Abstract
Although an attentional bias for threat has been implicated in obsessive-compulsive disorder (OCD), evidence supporting such a bias has been inconsistent. Furthermore, few studies have made distinctions between attentional capture vs. attentional disengagement and the extent to which different emotional content modulates attention in OCD also remains unclear. To address these issues, we examined patients with OCD (n = 30) and controls (n = 30) during an emotional attentional blink paradigm in which participants searched for a target embedded within a series of rapidly presented images. Critically, an erotic, fear, disgust, or neutral distracter image appeared 200 ms or 800 ms before the target. Impaired target detection was observed among OCD patients relative to controls following erotic distracters, but only when presented 800 ms, and not 200 ms, prior to the target, indicating difficulty with attentional disengagement. Difficulty disengaging from erotic images was significantly correlated with OCD symptoms in the full sample but not with symptoms of trait anxiety. These data delineate a specific information processing abnormality in OCD.
Keywords: OCD, Erotica, Attention, Disengagement
A growing body of research has implicated an attentional bias favoring threatening information in the development of various anxiety disorders (Bar-Haim et al 2007; Cisler & Koster, 2010). The modal finding in such research is increased allocation of attention to threatening stimuli, through biases in the orienting of attention (vigilance; Mogg & Bradley, 1998), or in the continued engagement of attention (maintenance; Weierich et al 2008). Although obsessive-compulsive disorder (OCD) appears to also be associated with an attentional bias favoring threatening information, as well as reduced levels of cognitive inhibition (Muller & Roberts, 2005), the experimental demonstration of such biases has been less consistent relative to other anxiety disorders (Moritz & Mühlenen, 2008; Summerfeldt & Endler, 1998). Indeed, the specific components of the attentional bias that occur in OCD remain unclear, as it has yet to be determined if attentional biases in OCD are comprised of facilitated attention to threat and/or difficulty disengagement from threat.
Neurobehavioral research has begun to illuminate the neural substrates of attentional biases in the anxiety disorders (Bishop, 2008; Cisler & Koster, 2010). This literature has suggested that amygdala activity may mediate facilitated attention to threat. However, higher-order cortical structures centered around prefrontal cortex (PFC) activity may account for difficulty disengaging from threat via individual differences in the ability to down-regulate the influence of sub-cortical fear structures (i.e., attentional control) and maintain attention on task-relevant stimuli (Eldar & Bar-Haim, 2010). OCD is characterized by dysfunction of fronto-striatal-thalamic circuitry and this dysfunction may account for difficulty with attentional disengagement in OCD (van den Heuvel et al., 2005).
Despite important advances in the neural bases of attention, a clear understanding of the inconsistency in demonstrating an attentional bias in OCD remains elusive. The inconsistency may be partially attributed due to the heterogeneous and idiosyncratic nature of OCD (Summerfeldt & Endler, 1998). Inconsistencies in demonstrating an attentional bias may also be partially due to use of stimuli that inadequately access core beliefs in OCD. Most attentional bias studies have focused on the manipulation of the emotional valence of the stimuli. However, emerging research suggests that the arousal value of a stimulus, and not necessarily its valence (negative versus positive versus neutral) is more important for modulating attention (Anderson, 2005; Most et al 2007). Erotic (i.e., sexually explicit) stimuli in particular, which are often associated with high arousal value but minimal valence, have been found to affect attention to a greater degree than stimuli with negative valence (Arnell et al 2007). Thus, erotic stimuli may be excellent stimuli to employ in attentional bias research among anxious populations to control for arousal levels when examining the effects of valenced stimuli on attention. Although the attentional capture by erotica has been found to be robust in past research (Ciesielski, Armstrong, Olatunji, & Zald, 2010), this attentional capture may be moderated by individual differences in attentional control (Derryberry & Reed, 2002). Attentional control consists of one’s ability to maintain attentional engagement in the face of distraction and one’s ability to execute attentional disengagement, in order to shift attention away from a distraction or towards a new task. These attentional processes may be limited among those high in OCD symptoms.
