Abstract
Soluble isoforms of three human IgE Fc receptors, namely FcεRI, FcεRII and galectin-3, can be found in serum. These soluble IgE receptors are a diverse family of proteins unified by the characteristic of interacting with IgE in the extracellular matrix. A truncated form of the alpha-chain of FcεRI, the high affinity IgE receptor, has recently been described as a soluble isoform (sFcεRI). Multiple soluble isoforms of CD23 (sCD23), the low affinity IgE receptor also known as FcεRII, are generated via different mechanisms of extracellular and intracellular proteolysis. The second low affinity IgE receptor, galectin-3, only exists as a secretory protein. We here discuss the physiological roles of these three soluble IgE receptors as elements of the human IgE network. Additionally, we review the potential and current use of sFcεRI, sCD23 and galectin-3 as biomarkers in human disease.
Keywords: IgE, Fc receptors, FcεRI, CD23, FcεRII, galectin-3
Antibodies of the immunoglobulin E isotype (IgE) are key regulators of host defense against parasitic infections. Over the last three decades, IgE additionally gained undesirable fame as a central mediator of allergic responses. Allergic responses, however, are not regulated by IgE alone, but rather by a complex protein network including transmembrane and soluble IgE receptors and a variety of co-receptors that do not even bind IgE directly (for a detailed review on the human IgE network see Gould et al. [1]).
Soluble IgE receptors are constituents of the human IgE network and are part of feedback mechanisms that regulate IgE production. Therefore, the physiology of these serum components is highly interesting as they are potential in vivo modulators of allergic responses. Thus far, three human soluble IgE receptors have been described, namely, soluble FcεRI (sFcεRI), soluble CD23 (sCD23), and galectin-3 (Table 1 and Figure 1). The focus of this review is to compare and contrast the role of these three soluble IgE receptors in the context of the human IgE network. We discuss the possible physiological roles of the soluble IgE receptors, clinical implications, and elaborate on the potential and current use of soluble IgE receptors as biomarkers of disease.
Table 1.
Soluble IgE receptors in human serum
| Soluble IgE receptor | Main source in vivo | Regulation of production |
|---|---|---|
| sFcεRI – soluble alpha- chain of FcεRI | Not defined | IgE-mediated FcεRI activation |
| sCD23 | B cells | Surface expression of membrane CD23 and accessibility of cleavage sites Expression and activity of shedding enzymes ADAM10, ADAM8, ADAM33, MMP9 |
| Galectin-3 | Macrophages | Induction via IL-4 and IL-13 Inhibition by LPS and INF-γ |
Figure 1.
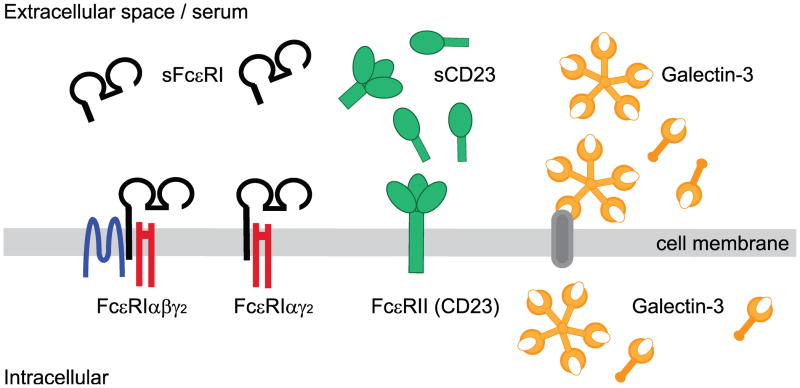
Human IgE Fc-receptors and their soluble isoforms.
The high affinity IgE Fc receptor, Fc-epsilon-RI (FcεRI), has two transmembrane isoforms, FcεRIαβγ2 and FcεRIαγ2. The soluble isoform, sFcεRI, is a single chain receptor consisting of a truncated version of the IgE-binding FcεRIα subunit. Several different soluble isoforms of the transmembrane low affinity IgE Fc receptor, FcεRII or CD23, have been described. A detailed summary of soluble CD23 (sCD23) isoforms and their cleavage sites is provided in Table 2. Galectin-3 is a secretory IgE Fc receptor. After secretion, galectin-3 can attach to cell membranes via interacting with a large number of carbohydrate structures displayed by cell surface proteins. Additionally, an intracellular pool of galectin-3 can be found in the cytoplasm and the nucleus.
1 - Generation of soluble IgE receptors
1.1 - Soluble FcεRI, sFcεRI
sFcεRI is a single-chain receptor isoform of FcεRI, the high affinity IgE receptor. In humans and mice, robust levels of tetrameric FcεRIαβγ2 are constitutively expressed on the cell surface of mast cells and basophils. This receptor isoform is well known for its function as a key regulator of allergic responses [2]. Under physiological conditions, FcεRIαβγ2 is preloaded with IgE. When IgE-specific antigen crosslinks the receptor, the release of preformed inflammatory mediators and cytokines is triggered. Thus, IgE-FcεRI mediated activation of mast cells and basophils is considered a hallmark of immediate allergic reactions [2]. The following subunits assemble cotranslationally to form tetrameric FcεRI [3]: an IgE-binding α-chain, FcεRIα, and two signaling subunits, FcεRIβ and FcεRIγ; the latter is commonly referred to as the common FcRγ-chain and dimerizes. In addition to the tetrameric isoform, human antigen presenting cells (APCs), such as Langerhans cells of the skin and various other peripheral blood dendritic cell subpopulations, constitutively express a trimeric αγ2 isoform of the FcεRI [2,4–6]. In contrast, murine APCs lack constitutive expression of the receptors, but an inducible version of FcεRIαγ2 has been described in mice after viral infection or challenge with house dust mite ([7,8]). Trimeric FcεRIαγ2 is considered to be an antigen uptake receptor and has been shown to be involved in the regulation of Th2-type allergic tissue inflammation [5,9].
In allergic individuals, induction of FcεRI expression has also been described for many other cell types, including monocytes, eosinophils, platelets and gastrointestinal epithelial cell [10–14]. Immunoprecipitation studies from human serum show that sFcεRI consists of a smaller FcεRI alpha-chain with a molecular weight of ~40kDa compared to the ~60kDa full length protein [15]. The lower molecular weight is likely explained by the lack of the transmembrane and cytosolic domains [16]. This is also supported by that fact that FcεRIβ and FcεRIγ2 require the alpha-chain transmembrane region to form a receptor complex fail to co-immunoprecipitate with sFcεRI. Therefore, ultimately, mass spectrometric analysis is needed to precisely define the protein sequence of sFcεRI.
The alpha-chain of the multimeric FcεRI complex is a type I membrane protein that contains the receptor’s IgE-binding site [17]. The soluble alpha-chain, sFcεRI, likewise contains an IgE binding site as it is precipitated with IgE and forms IgE-complexes in serum [15]. Dissociation studies [18] as well as subsequent analysis of the crystal structure [19] of FcεRI-alpha and IgE revealed an extraordinarily high affinity of this ligand-receptor interaction. As the crystals analyzed were generated with a recombinant soluble version of FcεRI-alpha, it is likely that serum sFcεRI has a high affinity for IgE consistent with reports in the literature [19].
It has not yet been characterized how the production of sFcεRI is induced in vivo. In vitro data show that sFcεRI can be generated after IgE-mediated crosslinking of surface-expressed FcεRI when the trimeric isoform of the receptor is expressed in MelJuso cells [15], which are a common model for non-professional antigen presenting cells [20]. This set of data suggests that production of the soluble isoform is induced by FcεRI crosslinking-induced receptor activation. Since these data were generated with a stable cell line generated with full length FcεRI-alpha cDNA, sFcεRI could not be produced as a splice variant, but rather must be a product of a posttranslational modification such as cleavage by a protease. Nonetheless, several in vivo mechanisms for generating sFcεRI could be operating in parallel as discussed for the other sIgE receptors later in this review.
