Abstract
Objective
To develop an Radiation Therapy Oncology Group (RTOG) atlas delineating gross tumor volume (GTV), and clinical target volume (CTV) to be used for preoperative radiotherapy of primary extremity soft tissue sarcoma (STS).
Methods
A consensus meeting was held during the RTOG meeting in January 2010 to reach agreement about GTV and CTV delineation on CT images for preoperative radiotherapy of high-grade large extremity STS. Data were presented to address the local extension of STS. Extensive discussion ensued to develop optimal criteria for GTV and CTV delineation on CT images.
Results
A consensus was reached on appropriate CT-based GTV and CTV. GTV is gross tumor defined by T1 contrast-enhanced MRI images. Fusion of MRI and CT is recommended to delineate the GTV. CTV for high-grade large STS typically includes GTV plus 3 cm margins in the longitudinal directions. If this causes the field to extend beyond the compartment, the field can be shortened to include the end of a compartment. The radial margin from the lesion should be 1.5 cm including any portion of the tumor not confined by an intact fascial barrier, bone or skin surface.
Conclusion
The consensus on GTV and CTV for preoperative radiotherapy of high-grade large extremity STS is available as web-based images as well as descriptive format through the RTOG. This is expected to improve target volume consistency and allow for rigorous evaluation of the benefits and risks of such treatment.
Keywords: Sarcoma, target definition, radiotherapy
Introduction
Highly conformal image-guided radiation treatment (IGRT) is being used with increasing frequency in the clinic to minimize radiation to normal tissue by reducing planning target volume expansion while maintaining or improving radiation dose delivery to the tumor target(s). To achieve these potential advantages of dose conformality to tumor and decreased volume of normal tissue irradiated, accurate delineations and atlas contours of gross target volume (GTV) and clinical target volume (CTV) are critical (1-5). This may potentially result in improvement of clinical outcomes, reduction of side effects and improvement of patients’ quality of life (6-8). This hypothesis is being tested in the phase II Radiation Therapy Oncology Group (RTOG) trial 0630 for preoperative image-guided radiation treatment (IGRT) of primary soft tissue sarcoma of extremity. A total protocol dose of 50 Gy in 25 fractions is delivered to 95% or more of the primary CTV planning target volume using daily IGRT. The primary endpoint is to estimate the effect of reduced radiation volume through IGRT on late radiation morbidity (≥ Grade 2 lymphedema, subcutaneous fibrosis, or joint stiffness) at 2 years (window period of 21–27 months) from the start of radiation treatment.
We recently completed a comparative study of GTV and CTV in the treatment of extremity sarcoma by a group of sarcoma radiation oncologists (9). In this study, contours of GTV and CTV of extremity soft tissue sarcoma were reasonably consistent among radiation oncologists experienced in the treatment of extremity sarcoma, although, slightly larger variations in CTV contours were seen than in GTV contours and in upper extremity compared to lower extremity target volumes (9). Here, radiation oncologists participating in RTOG sarcoma trials have developed consensus definitions for contouring GTV and CTV for large high grade STS.
Materials and Methods
Given the established variability in GTV and CTV for preoperative radiotherapy of large, high-grade extremity sarcoma by the RTOG Sarcoma Radiation Oncologists (9), a consensus meeting was held during the RTOG meeting in January 2010. All physicians/institutions involved in the original contouring project were invited to attend. Seven physicians, who contributed contours to the original study, were actually present for this consensus conference. One of the investigators (D.W.) presented two extremity sarcoma cases including clinical history, details of the pathology (histology and grade), diagnostic MR images, the nature of disease extension, and data about agreement and variations of GTV and CTV contoured by all ten investigators experienced in sarcoma radiotherapy. The lower extremity case with fused images of simulation CT and diagnostic MR was then reviewed. Using candidate consensus contours computed with the Simultaneous Truth And Performance Level Estimation (STAPLES) algorithm (10) as the starting point, consensus contours were determined. The algorithm uses an estimation-maximization approach to find probabilistic estimate of the true segmentation from a collection of image segmentations. It estimates the conditional probability of each pixel belonging to the structure (CTV, GTV), given the collection of contours drawn by the physicians, generating 95% and 100% contours representing the set of pixels belonging to each target volume with that (conditional) probability.
After the completion of this consensus conference, we reviewed and approved the recommended target volume definitions and representative images of GTV and CTV in the Sarcoma Working Group meeting of RTOG in June 2010.
