Abstract
Background
The association between buprenorphine taper duration and treatment outcomes is not well understood. This review evaluated whether duration of outpatient buprenorphine taper is significantly associated with treatment outcomes.
Methods
Studies that were published in peer-reviewed journals, administered buprenorphine as an outpatient taper to opioid-dependent participants, and provided data on at least one of three primary treatment outcome measures (opioid abstinence, retention, peak withdrawal severity) were reviewed. Primary treatment outcomes were evaluated as a function of taper duration using hierarchical linear regressions using pre-taper maintenance as a cofactor.
Results
Twenty-eight studies were reviewed. Taper duration significantly predicted percent of opioid-negative samples provided during treatment, however pre-taper maintenance period predicted percent participants abstinent on the final day of treatment. High rates of relapse were reported. No significant association between taper duration and retention in treatment or peak withdrawal severity was observed.
Conclusion
The data reviewed here suggest taper duration is associated with opioid abstinence achieved during detoxification but not with other markers of treatment outcome. The reviewed studies varied widely on several parameters (e.g., frequency of urinalysis testing, provision of ancillary medications) that may influence treatment outcome and thus could have interfered with the ability to identify relationships between taper duration and outcomes. Future studies evaluating opioid detoxification should utilize rigorous experimental methods and report a wider range of outcome measures in order to help advance our understanding of the association between taper duration and treatment outcomes.
Keywords: opioid, buprenorphine, taper, detoxification, withdrawal, outpatient
1. Introduction
Abuse of opioids (e.g., heroin; prescription opioids) is a significant national and international public health problem (European Monitoring Centre for Drugs and Drug Addiction, 2010; Substance Abuse and Mental Health Administration, 2010a). Illicit use of opioids has been associated with considerable societal costs, including increased rates of emergency department visits, drug overdoses, criminal activity, lost work days and general medical and psychiatric consequences (Becker et al., 2008; Clausen et al., 2009; Shah et al., 2008; Wisniewski et al., 2008). Treatment admissions for opioid abuse and dependence in the U.S. and across Europe have increased dramatically in recent years (European Monitoring Centre for Drugs and Drug Addiction, 2010). For example, in the U.S. between 1992 and 2008, treatment admissions for heroin and prescription opioid (e.g., Oxycontin, Vicodin, Percocet) dependence increased by 57% and 717% respectively (Substance Abuse and Mental Health Administration, 2010b). More than one million people in the U.S. sought treatment for opioid abuse or dependence in 2009 (Substance Abuse and Mental Health Administration, 2010a), and need for treatment has increased in Europe as well (European Monitoring Centre for Drugs and Drug Addiction, 2010).
Buprenorphine-naloxone (Suboxone®) is prescribed in the U.S. and internationally as a treatment for opioid dependence (European Monitoring Centre for Drugs and Drug Addiction, 2010). Buprenorphine is approved in the U.S. as a Schedule-III drug, and is the first opioid agonist that can be prescribed to treat opioid dependence from a primary care setting (Johnson et al., 2003). The importance of prescribing buprenorphine directly from a primary care setting cannot be understated. Up to 50% of patients visiting a primary care provider are believed to require some form of substance abuse treatment; therefore, office-based buprenorphine treatment may represent an important first point of contact for opioid-dependent patients in need of treatment (Miller and Gold, 1998; Prater et al., 1999). The number of prescriptions for buprenorphine has increased steadily since its approval and office-based buprenorphine has been associated with bringing new users into treatment. Buprenorphine may appeal to patients who are unwilling or unable to access methadone treatment, particularly those residing in rural areas where access to methadone treatment may be limited (Apple et al., 2004; Becker and Fiellin, 2005; Cicero et al., 2005; Havens et al., 2007; Fiellin, 2007; Korthius et al., 2010; Pinto et al., 2010; Rosenblum et al., 2007; Schnoll, 2001; Schwartz et al., 2008; Sullivan et al., 2005; Winstock et al., 2009; Zacny et al., 2003).
Maintenance on methadone or buprenorphine is the most common form of treatment for opioid-dependence, however detoxification (i.e., medically supervised withdrawal) is a common treatment option that is an important alternative to maintenance for several reasons (Amato et al., 2005; Drug and Alcohol Services Information System, 2006; Gruber et al., 2008; Substance Abuse and Mental Health Administration, 2008). First, some opioid users prefer detoxification to maintenance (Apple et al., 2004; Luty, 2004; Pinot et al., 2010; Zacny et al., 2003). Second, the advent of prescription opioid abuse has resulted in a subgroup of opioid-dependent patients who are younger and have briefer and less severe opioid use histories compared to traditional heroin-dependent patients, which could make them good candidates for detoxification, or even ineligible for agonist maintenance (Fiellin, 2007; Kissin et al., 2006; Sullivan et al., 2005). Finally, when maintenance availability is limited, a circumstance that is likely to occur in rural areas that are struggling with high rates of prescription opioid dependence, detoxification is a valuable and necessary treatment option (Booth and McLaughlin, 2000; Cicero et al., 2007; Cunningham et al., 2007; Fortney and Booth, 2001; Havens et al., 2007; Kissin et al., 2006; Lenardson and Gale, 2008; O'Connor et al., 1997; Rounsaville and Kosten, 2000).
Buprenorphine may be well-suited for use in opioid detoxification settings for several reasons. First, data indicates that buprenorphine produces a more limited withdrawal syndrome when compared to other full opioid agonists like methadone (Bickel et al., 1988a; Fudala et al., 1990; Jasinski et al., 1978). This may be related to buprenorphine’s long plasma half-life and slow dissociation from the receptor (Bickel et al., 1988a; Bullingham et al., 1980; Fudala et al., 1990; Hambrook and Rance, 1976; Jasinski et al., 1978). Second, buprenorphine blocks the effects of exogenously-administered opioids, and can potentially slow or prevent relapse to illicit opioids from occurring (Jasinski et al., 1978; Mello et al., 1982; Rosen et al., 1994; Walsh et al., 1995). Third, buprenorphine has a ceiling on its agonist effects that reduces the risk for buprenorphine-related overdose, making it a good candidate for use in outpatient settings (Banks, 1979; Jasinski et al., 1978; Lewis, 1985; Mello and Mendelson, 1980; Walsh et al., 1994; Walsh et al., 1995). Recent preliminary evidence has confirmed an advantage of buprenorphine over methadone in detoxification settings (Gowing et al., 2009).
