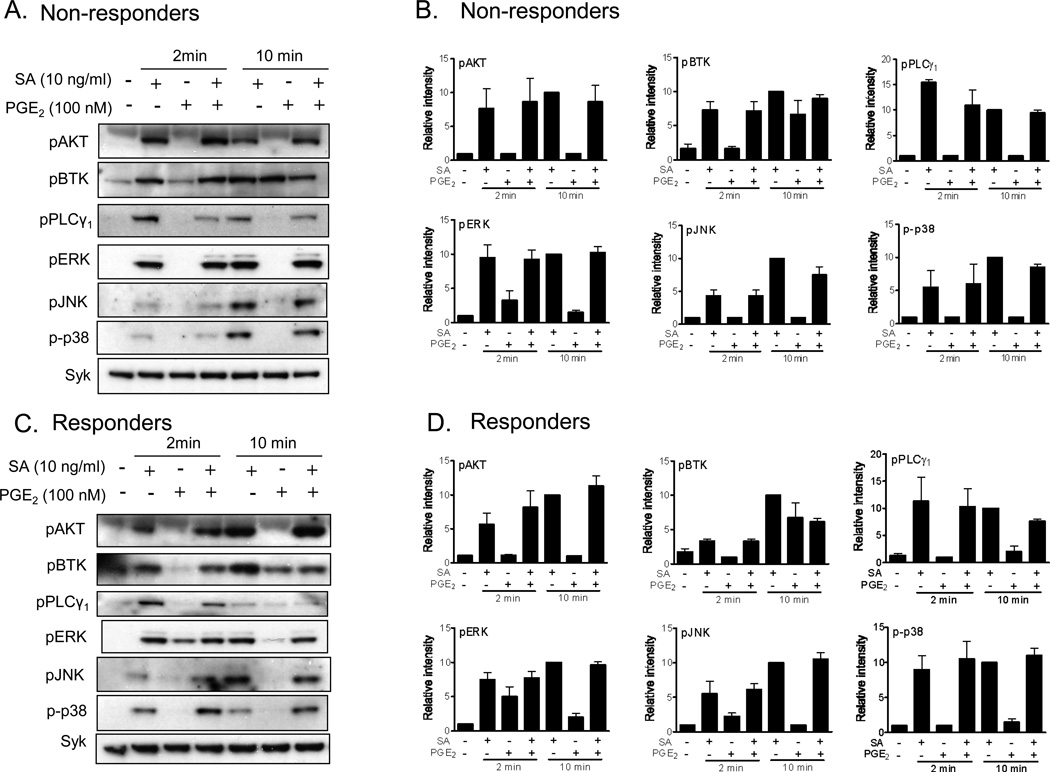
Downstream of early signaling events, namely phosphorylation of FcεRIβ and γ chains and activation of the tyrosine kinases Lyn, Fyn, and Syk [2,3,5], antigen-mediated degranulation is regulated by two key enzymes, PLCγ and PI3K [5], whereas the MAP kinases ERK1/2, JNK and p38 regulate cytokine gene transcription. To detect whether these signaling events were intact or not in non-responder HuMCs, cells of both phenotypes (responders and non-responders) were stimulated with PGE2 and/or antigen and the key signaling molecules were probed for their phosphorylation status in cell lysates. As shown in Figure 6, there was no obvious deficiency in PI3K activation induced by antigen and PGE2 or the combination thereof, as monitored by the phosphorylation of AKT, a surrogate marker for PI3K activation, in the non-responder population. In addition, there was no apparent deficiency in phosphorylation of Btk, a PI3K regulated TEC kinase responsible for mediating amplification signals for enhancing antigen-mediated mast cell activation. Furthermore there were little obvious differences in the phosphorylation of PLCγ1, the major PLCγ isoform for human mast cell activation, nor in the phosphorylation of ERK1/2, JNK or p38 between the non-responder and responder mast cell populations (Figure 6). Therefore defects in the activation of PI3K, PLCγ1 and MAP kinases did not appear to account for the differences between the non-responder and responder HuMC phenotypes. These data also again support the conclusion that differences in cell surface PGE2 receptor expression and immediate downstream signals were not responsible for the relative inability of the non-responder populations to show enhanced antigen-mediated degranulation in response to PGE2.
Figure 6.
Cell signaling in response to antigen and/or PGE2. Sensitized HuMCs were washed and stimulated with the indicated agonist for 2 or 10 min. A–B. Total cell lysates were prepared from HuMCs and proteins separated by gel electrophoresis. The proteins were probed with indicated antibodies. Protein loading of the samples was normalized by stripping and then probing for Syk. Indicated protein levels were normalized to Syk, then to antigen responses at 10 min to determine the relative intensities presented under each blot. C,D. To quantitate changes in protein level, the ECL films were scanned using a BioRad Quantity One scanner. Data are represented as the mean ±S.E. from three donors. The blots are representative of three responder or non-responder donors.