Cognitive behavioral models posit that OCD emerges as a function of inflated responsibility composed of the following beliefs: (1) the threat of a negative outcome, which may be either a manifest threat (e.g. a car accident) or a moral threat (e.g. “Having unacceptable thoughts means that I am a bad person”); (2) the prevention of a negative outcome as the primary goal; and (3) the belief in one’s personal power to prevent the negative outcome (e.g., Rachman, 1998; Salkovskis, 1999). Theoretical and empirical extensions of this model contend that appraisals of responsibility, which are characteristic of OCD, are mediated by fear of behaving badly and associated feelings of guilt (Mancini & Gangemi, 2004; Niler & Beck, 1989). Employing stimuli that more directly evoke fear of behaving badly and associated guilt feelings may reveal more disorder-unique attentional biases in OCD. Erotica are of interest in this regard, as they can evoke feelings of guilt because of their taboo nature, and the moral/religious concerns which they evoke. Indeed, such stimuli may be perceived as either rewarding or punishing depending upon one’s level of guilt (Griffitt & Kaiser, 1978). Prior research has also shown that erotica rated as unpleasant still evinced inhibited startle, which was interpreted as indicating pleasant affect to those same stimuli (Bradley et al 2001). Such findings may highlight the potential utility of erotic content in accessing the overactive conflict monitoring system that may be central to OCD (Pitman, 1987). Although exposure to erotic content, relative to other emotional stimuli, may more directly stimulate underlying processes implicated in OCD, no study to date has employed such stimuli in the examination of attentional biases in OCD.
A more precise understanding of the components underlying attentional biases in OCD may be informed by a more precise assessment of the specific affective content that may modulate attention as well as employment of tasks where components of attention can be clearly operationalized. The emotional attentional blink task (Most et al 2005), provides a robust paradigm for examining different components of attention. On each trial participants view a rapid serial visual presentation (RSVP) of stimuli and attempt to detect a rotated target image, which occurs either 200 ms (Lag 2) or 800 ms (Lag 8) after an emotional distractor allowing for a measurement of attentional capture (Lag 2) and disengagement recovery (Lag 8). It was predicted that OCD patients would show deficits at Lag 8 following emotional distractors. Prior research with non-clinical participants found impaired target detection at early lags, but not late lags, for erotic stimuli (Most et al 2007). Similar effects have been observed for sexual words in a word version of the task (Arnell et al 2007). Consistent with deficits in attentional disengagement and inhibition in OCD (Chamberlain et al 2005; Cisler & Olatunji, 2010), it predicted that OCD patients would be especially less accurate than controls when erotic emotional distractors appeared at Lag 8, whereas all subjects were expected to perform poorly when these images appeared at Lag 2.
Method
Participants
Participants consisted of 30 adults who met diagnostic criteria for OCD and 30 non-clinical controls (NCC) with no current diagnoses. The Structured Clinical Interview for the DSM–IV (SCID-IV; First et al 1994) was administered by a trained clinical psychologist to confirm diagnosis for all participants, with exclusionary criteria for the OCD groups including a diagnosis of substance abuse, attention deficit hyperactivity disorder, pervasive developmental disorders, mental retardation, or current or past neurological diseases. A Yale-Brown Obsessive Compulsive Scale (YBOCS; Goodman et al 1989a, 1989b) was also administered to those meeting diagnostic criteria for OCD to ensure that presenting symptoms were at least moderate in severity (minimum score of 16). Many OCD patients had additional current Axis I diagnoses (46%), including 21% with major depressive disorder and 18% with an anxiety disorder.
Procedure
All participants completed written informed consent approved by the Vanderbilt Institutional Review Board, Participants were then seated at a computer where they first completed several self-report questionnaires to assess OCD symptoms, anxiety and self-reported attentional ability, after which they completed the emotional attentional blink task.
Symptom Assessment
The Dimensional Obsessive-Compulsive Scale (DOCS; Abramowitz et al 2010) is a 20-item measure of the severity of obsessive-compulsive symptoms across four dimensions: (1) contamination, (2) responsibility for harm, injury, or bad luck, (3) unacceptable obsessional thoughts, and (4) symmetry, completeness, and exactness. The DOCS had excellent internal consistency in the present study (α = .96).
The State-Trait Anxiety Inventory - Trait (STAI-T; Spielberger et al 1983) is a 20-item measure of proneness towards experiencing anxiety and distress (trait anxiety). The STAI-T had good internal consistency in the present study (α = .94).
The Attentional Control Scale (ACS; Derryberry & Reed, 2002) is a 20-item measure of control of attention across two domains; focusing, the ability to maintain attention on a given task, and shifting, the ability to reallocate attention to a new task or to engage attention on multiple tasks. The ACS had adequate internal consistency (α = .86).