The use of buprenorphine for opioid detoxification is increasing (Gowing and Ali, 2006; Helm et al., 2008; Horspool et al., 2008; Ridge et al., 2008), yet there is little standardization of buprenorphine detoxification designs. As a result, detoxifications vary widely and limited scientific attention has been paid to the role that various treatment components may have on treatment outcomes. The duration of a buprenorphine detoxification and its association with treatment outcomes is one important component that warrants further empirical consideration. Methadone detoxification studies have reported significant associations between detoxification duration and treatment outcome (Fudala et al., 1990; Gossop et al., 1989; Senay et al., 1977), however it remains unclear whether these results will generalize to buprenorphine given its unique pharmacological profile. Although there have been some scientific efforts to parametrically compare different buprenorphine taper durations (Amass et al., 1994; Katz et al., 2009; Ling et al., 2009, Woody et al., 2008), the results of these studies have been mixed and the majority of studies on this topic are uncontrolled reports of a single taper duration. Therefore, the aim of this report is to review the current literature on outpatient buprenorphine detoxification and examine whether taper duration is associated with three commonly reported measures of treatment outcome: biochemically-verified opioid abstinence, treatment retention and opioid withdrawal severity. This review also highlights the heterogeneity of the scientific literature on this topic and makes recommendations for future research considerations.
2. Methods
2.1 Search strategy
Studies meeting inclusion criteria (see below) were identified by searching PsychInfo, Pubmed and MEDLINE (1973-September 2010) using a combination of keywords: buprenorphine, buprenorphine detoxification, buprenorphine taper, opiate treatment, opioid treatment, opiate detoxification, opioid detoxification, opiate taper and opioid taper. The reference sections of identified articles were also searched for additional reports that met the review criteria.
2.2 Inclusion criteria
Peer-reviewed journal articles were selected for inclusion if they specified buprenorphine as a primary pharmacological agent, sought to detoxify participants with a primary end goal of opioid abstinence and took place in an outpatient setting for the duration of treatment. Studies that compared buprenorphine to other medications were included in this review; however, only data related to buprenorphine administration are reported here. Studies that parametrically compared multiple buprenorphine taper durations (Amass et al., 1988; Katz et al., 2009; Ling et al., 2009; Woody et al., 2008) were divided such that individual taper durations were included in the analyses as independent observations. Studies varied widely with respect to design, primary outcomes and other treatment components that may have influenced treatment outcome, including the duration of the pre-taper maintenance period (Table 1). Only studies that reported one or more of the three primary outcomes measures reviewed here (i.e., biochemically-verified abstinence, treatment retention, peak withdrawal severity) were included in this review.
Table 1.
Details of Reviewed Studies
| Reference | Participants (#) |
Opioida | Buprenorphine Type |
Buprenorphine Maintenance Period prior to Taper (days) |
Mean Taper Duration (days) |
Mean Starting Buprenorphine Dose (mgs)b |
Urinalysis testing |
Approximate # urine samples collected during taper |
Counseling | Withdrawal Assessment |
Ancillary Medications |
Naltrexone encouraged |
Follow-up Assessment (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amass et al., 1994 | 5 | Opioid | SL Liquid | 28 | 12 | 8 | Y | 4 | Y | Y | NS | NS | NS |
| 3 | Opioid | SL Liquid | 28 | 36 | 8 | Y | 12 | Y | Y | NS | NS | NS | |
| Becker et al., 2001 | 17 | Heroin | SL Liquid | 7 | 19 | 8 | Y | 6 | NS | Y | NS | NS | NS |
| Bickel et al., 1988 | 45 | Opioid | SL Liquid | 21 | 28 | 2 | Y | 11 | NS | Y | NS | NS | NS |
| Blondell et al., 2010 | 6 | Prescription | Suboxone | 2 | 120 | 9.8 | Y | 4 | Y | NS | NS | NS | NS |
| Breen et al., 2003 | 55 | Methadone | NS | 19 | 77 | 8.8 | NS | NS | Y | Y | Y | Y | 30* |
| Cameron et al., 2001 | 13 | Opioid | NS | 7 | 28 | 8 | Y | 5 | NS | Y | NS | NS | NS |
| Diamont et al., 1998 | 50 | Opioid | SL (Temgesic) | 0 | 8 | 2.58 | Y | 3 | NS | Y | Y | NS | NS |
| Fingerhood et al., 2001 | 65 | Opioid | Buprenex | 0 | 4 | 0.3 | NS | NS | Y | Y | Y | NS | NS |
| Galanter et al., 2003 | 52 | Heroin | Suboxone | 35 | 52 | 16.4 | Y | 15 | Y | Y | Y | Y | NS |
| Gandhi et al., 2003 | 119 | Heroin | SL Tablet or IM | 3 | 0 | 4 or 0.6 | Y | 1 | Y | Y | Y | NS | 30*, 60, 90 |
| Gibson et al., 2003 | 115 | Heroin | Sl Tablet | 3 | 2 | 12 | Y | 1 | NS | NS | NS | Y | 8* |
| Katz et al., 2009 | 364 | Heroin | Suboxone | 5 | 0 | 2 | Y | 1 | Y | NS | NS | NS | NS |
| 146 | Heroin | Suboxone | 22 | 7 | 14.6 | Y | 4 | Y | NS | NS | NS | NS | |
| Ling et al., 2005 | 157 | Opioid | Suboxone | 3 | 10 | 16 | Y | 10 | Y | Y | Y | NS | NS |
| Ling et al., 2009 | 255 | Opioid | Suboxone | 29 | 6 | 24 | Y | 1 | Y | Y | Y | NS | 30*, 90 |
| 261 | Opioid | Suboxone | 30 | 26 | 24 | Y | 4 | Y | Y | Y | NS | 30*, 90 | |
| Lintzeris, 2002 | 18 | Heroin | Subutex | 4 | 1 | 10.1 | Y | 1 | NS | Y | NS | Y | NS |
| Lintzeris et al., 2002 | 58 | Heroin | Subutex | 3 | 2 | 9.6 | Y | 1 | NS | Y | NS | NS | 35 |
| Marsch et al., 2005 | 18 | Opioid | Subutex | 0 | 28 | 8 | Y | 12 | Y | Y | Y | Y | NS |
| O'Connor et al. 1997 | 53 | Heroin | NS | 3 | 0 | 3 | NS | NS | NS | Y | Y | Y | NS |
| Quigley et al., 1987 | 15 | Heroin | SL Liquid | 28 | 30 | 4 | Y | 10 | NS | Y | Y | NS | NS |
| Raistrick et al., 2005 | 107 | Heroin | Subutex | 2 | 5 | 8 | Y | 1 | NS | Y | NS | Y | 30 |
| Resnick et al., 1992 | 51 | Heroin | SL Liquid | 12 – 365 | 35 | 8 | Y | 3 | Y | Y | Y | NS | NS |
| Resnick et al., 2001 | 40 | Heroin | Buprenex | NS | 18 | 16 | NS | NS | NS | NS | NS | Y | 90*, 365 |
| Sigmon et al., 2009 | 14 | Prescription | Suboxone | 12 | 14 | 9 | Y | 6 | Y | Y | Y | Y | NS |
| Summers & Stone, 2002 | >35 | Opioid | Subutex | 5 | 15 | 21.3 | NS | NS | NS | NS | Y | Y | 90 |
| White et al., 2001 | 38 | Opioid | Subutex | NS | 19 | 8 | Y | NS | NS | Y | Y | NS | NS |
| Williams et al., 2002 | 60 | Heroin | NS | 5 | 17 | 10 | Y | NS | NS | NS | NS | NS | NS |
| Woody et al., 2008 | 78 | Opioid | Suboxone | 3 | 11 | 8 | Y | 1 | Y | NS | NS | NS | 30*, 60, 90, 180, 270, 365 |
| 74 | Opioid | Suboxone | 56 | 28 | 14 | Y | 3 | Y | NS | NS | NS | 180*, 270, 365 | |
| Wright et al., 2007 | 28 | Opioid | NS | 3 | 12 | 24 | Y | 1 | NS | NS | NS | NS | NS |
Studies that did not specify heroin vs. prescription opioids are are considered to have addressed general opioid dependence
When multiple dose values were available, mean highest maintenance dose was provided.