Effect of the PGE2 on membrane translocation of PLCγ1 and calcium flux in responder and non-responder HuMC populations
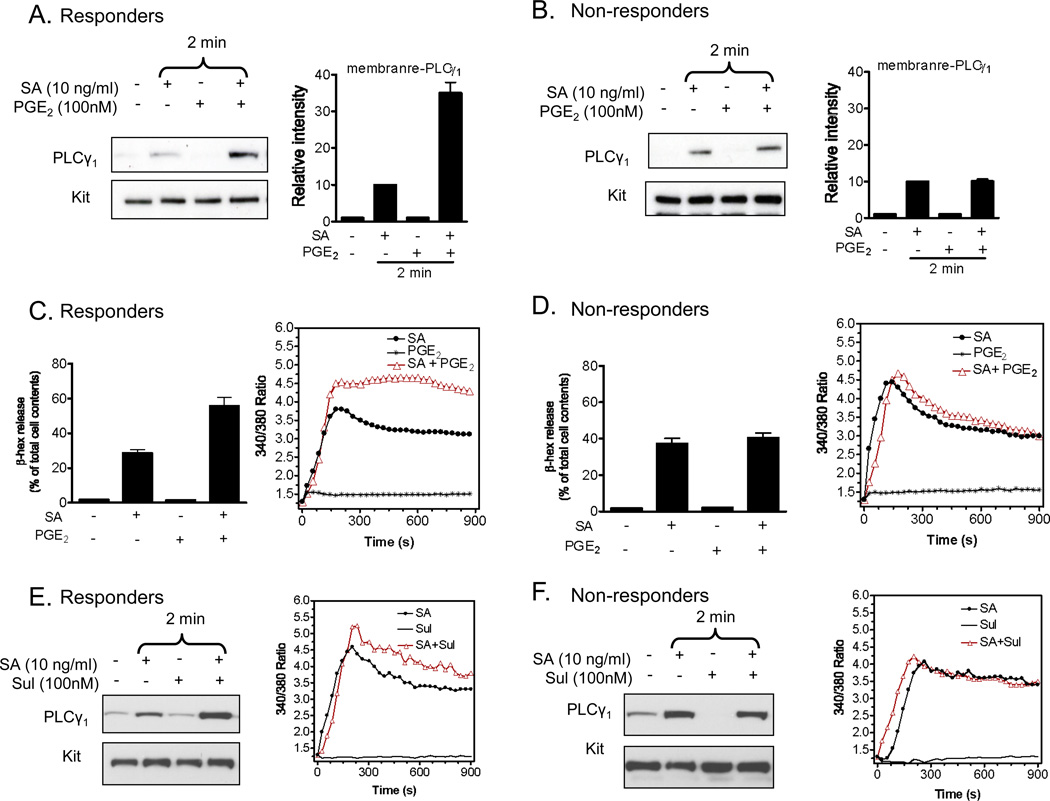
Our previous studies revealed that the ability of PGE2 to enhance antigen-mediated degranulation in BMMCs not only requires PLCγ1 activation but also the synergistic enhancement of PLCγ1 translocation to the cell membrane and the associated calcium signal [20]. As reported previously for BMMCs [20], PGE2 by itself did not induce membrane translocation of PLCγ1 but it markedly augmented antigen-mediated membrane translocation of PLCγ1 in the responder HuMC populations (Figure 7A). In contrast, PGE2 did not augment antigen-mediated membrane translocation of PLCγ1 in the non responder population, although antigen on its own induced PLCγ1 translocation (Figure 7B).
Figure 7.