Rapid Serial Visual Presentation (RSVP) Task
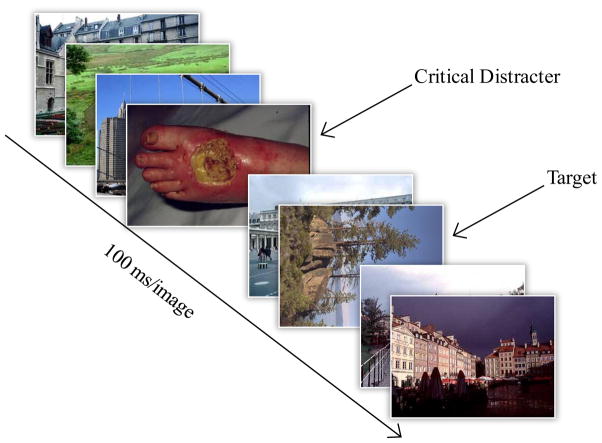
The visual stimuli were images consisting of 168 distractor images drawn from four categories of emotional images (42 disgusting, 42 erotic, 42 fear evoking, 42 neutral), 252 upright landscapes/architectural filler images (appearing before the distractor, between the distractor and the target, and after the target) and 80 target images consisting of landscape/architectural photos 40 rotated 90° degrees to the left and 40 rotated 90° to the right. One trial consisted of 17 images, including one distractor image and one target image that was rotated 90° to the left or right (see Figure 1). Each image was presented for 100ms. Each trial consisted of a disgust (contaminated or diseased items including roaches, feces, and maggot-ridden food products), fear (animals bearing teeth in a threatening manner, humans brandishing weapons, and explosions), erotic (nude male-female couples engaging in sexual scenarios), or neutral (scenic in style and including both animals and humans) distractor image that appeared 200ms (lag 2) or 800ms (lag 8) before the rotated image.
Figure 1.
The trial procedure for the emotional attentional-blink paradigm. Note that the distracter consisted of four distinct categories (disgust, erotic, fear, and neutral) presented at 200 and 800ms time lags.
Fear, disgust, and neutral pictures were partially drawn from the International Affective Picture System (IAPS; Lang et al 1999) and were supplemented with similar images found from publicly available sources.1 Erotic images were mainly obtained from publicly available sources and have been employed in previous research (Most et al 2007). Participants completed 6 blocks with 28 trials per block. Of the total 168 trials, each distractor type was presented 42 times with 2 trials per distractor type containing no target; the 2 lags were equally distributed for 40 trials with targets present per distractor type. The position of the distractors was equally distributed by emotion category and lag positions in the visual stream. Participants were instructed to indicate by key press if they saw a rotated image (yes, no; detection) and which direction it was rotated (right, left; accuracy). Participants received 16 practice trials to ensure mastery of the task with 4 of the trials containing no rotated target image, 6 trials with the target image rotated to the right, and 6 trials with the target mage rotated to the left. A response was considered accurate if the participant both said they saw the target, and correctly identified the direction of its rotation.
Data Analysis
Demographic and clinical symptoms were compared between patients with OCD and NCCs. A 2 (Group; OCD, NCC) × 2 (Lag; 2, 8) × 4 (Emotion; disgust, fear, erotic, neutral) mixed model Analysis of Variance (ANOVA) was then conducted on percent accuracy on the RSVP. Consistent with prior research that have computed a difference score between Lags in the RSVP to assess the magnitude of the attentional blink (i.e., Dux & Marois, 2008), a ‘disengagement efficiency score’ (DES) was computed by subtracting performance at Lag 2 from performance at Lag 8, with higher scores indicating greater disengagement efficiency. The DES was then subjected to a 2 (Group; OCD, NCC) × 4 (Emotion; disgust, fear, erotic, neutral) mixed model ANOVA. Lastly, Pearson correlation coefficients were computed to examine the association between the DES for each emotional distractor and various symptoms in the full sample.
