Abstract
Electrospray ionization mass spectrometry is a powerful technique to analyze lipid extracts especially for the identification of new lipid metabolites. A hurdle to lipid identification is the presence of solvent contaminants that hinder the identification of low abundance species or covalently modify abundant lipid species. We have identified several non-enzymatically derived minor lipid species in lipid extracts of Escherichia coli, phosphatidylmethanol, ethyl and methyl carbamates of PE and N-succinyl PE were identified in lipid extracts of Escherichia coli. Phosphatidylmethanol (PM) was identified by exact mass measurement and collision induced dissociation tandem mass spectrometry (MS/MS). Extraction in the presence of deuterated methanol leads to a 3 atomic mass unit shift in the [M-H]- ions of PM indicating its formation during extraction. Ethyl and methyl carbamates of PE, also identified by exact mass measurement and MS/MS, are likely to be formed by phosgene, a breakdown product of chloroform. Addition of phosgene to extractions containing synthetic PE significantly increases the levels of PE-MC detected in the lipid extracts by ESI-MS. Extraction in the presence of methylene chloride significantly reduced the levels of these lipid species. N-succinyl PE is formed from reaction of succinyl-CoA with PE during extraction. Interestingly N-succinyl PE can be formed in an aqueous reaction mixture in the absence of added E. coli proteins. This work highlights the reactivity of the amine of PE and emphasizes that careful extraction controls are required to ensure that new minor lipid species identified using mass spectrometry are indeed endogenous lipid metabolites.
Keywords: mass spectrometry, E. coli, lipids, chloroform, phosgene, artifacts
1. Introduction
The identification of new cellular metabolites can facilitate the identification of new biochemical pathways and lead to accurate genome annotation. High-resolution mass spectrometry is a superb tool for the identification of new metabolites especially lipid metabolites. The combination of accurate mass measurements and collision-induced dissociation MS/MS analysis facilitates the identification of known and novel lipid structures and has been used to characterize several new lipid metabolites in Gram-negative bacteria such as Escherichia coli and other organisms such as Clostridium [1-5].
In addition to being used to identify new minor lipid metabolites, ESI-MS/MS is extensively used to characterize the levels of the major lipid metabolites [6,7]. In nearly all of these studies lipids are extracted from cells or tissues using standard extraction conditions, such as Bligh-Dyer or Folch extractions [8, 9]. Many of these extractions are chloroform-methanol based. Through the course of our analysis of total lipid extracts from wild-type and phosphatidylglycerolphosphate synthase (pgsA) deficient E. coli [2,10] we have identified several minor lipid species that are formed from major lipid metabolites during extraction. In particular, phosphatidic acid (PA) and phosphatidylethanolamine (PE) derivatives have been identified in E. coli extracted by the method of Bligh and Dyer [8].
The structures of these non-enzymatically formed lipid derivatives were determined by accurate mass measurement and collision-induced dissociation tandem mass spectrometry (MS/MS) using an electrospray ionization (ESI) quadrupole time-of-flight (qTOF) mass spectrometer and by comparison to synthetic standards. We have identified one modification of PA and four modifications to PE. Investigations into the origin of these novel lipids revealed that these lipids are not present in the cell prior to extraction but are likely derived during extraction particularly in the presence of chloroform. Of particular interest is the fact that several of these non-enzymatically-generated lipids are isobaric with well-characterized lipids of the cell hampering structural identification and quantification.
2. Materials and Methods
2.1. Materials
Tryptone and yeast extract were from FisherBiotech (Fairlawn, New Jersey). Glass-backed Silica Gel 60 thin layer chromatography plates (0.25-mm) and high performance thin layer chromatography (HPTLC) plates were from E. Merck; solvents were reagent grade from Malinckrodt. CDCl3, CD3OD, 15N-NH4Cl, and phosgene (20% in toluene) were from Sigma. Other chemicals were purchased from VWR. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (PE(16:0/18:1)), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (PE(18:0/20:4)), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(succinyl), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-ethylcarbamate were from Avanti Polar Lipids, Alabaster, AL. L-α 1-palmitoyl-2-lineoyl phosphatidylethanolamine [palmitoyl-1-14C] ((50-62 mCi/mmol, 25 uCi/ml) was from Amersham.