Legend: Y = Yes, N= No, NS= Not specified,
percent abstinence at this follow-up visit was included in analysis
Twenty-eight studies, including 4 parametric comparisons of taper duration (resulting in 32 total observations), met criteria and are reviewed here. Overall, the above search terms resulted in 16 randomized trials (Amass et al., 1988; Bickel et al., 1988b; Blondell et al., 2010, Breen et al., 2003; Cameron et al., 2001; Gandhi et al., 2003; Gibson et al., 2003; Ling et al., 2005; Ling et al., 2009; Lintzeris et al., 2002; Marsch et al., 2005; O’Connor et al., 1997; Raistrick et al., 2005; Resnick et al., 1992; Woody et al., 2008; Wright et al., 2007), 10 unrandomized evaluations (Becker et al., 2001; Diamont et al., 1998; Galanter et al., 2003; Katz et al., 2009; Lintzeris, 2002; Quigley et al., 1987; Resnick et al., 2001; Sigmon et al., 2009; Summers and Stone, 2002; Williams et al., 2002) and 2 retrospective comparisons with other treatment medications (Fingerhood et al., 2001; White et al., 2001).
2.3 Data analysis
Studies were categorized by taper duration, defined here as the number of days during which the buprenorphine dose was reduced (excluding the initial induction onto buprenorphine or any maintenance period that preceded the taper). Due to the potential for duration of pre-taper maintenance period to impact treatment outcomes, hierarchical linear regressions that held maintenance period constant were conducted to determine whether taper duration contributed unique variance to the regression model. Opioid abstinence, retention and self-reported withdrawal were evaluated as dependent variables. Opioid abstinence was generally reported in one of two ways: mean percent opioid-negative samples collected during treatment (n=16) and percent participants opioid-negative on the final day of treatment (n=9). Both abstinence outcomes are evaluated here. Withdrawal was measured with several different assessment tools (see Table 2). To facilitate comparison across different measures and studies, withdrawal scores were converted to peak withdrawal severity (represented as percent of the maximum possible scale value) and evaluated using hierarchical linear regressions. Data were generally skewed towards briefer taper durations so measures of central tendency are presented as median (range). All statistical analyses were conducted using SPSS 19.0 (Chicago, IL). A p-value of <0.05 was considered significant.
Table 2.
Questionnaire utilization among studies assessing withdrawal
| % used | |
|---|---|
| Self-Report | 35 |
| Adjective Rating Scale | 44 |
| Short Opiate Withdrawal Scale (SOWS) | 25 |
| Subjective Opiate Withdrawal Scale (SOWS) | 25 |
| Visual Analog Scales (VAS) | 44 |
| Craving | 13 |
| Withdrawal Severity | 19 |
| Drug effects | 13 |
| Observer-Rated | 10 |
| Clinical Instutute Narcotic Assessment (CINA) | 18 |
| Clinical Opiate Withdrawal Scale (COWS) | 6 |
| Opiate Objective Withdrawal Scale (OOWS) | 18 |
| Physiological Measures (e.g. blood pressure, pupil) | 6 |
| Wang Scale | 6 |
| Unspecified observer-report | 13 |
3. Results
3.1 Taper duration
Taper duration varied substantially across the studies reviewed, ranging from abrupt discontinuation of buprenorphine (e.g., 0 taper days) to a 120-day taper. The median (range) number of taper days was 17 (0–132). The duration of the pre-taper maintenance period was specified for 29 (91%) of the studies and also varied substantially (Median: 5 days, range: 0–56). Additional study characteristics are presented in Table 1.
3.2 Biochemically-verified opioid abstinence
Twenty-six (81%) of the studies reported results of urine toxicology testing. The median percent opioid-negative samples provided during treatment was 41% (range: 1%–94%) and the median percent participants who were opioid-negative on the final day of treatment was 30% (range: 22%–41%). A hierarchical regression model revealed that taper duration was a significant predictor of percent opioid-negative samples collected during treatment when maintenance period was held constant (Table 3), however the duration of maintenance period was a better predictor of the percent of participants negative on the final day of treatment than was taper duration (Table 3).
Table 3.
Hierarchical Regression Analyses
| Outcome | Variable | B | SE B | Beta | R2 | ĘR2 | p-value | |
|---|---|---|---|---|---|---|---|---|
| Percent Opioid-negative Samples Provided During Treatment | ||||||||
| Step 1 | Maintenance Duration | 0.608 | 0.569 | 0.275 | 0.076 | 0.076 | 0.303 | |
| Step 2 | Maintenance Duration | 0.467 | 0.511 | 0.211 | 0.318 | 0.243 | 0.377 | |
| Taper Duration | 0.442 | 0.205 | 0.497 | 0.051 | ||||
| Percent Participants Opioid-negative on the Final Day | ||||||||
| Step 1 | Maintenance Duration | 0.230 | 0.105 | 0.640 | 0.409 | 0.409 | 0.063 | |
| Step 2 | Maintenance Duration | 0.491 | 0.108 | 1.362 | 0.776 | 0.366 | 0.004 | |
| Taper Duration | −0.733 | 0.234 | −9.420 | 0.020 | ||||
| Percent Participants Opioid-negative at Follow-up | ||||||||
| Step 1 | Maintenance Duration | 0.347 | 0.287 | 0.476 | 0.226 | 0.226 | 0.281 | |
| Step 2 | Maintenance Duration | 0.314 | 0.328 | 0.431 | 0.251 | 0.024 | 0.392 | |
| Taper Duration | 0.079 | 0.219 | 0.162 | 0.737 | ||||
| Percent Participants Retained on the Final Day | ||||||||
| Step 1 | Maintenance Duration | −0.049 | 0.350 | −0.029 | 0.001 | 0.001 | 0.889 | |
| Step 2 | Maintenance Duration | 0.031 | 0.356 | 0.018 | 0.055 | 0.054 | 0.932 | |
| Taper Duration | −0.213 | 0.189 | −0.238 | 0.273 | ||||
| Peak Self-reported Withdrawal | ||||||||
| Step 1 | Maintenance Duration | −0.619 | 0.355 | −0.550 | 0.302 | 0.302 | 0.125 | |
| Step 2 | Maintenance Duration | −0.644 | 0.422 | −0.571 | 0.305 | 0.002 | 0.178 | |
| Taper Duration | 0.062 | 0.452 | 0.052 | 0.895 | ||||
Three studies used contingency management (CM), a behavioral intervention that provides monetary-based incentives for urinalysis-verified drug abstinence, to increase opioid abstinence (Amass et al., 1994; Becker et al., 2001; Marsch et al., 2005). Percent opioid abstinence among participants who earned monetary rewards in exchange for providing opioid-negative urine samples were generally higher (Median: 64%; range: 39%–78%) when compared to studies that did not use CM (Median: 23%; range: 1%–94%).