Results
Participant Characteristics
As shown in Table 1, OCD participants and NCCs were well-matched on all demographic characteristics with no significant differences between the two groups. As expected, Table 2 shows that OCD participants reported significantly more severe symptoms of OCD, trait anxiety, depression, and difficulty with attentional control than NCCs (ps < .001). Examination of subscale scores on the DOCS suggests that the OCD sample is well characterized by heterogeneous symptoms. Scores on the four dimensions of contamination (M = 7.73, SD = 6.35), responsibility for harm, injury, or bad luck (M = 8.40, SD = 5.53), unacceptable obsessional thoughts (M = 9.83, SD = 4.72), and symmetry, completeness, and exactness (M = 5.96, SD = 5.24) generally did not significant differ from each other with the exception of symmetry, completeness, and exactness symptoms being significantly less endorsed than responsibility for harm, injury, or bad luck symptoms [t (29) = 2.17, p < .05] and unacceptable obsessional thoughts [t (29) = 4.47, p < .001].
Table 1.
Demographic information by diagnostic group
| OCD | NCC | |
|---|---|---|
| N | 30 | 30 |
| % female | 50 | 50 |
| Age | 39.23 (11.91) | 39.50 (10.29) |
| % Caucasian | 96.7 | 73.3 |
| % Income | ||
| <$39,999 | 63.3 | 66.7 |
| $40,000–$69,999 | 16.7 | 30.0 |
| >$70,000 | 20.0 | 3.3 |
| Marital Status | ||
| % Married | 30.0 | 30.0 |
| % Single | 56.7 | 50.0 |
| % Divorced | 13.3 | 20.0 |
| Highest Education Level | ||
| % High School | 43.3 | 26.7 |
| % College Degree | 43.3 | 46.6 |
| % Masters/Doctorate | 13.4 | 26.7 |
Note: OCD = Obsessive-compulsive disorder; NCC = Non-clinical control.
Table 2.
Means and standard deviation by group on symptom measures
| Symptom Measures | OCD M (SD) |
NCC M (SD) |
t | d |
|---|---|---|---|---|
| DOCS | 31.93 (15.95) | 5.40 (4.71) | 8.73 | 1.50 |
| STAI - T | 53.66 (10.41) | 35.50 (8.73) | 7.32 | 1.37 |
| ACS-Focus | 20.06 (5.35) | 26.00 (4.08) | 4.77 | 1.06 |
| ACS-Shift | 27.26 (5.22) | 32.51 (4.22) | 4.23 | 0.97 |
Note: all t-values significant at p < .001. OCD = Obsessive Compulsive Disorder; NCC = Non-clinical control; DOCS = Dimensional Obsessive-Compulsive Scale; STAI–T = State Trait Anxiety Inventory – Trait Subscale; ACS = Attention Control Scale. Cohen’s d was calculated as the difference between the mean scores in each group divided by the pooled standard deviation.
RSVP Task Accuracy
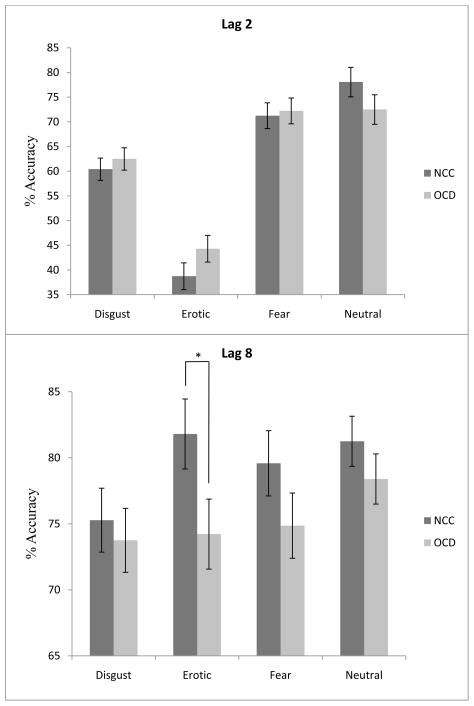
A 2 (Group) × 2 (Lag) × 4 (Emotion) mixed model ANOVA on percent accuracy2 revealed a significant main effect of Lag [F (1, 58) = 209.78, p < .001, partial η2 = .78], reflecting higher accuracy at Lag 8 than Lag 2, and Emotion [F (3, 174) = 78.68, p < .001, partial η2 = .58], reflecting differential performance across stimulus categories. These main effects were qualified by a significant Group × Lag [F (1, 58) = 5.75, p < .03, partial η2 = .09] and Lag × Emotion [F (3, 174) = 74.69, p < .001, partial η2 = .56] interaction. The predicted Group × Lag × Emotion interaction was also