Currently, many more questions about sFcεRI remain open. For example, the cell type(s) that release or shed this protein in humans remain to be defined. No in vivo modulators of sFcεRI production are as of yet known. Furthermore, experiments are needed to investigate whether activation of tetrameric FcεRI also induces the release of sFcεRI. Such experiments will answer the question as to whether mast cells and basophils contribute to the generation of the serum pool of sFcεRI. Another important issue not yet resolved is whether sFcεRI exists in mice. If it does not, murine models might be inadequate for studying the potential physiological role of this receptor isoform. Initial experiments to detect sFcεRI from supernatants of IgE-activated RBL-2H3 cells, a rat basophilic leukemia cell line that expresses the tetrameric isoform of the receptor, and murine dendritic cells from a human FcεRIα-transgenic animal that express a chimeric form of FcεRIαγ2 have failed (Fiebiger lab, unpublished observation and [9]). It is conceivable that expression of the sFcεRI isoform is different between humans and mice, comparable to the dissimilar expression patterns of trimeric sFcεRI between the two species [21]. However, more studies on the topic need to be performed before any conclusions are warranted.
1.2 - Soluble FcεRII, sCD23
Soluble CD23 (sCD23) molecules result from proteolytic cleavage of the 45 kDa transmembrane form of the low affinity receptor for IgE, FcεRII (CD23). Unlike FcεRI, this IgE Fc receptor does not belong to the immunoglobulin receptor family. Its large extracellular globular C-type lectin domain places CD23 in the C-type lectin superfamily. Various cell types including B cells, T cells, NK cells, monocytes, macrophages, follicular dendritic cells, Langerhans cells, bone marrow stromal cells, neutrophils, eosinophils, platelets and epithelial cells express CD23 at the cell surface. For a more detailed insight to the biology of transmembrane CD23, we refer the reader to Gould et al. and Acharya et al. who discussed this topic in their reviews in great detail [1,22].
Based on distinct molecular weights, several sCD23 isoforms have been described (Table 2). The cleavage sites for all forms of sCD23 are located within the extracellular αhelical stalk region of the transmembrane protein. All cleavage products posses the globular lectin head domain, but retain stalk regions of different lengths. Since the IgE binding domain of CD23 resides in the globular lectin head domain, all isoforms of sCD23 have the capacity to interact with IgE.
Table 2.
Soluble CD23 isoforms
| Molecular weight (kDa) | Origin | Enzyme |
|---|---|---|
| 37 | Extracellular shedding of membrane CD23 at position Ala80 |
in vivo: ADAM10 and MMP9 in vitro: ADAM10, ADAM8 and ADAM 33 |
| 33 | Extracellular shedding of membrane CD23 at position Arg101 |
in vivo: ADAM10 and MMP9 in vitro: ADAM10, ADAM8 and ADAM 33 |
| 28–29 | Intracellular processing of newly synthesized CD23 protein | Not defined |
| 25–27 | Proteolytic cleavage/degradation products of 33kDa and 37kDa sCD23 | Not defined |
| 16–17 | Extracellular cleavage of membrane CD23 at Ser155 and Glu298 | Der p1 |
sCD23 can be produced by intracellular proteolytic processing of newly synthesized CD23 molecules, which are subsequently secreted [23]. This intracellular cleavage event is responsible for the generation of sCD23 with a molecular weight of 28–29 kDa. Additionally, several enzymes have been described to generated sCD23 via extracellular processing. Metalloproteases of the ADAM family were identified as cleavage enzymes for the generation of 37 kDa as well as 33 kDa sCD23 fragments. In vivo, ADAM10 has also been found as the predominant sCD23-releasing enzyme [24,25]. B cell-specific deletion of ADAM10 reduces sCD23 levels by 70% [24], indicating that this cell type is the main source of sCD23 in serum. Additionally, ADAM8 and ADAM33 can generate sCD23 in vitro [26,27]. The contribution of both proteases for production of sCD23 in vivo is still debated, since experiments performed with knock-out animals showed that sCD23 production was not altered in the absence of either protease [25]. The finding that B cells express only very low levels of ADAM8 [28] and no ADAM33 [29] are in line with the observations seen in knock-out animals. Interestingly, ADAM8 is highly expressed on lung epithelial cells of asthmatic patients and upregulated in models of murine allergic airway inflammation [30–32]. Increased expression of ADAM33 in lung tissue has also been linked to the pathogenesis of asthma [33]. These findings imply that sCD23 production by ADAM8 and ADAM33 could be predominantly tissue-specific and restricted to the lung. As discussed later in this review in detail (2.2), increased levels of sCD23 can foster IgE production as well as enhance production of inflammatory cytokines. As a result, sCD23 generated by ADAM8 or ADAM33 in the lung could be a local mediator that regulates asthma development or, more generally, the pathology of IgE-mediated asthmatic reactions. Additionally, smaller proteolytic cleavage products of sCD23 with molecular weights of 25–27 kDa are described. These isoforms of sCD23 are most likely extracellular degradation intermediates and can be found in cell culture supernatants [34,35], as well as in human serum of patients with chronic lymphocytic leukemia [36]. The house dust mite protease Der p1 has been shown to generate a 17kDa sCD23 fragment [37,38]. It is speculated that, at least in part, the high allergenicity of the mites is a result of the Der p1– mediated increase in serum sCD23 levels, which in turn modulates IgE production.
Recently, it was shown that membrane CD23 and ADAM10 can be found in exosomes of B cells. [39]. The authors of this study suggest that membrane CD23 is internalized from the cell surface and traffics into endosomal compartments. Endosome-derived exosomes are then secreted and CD23 is cleaved extracellularly. Exosome-derived sCD23 could be an additional source of serum sCD23 in humans.
Production of sCD23 is dictated by the expression levels of transmembrane CD23, as well as the rate of proteolytic cleavage from the cell surface. Surface expression of CD23 is regulated by many cytokines including IL-4, IL-13, IL-5, IL-9, GM-CSF, INF-γ and CD40 (reviewed in [27]) and accordingly these immune mediators influence the generation of sCD23. Along this line, activation of murine splenic B cells and human peripheral blood B cells via lipopolysaccharides (LPS) induces release of sCD23. This LPS-induced increase in sCD23 production is mediated by both induction of de novo synthesis of CD23 and by enhanced CD23 cleavage by the matrix metalloproteinase MMP9 [40]. Supernatants from MMP9 −/− splenocytes fail to induce sCD23 release from B cells and MMP9 expression itself is upregulated following LPS stimulation [40]. The cleavage sites and cleavage products of membrane CD23 by MMP9 are currently unknown. Recently, it was shown that glutamate, a major excitatory neurotransmitter in the central nervous system, also enhances sCD23 release by B cells. Activity of the glutamate-specific kainate receptor (KAR) mediates the glutamate-dependent release of sCD23 by increasing ADAM10 expression [41].
Membrane cleavage of sCD23 is primarily regulated via the accessibility of the cleavage sites for proteolysis. Ligation of membrane CD23 by IgE induces a conformational change that inhibits sCD23 shedding by ADAM10 [42]. Using a comparable mechanism, CD23-specific monoclonal antibodies (mAb) can either enhance or limit the release of sCD23 [43]. Binding of 19G5 mAb to murine sCD23 changes the topology of the α-helical coiled coil stalk region in favor of proteolytic cleavage [44]. Interestingly, the 19G5 mAb not only facilitates proteolytic cleavage of sCD23 from the cell surface but also induces internalization of CD23 into endosomal compartments [39]. This reagent could thus also induce production of sCD23 via the exosomal pathway as described earlier in the review. The anti-CD23 antibody Lumiliximab has been shown to decreases serum IgE, likely by inhibiting production of sCD23. It is speculated that Lumiliximab stabilizes surface CD23 by preventing its proteolytic cleavage [45].