The effect of PGE2 on PLCγ1 translocation and Ca2+ mobilization. A–B. HuMCs were sensitized and stimulated SA or PGE2 or both for 2 min. Membrane fractions were prepare as described in ‘Materials and Methods’ followed by gel electrophoresis. The proteins were probed with PLCγ1 antibodies. Protein loading of the samples was normalized by stripping and then probing for the membrane protein KIT. PLCγ1 levels were normalized to KIT, then to antigen responses, to determine the relative intensities presented under each blot. C–D. HuMCs were sensitized overnight and then challenged with PGE2 (100 nM) or antigen (streptavidin (SA), 10 ng/ml) or antigen with PGE2 added concurrently for 30 min prior to determination of β-hexosaminidase (β-hex) release (left panel). HuMCs were loaded with Fura 2-AM, then changes in intracellular Ca2+ concentrations in response to SA (10 ng/ml) with or without PGE2 (100 nM) were determined at the indicated time points (right panel). E, F. Sensitised HuMCs were stimulated SA or sulprostone or both for 2 min. Membrane fractions were prepare as described in ‘Materials and Methods’ followed by gel electrophoresis. HuMCs were loaded with Fura 2-AM, then changes in intracellular Ca2+ concentrations in response to SA (10 ng/ml) with or without sulprostone (Sul: 100 nM) were determined at the indicated time points (right panel). Densitometer data from A and B are presented as the mean ±S.E. from three donors. The blots in A and B are representative of three responder or non-responder donors, and in E and F of 2 responder and 1 non-responder. The degranulation and Ca2+ data are presented as the mean ±S.E. from n=2–3 experiments from four different donors, conducted in duplicate.
With respect to the calcium signal, PGE2 enhanced the antigen-induced rise in intracellular calcium levels in the HuMC responder populations (Figure 7C) as previously observed in mouse BMMCs [20,21]. However, PGE2 had a marginal effect on the antigen-mediated calcium signal in the non-responder populations (Figure 7D). Similarly, the EP3 agonist, sulprostone, potentiated antigen-mediated PLCγ1 translocation and calcium flux in the responder mast cells but not in the non-responder mast cells (Figure 7E and F). Taken together these data provide supporting evidence that the underlying defect in the non-responder HuMC population is a failure of PGE2 to enhance antigen-mediated membrane translocation of PLCγ1 and the ensuing calcium signal.
Discussion
In this study we sought to understand the disparity in the ability of the GPCR agonist, PGE2, to modulate antigen-mediated degranulation in mouse and human mast cells. Our new data provide an explanation for previous reports that PGE2 potentiates antigen-mediated degranulation and cytokine production in the mouse but not in the human [20]. We now show that, unlike mouse BMMCs, the inability of PGE2 to modulate antigen-mediated degranulation (Figure 1) and cytokine production (Figure 5) in human mast cells was restricted to a subset of the human donors tested. As the results were consistent from one culture to another for each donor, they likely reflect a pre-programmed phenotype rather than cryptic influences of culture conditions on cell differentiation or maturation during the six weeks of culture.
Unlike the SCF receptor, KIT, there are multiple PGE2 receptors which have different effects on mast cell responses to antigen. Specifically, the EP2 receptor has the capacity to downregulate antigen-mediated mast cell responses through Gαs-dependent production of cAMP, whereas the EP3 receptor can up-regulate antigen-mediated mast cell responses through enhanced calcium-dependent signaling [10,30]. It could be argued that differences in expression of the EP2 and EP3 receptors in mast cells could dictate the ability of PGE2 to up- or down-regulate antigen-mediated responses. However, the relative expression of either receptor at the mRNA levels and protein levels (Figure 2) and the production of cAMP (Figure 3) were similar in the non responder and responder populations. In addition, the ineffectiveness of the EP2 agonist, butaprost (Figure 4), to modify antigen-mediated responses indicate in non-responders and responder populations indicate that EP2 receptors and the associated activation of adenylate cyclase does not account for the differences in these two populations. Also, the comparable expression of the EP3 receptor (Figure 2) and downstream activation of PI3K, Btk, PLCγ1 and MAP kinases (Figure 6) by PGE2 would argue against differential expression and sensitivities of the EP3 receptor in these two populations of mast cells.
The similarity in the actions of the selective EP3 receptor agonist sulprostone and PGE2 point instead to diminished signaling processes downstream of the EP3 receptor in the non-responder population as compared to the responder HuMC population. Nevertheless, as noted above, early EP3-mediated signaling events appeared intact in both populations (Figure 6). However, diminished signaling was apparent with respect to translocation of PLCγ1 and calcium mobilization (Figure 7) in the non-responder population. In our studies, these two signaling events appeared to be critical for the ability of PGE2 to enhance antigen-mediated mast cell activation in BMMCs [20]. Thus, it is likely that this signaling node is active in the PGE2-responder phenotype but not in the PGE2-non-responder phenotype.
The reason for the diminished translocation of PLCγ1 in the non-responding HuMCs is unclear in that PI3K activation, which can regulate PLCγ1 translocation, is still apparent in these cells and, in any case, PI3K does not appear to be essential for the synergistic interactions between PGE2 and antigen, in BMMC [20] and HuMCs (Kuehn and Gilfillan, unpublished observations). Whether differences lie in variation in the composition of the lipid anchoring sites within the membrane, or in the binding of PLCγ to adaptor proteins and assembly of signaling complexes [31], warrant further investigation. We cannot rule out entirely the possibility that synergistic signals through PLC are down-regulated via cAMP-dependent inhibitory signals because gross measurement of cellular levels of cAMP may mask discrete localized production and inactivation of cAMP by adenylate cyclase and phosphodiesterases [24]. However, this scenario is unlikely as we observe no diminution of responses to antigen in non-responder cells as compared to cells that respond to PGE2.