significant [F (3, 174) = 3.59, p < .02, partial η2 = .06].3 To examine this 3-way interaction, a 2 (Group) × 2 (Lag) mixed model ANOVA was conducted for percent accuracy for each emotion. This analysis revealed a significant main effect of Lag for disgust [F (1, 58) = 44.15, p < .001, partial η2 = .43], fear [F (1, 58) = 12.70, p < .01, partial η2 = .18], erotic [F (1, 58) = 329.58, p < .001, partial η2 = .85], and neutral [F (1, 58) = 7.21, p < .01, partial η2 = .11] distractors. However, the Group × Lag interaction was significant for target accuracy only when erotic distractors were present [F (1, 58) = 10.68, p < .01, partial η2 = .16]. As depicted in Figure 2, examination of this interaction revealed no significant group differences in percent accuracy in identifying the target when erotic images were distractors at Lag 2 [t (58) = 1.45, p = .15]. However, percent accuracy at Lag 8 was significantly lower for OCD patients relative to NCCs when erotic images were distractors [t (58) = 2.02, p < .05]. The Group × Lag interaction when fear distractors were present did approach significance [F (1, 58) = 3.41, p = .07, partial η2 = .06]. However, there were no significant group differences in percent accuracy in identifying the target when fear images were distractors at Lag 2 [t (58) = −.263, p = .79] and Lag 8 [t (58) = 1.35, p = .18]. Means and standard deviations of percent accuracy on the RSVP by emotion, lag, and group are presented in Table 3.
Figure 2.
Percent accuracy by group and emotion at lag 2 (left panel) and lag 8 (right panel) on the rapid serial visual presentation task. Bars represent standard error. *p < .05
Table 3.
Means and standard deviations of percent accuracy by group, emotion, and lag on the rapid serial visual presentation task
| Group | OCD | NCC | ||||||
|---|---|---|---|---|---|---|---|---|
| Emotion | Disgust M (SD) |
Erotic M (SD) |
Fear M (SD) |
Neutral M (SD) |
Disgust M (SD) |
Erotic M (SD) |
Fear M (SD) |
Neutral M (SD) |
| Lag 2 | 62.49 (12.14) | 44.31 (16.36) | 72.22 (16.28) | 72.50 (18.72) | 60.42 (12.61) | 38.75 (13.05) | 71.25 (12.05) | 78.05 (13.58) |
| 8 | 73.75 (14.81) | 74.22 (17.02) | 74.86 (15.42) | 78.40 (12.24) | 75.28 (11.52) | 81.80 (11.50) | 79.58 (11.34) | 81.25 (8.17) |
Note: OCD = Obsessive-compulsive disorder; NCC = Non-clinical control.
Disengagement Efficiency
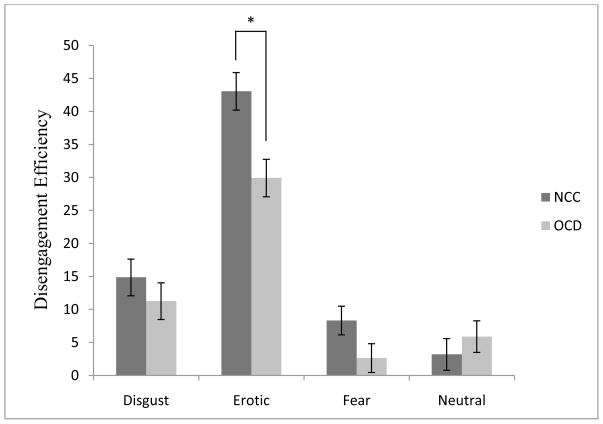
A 2 (Group) × 4 (Emotion) mixed model ANOVA on the DES revealed a significant main effect of Group [F (1, 58) = 5.75, p < .03, partial η2 = .09] and Emotion [F (3, 174) = 74.69, p < .001, partial η2 = .56]. These main effects were also qualified by a significant Group × Emotion interaction [F (3, 174) = 3.59, p < .02, partial η2 = .06]. Figure 3 shows that examination of this interaction revealed no significant group differences on the DES for fear [t (58) = 1.84, p = .07], disgust [t (58) = 0.91, p = 36], or neutral [t (58) = −0.79, p = .42]. However, the DES for erotica was significantly larger for NCCs compared to OCD patients [t (58) = 3.26, p < .01].