1.3 – Galectin-3
Galectin-3 is a secretory protein of ~30 kDa and belongs to a family of β-galactoside-binding animal lectins [46]. This protein was formerly named ε-binding protein because of its ability to interact with IgE as well as FcεRI [47]. In contrast to FcεRI and CD23, galectin-3 does not exist as a transmembrane protein. The structure of galectin-3 consists of a carbohydrate-recognition domain (CRD) linked to a non-lectin region of proline- and glycine-rich tandem repeats. Galectin-3 can form pentamers via the non-CRD domain which strongly resemble pentameric IgM; this is a unique structural feature of galectin-3 among the 15 members of the galectin family. Similar to all other galectin family members, galectin-3 lacks a classical signal for the secretory pathway. Therefore, the mechanism of galectin-3 secretion is currently poorly understood [46].
Galectin-3 resides in the cytosol or the nucleus, but has also been shown to associate with intracellular vesicles [48] and has been found in exosomes from dendritic cells [49]. Following secretion, galectin-3 is detected in the extracellular space from where it can attach to cell surfaces. IgE and FcεRI were among the first described binding partners at the cell surface of mast cells [50]. Interestingly, differentially glycosylated IgE isoforms have been found that display distinct binding capacities for galectin-3 [51,52]. Additionally, galectin-3 appears to interact also with a large variety of other cell surface and extracellular matrix proteins. Several intracellular proteins were also described as galectin-3 binding partners (reviewed in detail in [46,48]). Because of the promiscuous binding pattern of galectin-3, it is important to note that the detection of this molecule at the cell surface does indicate whether the cell type by itself produces the protein.
Galectin-3 has been found on the cell surface of eosinophils, neutrophils, mast cells, dendritic cells, macrophages, T cells and B cells [46,47,53–57]. Macrophages have been shown to be a key source of extracellular soluble galectin-3. When monocytes differentiate into macrophages increased galectin-3 expression is observed [57]. Alveolar macrophages release galectin-3 into the alveolar space after infection with Streptococcus pneumoniae [58,59]. Furthermore, alternative macrophage activation with the Th2 response associated cytokines, IL-4 and IL-13, increase expression and release of galectin-3. In contrast, LPS or INF-γ induced macrophage activation can inhibit galectin-3 release [60]. In line with the finding that Th2 cytokines regulate galectin-3 expression in macrophages, eosinophils from allergic donors show increased levels of galectin-3 [61]. Additionally, various fibrotic conditions in humans including liver cirrhosis and pulmonary fibrosis are characterized by increased levels of galectin-3 [62,63].
Interestingly, galectin-3 expression appears to be species specific and differences between humans and mice are described. Similar to FcεRI, galectin-3 was found on human but not murine eosinophils [64]. Galectin-3 might thus be another IgE Fc-receptors for which species-specific expression patterns might hamper interpretation of murine studies.
2 - Functions of the soluble IgE receptors in the IgE network
2.1 – sFcεRI
Attempts to modulate IgE-mediated immune responses by recombinant FcεRI [65,66] well precede the description of sFcεRI in human serum. Recombinant soluble forms of the extracellular domain of FcεRI alpha-chain that interact with the Fc-portion of IgE were described as inhibitors of cytokine release from human basophils [66] and RBL-2H3 release assays [65]. In vivo models of passive cutaneous anaphylaxis confirmed that such soluble IgE receptors indeed blunt IgE-mediated immune responses [65]. Omalizumab is an anti-IgE specific mAb that reacts with the Fc-portion of the immunoglobulin and is successfully used to clinically modulate serum IgE levels in several types of allergic diseases [67]. Based on its binding domains on IgE, a recombinant form of sFcεRI could be used in a similar manner as Omalizumab to modulate IgE-mediated allergy without the disadvantages of antibody therapy.
In vivo, sFcεRI has several potential binding partners in serum as well as on the surface of peripheral blood or tissue cells (Figure 2). As a free IgE receptor, sFcεRI can form a complex with IgE or form complexes of higher order that include antigen. Free sFcεRI can additionally bind to membrane IgE (mIgE) expressed by B-cells. Based on a one-to-one ligand-receptor ratio, no crosslinking and activation of the B cell could occur. By blocking the Fc-region of IgE, free sFcεRI could prevent IgE from interacting with other FcεRI receptors expressed on the cell surface [15]. On effector cells, such as basophils and mast cells, this would impair IgE-mediated degranulation and release of cytotoxic mediators. Thus, sFcεRI has the potential to blunt the acute phase of an allergic response. If sFcεRI-IgE-antigen complexes of higher order interact with surface-expressed FcεRI, however, such complexes could activate cells via FcεRI-crosslinking, provided the complexes contain free IgE Fc-domains. Via such an activation pathway, sFcεRI could exacerbate immediate type allergic responses. On dendritic cells, IgE-mediated antigen presentation might be down-regulated when free sFcεRI blocks IgE from binding to surface expressed receptors, which are used for antigen-sampling. Consequently, the sensitization phase towards allergens as well as the Th2-type immune responses of chronic allergic reactions might be modified [9]. If sFcεRI is internalized as part of a sFcεRI-IgE-antigen complex by antigen presenting cells, the endogenous alpha-chain could be presented as an exogenous allergen. Such a process might provide a mechanistic explanation for how autoantibodies against FcεRI-alpha are generated [68,69]. Finally, it is conceivable that as of yet unidentified binding partners for sFcεRI exist in human serum.
Figure 2.
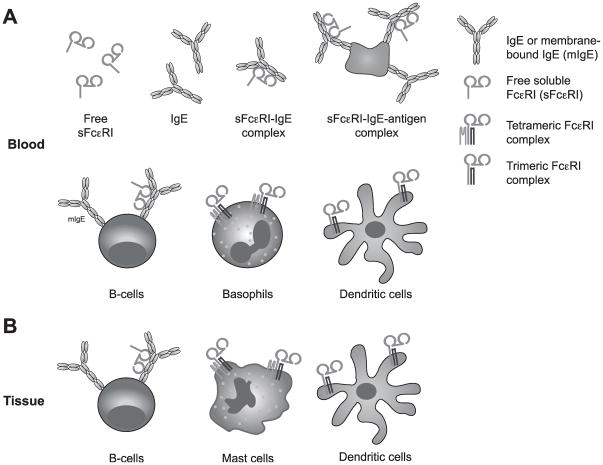
Possible interaction partners of soluble Fc-epsilon-RI (sFcεRI) in vivo. A) FcεRI exists as a membrane bound as well as a soluble isoform in human blood. In serum, sFcεRI is found as a true soluble form or as a sFcεRI-IgE complex when bound to its natural ligand. Potentially, sFcεRI-IgE complexes can interact with antigens and form immune complexes of higher order. Free sFcεRI can additionally interact with membrane IgE expressed on B cells. sFcεRI-IgE complexes cannot bind to trimeric or tetrameric FcεRI expressed on the cell surface of peripheral blood cells, because the binding site of IgE and the cellular receptor is blocked by sFcεRI in solution.
B) In tissue, all interactions described for peripheral blood in (A) are theoretically possible. It remains to be defined whether the local concentration of sFcεRI in tissue is comparable to sFcεRI serum levels. Cells migrating from the periphery blood could also serve as vehicles to transport sFcεRI into tissue.