Eleven (34%) studies included a post-taper follow-up assessment, ranging from 8–365 days after the taper (Table 1), and 8 (25%) of those reported urinalysis results from these visits. Median opioid-negative samples collected at the first post-taper follow-up visit (e.g., samples that occurred in closest proximity to the final taper day, marked by an asterisk in Table 1) were 23% (range: 8.4% – 52%). Regression analyses revealed no significant relationship between maintenance period and/or taper duration at the first post-detoxification follow-up (Table 3). Follow-up abstinence was further examined among only those studies that reported urinalysis outcomes on the final day of treatment and a follow-up visit (n=5). Among these studies, median percent participants opioid-negative on the final day of treatment and at the first follow-up assessment were 30% (range: 25%–44%) and 29% (range: 17.6% – 52%), respectively.
3.3. Retention
Twenty-nine (91%) of the studies reported treatment retention as an outcome variable. Median percent participants retained at the end of treatment was 65.5% (range: 4%–100%). In contrast to data regarding opioid abstinence achieved during treatment, neither maintenance nor taper duration significantly predicted the number of participants retained at the end of treatment (Table 3).
3.4. Withdrawal severity
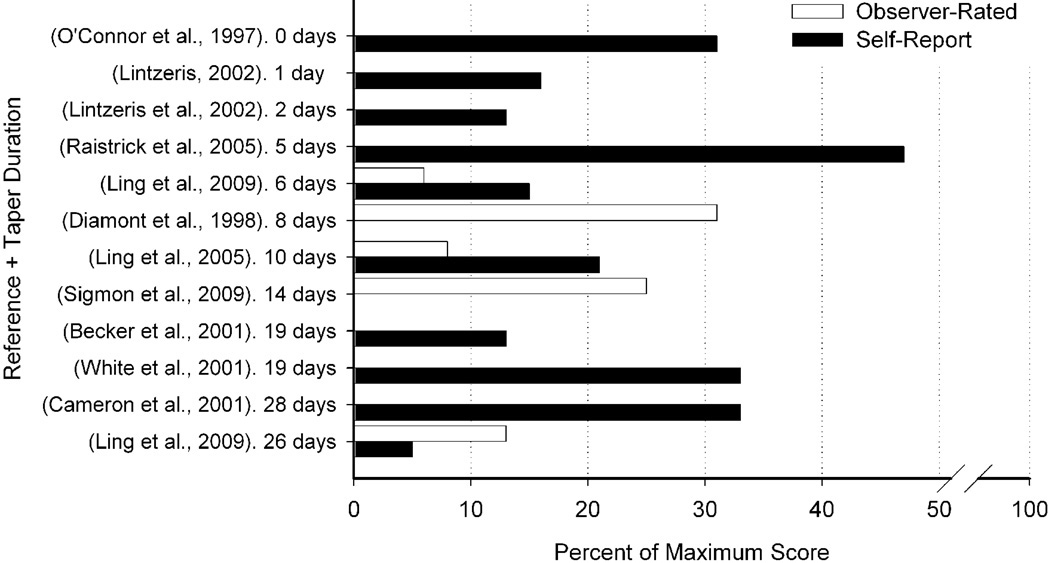
The studies employed a wide variety of self-report and observer-rated measures to assess opioid withdrawal (Table 2). Of the studies reviewed, 17 (53%) reported using at least one withdrawal assessment during detoxification. With the exception of 4 studies that measured withdrawal but did not provide specific data (Amass et al., 1994; Bickel et al., 1988a; Quigley et al., 1987; Resnick et al., 1992), data from peak withdrawal severity are presented in Figure 1. Overall, withdrawal from buprenorphine was relatively mild (e.g., represented a small percentage of the maximum possible score), with median peak self-reported and observer ratings of withdrawal at 18.5% (range: 5%–47%) and 13% (range: 6%–31%) of maximum scale values, respectively. Neither maintenance period or taper duration were significantly predictive of peak self-reported withdrawal severity (Table 3).
Fig 1.
Peak buprenorphine withdrawal during taper, presented as percent of maximum scale value. Only studies from which peak withdrawal severity could be calculated are presented here. Black bars represent self-report measures and open bars represent observer-rated measures. Studies are organized by taper durations on the y-axis and percent peak withdrawal is presented on the x-axis.
Interestingly, several of the reviewed studies indicated that peak buprenorphine withdrawal occurred at the very end of the detoxification or even after the final buprenorphine dose had been administered. Five studies reported that withdrawal severity peaked after the buprenorphine dose decreased below 2 mg (Cameron et al., 2001; Quigley et al., 1987; Resnick et al., 1992; Resnick et al., 2001; White et al., 2001), and 7 studies reported that withdrawal severity peaked 3 to 15 days after the last dose was administered (Amass et al., 1994; Becker et al., 2001; Cameron et al, 2001; Lintzeris, 2002; Raistrick et al., 2002; Sigmon et al. 2009; White et al., 2001).
4. Discussion
This review evaluated the association between taper duration and treatment outcome following outpatient detoxification using buprenorphine. Twenty-seven studies that administered buprenorphine as a primary pharmacological agent for opioid detoxification in an outpatient setting were reviewed.
Analysis of abstinence outcomes revealed interesting results. First, taper duration was associated with the amount of opioid abstinence achieved during the taper and this association was not influenced by the preceding duration of buprenorphine maintenance. An association between taper duration and percent negative samples provided during treatment has also been observed following detoxification using methadone (Senay et al., 1977). Second, duration of pre-taper buprenorphine maintenance was a better predictor than taper duration of the percent participants who were opioid-negative on the final day. Buprenorphine maintenance has been associated with reductions in illicit drug use, which has itself been associated with better inpatient detoxification outcomes (Franken and Hendriks, 1999; Mattick et al., 2008). Thus, it is possible that longer maintenance periods may provide more opportunities to become stable in treatment, thus making participants good candidates for a detoxification; however, more research on the relationship between maintenance duration and detoxification outcomes is needed.
None of the studies reviewed here reported results for both of the urinalysis outcomes reviewed (i.e., percent negative samples collected during treatment and percent participants who were opioid-negative on the final day of treatment); therefore, it is not possible from this review to evaluate whether an association between these outcome measures exists. However, the fact that duration of taper is significantly predictive of one urinalysis outcome (i.e., percent negative samples collected during treatment) but not another (i.e., percent participants who were opioid-negative on the final day of treatment) may help explain, in part, the lack of consensus in the literature regarding the optimal duration for outpatient buprenorphine detoxification. For example, of the 4 parametric comparisons of buprenorphine taper duration, the two studies that defined abstinence as percent negative samples during treatment reported an advantage of longer duration detoxifications (Amass et al., 1994; Katz et al., 2009), while the two studies that defined abstinence as the percent of participants opioid-negative on the final day of treatment reported no advantage of longer duration detoxifications (Ling et al., 2009; Woody et al., 2008). Since no study reported both abstinence outcomes, it is not possible to determine which outcome is more clinically significant, therefore future studies are encouraged to report outcomes to enable this comparison (Table 4).