Figure 3.
Disengagement efficiency score (Lag 8 – Lag 2) by emotion and group. Bars represent standard error. *p < .01
Symptom Correlates
After a Bonferroni correction for multiple correlations (p < .003), Table 4 shows that only the OCD symptoms as assessed by the DOCS correlated inversely with the DES when erotic images served as the distractor (r = −.43, p < .001), indicating that subjects with greater OCD symptoms showed a weaken ability to disengage their attention.
Table 4.
Pearson correlation coefficients for symptom measures and disengagement efficiency scores for each emotional distracter for the full sample
| Disengagement Efficiency Score | ||||
|---|---|---|---|---|
| Symptom Measures | Disgust | Erotic | Fear | Neutral |
| DOCS | −.04 | −.43* | −.28 | .09 |
| STAI - T | −.06 | −.25 | −.15 | .04 |
| ACS-Focus | .11 | .28 | .02 | .00 |
| ACS-Shift | .21 | .06 | .01 | −.11 |
| M (SD) | 13.06 (15.20) | 36.48 (16.79) | 5.48 (12.16) | 4.54 (13.06) |
Note:
p < .001.
DOCS = Dimensional Obsessive-Compulsive Scale; STAI–T = State Trait Anxiety Inventory – Trait Subscale; ACS = Attention Control Scale.
Discussion
This investigation examined the extent to which erotic stimuli modulates attention in OCD on a RSVP task. The present findings showed that performance on the RSVP task varied significantly between patients with OCD and NCC as a function of lag. That is, patients with OCD and NCCs did not significantly differ in target detection accuracy when Lag 2 distractors were present. However, target detection accuracy was significantly lower for OCD patients compared to NCCs when Lag 8 distractors were present. This finding helps delineate the components of attention contributing to the attentional bias in OCD. Different components of attention and associated mechanisms are thought to distinctly reflect automatic vs. strategic stages of information processing (Shiffrin & Schneider, 1977). Automatic processing typically reflects processing that is capacity free and occurs without intent, control, or awareness, whereas strategic processing generally refers to processing that is intentional, controllable, capacity-limited, and dependent on awareness. Lag 2 deficits appear to heavily reflect bottom-up (i.e., stimulus driven) automatic processes. In contrast, as one moves further out in time from the attention capturing stimulus, strategic processes allowing a refocusing of attention become more prominent (Dux & Marois, 2009). As such, the disengagement difficulty observed in OCD patients, indexed by poorer target detection accuracy when distractors occurred at Lag 8 compared to Lag 2 suggests that attentional deficits in OCD may occur at the strategic, rather than automatic, stage of information processing.
The present findings also show that the emotional content of the distractor modulates group differences in target detection accuracy at Lag 8. Specifically, NCCs attention is impaired by erotica (as indicated by Lag 2 effects) but they appear to recover (no Lag 8 effect). In contrast, patients with OCD appear to be impaired at Lag 2 and Lag 8, suggesting that what is impaired is “disengagement recovery time”, which may be necessary to recoup attentional control. The specificity of the effects to erotica suggests that traditionally ‘threatening’ stimuli may not always access deficits that define OCD. Indeed, the overall effects in the present study appear to be driven by difficulty disengaging from erotica, while threat and disgust disengagement did not differ between groups (albeit there was a modest association between fear disengagement and OCD symptoms).
While the findings indicate that difficulty disengaging from erotic stimuli differentiates OCD patients from NCCs, Figure 2 also shows higher target detection accuracy after erotic images at Lag 8 among NCCs rather than a deficit in target processing among OCD patients per se. This view raises the interesting possibility that NCCs are able to employ a compensatory mechanism in response to a strong attention grabbing arousing stimulus, which enhances information processing in the period after an attentional blink. This compensatory mechanism may indeed be the adaptive default mode. In contrast, OCD patients may be unable to employ such a strategic compensatory mechanism. This can be construed as a deficit in a ‘top-down’ regulatory ability (Posner & Rothbart, 2000) that inhibits the ‘bottom-up’ influence of emotional distracters (Eysenck et al 2007). Difficulty in one’s ability to apply this regulation may account for the likelihood that erotic content will intrude into consciousness and interfere with target detection accuracy.