2.2 - sCD23
IgE-mediated ligation of membrane CD23 inhibits IgE production in B cells via a negative feedback loop [1]. In contrast, sCD23 increases the production of IgE through co-ligation of CD21 and mIgE [70,71]. The size of clusters induced by sCD23-mediated crosslinking at the cell surface defines the strength of the IgE-inducing signal. Since the oligomerization capacity of sCD23 depends on the length of its stalk region, not all forms of sCD23 are equally potent at inducing IgE-production [72]. In this context, McCloskey et al. have reported that short recombinant sCD23 fragments corresponding to short sCD23 forms generated by Der p1 can form only small complexes, which were even inhibitory for IL-4 induced synthesis of IgE in their experimental settings [73]. This finding argues against the assumption that the high allergenicity of the house dust mite derives from increased production of sCD23 through Der p1. In addition to regulating IgE synthesis, sCD23 has been shown to promote B cell differentiation, survival of germinal center B cells as well as differentiation towards B cell blasts in vitro in the presence of IL-1α [74–76]. Aside from acting on the B cell compartment, combinations of sCD23 and IL-1α also promote proliferation of human myeloid bone marrow precursors, differentiation of thymic T cell precursors and CD4 T cell responses [77–79].
sCD23 also magnifies allergic diseases by enhancing the production of inflammatory cytokines. On human monocytes, binding of sCD23 to CD11b/CD18 and CD11c/CD18 has been reported to activate nitric oxide synthase and to induce the production of inflammatory cytokines, including TNF-α, IL-6, IL-8, MIP1-α and MIP1-β, as well as IL-1β [80–83]. Similarly, murine monocytes and macrophages produce IL-6 after incubation with recombinant sCD23 [84]. Activation of vitronectin receptors by sCD23 is an additional pathway for the induction of pro-inflammatory cytokines [85]. Peripheral blood mononuclear cells of patients with hyper-IgE syndrome are particularly reactive to treatment with recombinant sCD23, producing high levels of IL-1β and TNFα [86].
2.3 – Galectin-3
Galectin-3 is a versatile player of the immune system [46,87,88]. Secreted galectin-3 can activate cells directly by binding to cell surface receptors. Alternatively, galectin-3 is endocytosed, permitting it to modify intracellular signalling pathways. The large number of intracellular as well as extracellular galectin-3 binding partners, therefore, allows this protein to play a role in a large variety of inflammatory responses, including neutrophil activation, chemoattraction of monocytes and macrophages, adhesion and migration of neutrophils and dendritic cells as well as regulation of apoptosis in immune cells [46,89–91]. Recently it was found that galectin-3 even displays antimicrobial functions against the fungus Candida albicans [92]. In the context of allergic reactions, it is important to note that extracellular galectin-3 is a potent activator of mast cells via crosslinking of IgE-loaded FcεRI or via crosslinking FcεRI directly in an IgE-independent manner. Galectin-3 was shown to induce inflammatory mediator release from IgE-sensitized as well as non-sensitized mast cells and human eosinophils [47,55]. Since membrane FcεRI is a binding partner, it is likely that galectin-3 can also interact with sFcεRI in serum. Such an interaction could potentially modulate the functions of sFcεRI in vivo.
3. Soluble IgE receptors as disease biomarkers
As detailed below, the soluble IgE receptors CD23 and galectin-3 are upregulated in a variety of diseases and have made their way into clinical practice as diagnostic biomarkers. We speculate here that sFcεRI might also prove itself as a biomarker, although it should be noted that solid evidence for this role is so far lacking.
3.1. sFcεRI – a potential new biomarker?
Development of a standardized assay that allows for the comparative analysis of serum levels of sFcεRI in health and disease will facilitate an understanding of the clinical relevance of sFcεR in serum. Currently, it is known only that serum levels of sFcεRI correlate with serum IgE in a pediatric patient cohort with elevated IgE [88][93]. Interestingly, sFcεRI was also found in serum of individuals with normal IgE levels. In fact the highest levels of sFcεRI were actually described in this subpopulation [93]. More detailed studies investigating the possible link between serum sFcεRI to clinical symptoms of allergy are urgently needed.
3.2. sCD23 as a biomarker
sCD23 levels appear to be upregulated in a plethora of diseases. Less frequently, a decrease of serum sCD23 has been described (Table 3). Elevated sCD23 levels have been found in association with allergic diseases, including asthma and atopic dermatitis [94–97]. There is some debate amongst allergists, however, as to whether sCD23 serum levels have a predictive value for the diagnosis of allergic reactions [98]. Clearly, further investigations are needed.
Table 3.
Serum levels of sCD23 and galectin-3 are modulated in various pathologies
| Pathology | sCD23 | Galectin-3 |
|---|---|---|
| Cancer | Chronic lymphocytic leukemia ↑a Pancreatic cancer ↑ AIDS-associated Non-Hodgkin’s lymphoma ↑ Glioma ↓b |
Thyroid cancer ↑ [125–127] c Melanoma ↑ [128–130] Colorectal Cancer ↑ [128] Head and neck squamous cell carcinomas ↑ [131] Bladder cancer ↑ [132] Breast and Ovarian cancer ↑ [128] Non-Hodgkin’s lymphoma ↑ [128] |
| Auto-immune diseases | Arthritis ↑ and ↓ Systemic lupus erythematosus ↑ [133] Primary Sjogren’s disease ↑ [133] Autoimmune thyrioditis ↑ [134] Myasthenia gravis ↑ [134,135] Crohn’s disease ↓ [134] |
Rheumatoid arthritis ↑ [136,137] Systemic lupus erythematosus ↑ [137] Behçet’s disease ↑ [137] Inflammatory bowel disease ↑ [138] |
| Allergic diseases | Asthma ↑ Atopic dermatitis ↑ |
|
| Other | Chronic heart failure ↑ [123,139–141] Liver fibrosis ↑ Pulmonary fibrosis ↑ |
↑ serum levels are found to be upregulated
↓ serum levels are found to be downregulated
References are given only if not cited in the text
Chronic lymphocytic leukemia (CLL) is currently the only disease in which sCD23 is used as a clinical biomarker. CLL patients show significantly higher levels of serum sCD23 when compared to healthy controls [99,100]. Plasma levels of sCD23 correlate with disease outcome; high sCD23 concentrations indicate a more severe disease stage, a more rapid median progression time and shorter median survival time [99,101–105]. Other parameters of CLL which correspond to a negative prognosis, such as a short doubling time of lymphocyte and diffuse bone marrow histology, also correlate with increased levels of sCD23 [99,105].
In AIDS patients, sCD23 appears to be a predictive biomarker for the development of Non-Hodgkin lymphoma. sCD23 is specifically elevated in HIV patients prior to the diagnosis of AIDS-associated Non-Hodgkin lymphoma [106–108]. Recently, elevated serum levels of sCD23 were described in patients with pancreatic cancer [109], but additional studies are required to evaluate whether sCD23 can function as a biomarker in this disease. Several reports describe elevated sCD23 levels in serum and synovial fluid of patients with rheumatoid arthritis [110–117]. In contrast, Singh et al. showed recently that sCD23 levels were slightly decreased in patients with juvenile arthritis [118]. More extensive studies are needed to clarify this contradiction.
Low serum sCD23 levels are described for glioma patients [119]. The reason for this decrease of serum sCD23 is unknown as of yet. Glioma patients commonly do not have elevated serum IgE and suffer less often from allergies. Conversely, the survival time of glioma patients is prolonged if they show elevated serum IgE [120]. In these studies, the authors speculate that elevated IgE levels correlated with more effective anti-tumor response or less aggressive tumors. A study by Merril et al. found that patients with allergy actually have a decreased risk for glioma [121]. More research is needed to understand how and if sCD23 and IgE have protective functions in this type of cancer. This topic is a focus of attention in the nascent field of allergooncology [122].
3.3. Galectin-3
Elevated serum levels of galectin-3 are found in many diseases including various types of autoimmune diseases and cancer (Table 3). So far, the only FDA approved application of galectin-3 as a biomarker is for the prognosis of chronic heart failure [123]. However, Galectin-3 has recently emerged also as a promising diagnostic marker for thyroid cancers [124].