Table 4.
Recommendations for Outcome Measures and Methodology
| Outcome Measure Recommendations |
| Abstinence Outcomes |
| Percent opioid abstinence achieved during treatment |
| Percent of participants opioid-negative on the final day of treatment |
| Percent opioid abstinence at follow-up among participants who completed the detoxification without relapse |
| Retention Outcomes |
| Percent of scheduled treatment days attended |
| Percent of participants retained on the final day of treatment |
| Withdrawal Outcomes |
| Dose at which withdrawal severity peaked. |
| Methodological Recommendations |
| Withdrawal Methods |
| Describe dose tapering schedule |
| Indicate whether tapering schedule was fixed or flexible |
| Evaluate withdrawal after completion of the taper |
| Ancillary Medications and Counseling |
| Indicate manner in which medications are provided (e.g., protocol-driven vs. patient request) |
| Indicate presence of ancillary counseling procedures |
Only 34% of the reviewed studies reported urinalysis-testing outcomes from follow-up assessments, and the median opioid abstinence rate at follow-up was 23%. It is important to evaluate whether participants who completed the detoxification without relapse remained abstinent at the first post-detoxification follow-up assessment to help judge the efficacy of an intervention. Among studies that reported both percent abstinence on the final day of treatment and at the first follow-up assessment (n=5), median abstinence rates were 30% and 29% respectively. These data are encouraging and suggest abstinence may have persisted among those participants who completed the detoxification without relapse. It will be important for future studies to report the percent abstinence at follow-up as a function of those participants who completed the treatment successfully, to help evaluate whether those participants continued to abstain from opioids (Table 4).
Nevertheless, the high rate of relapse observed here is consistent with the larger literature that suggests only a minority (41% and 20%) of patients leaving a residential detoxification remain abstinent from illicit opioid use at 7 and 30 days post-detoxification, respectively (Smyth et al., 2010). Similar relapse rates have been reported following detoxification from methadone maintenance, wherein approximately 17–37% of patients who complete detoxification remain abstinent from opioids 2 – 6 years later (Cushman, 1978; Gossop et al., 1986; Stimmel and Rabin, 1974; Stimmel et al., 1977). In order for opioid detoxification to be successful, additional research is needed to evaluate strategies for maintaining opioid abstinence following detoxification. Use of the opioid antagonist naltrexone, which has recently been approved for use in a sustained release formulation, represents one promising method for reducing relapse that warrants additional research consideration (Comer et al., 2002; Comer et al., 2006; Foster et al., 2003).
Neither taper duration nor maintenance period significantly predicted the percent of participants retained at the end of treatment. Longer taper durations can provide more opportunities for participants to drop out of treatment, whereas shorter taper durations require more limited engagement and may result in higher, albeit limited, retention. However, this was not supported by results from the four parametric studies reviewed here, in which 63% and 35% of participants in long vs. short taper durations completed the intervention, respectively. The type or intensity of ancillary services provided during treatment has also been shown associated with better retention outcomes during opioid detoxification treatment (Bickel et al., 1997). Yet, the lack of information on treatment services in the reviewed studies prevents any firm conclusions from being drawn. Therefore, to facilitate a better understanding of the relationship between taper duration and retention, it would be helpful for future studies to report retention outcomes as both percent of scheduled days attended and percent of participants retained at the end of treatment (Table 4). It would also be important to evaluate retention in the context of withdrawal severity and buprenorphine dose, to determine whether study attrition is most likely to occur once a specific withdrawal threshold is crossed. This information can be used to help target effectively ancillary services that could increase retention and completion of the detoxification.
Withdrawal during buprenorphine detoxification was assessed by 53% of studies. Peak withdrawal severity was generally mild and no significant association between taper duration and peak withdrawal severity was observed. However, withdrawal reports may have been confounded by continued illicit opioid use, study attrition that may be complicated by differential group dropout (e.g., patients with high withdrawal severity) or provision of ancillary medications to treat withdrawal symptoms. To better evaluate withdrawal during outpatient buprenorphine detoxifications, it will be important to know the manner in which ancillary medications are administered (e.g., according to protocol guidelines such as reaching a numerical threshold on a withdrawal self-report or observer-rated inventory, or by participant request; Table 4).
Interpretation of withdrawal is further complicated by the wide range of self-report and observer-rated measures used (Table 2), which limits sensitive comparisons of withdrawal across studies. In general, buprenorphine appeared to produce a relatively mild withdrawal syndrome compared to the withdrawal expected following detoxification using methadone or clonidine. This is consistent with the literature on buprenorphine withdrawal (Bickel et al., 1988a; Bullingham et al., 1980; Fudala et al., 1990; Hambrook and Rance, 1976; Jasinski et al., 1978). Review of these studies suggests several potential areas for future research. First, in several studies, peak withdrawal symptoms emerged once the dose dropped below 2mg, and withdrawal symptom severity peaked 3–15 days after the final dose was administered. This suggests patients may be experiencing the most severe withdrawal in the days following a detoxification, presumably when treatment resources may no longer be available to help manage the withdrawal, and therefore may benefit most from ancillary medications and other resources when they are provided at the end of the detoxification. This information can be useful for designing efficient and cost-effective detoxification treatments. Second, it was not possible to determine from the studies reviewed here whether the magnitude of the buprenorphine dose decrease was associated with withdrawal syndrome severity and/or duration. Magnitude decrease has been associated with differential outcomes following methadone detoxification, yet it is not clear this finding will generalize given buprenorphine’s unique pharmacological profile (Strang and Gossop, 1990). The relationship between magnitude decrease and withdrawal is an important factor to consider when designing tapering schedules. Third, studies reviewed here administered a wide variety of buprenorphine formulations that may have different absorption and excretion patterns (Table 1; Chawarski et al., 2005). The degree to which buprenorphine formulation influenced treatment outcomes was not evaluated. Although the majority of buprenorphine products being administered for opioid dependence today are in sublingual tablet form (e.g., Suboxone, Subutex), new abuse-deterrent (e.g., Suboxone soluble film strip) and sustained-release products are becoming available that may have unique excretion patterns (Ling et al., 2010; Strain et al., 2011; White et al., 2009). Ultimately, additional research is needed to more rigorously evaluate buprenorphine withdrawal, including the relationship between buprenorphine dose and withdrawal severity, the duration of the withdrawal syndrome, the severity of withdrawal that persists after the final buprenorphine dose has been administered, the severity of withdrawal following detoxification with alternative formulations of buprenorphine and the association between withdrawal and other treatment outcomes, including abstinence and retention.