Results
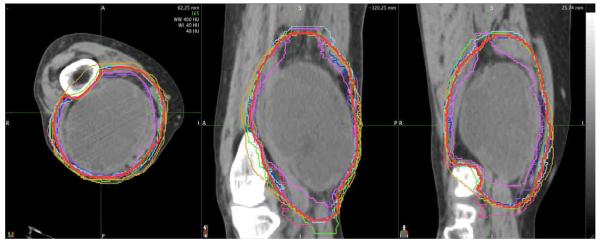
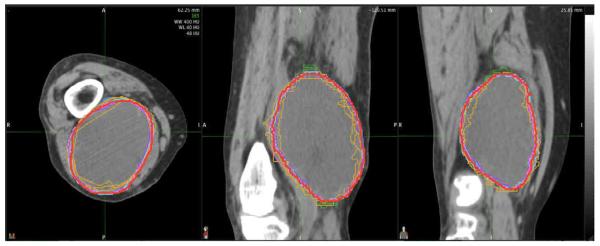
Clinical synopses of the lower extremity case were discussed (Table 1). We reviewed the STAPLES contours of GTV and CTV at both 100% and 95% confidence levels in the computed tomography (CT) images slice by slice. Consensus was obtained from the physicians present after extensive discussions over the contoured CT images. Anatomical description of the GTV and CTV were created along with caveats for specific clinical scenarios. Figure 1 and Figure 2 show representative GTV contours and representative CTV contours drawn on the CT consensus scan. A written description was developed as follows:
Table 1.
Clinical synopses
| Case 1 with soft tissue sarcoma of lower extremity |
|---|
| 55-year-old male with 16 cm mass in the posterior aspect of right distal thigh. The core needle biopsy of this lesion demonstrated a high-grade round cell liposarcoma. The clinical stage (AJCC) is III T2bN0M0G3 |
| Description of the diagnostic MRI of right distal thigh: A large well circumscribed heterogeneous, multiloculated mass located within the posterior distal thigh. The tumor measures 14.8 cm in craniocaudad dimension, 7.8 cm in AP dimension, and 11.3 cm in maximal medial-lateral dimension. There is a small loculated component located at its midaspect, anterolaterally, which measures 2.4 × 1.2 cm. There is irregular and discontinuous enhancement lining this mass which appears slightly less intense and less nodular when compared to the prior study. There are multiple thin enhancing septae traversing this mass as well. Overall the enhancing component of this mass is estimated to represent less than 10% of the mass volume. The anterior margin of this mass abuts the posterior margin of the popliteus artery for several centimeters along its course. This mass abuts the posterior tibial and common peroneal nerves which are deviated laterally. This mass has a large area of contact with the semimembranosis muscle and focally abuts the semi tendinosis muscle. There is a distinct fat plane between this mass and the biceps femoris muscles. |
| There is mild increased signal within the inferior aspect of the vastus medialis muscle and a tiny amount of fluid along its medial margin. These findings have not significantly changed from prior study and are nonspecific. Incidental note is made of degenerative disease involving the patellofemoral joint. |
| The patient underwent a simulation CT scan in a supine position with right thigh (affected thigh) straight and left thigh (unaffected thigh) in frog leg position, and then MR of right thigh in the same position as for the simulation CT scan. |
Figure 1.
Example of individual and consensus (red) contours of GTV on axial computed tomography (CT) for patient with large high-grade sarcoma of right distal thigh.
Figure 2.
Example of individual and consensus (red) contours of CTV on axial computed tomography (CT) for patient with large high-grade sarcoma of right distal thigh.
Definition of Gross Target Volume (GTV) for preoperative radiotherapy: Gross tumor defined by MRI T1 plus contrast images (MRI with contrast is required). Fusion of MRI and CT is recommended to delineate the GTV for radiotherapy planning. Intravenous contrast is recommended, particularly for upper extremity lesions, where there is a greater rotational mobility and positioning fidelity between the diagnostic MRI and the planning CT may be more difficult to achieve
Definition of Clinical Target Volume (CTV) for intermediate-to-high grade sarcoma ≥ 5 cm: Include gross tumor and clinical microscopic margins. Typically CTV = GTV plus 3 cm margins in the longitudinal (proximal and distal) directions. If this causes the field to extend beyond the compartment, the field can be shortened to include the end of a compartment. The radial margin from the lesion should be 1.5 cm including any portion of the tumor not confined by an intact fascial barrier, bone or skin surface. The suspicious edema defined on MRI T2 images is often included within the above margins. However, clinical judgment is required to make sure if the above margins need to be extended to cover the T2 edema defined on MRI T2 images. For example, the extensive T2 edema may be excluded if clinical judgment suggests that the risk of the edema harboring sarcoma many cm beyond the GTV is low or if extending the radiation field to include all of the edema would cause excessive toxicity.