4. Summary and perspective
Soluble IgE receptors in human serum are a diverse group of proteins with the unifying characteristic of interacting with IgE. We here discussed the production and physiological roles of these receptors and compared them to their parental transmembranous receptor isoforms (Table 4). Further studies on how the generation of soluble IgE receptors is regulated, how these proteins function within the human IgE network and how they are connected to various disease pathologies will close large gaps in our understanding of IgE-mediated immune responses. In addition, a better understanding of the patho-physiology of soluble IgE receptors might point us towards novel intervention strategies for IgE-mediated allergies.
Table 4.
Major physiological roles of transmembrane and soluble IgE Fc receptors.
| IgE receptor | Transmembrane form | Soluble form |
|---|---|---|
| FcεRI | Mast cells, basophils: release of inflammatory mediators Dendritic cells: antigen uptake receptor |
Not defined |
| CD23 | Regulation of IgE sythesis Transport of IgE at mucosal surfaces |
Promotion of IgE sythesis Induction of inflammatory cytokine production from monocytes and macrophages Promotion of T and B cell differentation as well as B cell survival |
| Galectin-3a | Induction of inflammatory mediator release from mast cells via binding of FcεRI and IgE Promotion of adhesion, migration and respiratory burst of neutrophils Chemoattraction of monocytes and macrophages Promotion of Th2 responses and alternative macrophage activation Regulation of apoptosis in immune cells Anti-microbial functions against Candida albicans Activation and growth induction of tissue fibroblasts |
|
No transmembrane form of galectin-3 exists. Next to the secreted pool, galectin-3 is found intracellularly in a non-secreted form.
Research highlights.
Soluble isoforms of three different IgE Fc receptors are found in human serum.
Soluble IgE receptors are potential modulator of IgE-mediated immune responses.
Soluble IgE receptors are potential biomarkers for various diseases.
Acknowledgments
We apologize to colleagues whose work was not cited in this review due to space limitations and thank Bonny Dickinson and Michael Pardo for critically reading this manuscript. This work was supported by the Gerber Foundation and the National Institutes of Health grant AI075037 (both to E.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 2.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 3.Fiebiger E, Tortorella D, Jouvin MH, Kinet JP, Ploegh HL. Cotranslational endoplasmic reticulum assembly of FcεRI controls the formation of functional IgE-binding receptors. J Exp Med. 2005;201:267–277. doi: 10.1084/jem.20041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer D, Fiebiger E, Ebner C, Reininger B, Fischer GF, Wichlas S, et al. Peripheral blood dendritic cells express FceRI as a complex composed of FceRIa- and FceRIg-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–613. [PubMed] [Google Scholar]
- 6.Bieber T, de la Salle H, Wollenberg A, Hakimi J, Chizzonite R, Ring J, et al. Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (FcεRI) J Exp Med. 1992;175:1285–1290. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallmann E, Reininger B, Brandt S, Duschek N, Hoflehner E, Garner-Spitzer E, et al. High-Affinity IgE Receptors on Dendritic Cells Exacerbate Th2-Dependent Inflammation. J Immunol. 2011 doi: 10.4049/jimmunol.1003392. [DOI] [PubMed] [Google Scholar]
- 10.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin M-H, Kilgus O, et al. Expression of functional high affinity immunoglobulin E receptors (FceRI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 12.Kayaba H, Dombrowicz D, Woerly G, Papin JP, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J Immunol. 2001;167:995–1003. doi: 10.4049/jimmunol.167.2.995. [DOI] [PubMed] [Google Scholar]
- 13.Joseph M, Gounni AS, Kusnierz JP, Vorng H, Sarfati M, Kinet JP, et al. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. European journal of immunology. 1997;27:2212–2218. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- 14.Untersmayr E, Bises G, Starkl P, Bevins CL, Scheiner O, Boltz-Nitulescu G, et al. The high affinity IgE receptor FcεRI is expressed by human intestinal epithelial cells. PLoS ONE. 5:e9023. doi: 10.1371/journal.pone.0009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehlink E, Platzer B, Baker AH, Larosa J, Pardo M, Dwyer P, et al. A Soluble Form of the High Affinity IgE Receptor, Fc-Epsilon-RI, Circulates in Human Serum. PLoS ONE. 2011;6:e19098. doi: 10.1371/journal.pone.0019098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platzer B, Fiebiger E. The signal peptide of the IgE receptor alpha-chain prevents surface expression of an immunoreceptor tyrosine-based activation motif-free receptor pool. J Biol Chem. 2010;285:15314–15323. doi: 10.1074/jbc.M110.104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 18.Ortega E, Schweitzer-Stenner R, Pecht I. Kinetics of ligand binding to the type 1 Fc epsilon receptor on mast cells. Biochemistry. 1991;30:3473–3483. doi: 10.1021/bi00228a018. [DOI] [PubMed] [Google Scholar]
- 19.Garman SC, Kinet JP, Jardetzky TS. Crystal structure of the human high-affinity IgE receptor. Cell. 1998;95:951–961. doi: 10.1016/s0092-8674(00)81719-5. [DOI] [PubMed] [Google Scholar]
- 20.Paul P, van den Hoorn T, Jongsma ML, Bakker MJ, Hengeveld R, Janssen L, et al. A Genome-wide multidimensional RNAi screen reveals pathways controlling MHC class II antigen presentation. Cell. 145:268–283. doi: 10.1016/j.cell.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Dehlink E, Fiebiger E. The role of the high-affinity IgE receptor, FcεRI, in eosinophilic gastrointestinal diseases. Immunol Allergy Clin North Am. 2009;29:159–170. xii. doi: 10.1016/j.iac.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Acharya M, Borland G, Edkins AL, Maclellan LM, Matheson J, Ozanne BW, et al. CD23/FcεRII: molecular multi-tasking. Clinical and experimental immunology. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BW, Simmons CF, Jr, Wileman T, Geha RS. Intracellular cleavage of newly synthesized low affinity Fcε receptor (FcεR2) provides a second pathway for the generation of the 28-kDa soluble FcεR2 fragment. J Immunol. 1989;142:1614–1620. [PubMed] [Google Scholar]
- 24.Gibb DR, El Shikh M, Kang DJ, Rowe WJ, El Sayed R, Cichy J, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J Exp Med. 207:623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weskamp G, Ford JW, Sturgill J, Martin S, Docherty AJ, Swendeman S, et al. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat Immunol. 2006;7:1293–1298. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- 26.Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- 27.Rosenwasser LJ, Meng J. Anti-CD23. Clin Rev Allergy Immunol. 2005;29:61–72. doi: 10.1385/CRIAI:29:1:061. [DOI] [PubMed] [Google Scholar]
- 28.Richens J, Fairclough L, Ghaemmaghami AM, Mahdavi J, Shakib F, Sewell HF. The detection of ADAM8 protein on cells of the human immune system and the demonstration of its expression on peripheral blood B cells, dendritic cells and monocyte subsets. Immunobiology. 2007;212:29–38. doi: 10.1016/j.imbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Umland SP, Garlisi CG, Shah H, Wan Y, Zou J, Devito KE, et al. Human ADAM33 messenger RNA expression profile and post-transcriptional regulation. Am J Respir Cell Mol Biol. 2003;29:571–582. doi: 10.1165/rcmb.2003-0028OC. [DOI] [PubMed] [Google Scholar]