Review of these studies suggested several areas that warrant additional research consideration. First, do specific characteristics of opioid-dependent patients exist (e.g., amount of opioids used per day, primary route of administration) that predict success in outpatient buprenorphine detoxification? This information could help match patients to appropriate treatments and reduce the potential that an individual will not complete a detoxification. Second, what additional contribution will concomitant therapies (including CM) have on abstinence rates during and after detoxification? CM produced promising outcomes in the studies reviewed here, consistent with studies that have used CM with opioid-dependent patients (Bickel et al., 1997; Gross et al., 2006; Kosten et al., 2003), and that have addressed other drug use more broadly (Lussier et al., 2006). Third, is it more advantageous to use a fixed or a flexible dosing schedules, or are results comparable? Fixed dosing schedules use guidelines to determine the frequency and magnitude of a dose decrease and are common among research interventions. Flexible dosing schedules allow participants to dictate the frequency and magnitude of the dose decreases and are common in “real-world” practice (Connock et al., 2007; Mattick et al., 2008). Though we know of no studies that have experimentally evaluated opioid abstinence outcomes following fixed vs. flexible buprenorphine detoxification schedules, flexible dosing has been associated with poorer retention in treatment following methadone detoxification (Dawe et al., 1991). Five of the studies reviewed here (Breen et al., 2003; Resnick et al, 2001; Summers and Stone, 2002; White et al., 2001; Williams et al., 2002) utilized a flexible dosing strategy, yet only 1 reported the percent of samples that were opioid abstinent (23%; Williams et al., 2002). Additional research that evaluates whether an advantage exists for fixed vs. flexible dosing schedules will help guide clinical decisions about detoxification.
In conclusion, opioid abuse and dependence continue to be serious national and international public health problems. Administration of buprenorphine in outpatient settings is an important treatment option. The data reviewed here suggest the duration of an outpatient buprenorphine detoxification is positively associated with opioid abstinence achieved during treatment, and the duration of the preceding maintenance period is positively associated with the percent of participants who were opioid-negative on the final day of treatment. There was high concordance between participants who were negative on the final day of treatment and at the first follow-up visit, suggesting a persisting effect of the intervention, however this observation is based on a small sample size and warrants further research consideration. Although these data have important scientific and clinical implications, this review was based on several uncontrolled observations of a single taper duration that employed a wide range of study designs and reported a diverse array of outcome measures, which ultimately restricted sensitive comparisons across studies. Therefore, this review should be considered a first evaluation into the association between taper duration and clinical treatment outcomes and additional research is needed to further investigate these results. Report more standardized outcome measures will facilitate better comparisons across studies (Table 4), permit meta-analytic reviews and promote development of recommendations for clinical practice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly E. Dunn, Johns Hopkins University School of Medicine, 5200 Eastern Avenue, Suite 142 West, Baltimore, MD 21224. Phone: 410-550-5370, Fax: 410-550-7495, kdunn9@jhmi.edu
Stacey C. Sigmon, University of Vermont. UHC-SATC Room 1415, 1 South Prospect Street, Burlington, VT 05401, ssigmon@uvm.edu
Eric C. Strain, Johns Hopkins University. 5500 Nathan Shock Drive, Baltimore MD, 21224. ecsgss@aol.com
Sarah H. Heil, University of Vermont. UHC-SATC Room 1415, 1 South Prospect Street, Burlington, VT 05401, sheil@uvm.edu
Stephen T. Higgins, University of Vermont. UHC-SATC Room 1415, 1 South Prospect Street, Burlington, VT 05401, shiggins@uvm.edu
References
- Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J. Addict. Dis. 1994;13:33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J. Subst. Abuse Treat. 2005;28:321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Apple PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am. J. Drug Alcohol Abuse. 2004;30:129–153. doi: 10.1081/ada-120029870. [DOI] [PubMed] [Google Scholar]
- Banks CD. Overdosage of buprenorphine: case report. N.Z. Med. J. 1979;89:255–256. [PubMed] [Google Scholar]
- Becker WC, Fiellin DA. Provider satisfaction with office-based treatment of opioid dependence: a systematic review. Subst. Abuse. 2005;26:15–22. doi: 10.1300/j465v26n01_02. [DOI] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, Merrill JO, Schulman B, Finkelstein R, Olsen Y, Busch SH. Opioid use disorder in the United States: insurance status and treatment access. Drug Alcohol Depend. 2008;94:207–213. doi: 10.1016/j.drugalcdep.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. J. Consult. Clin. Psychol. 1997;65:803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J. Pharmcol. Exp. Ther. 1988a;247:47–53. [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin. Pharm. Ther. 1988b;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A clinical trial comparing tapering doses of buprenorphine with steady doses for chronic pain and coexistent opioid addiction. J. Addict. Med. 2010;4:140–146. doi: 10.1097/ADM.0b013e3181ba895d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BM, McLaughlin YS. Barriers to and need for alcohol services for women in rural populations. Alcohol Clin. Exp. Res. 2000;24:1265–1275. [PubMed] [Google Scholar]
- Breen CL, Harris SJ, Lintzeris N, Mattick RP, Hawken L, Bell J, Ritter AJ, Lenne M, Mendoza E. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend. 2003;71:49–55. doi: 10.1016/s0376-8716(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Bullingham RES, McQuay HJ, Moore A, Bennett MRD. Buprenorphine kinetics. Clin. Pharm. Ther. 1980;28:667–672. doi: 10.1038/clpt.1980.219. [DOI] [PubMed] [Google Scholar]
- Cameron D, Allen D, Galway K. A pilot study of the effectiveness of buprenorphine and methadone as detoxification agents when choice is given to the consumer. J. Subst. Use. 2001;6:101–109. [Google Scholar]
- Chawarski MC, Moody DE, Pakes J, O’Connor PG, Schottenfield RS. Buprenorphine tablet versus liquid: a clinical trial comparing plasma levels, efficacy and symptoms. J. Subst. Abuse Treat. 2005;29:307–312. doi: 10.1016/j.jsat.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J. Pain. 2005;6:662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Surratt H, Inciardi JA, Munoz A. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol. Drug Saf. 2007;16:827–840. doi: 10.1002/pds.1452. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opioid users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol. Assess. 2007;11:1–171. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Cushman P. Abstinence following detoxification and methadone maintenance treatment. Am. J. Med. 1978;65:46–52. doi: 10.1016/0002-9343(78)90691-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CO, Kunis HV, Roose RJ, Elam RT, Sohler NL. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction among HIV physicians. J. Gen. Intern. Med. 2007;22:1325–1329. doi: 10.1007/s11606-007-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Griffiths P, Gossop M, Strang J. Should opiate addicts be involved in controlling their own detoxification? A comparison of fixed versus negotiable schedules. Br. J. Addict. 1991;86:977–982. doi: 10.1111/j.1360-0443.1991.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Diamont K, Fischer G, Schneider C, Lenziger E, Pezawas L, Schindler S, Eder H. Outpatient opiate detoxification treatment with buprenorphine: preliminary investigation. Eur. Addict. Res. 1998;4:198–202. doi: 10.1159/000018953. [DOI] [PubMed] [Google Scholar]