Discussion and Conclusion
There is a growing body of evidence supporting the use of preoperative radiation therapy for patients with soft tissue sarcoma of extremity (6-8, 11-14). In many earlier clinical trials and retrospective studies of soft tissue sarcomas, 2D radiation treatment techniques were used to administer the radiation therapy. Advanced image-guided dosing and delivery technology such as image-guided intensity modulated radiation treatment may conceivably allow a reduction in the high-dose irradiated volume of adjacent normal tissue structures including soft tissue, joints, bone and internal organs in the preoperative setting. This may significantly reduce the radiation-related toxicities. Currently, the Radiation Therapy Oncology Group (RTOG), supported by the National Cancer Institute (NCI), is conducting a prospective clinical trial to investigate the impact of preoperative, advanced image-guided radiation technology (IGRT) on radiation-related toxicities in patients with extremity soft tissue sarcoma. The results of this study should provide important information on the use of IGRT for extremity soft tissue sarcomas. Histopathology has demonstrated that suspicious edema adjacent to the grossly evident sarcoma in the T2 images of MR can harbor microscopic tumor cells (15). Therefore, we agreed that the clinical target volume should cover the suspicious tumor-associate edema for sarcoma radiotherapy, except when there is generalized edema of the extremity from tumor compressing lymphatics. After reviewing the diagnostic MR images and the consensus contours of the representative cases, we believe that it would be very reasonable to define the CTV as GTV plus 3 cm margins in the longitudinal (proximal and distal) directions and 1.5 cm radial margins.
It is important to note that the above target definitions might not be suitable for sarcomas of specific histologies including rhabdomyosarcoma, extraosseous primitive neuroectodermal tumor (PNET) or soft tissue Ewing’s sarcoma, Kaposi’s sarcoma, angiosarcoma, aggressive fibromatosis (desmoid tumor), dermatofibrosarcoma protuberans or subcutaneous myxofibrosarcoma. Indeed it may be challenging to identify the extent of disease of any subcutaneous sarcoma with or without cutaneous involvement using MRI. For these sarcoma histologies and anatomical sites, additional studies are required to determine the optimal target definitions for preoperative radiotherapy.
In conclusion, this report reflects the consensus among RTOG sarcoma radiation oncologists to define GTV and CTV for preoperative radiotherapy for large, deep sarcomas. We believe these definitions of GTV and CTV for preoperative radiotherapy of extremity soft tissue sarcoma will be useful for prospective RTOG trials. In addition, the RTOG will make available the contouring atlas as a web-based set of images. If these target definitions are adopted widely, they may improve target volume consistency between centers. Moreover, in future prospective studies they may allow for more rigorous evaluation of the benefits and risks of sarcoma radiotherapy, as well as incorporation of new treatment techniques and strategies.
Acknowledgments
This study is supported by RTOG grant U10CA21661 and ATC Grant U24 CA81647 from the NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: This study was presented at the 2010 American Society Therapeutic Radiation Oncology (ASTRO) meeting in San Diego, CA and at the 2010 Connective Tissue Oncology Society (CTOS) Meeting, Paris, France.
Conflict of Interest Notification: None
Reference
- 1.Mackie TR, Kapatoes J, Ruchala K, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:89–105. doi: 10.1016/s0360-3016(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 2.Yan D, Lockman D, Martinez A, et al. Computed tomography guided management of interfractional patient variation. Semin Radiat Oncol. 2005;15:168–79. doi: 10.1016/j.semradonc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9:108–17. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 4.Mohan R, Zhang X, Wang H, et al. Use of deformed intensity distributions for on-line modification of image-guided IMRT to account for interfractional anatomic changes. Int J Radiat Oncol Biol Phys. 2005;61:1258–66. doi: 10.1016/j.ijrobp.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Jaffray DA. Emergent technologies for 3-dimensional image-guided radiation delivery. Semin Radiat Oncol. 2005;15:208–16. doi: 10.1016/j.semradonc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan B, Ward I, Haycocks T, et al. Techniques to modulate radiotherapy toxicity and outcome in soft tissue sarcoma. Curr Treat Options Oncol. 2003;4:453–464. doi: 10.1007/s11864-003-0046-3. [DOI] [PubMed] [Google Scholar]
- 7.Davis AM, O’Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 8.Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Bosch W, Kirsch D, Lozi R, Naqa I, Roberge D, Finkelstein S, Petersen I, Saito N, DeLaney T. Variations in the gross target volume and clinical target volume evaluated by RTOG sarcoma radiation oncologists for preoperative radiotherapy of primary extremity sarcoma. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Sullivan MB, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan B, Ward I Iain, Catton C. Recent advances in radiotherapy for soft tissue sarcoma. Current Oncology Reports. 2003;5:274–281. doi: 10.1007/s11912-003-0066-y. [DOI] [PubMed] [Google Scholar]
- 13.Maples WJ, Buskirk SJ. Multimodality treatment of upper extremity bone and soft tissue sarcomas. Hand Clinic. 2004;20:221–225. doi: 10.1016/j.hcl.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson P, Ferguson PC. Primary multidisciplinary management of extremity soft tissue sarcomas. An excellent and in-depth overview of the most recent therapeutic approaches concerning ESTS. Current Options Oncol. 2004;5:451–462. doi: 10.1007/s11864-004-0034-2. [DOI] [PubMed] [Google Scholar]
- 15.White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1439–1455. doi: 10.1016/j.ijrobp.2004.08.036. [DOI] [PubMed] [Google Scholar]