- 30.Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol. 2007;119:863–871. doi: 10.1016/j.jaci.2006.12.665. [DOI] [PubMed] [Google Scholar]
- 31.Knolle MD, Owen CA. ADAM8: a new therapeutic target for asthma. Expert Opin Ther Targets. 2009;13:523–540. doi: 10.1517/14728220902889788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 33.Haitchi HM, Powell RM, Shaw TJ, Howarth PH, Wilson SJ, Wilson DI, et al. ADAM33 expression in asthmatic airways and human embryonic lungs. American journal of respiratory and critical care medicine. 2005;171:958–965. doi: 10.1164/rccm.200409-1251OC. [DOI] [PubMed] [Google Scholar]
- 34.Letellier M, Nakajima T, Pulido-Cejudo G, Hofstetter H, Delespesse G. Mechanism of formation of human IgE-binding factors (soluble CD23): III. Evidence for a receptor (FcεRII)-associated proteolytic activity. J Exp Med. 1990;172:693–700. doi: 10.1084/jem.172.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letellier M, Sarfati M, Delespesse G. Mechanisms of formation of IgE-binding factors (soluble CD23)--I. Fcε RII bearing B cells generate IgE-binding factors of different molecular weights. Mol Immunol. 1989;26:1105–1112. doi: 10.1016/0161-5890(89)90054-0. [DOI] [PubMed] [Google Scholar]
- 36.Sarfati M, Bron D, Lagneaux L, Fonteyn C, Frost H, Delespesse G. Elevation of IgE-binding factors in serum of patients with B cell-derived chronic lymphocytic leukemia. Blood. 1988;71:94–98. [PubMed] [Google Scholar]
- 37.Schulz O, Laing P, Sewell HF, Shakib F. Der p I, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) European journal of immunology. 1995;25:3191–3194. doi: 10.1002/eji.1830251131. [DOI] [PubMed] [Google Scholar]
- 38.Schulz O, Sutton BJ, Beavil RL, Shi J, Sewell HF, Gould HJ, et al. Cleavage of the low-affinity receptor for human IgE (CD23) by a mite cysteine protease: nature of the cleaved fragment in relation to the structure and function of CD23. European journal of immunology. 1997;27:584–588. doi: 10.1002/eji.1830270303. [DOI] [PubMed] [Google Scholar]
- 39.Mathews JA, Gibb DR, Chen BH, Scherle P, Conrad DH. CD23 Sheddase A disintegrin and metalloproteinase 10 (ADAM10) is also required for CD23 sorting into B cell-derived exosomes. J Biol Chem. 2010;285:37531–37541. doi: 10.1074/jbc.M110.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson L, Cady CT, Cambier JC. TLR4-mediated signaling induces MMP9-dependent cleavage of B cell surface CD23. J Immunol. 2009;183:2585–2592. doi: 10.4049/jimmunol.0803660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturgill JL, Mathews J, Scherle P, Conrad DH. Glutamate signaling through the kainate receptor enhances human immunoglobulin production. Journal of neuroimmunology. 2011;233:80–89. doi: 10.1016/j.jneuroim.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Current allergy and asthma reports. 2007;7:331–337. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 43.Kilmon MA, Shelburne AE, Chan-Li Y, Holmes KL, Conrad DH. CD23 trimers are preassociated on the cell surface even in the absence of its ligand, IgE. J Immunol. 2004;172:1065–1073. doi: 10.4049/jimmunol.172.2.1065. [DOI] [PubMed] [Google Scholar]
- 44.Kilmon MA, Ghirlando R, Strub MP, Beavil RL, Gould HJ, Conrad DH. Regulation of IgE production requires oligomerization of CD23. J Immunol. 2001;167:3139–3145. doi: 10.4049/jimmunol.167.6.3139. [DOI] [PubMed] [Google Scholar]
- 45.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol. 2003;112:563–570. doi: 10.1016/s0091-6749(03)01861-x. [DOI] [PubMed] [Google Scholar]
- 46.Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 47.Frigeri LG, Zuberi RI, Liu FT. εBP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (FcεRI) and activates mast cells. Biochemistry. 1993;32:7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- 48.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 50.Liu FT, Frigeri LG, Gritzmacher CA, Hsu DK, Robertson MW, Zuberi RI. Expression and function of an IgE-binding animal lectin (epsilon BP) in mast cells. Immunopharmacology. 1993;26:187–195. doi: 10.1016/0162-3109(93)90034-n. [DOI] [PubMed] [Google Scholar]
- 51.Robertson MW, Albrandt K, Keller D, Liu FT. Human IgE-binding protein: a soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry. 1990;29:8093–8100. doi: 10.1021/bi00487a015. [DOI] [PubMed] [Google Scholar]
- 52.Robertson MW, Liu FT. Heterogeneous IgE glycoforms characterized by differential recognition of an endogenous lectin (IgE-binding protein) J Immunol. 1991;147:3024–3030. [PubMed] [Google Scholar]
- 53.Truong MJ, Gruart V, Kusnierz JP, Papin JP, Loiseau S, Capron A, et al. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/εBP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med. 1993;177:243–248. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Truong MJ, Gruart V, Liu FT, Prin L, Capron A, Capron M. IgE-binding molecules (Mac-2/εBP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. European journal of immunology. 1993;23:3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- 55.Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–867. [PubMed] [Google Scholar]
- 56.Wollenberg A, de la Salle H, Hanau D, Liu FT, Bieber T. Human keratinocytes release the endogenous beta-galactoside-binding soluble lectin immunoglobulin E (IgE-binding protein) which binds to Langerhans cells where it modulates their binding capacity for IgE glycoforms. J Exp Med. 1993;178:777–785. doi: 10.1084/jem.178.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 58.Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 59.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 60.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 61.Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, et al. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol. 2007;179:7800–7807. doi: 10.4049/jimmunol.179.11.7800. [DOI] [PubMed] [Google Scholar]
- 62.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, et al. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 64.de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, et al. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 65.Ra C, Kuromitsu S, Hirose T, Yasuda S, Furuichi K, Okumura K. Soluble human high-affinity receptor for IgE abrogates the IgE-mediated allergic reaction. Int Immunol. 1993;5:47–54. doi: 10.1093/intimm/5.1.47. [DOI] [PubMed] [Google Scholar]
- 66.Vangelista L, Laffer S, Turek R, Gronlund H, Sperr WR, Valent P, et al. The immunoglobulin-like modules Cepsilon3 and alpha2 are the minimal units necessary for human IgE-FcepsilonRI interaction. J Clin Invest. 1999;103:1571–1578. doi: 10.1172/JCI6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopp MV. Omalizumab: Anti-IgE Therapy in Allergy. Current allergy and asthma reports. 2011 doi: 10.1007/s11882-010-0173-4. [DOI] [PubMed] [Google Scholar]
- 68.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998;101:243–251. doi: 10.1172/JCI511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiebiger E, Stingl G, Maurer D. Anti-IgE and anti-Fc epsilon RI autoantibodies in clinical allergy. Curr Opin Immunol. 1996;8:784–789. doi: 10.1016/s0952-7915(96)80005-7. [DOI] [PubMed] [Google Scholar]
- 70.Bonnefoy JY, Gauchat JF, Life P, Graber P, Aubry JP, Lecoanet-Henchoz S. Regulation of IgE synthesis by CD23/CD21 interaction. Int Arch Allergy Immunol. 1995;107:40–42. doi: 10.1159/000236924. [DOI] [PubMed] [Google Scholar]
- 71.Hibbert RG, Teriete P, Grundy GJ, Beavil RL, Reljic R, Holers VM, et al. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med. 2005;202:751–760. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beavil RL, Graber P, Aubonney N, Bonnefoy JY, Gould HJ. CD23/Fc epsilon RII and its soluble fragments can form oligomers on the cell surface and in solution. Immunology. 1995;84:202–206. [PMC free article] [PubMed] [Google Scholar]