- Drug and Alcohol Services Information System (DASIS) 2006. Substance Abuse and Mental Health Services Association (SAMHSA), Office of Applied Studies. Admissions For Detoxifications: 2001. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Luxembourg: Publications Office of the European Union; 2010. Annual report 2010: The State of the Drugs Problem in Europe. [Google Scholar]
- Fiellin DA. The first three years of buprenorphine in the United States: experience to date and future directions. J. Addict. Med. 2007;1:62–67. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- Fingerhood MI, Thompson MR, Jasinski DR. A comparison of clonidine and buprenorphine in the outpatient treatment of opiate withdrawal. Subst. Abuse. 2001;22:193–199. doi: 10.1080/08897070109511459. [DOI] [PubMed] [Google Scholar]
- Fortney J, Booth BM. Access to substance abuse services in rural areas. Recent Dev. Alcohol. 2001;15:177–197. doi: 10.1007/978-0-306-47193-3_10. [DOI] [PubMed] [Google Scholar]
- Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1-month) relapse after opiate detoxification: a pilot study of two cohorts totaling 101 patients with a note on naltrexone blood levels. Addict. Biol. 2003;8:211–217. doi: 10.1080/1355621031000117446. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM. Predicting outcome of inpatient detoxification with substance abusers. Psychiatr. Serv. 1999;50:813–817. doi: 10.1176/ps.50.6.813. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin. Pharm. Ther. 1990;47:525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- Gandhi DH, Jaffe JH, McNary S, Kavanagh GJ, Hayes M, Currens M. Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users. Addiction. 2003;98:453–462. doi: 10.1046/j.1360-0443.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- Galanter M, Dermatis H, Resnick R, Maslansky R, Neumann E. Short-term buprenorphine maintenance: treatment outcome. J. Addict. Dis. 2003;22:39–49. doi: 10.1300/J069v22n03_04. [DOI] [PubMed] [Google Scholar]
- Gibson AE, Doran CM, Bell JR, Ryan A, Lintzeris N. A comparison of buprenorphine treatment in clinic and primary care settings: a randomised trial. Med. J. Aust. 2003;179:38–42. doi: 10.5694/j.1326-5377.2003.tb05417.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez JP, Brogden RN. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Bradley B, Strang J. Opiate withdrawal symptoms in response to 10-day and 21-day methadone withdrawal programs. Br. J. Psychiatry. 1989;54:360–363. doi: 10.1192/bjp.154.3.360. [DOI] [PubMed] [Google Scholar]
- Gossop M, Johns A, Green L. Opiate withdrawal: inpatient versus outpatient programmes and preferred versus random assignment to treatment. Br. Med. J. 1986;293:103–104. doi: 10.1136/bmj.293.6539.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing LR, Ali RL. The place of detoxification in the treatment of opioid dependence. Curr. Opin. Psych. 2006;19:266–270. doi: 10.1097/01.yco.0000218596.54054.a1. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Ali RL, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2009;8 doi: 10.1002/14651858.CD002025.pub4. CD002025. [DOI] [PubMed] [Google Scholar]
- Gross A, Marsch LA, Badger GJ, Bickel WK. A comparison between low-magnitude voucher and buprenorphine medication contingencies in promoting abstinence from opioids and cocaine. Exp. Clin. Psychopharm. 2006;14:148–156. doi: 10.1037/1064-1297.14.2.148. [DOI] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrook JM, Rance MJ. The interaction of buprenorphine with the opiate receptor: lipophilicity as a determining factor in drug-receptor kinetics. In: Kosterlitz HW, editor. Opiates and Endogenous Opioid Peptides. Amsterdam: Elsevier/North Holland, Biomedical Press; 1976. pp. 295–301. [Google Scholar]
- Havens JR, Oser CB, Leukefeld CG. Increasing prevalence of prescription opiate misuse over time among rural probationers. J. Opioid Manag. 2007;3:107–111. doi: 10.5055/jom.2007.0047. [DOI] [PubMed] [Google Scholar]
- Helm S, Trescot AM, Colson J, Sehgal N, Silverman S. Opioid antagonists, partial agonists and agonists/antagonists: the role of opioid detoxification. Pain Physician. 2008;11:225–235. [PubMed] [Google Scholar]
- Horspool MJ, Seivewright N, Armitage CJ, Mathers N. Post-treatment outcomes of buprenorphine detoxification in community settings: a systematic review. Eur. Addict. Res. 2008;14:179–185. doi: 10.1159/000141641. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch. Gen. Psych. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Katz EC, Schwartz RP, King SD, Highfield DA, O’Grady KE, Billings T, Gandhi D, Weintraub E, Glovinsky D, Barksdale W, Brown BS. Brief vs. extended buprenorphine detoxification in a community treatment program: engagement and short-term outcomes. Am. J. Drug Alcohol Abuse. 2009;35:63–67. doi: 10.1080/00952990802585380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J. Addict. Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Korthius PT, Gregg J, Rogers WE, McCarty D, Nicolaidis C, Boverman J. Patient’s reasons for choosing office-based buprenorphine: preference for patient-centered care. J. Addict. Med. 2010;4:204–210. doi: 10.1097/ADM.0b013e3181cc9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Lenardson J, Gale JA. U.S. Department of Health and Human Services. Portland, ME: Federal Office of Rural Health Policy; 2008. Distribution of substance abuse treatment facilities across the rural-urban continuum. CA#U1CRH03716. [Google Scholar]
- Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14:363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhoise M, Babcock D, Brigham G, Harrer J, Reid M, Muir J, Buchan B, Orr D, Woody G, Krejci J, Ziedonis D. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Casadonte P, Bigelow G, Kampman KM, Parkar A, Bailey GL, Rosenthal RN, Beebe KL. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304:1576–1583. doi: 10.1001/jama.2010.1427. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, Jenkins J, Hasson A, Annon J, Saxon A, Selzer J, Boverman J, Bilangi R. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104:256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N. Buprenorphine dosing regime in the management of out-patient heroin withdrawal. Drug Alcohol Rev. 2002;21:39–45. doi: 10.1080/09595230220119309. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Bell J, Bammer G, Jolley DJ, Rushworth L. A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction. 2002;97:1395–1404. doi: 10.1046/j.1360-0443.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Luty J. Treatment preferences of opiate-dependent patients. Psych. Bulletin. 2004;28:47–50. [Google Scholar]