The present study also found that symptoms of OCD, but not general trait anxiety, correlated with difficulty disengaging from erotic images. This finding suggests that difficulty disengaging from erotic images is uniquely linked to OCD symptoms. However, alternative explanations for the specific effects of erotic images on attention in OCD do warrant consideration. For example, erotica may come to serve different functions for OCD patients and NCCs subsequent to cognitive elaboration. Specifically, erotic images may function more as a ‘reward’ in NCCs, and more as a ‘punisher’ for patients with OCD. A negative semantic shift in mental representations of erotic images among patients with OCD relative to NCCs and difficulty disengaging from such images may reflect underlying beliefs of inflated responsibility (e.g. “Enjoying erotic images means that I am a bad person”) and subsequent feelings of guilt that have been proposed to give rise to OCD (e.g., Mancini & Gangemi, 2004; Salkovskis, 1999). As such, differences in meanings may dramatically alter the effects of such stimuli in OCD.
This is the first investigation, to our knowledge, demonstrating that erotic content differentiates attention disengagement difficulty in OCD relative to controls. However, inferences based on these findings must be considered within the context of the study limitations. For example, psychological processes (inflated responsibility, guilt) that characterized OCD which are hypothesized to explain poorer target detection among OCD patients compared to NCCs when Lag 8 distractors consist of erotic images were not assessed. This presents an opportunity for future research to delineate if psychological processes that are transdiagnostic across various OCD subtypes account for emotion modulation of attention among patients with OCD. The amount of time spent viewing erotica prior to arrival at the lab was also not assessed. Given that the amount of guilt or perception of taboo regarding erotic images is highly variable and value laden, individual differences along these lines may be a moderator for future consideration. Although both men and women rate erotic images as appealing, men tend to do so to a greater degree and show greater levels of physiological reactivity to them (Most et al., 2007). Accordingly, future research with ample sample size may also consider gender as a potential moderator of these attentional effects. Although the association between OCD symptoms and difficulty disengaging from erotica was not accounted for by trait anxiety or depression, inclusion of a psychiatric control group (that does not overlap with OCD in symptom phenomenology) in future research may further clarify the extent to which these findings are unique to OCD (and associated beliefs of inflated responsibility). Research along these lines may further elucidate causal mechanisms that are specific to OCD that can be directly targeted during treatment.
Research Highlights.
Impaired target detection was observed among OCD patients, relative to controls, following only erotic distracters.
The target interference in OCD patients, relative to controls, occurred when erotic distracters were presented at 800 ms but not 200 ms.
The pattern of attention findings indicate a difficulty with attentional disengagement in OCD.
Abbreviations
- OCD
obsessive-compulsive disorder
- PFC
prefrontal cortex
- RSVP
rapid serial visual presentation
- SCID-IV
Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- YBOCS
Yale-Brown Obsessive Compulsive Scale
- DOCS
Dimensional Obsessive-Compulsive Scale
- STAI-T
State-Trait Anxiety Inventory – Trait
- ACS
Attentional Control Scale
- IAPS
International Affective Picture System
- NCC
non-clinical controls
- ANOVA
Analysis of Variance
- DES
disengagement efficiency score’
Footnotes
An independent sample of participants (n = 23; 65.2% female; 65.2% Caucasian, mean age = 20.35, SD = 2.57) rated each Disgust (valence = −24.69, SD = 7.29; arousal = 46.26, SD = 14.65), Erotic (valence = 4.45, SD = 15.59; arousal = 41.77, SD = 20.42), Fear (valence = −15.83, SD = 7.17; arousal = 31.98, SD =10.36), and Neutral (valence = 4.87, SD = 3.66; arousal = 6.18, SD = 5.05) image for valence (−50 = extremely negative, +50 = extremely positive, 0 = being no positive or negative valence/neutral) and arousal (0 = none to 100 = extremely/most imaginable). A significant difference for valence ratings between disgust images and all other categories was found such that disgust images were rated the most negative (ps < .001). Fear images were rated as significantly more negative than erotic and neutral images (ps < .001). However, the valence of erotic and neutral images did not significantly differ from each other (p > .90). Neutral images were rated significantly less arousing than all other images (ps < .001). Fear images were significantly less arousing than disgust images (p < .001), but not erotic images (p > .05). Lastly, arousal ratings for disgust and erotic images did not significantly differ from each other (p > .05).