- 73.McCloskey N, Hunt J, Beavil RL, Jutton MR, Grundy GJ, Girardi E, et al. Soluble CD23 monomers inhibit and oligomers stimulate IGE synthesis in human B cells. J Biol Chem. 2007;282:24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 74.Kagan JM, Champlin RE, Saxon A. B-cell dysfunction following human bone marrow transplantation: functional-phenotypic dissociation in the early posttransplant period. Blood. 1989;74:777–785. [PubMed] [Google Scholar]
- 75.Liu YJ, Mason DY, Johnson GD, Abbot S, Gregory CD, Hardie DL, et al. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991;21:1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- 76.Liu YJ, Cairns JA, Holder MJ, Abbot SD, Jansen KU, Bonnefoy JY, et al. Recombinant 25-kDa CD23 and interleukin 1 alpha promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur J Immunol. 1991;21:1107–1114. doi: 10.1002/eji.1830210504. [DOI] [PubMed] [Google Scholar]
- 77.Mossalayi MD, Merle-Beral H, Dalloul AH, Arock M, Michel A, Hofstetter H, et al. Inhibition of interleukin-3-dependent growth of CD34+ acute myelogenous leukemia cells by recombinant soluble CD23. Ann N Y Acad Sci. 1991;628:362–367. doi: 10.1111/j.1749-6632.1991.tb17269.x. [DOI] [PubMed] [Google Scholar]
- 78.Mossalayi MD, Arock M, Bertho JM, Blanc C, Dalloul AH, Hofstetter H, et al. Proliferation of early human myeloid precursors induced by interleukin-1 and recombinant soluble CD23. Blood. 1990;75:1924–1927. [PubMed] [Google Scholar]
- 79.Mossalayi MD, Lecron JC, Dalloul AH, Sarfati M, Bertho JM, Hofstetter H, et al. Soluble CD23 (Fc epsilon RII) and interleukin 1 synergistically induce early human thymocyte maturation. J Exp Med. 1990;171:959–964. doi: 10.1084/jem.171.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aubry JP, Dugas N, Lecoanet-Henchoz S, Ouaaz F, Zhao H, Delfraissy JF, et al. The 25-kDa soluble CD23 activates type III constitutive nitric oxide-synthase activity via CD11b and CD11c expressed by human monocytes. J Immunol. 1997;159:614–622. [PubMed] [Google Scholar]
- 81.Lecoanet-Henchoz S, Gauchat JF, Aubry JP, Graber P, Life P, Paul-Eugene N, et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995;3:119–125. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 82.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868–3877. [PubMed] [Google Scholar]
- 83.Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001;97:2932–2940. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- 84.Lecoanet-Henchoz S, Plater-Zyberk C, Graber P, Gretener D, Aubry JP, Conrad DH, et al. Mouse CD23 regulates monocyte activation through an interaction with the adhesion molecule CD11b/CD18. European journal of immunology. 1997;27:2290–2294. doi: 10.1002/eji.1830270924. [DOI] [PubMed] [Google Scholar]
- 85.Hermann P, Armant M, Brown E, Rubio M, Ishihara H, Ulrich D, et al. The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J Cell Biol. 1999;144:767–775. doi: 10.1083/jcb.144.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daniels BB, Askew SL, van de Venter M, Oosthuizen V. Production of biologically active recombinant human soluble CD23 and its effect on PBMCs isolated from hyper-IgE blood. Cellular immunology. 2005;234:146–153. doi: 10.1016/j.cellimm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Chen HY, Liu FT, Yang RY. Roles of galectin-3 in immune responses. Arch Immunol Ther Exp (Warsz) 2005;53:497–504. [PubMed] [Google Scholar]
- 88.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 89.Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 91.Hsu DK, Chernyavsky AI, Chen HY, Yu L, Grando SA, Liu FT. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J Invest Dermatol. 2009;129:573–583. doi: 10.1038/jid.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 93.Dehlink E, Baker AH, Yen E, Nurko S, Fiebiger E. Relationships between levels of serum IgE, cell-bound IgE, and IgE-receptors on peripheral blood cells in a pediatric population. PLoS ONE. 2010;5:e12204. doi: 10.1371/journal.pone.0012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Lorenzo G, Drago A, Pellitteri ME, Candore G, Colombo A, Potestio M, et al. Serum levels of soluble CD23 in patients with asthma or rhinitis monosensitive to Parietaria. Its relation to total serum IgE levels and eosinophil cationic protein during and out of the pollen season. Allergy Asthma Proc. 1999;20:119–125. doi: 10.2500/108854199778612590. [DOI] [PubMed] [Google Scholar]
- 95.Rogala B, Rymarczyk B. Soluble CD23 in allergic diseases. Arch Immunol Ther Exp (Warsz) 1999;47:251–255. [PubMed] [Google Scholar]
- 96.Takigawa M, Tamamori T, Horiguchi D, Sakamoto T, Yamada M, Yoshioka A, et al. Fc epsilon receptor II/CD23-positive lymphocytes in atopic dermatitis. I. The proportion of Fc epsilon RII+ lymphocytes correlates with the extent of skin lesion. Clinical and experimental immunology. 1991;84:275–282. [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaka A, Ohashi Y, Nakai Y. Decrease of serum levels of soluble CD23 during immunotherapy in patients with perennial allergic rhinitis. Ann Otol Rhinol Laryngol. 1999;108:193–200. doi: 10.1177/000348949910800216. [DOI] [PubMed] [Google Scholar]
- 98.Ott H, Wilke J, Baron JM, Hoger PH, Folster-Holst R. Soluble immune receptor serum levels are associated with age, but not with clinical phenotype or disease severity in childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 24:395–402. doi: 10.1111/j.1468-3083.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 99.Saka B, Aktan M, Sami U, Oner D, Sanem O, Dincol G. Prognostic importance of soluble CD23 in B-cell chronic lymphocytic leukemia. Clin Lab Haematol. 2006;28:30–35. doi: 10.1111/j.1365-2257.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 100.Sarfati M, Delespesse G. Possible role of human lymphocyte receptor for IgE (CD23) or its soluble fragments in the in vitro synthesis of human IgE. J Immunol. 1988;141:2195–2199. [PubMed] [Google Scholar]
- 101.Sarfati M, Chevret S, Chastang C, Biron G, Stryckmans P, Delespesse G, et al. Prognostic importance of serum soluble CD23 level in chronic lymphocytic leukemia. Blood. 1996;88:4259–4264. [PubMed] [Google Scholar]
- 102.Reinisch W, Willheim M, Hilgarth M, Gasche C, Mader R, Szepfalusi S, et al. Soluble CD23 reliably reflects disease activity in B-cell chronic lymphocytic leukemia. J Clin Oncol. 1994;12:2146–2152. doi: 10.1200/JCO.1994.12.10.2146. [DOI] [PubMed] [Google Scholar]
- 103.Schwarzmeier JD, Shehata M, Hilgarth M, Marschitz I, Louda N, Hubmann R, et al. The role of soluble CD23 in distinguishing stable and progressive forms of B-chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:549–554. doi: 10.1080/10428190210323. [DOI] [PubMed] [Google Scholar]
- 104.Del Principe MI, Del Poeta G, Buccisano F, Maurillo L, Venditti A, Zucchetto A, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood. 2006;108:853–861. doi: 10.1182/blood-2005-12-4986. [DOI] [PubMed] [Google Scholar]
- 105.Meuleman N, Stamatopoulos B, Dejeneffe M, El Housni H, Lagneaux L, Bron D. Doubling time of soluble CD23: a powerful prognostic factor for newly diagnosed and untreated stage A chronic lymphocytic leukemia patients. Leukemia. 2008;22:1882–1890. doi: 10.1038/leu.2008.190. [DOI] [PubMed] [Google Scholar]
- 106.Yawetz S, Cumberland WG, van der Meyden M, Martinez-Maza O. Elevated serum levels of soluble CD23 (sCD23) precede the appearance ofacquired immunodeficiency syndrome--associated non-Hodgkin’s lymphoma. Blood. 1995;85:1843–1849. [PubMed] [Google Scholar]