- Marsch L, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, Brooklyn J. Comparison of pharmacological treatments for opioid-dependent adolescents. Arch. Gen. Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone for opioid dependence. Cochrane Database Sys. Rev. 2008;16 doi: 10.1002/14651858.CD002207.pub3. CD002207. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on heroin self-administration: an operant analysis. J. Pharmacol. Exp. Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Miller NS, Gold MS. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am. Fam. Physician. 1998;58:139–146. [PubMed] [Google Scholar]
- O'Connor PG, Carroll K, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ. Three methods of opioid detoxification in a primary care setting. Ann. Intern. Med. 1997;127:526–530. doi: 10.7326/0003-4819-127-7-199710010-00004. [DOI] [PubMed] [Google Scholar]
- Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone treatment. J. Subst. Abuse Treat. 2010;39:340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Prater CD, Miller KE, Zylstra RG. Outpatient detoxification of the addicted family or alcoholic patient. Am. Fam. Physician. 1999;60:1175–1183. [PubMed] [Google Scholar]
- Quigley AJ, Seow SSW, Ilett KF, Dusci L, Swensen G, Harrison-Stewart A, Rappeport L. Buphrenorphine: detoxification after maintenance treatment. Aust. Drug Alcohol Rev. 1987;6:5–10. [Google Scholar]
- Raistrick D, West D, Finnegan O, Thistlewaite G, Brearley R, Banberry J. A comparison of buprenorphine and lofexidine for community opiate detoxification: results from a randomized clinical trial. Addiction. 2005;100:1860–1867. doi: 10.1111/j.1360-0443.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Galanter M, Pycha C, Cohen A, Grandison P, Flood N. Buprenorphine: an alternative to methadone for heroin dependence treatment. Psychopharmacol. Bull. 1992;28:109–113. [PubMed] [Google Scholar]
- Resnick RB, Galanter M, Resnick E, Pycha C. Buprenorphine treatment of heroin dependence (detoxification and maintenance) in a private practice setting. J. Addict. Dis. 2001;20:75–83. doi: 10.1300/j069v20n02_06. [DOI] [PubMed] [Google Scholar]
- Ridge G, Gossop M, Lintzeris N, Witton J, Strang J. Factors associated with the prescribing of buprenorphine or methadone for treatment of opiate dependence. J. Subst. Abuse Treat. 2008;37:95–100. doi: 10.1016/j.jsat.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Wallace EA, McMahon TJ, Pearsall R, Woods SW, Price LH, Kosten TR. Buprenorphine: duration of blockade of effects of intramuscular hydromorphone. Drug Alcohol Depend. 1994;35:141–149. doi: 10.1016/0376-8716(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR. Treatment for opioid dependence: quality and access. JAMA. 2000;283:1337–1339. doi: 10.1001/jama.283.10.1337. [DOI] [PubMed] [Google Scholar]
- Schnoll SH. Treatment of opioid addiction in physicians’ offices: it is about time. J. Addict. Dis. 2001;20:1–3. doi: 10.1300/J069v20n02_01. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisingehr HS, Agar MH, Brown BS. Attitudes toward buprenorphine and methadone among opioid-dependent individuals. Am. J. Addict. 2008;17:396–401. doi: 10.1080/10550490802268835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senay EC, Dorus W, Goldberg F, Thornton W. Withdrawal from methadone maintenance. Rates of withdrawal and expectation. Arch. Gen. Psychiatry. 1977;34:361–367. doi: 10.1001/archpsyc.1977.01770150119014. [DOI] [PubMed] [Google Scholar]
- Shah NG, Lathrop SL, Reichard RR, Landen MG. Unintentional drug overdose death trends in New Mexico, USA, 1990–2005: combinations of heroin, cocaine, prescription opioids and alcohol. Addiction. 2008;103:126–136. doi: 10.1111/j.1360-0443.2007.02054.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict. Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir. Med. J. 2010;103:176–179. [PubMed] [Google Scholar]
- Stimmel B, Rabin J. The ability to remain abstinent upon leaving methadone maintenance: a prospective study. Am. J. Drug Alcohol Abuse. 1974;1:379–391. doi: 10.3109/00952997409011031. [DOI] [PubMed] [Google Scholar]
- Stimmel B, Goldberg J, Rotkopf E, Cohen M. Ability to remain abstinent after methadone detoxification: a six-year study. JAMA. 1977;237:1216–1220. [PubMed] [Google Scholar]
- Strain EC, Harrison JA, Bigelow GE. Induction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble-films. Clin. Pharmacol. Ther. 2011;89:443–449. doi: 10.1038/clpt.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Gossop M. Comparison of linear versus inverse exponential methadone reduction curves in the detoxification of opiate addicts. Addict. Behav. 1990;15:541–547. doi: 10.1016/0306-4603(90)90054-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Volume 1. Rockville, MD: 2010a. Office of Applied Studies Results from the 2009 National Survey on Drug Use and Health. Summary of National Findings (NSDUH Series H-38A, HHS Publication No. SMA 10-4856 Findings) [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Treatment Episode Data Set Admissions (TEDS-A) Concatenated, 1992 to present. 2010b Retrieved online (8/20/2010) at: http://www.icpsr.umich.edu/icpsrweb/SAMHDA/series/00056/studies/25221?sortBy=7.
- Substance Abuse and Mental Health Services Administration. National Survey of Substance Abuse Treatment Services, 2008. 2008 Retrieved online (3/01/2011) at: http://www.oas.samhsa.gov/2k3/NSSATS/NSSATS.cfm.
- Sullivan LE, Charwarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioids dependence: is it associated with new patients entering treatment? Drug Alcohol Depend. 2005;79:113–116. doi: 10.1016/j.drugalcdep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Summers A, Stone S. A description of opiate detoxification using buprenorphine in a community setting. J. Subst. Use. 2002;7:96–99. [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J. Pharmacol. Exp. Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin. Pharmacol. Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Williams H, Remedios A, Oyefeso A, Bennett J. Buprenorphine detoxification treatment for heroin dependence: a preliminary experience in an outpatient setting. Ir. J. Psychol. Med. 2002;19:80–83. doi: 10.1017/S0790966700007114. [DOI] [PubMed] [Google Scholar]
- White J, Bell J, Saundes JB, Williamson P, Makowska M, Farquharson A, Beebe KL. Open-label dose-finding trial of buprenorphine implants (Probuphine) for treatment of heroin dependence. Drug Alcohol Depend. 2009;103:37–43. doi: 10.1016/j.drugalcdep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- White R, Alcorn R, Feinmann C. Two methods of community detoxification from opiates: an open-label comparison of lofexidine and buprenorphine. Drug Alcohol Depend. 2001;65:77–83. doi: 10.1016/s0376-8716(01)00149-1. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lintzeris N, Lea T. Why do patients report transferring between methadone and buprenorphine. Drug Alcohol Rev. 2009;28:686–687. doi: 10.1111/j.1465-3362.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use and emergency department visits. J. Addict. Dis. 2008;27:1–11. doi: 10.1300/J069v27n01_01. [DOI] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, Parkar A, Publicker M, McCain K, Potter JS, Forman R, Vetter V, McNicholas L, Blaine J, Lynch KG, Fudala P. Extended vs. short-term buprenorphine-naloxone for treatment of opioid-addicted youth. JAMA. 2008;300:2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NMJ, Sheard L, Tompkins CNE, Adams CE, Allgar VL, Oldham NS. Buprenorphine versus dihydrocodeine for opiate detoxification in primary care: a randomised controlled trial. BMC Fam. Pract. 2007;8:1–9. doi: 10.1186/1471-2296-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J, Bigelow C, Compton P, Foley K, Iguchi M, Sannerud C. College on the problems of drug dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]