Our observations that mast cells derived from different donors react differently to PGE2 raises the question as to the potential implications for mast cell-driven diseases. We can conjecture, for example, that the extent to which mast cells may trigger in vivo, in specific atopic individuals, in part may be a factor of their ability to respond or not respond to circulating co-activators. It is plausible that such interactions do exist in a physiological setting. For example it is assumed that mast cells would be continually exposed to SCF in vivo to maintain their viability [32]. PGE2 is also released at sites of inflammation. We also note that, despite numerous reports of adenosine influencing mast cell activation in rodent mast cells, we have been unable to detect any effects of adenosine in all human mast cells cultures including the LAD2 cells despite a marked enhancement in the mouse BMMCs (data not shown). Thus, the potential may still exist where particular individuals may be more sensitive to adenosine or other co-activators in vivo thus exacerbating ongoing antigen-mediated mast cell activation.
In summary, we have demonstrated that human mast cells derived from blood CD34+ cells from different donors under similar culture conditions exhibit heterogeneity in their responsiveness to PGE2 and the selective EP3 agonist sulprostone. If PGE2 production is a significant factor in allergic inflammation, the finding has obvious implications in the use of EP3 antagonists as therapeutic agents in patients who might express PGE2-insensitive mast cells. The finding also raises the question of whether such heterogeneity extends to other potential co-activating endogenous agents that have been identified in studies with BMMCs and that are known to accumulate in inflamed tissues. Although our findings indicate caution in extrapolating data from animal to human models, especially where differences may be donor dependent, they may serve as a basis for investigating the potential roles of mast cell co-activators in the etiology of mast cell-driven disorders.
Research Highlights.
> We examined PGE2 enhancement of antigen-mediated degranulation in human mast cells. > Cells from only half of donors responded to PGE2. > The differences in responses were not due to differences in receptor expression. > The differences reflect defective enhancement of calcium flux in non-responders. > Results may have relevance to role of co-activating receptors in allergic disorders.
Acknowledgements
Financial support for this work was provided by the Division of Intramural Research of NIAID and NHLBI within the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
All of the authors (Hye Sun Kuehn, Michael A. Beaven, Dean D. Metcalfe and Alasdair M. Gilfillan) declare no financial or commercial conflict of interest.
Hye Sun Kuehn planned and conducted studies and contributed to the preparation of the manuscript. Mi-Yeon Jung conducted studies. Michael A Beaven helped conduct studies and contributed to the preparation of the manuscript. Dean D. Metcalfe helped plan studies and contributed to the preparation of the manuscript. Alasdair M. Gilfillan planned and conducted studies and contributed to the preparation of the manuscript.
References
- 1.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 3.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 4.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 647-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 7.Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 8.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drube S, Heink S, Walter S, Lohn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, Grotha S, Reich D, Rudeschko O, Norgauer J, Hartmann K, Roers A, Kamradt T. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 115:3899–3906. doi: 10.1182/blood-2009-10-247411. [DOI] [PubMed] [Google Scholar]
- 10.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcvarepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 128:36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsythe P, Ennis M. Adenosine, mast cells and asthma. Inflamm Res. 1999;48:301–307. doi: 10.1007/s000110050464. [DOI] [PubMed] [Google Scholar]
- 13.Jensen BM, Metcalfe DD, Gilfillan AM. Targeting kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflamm Allergy Drug Targets. 2007;6:57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- 14.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 16.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 17.Iwaki S, Spicka J, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, Kovarova M, Rivera J, Horejsi V, Metcalfe DD, Gilfillan AM. Kit- and FcεRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pushparaj PN, Tay HK, H'Ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 20.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, Dombrowicz D, Koller BH. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169:4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 22.Gomi K, Zhu FG, Marshall JS. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–6552. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- 23.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 26.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 27.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 28.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 29.Kuehn HS, Radinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA, Metcalfe DD, Gilfillan AM. Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced mast cell chemotactic responses. Journal of Cell Science. 2010;123:2576–2585. doi: 10.1242/jcs.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung KF. Evaluation of selective prostaglandin E2 (PGE2) receptor agonists as therapeutic agents for the treatment of asthma. Sci STKE. 2005;2005:pe47. doi: 10.1126/stke.3032005pe47. [DOI] [PubMed] [Google Scholar]
- 31.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]