- 107.Schroeder JR, Saah AJ, Hoover DR, Margolick JB, Ambinder RF, Martinez-Maza O, et al. Serum soluble CD23 level correlates with subsequent development of AIDS-related non-Hodgkin’s lymphoma. Cancer Epidemiol Biomarkers Prev. 1999;8:979–984. [PubMed] [Google Scholar]
- 108.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-Cell Stimulatory Cytokines and Markers of Immune Activation Are Elevated Several Years Prior to the Diagnosis of Systemic AIDS-Associated Non-Hodgkin B-Cell Lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu SL, Pierre J, Smith-Norowitz TA, Hagler M, Bowne W, Pincus MR, et al. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clinical and experimental immunology. 2008;153:401–409. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bansal AS, Haeney MR, Cochrane S, Pumphrey RS, Green LM, Bhavnani M, et al. Serum soluble CD23 in patients with hypogammaglobulinaemia. Clinical and experimental immunology. 1994;97:239–241. doi: 10.1111/j.1365-2249.1994.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chomarat P, Briolay J, Banchereau J, Miossec P. Increased production of soluble CD23 in rheumatoid arthritis, and its regulation by interleukin-4. Arthritis Rheum. 1993;36:234–242. doi: 10.1002/art.1780360215. [DOI] [PubMed] [Google Scholar]
- 112.Huissoon AP, Emery P, Bacon PA, Gordon J, Salmon M. Increased expression of CD23 in rheumatoid synovitis. Scand J Rheumatol. 2000;29:154–159. doi: 10.1080/030097400750002012. [DOI] [PubMed] [Google Scholar]
- 113.Kogure T, Itoh T, Shimada Y, Shintani T, Ochiai H, Terasawa K. Detection of serum soluble markers of immune activation in rheumatoid arthritis. Mediators Inflamm. 1996;5:262–265. doi: 10.1155/S0962935196000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kutukculer N, Caglayan S. Plasma and synovial fluid soluble CD23 concentrations in children with juvenile chronic arthritis. Autoimmunity. 1998;27:155–158. doi: 10.3109/08916939809003863. [DOI] [PubMed] [Google Scholar]
- 115.Massa M, Pignatti P, Oliveri M, De Amici M, De Benedetti F, Martini A. Serum soluble CD23 levels and CD23 expression on peripheral blood mononuclear cells in juvenile chronic arthritis. Clin Exp Rheumatol. 1998;16:611–616. [PubMed] [Google Scholar]
- 116.Rezonzew R, Newkirk MM. Impaired release of sCD23 by activated B-cells from RA patients. Clin Immunol Immunopathol. 1994;71:156–163. doi: 10.1006/clin.1994.1066. [DOI] [PubMed] [Google Scholar]
- 117.Ribbens C, Bonnet V, Kaiser MJ, Andre B, Kaye O, Franchimont N, et al. Increased synovial fluid levels of soluble CD23 are associated with an erosive status in rheumatoid arthritis (RA) Clinical and experimental immunology. 2000;120:194–199. doi: 10.1046/j.1365-2249.2000.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh A, Vastert SJ, Prakken BJ, Illges H. Decreased levels of sCD21 and sCD23 in blood of patients with systemic-juvenile arthritis, polyarticular-juvenile arthritis, and pauciarticular-juvenile arthritis. Rheumatol Int. doi: 10.1007/s00296-011-1830-1. [DOI] [PubMed] [Google Scholar]
- 119.Zhou M, Wiemels JL, Bracci PM, Wrensch MR, McCoy LS, Rice T, et al. Circulating levels of the innate and humoral immune regulators CD14 and CD23 are associated with adult glioma. Cancer Res. 2011;70:7534–7542. doi: 10.1158/0008-5472.CAN-10-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wrensch M, Wiencke JK, Wiemels J, Miike R, Patoka J, Moghadassi M, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66:4531–4541. doi: 10.1158/0008-5472.CAN-05-4032. [DOI] [PubMed] [Google Scholar]
- 121.Merrill RM, Isakson RT, Beck RE. The association between allergies and cancer: what is currently known? Ann Allergy Asthma Immunol. 2007;99:102–116. doi: 10.1016/S1081-1206(10)60632-1. quiz 117–109, 150. [DOI] [PubMed] [Google Scholar]
- 122.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiu CG, Strugnell SS, Griffith OL, Jones SJ, Gown AM, Walker B, et al. Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol. 176:2067–2081. doi: 10.2353/ajpath.2010.090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saussez S, Glinoer D, Chantrain G, Pattou F, Carnaille B, Andre S, et al. Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid. 2008;18:705–712. doi: 10.1089/thy.2007.0361. [DOI] [PubMed] [Google Scholar]
- 126.Inohara H, Segawa T, Miyauchi A, Yoshii T, Nakahara S, Raz A, et al. Cytoplasmic and serum galectin-3 in diagnosis of thyroid malignancies. Biochem Biophys Res Commun. 2008;376:605–610. doi: 10.1016/j.bbrc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 127.Sethi K, Sarkar S, Das S, Mohanty B, Mandal M. Biomarkers for the diagnosis of thyroid cancer. J Exp Ther Oncol. 8:341–352. [PubMed] [Google Scholar]
- 128.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- 129.Vereecken P, Awada A, Suciu S, Castro G, Morandini R, Litynska A, et al. Evaluation of the prognostic significance of serum galectin-3 in American Joint Committee on Cancer stage III and stage IV melanoma patients. Melanoma Res. 2009;19:316–320. doi: 10.1097/CMR.0b013e32832ec001. [DOI] [PubMed] [Google Scholar]
- 130.Vereecken P, Zouaoui Boudjeltia K, Debray C, Awada A, Legssyer I, Sales F, et al. High serum galectin-3 in advanced melanoma: preliminary results. Clin Exp Dermatol. 2006;31:105–109. doi: 10.1111/j.1365-2230.2005.01992.x. [DOI] [PubMed] [Google Scholar]
- 131.Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, Vertongen F, et al. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008;44:86–93. doi: 10.1016/j.oraloncology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 132.Sakaki M, Oka N, Nakanishi R, Yamaguchi K, Fukumori T, Kanayama HO. Serum level of galectin-3 in human bladder cancer. J Med Invest. 2008;55:127–132. doi: 10.2152/jmi.55.127. [DOI] [PubMed] [Google Scholar]
- 133.Bansal A, Roberts T, Hay EM, Kay R, Pumphrey RS, Wilson PB. Soluble CD23 levels are elevated in the serum of patients with primary Sjogren’s syndrome and systemic lupus erythematosus. Clinical and experimental immunology. 1992;89:452–455. doi: 10.1111/j.1365-2249.1992.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bansal AS, Ollier W, Marsh MN, Pumphrey RS, Wilson PB. Variations in serum sCD23 in conditions with either enhanced humoral or cell-mediated immunity. Immunology. 1993;79:285–289. [PMC free article] [PubMed] [Google Scholar]
- 135.Itoh M, Uchimura K, Hayakawa N, Makino M, Hayashi R, Nagata M, et al. Surface expression and release of soluble forms of CD8 and CD23 in CD40- and IL-4-activated mononuclear cells from patients with Graves’ disease (GD) Clin Exp Immunol. 1998;113:309–314. doi: 10.1046/j.1365-2249.1998.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ohshima S, Kuchen S, Seemayer CA, Kyburz D, Hirt A, Klinzing S, et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003;48:2788–2795. doi: 10.1002/art.11287. [DOI] [PubMed] [Google Scholar]
- 137.Lee YJ, Kang SW, Song JK, Park JJ, Bae YD, Lee EY, et al. Serum galectin-3 and galectin-3 binding protein levels in Behcet’s disease and their association with disease activity. Clin Exp Rheumatol. 2007;25:S41–45. [PubMed] [Google Scholar]
- 138.Frol’ova L, Smetana K, Jr, Borovska D, Kitanovicova A, Klimesova K, Janatkova I, et al. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res. 2009;58:503–512. doi: 10.1007/s00011-009-0016-8. [DOI] [PubMed] [Google Scholar]
- 139.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, et al. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. doi: 10.1016/j.cca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 141.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]