Analyses for accuracy, rather than detection, are presented as they reflect more precise performance on the RSVP. Furthermore, the pattern of findings did not differ when detection is employed as the dependent variable.
The mixed model ANOVA on percent accuracy was also conducted without the erotic trials, The predicted Group × Lag × Emotion interaction was no longer significant [F (2, 116) = 1.67, p = .19] suggesting that the observed group differences are accounted for by the erotic trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Deacon B, Olatunji B, Wheaton MG, Berman N, Losardo D, Timpano K, McGrath P, Riemann B, Adams T, Bjorgvinsson T, Storch EA, Hale L. Assessment of obsessive-compulsive symptom dimensions: Development and evaluation of the Dimensional Obsessive-Compulsive Scale. Psychological Assessment. 2010;22:180–198. doi: 10.1037/a0018260. [DOI] [PubMed] [Google Scholar]
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop paradigm: A generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: target misses follow attention capture by arousing distractors in the RSVP. Emotion. 2007;7:465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Ciesielski BG, Armstrong T, Olatunji BO, Zald DH. Content and temporal characteristics of emotion modulation of attention. PLoS ONE. 2010;5:e13860. doi: 10.1371/journal.pone.0013860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional bias towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO. Components of attentional biases in contamination fear: Evidence for difficulty in disengagement. Behaviour Research and Therapy. 2010;48:74–78. doi: 10.1016/j.brat.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: A review of data and theory. Attention, Perception and Psychophysics. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Marois R. Distractor inhibition predicts individual differences in the attentional blink. PLoS ONE. 2008;3:e3330. doi: 10.1371/journal.pone.0003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40:667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders. Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale: validity. Archives of General Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale: development, use, and reliability. Archives of General Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Griffitt W, Kaiser DL. Affect, sex guilt, gender, and the rewarding-punishing effects of erotic stimuli. Journal of Personality and Social Psychology. 1978;36:850–858. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1999. [Google Scholar]
- Mancini F, Gangemi A. Fear of guilt of behaving irresponsibly in obsessive– compulsive disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2004;35:109–120. doi: 10.1016/j.jbtep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Moritz S, Mühlenen A. Investigation of an attentional bias for fear-related material in obsessive-compulsive checkers. Depression and Anxiety. 2008;25:225–229. doi: 10.1002/da.20294. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin and Review. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, Zald DH. The naked truth: Positive, arousing distracters impair rapid target perception. Cognition and Emotion. 2007;21:964–981. [Google Scholar]
- Muller J, Roberts J. Memory and attention in obsessive-compulsive disorder: A review. Journal of Anxiety Disorders. 2005;19:1–28. doi: 10.1016/j.janxdis.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Niler ER, Beck SJ. The relationship among guilt, dysphoria, anxiety and obsessions in a normal population. Behaviour Research and Therapy. 1989;27:213–220. doi: 10.1016/0005-7967(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive–compulsive psychopathology. Comprehensive Psychiatry. 1987;28:334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Posner MR, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Rachman S. A cognitive theory of obsessions: Elaborations. Behaviour Research and Therapy. 1998;36:385–401. doi: 10.1016/s0005-7967(97)10041-9. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM. Understanding and treating obsessive-compulsive disorder. Behaviour Research and Therapy. 1999;37:S29–S52. [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Summerfeldt LJ, Endler NS. Examining the evidence for anxiety related cognitive biases in obsessive–compulsive disorder. Journal of Anxiety Disorders. 1998;12:579–598. doi: 10.1016/s0887-6185(98)00035-8. [DOI] [PubMed] [Google Scholar]
- Tata PR, Leibowitz JA, Prunty MJ, Cameron M, Pickering AD. Attentional bias in obsessional compulsive disorder. Depression and Anxiety. 1996;34:53–60. doi: 10.1016/0005-7967(95)00041-u. [DOI] [PubMed] [Google Scholar]
- van den Heuvel A, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC. Disorder-specific neuroanatomical correlates of attentional bias in obsessive–compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- Verwoerd JRL, Wessel I, de Jong PJ, Nieuwenhuis MMW. Preferential processing of visual trauma-film reminders predicts subsequent intrusive memories. Cognition and Emotion. 2009;23:1537–1551. [Google Scholar]
- Weierich MR, Treat TA, Hollingworth A. Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion. 2008;22:985–1018. [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]