2.2. Growth of E. coli
E. coli K-12 strain W3110 was cultured at 37 °C in Luria Broth (LB) consisting of 10 g of NaCl, 5 g of yeast extract, and 10 g of tryptone per liter [11]. The cells were grown overnight in LB medium at 37 °C and then diluted into LB medium to an A600 of 0.01. The culture was grown at 37 °C, shaking at 225 rpm until the A600 was about 1.0. Cells were harvested by centrifugation for 20 minutes at 2600 × g and washed with phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). pgsA deficient E. coli [10] was grown in LB as described above except at 30 °C instead of 37 °C.
2.3. Extraction of E. coli total lipids
The final cell pellet from a 1 L growth of E. coli was re-suspended in 40 mL of PBS and transferred to a Teflon-lined centrifuge bottle. The cellular lipids were extracted using the method of Bligh and Dyer [8] as described previously [1]. Briefly, 50 mL of chloroform and 100 mL of methanol were added to the cell suspension to generate a single-phase extraction mixture of CHCl3/CH3OH/PBS (1:2:0.8, v/v/v). After incubation at room temperature for 20 minutes, the mixture was centrifuged at 2600 × g for 15 minutes. The supernatant was transferred to a clean bottle and converted to a two-phase Bligh-Dyer extraction mixture (CHCl3/CH3OH/PBS, 2:2:1.8, v/v/v) by the addition of 50 mL of chloroform and 50 mL of PBS. The extraction mixture was centrifuged as above to resolve the phases. The upper phase, designated U1, was separated from the lower phase, designated L1, and transferred to a clean bottle. U1 was acidified with HCl (final concentration 0.2M), and washed with 100 mL pre-equilibrated acidic lower phase (generated from mixing CHCl3, CH3OH, 0.2M HCl (2:2:1.8, v/v/v)). The resulting lower phase was washed with 190 mL of pre-equilibrated acidic lower phase. The extraction mixture was centrifuged as above to resolve the phases and this lower phase, designated L2, was dried using rotary evaporation. L1 was washed with 190 mL of pre-equilibrated neutral upper phase and the resulting lower phase dried using rotary evaporation.
Wild type E. coli W3110 was extracted in the presence of CD3OD and CDCl3. A cell pellet from a 50 mL growth was re-suspended in 0.8 mL of PBS and divided into two glass tubes. To one tube 1 mL of CD3OD and 0.5 mL CDCl3 and to the second tube 1 mL of CH3OH and 0.5 mL CHCl3 to generate a single phase Bligh-Dyer extraction mixture. The tubes were incubated at room temperature for 20 minutes and then centrifuged for 15 minutes in a clinical centrifuge. The supernatant was transferred to a clean tube and then 0.5 mL CDCl3 or 0.5 mL of CHCl3 and 0.5 mL of PBS were added to generate a two-phase Bligh-Dyer extraction mixture. The resulting lower phase was transferred to a fresh tube and dried under N2. Samples were re-dissolved in 100 μL of either 2:1, CDCl3/CD3OD (v/v) or CHCl3/CH3OH (v/v) for mass spectrometry analysis. Cells were also extracted using Bligh-Dyer extraction mixtures in which the mixtures were acidified by the addition of HCl in the aqueous phase to a final concentration of 0.2 M and/or the CHCl3 was replaced with CH2Cl2.
2.4. Ion exchange chromatography
The total lipid extracts were fractionated on DEAE-cellulose as described previously [1,12].
2.5. Mass spectrometry
The dried lipid film was re-dissolved in CHCl3/CH3OH (2:1, v/v) to a concentration of ~ 1 μg/mL. This solution was directly infused into the Turbo electrospray ionization source of a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (ABI/MDS-Sciex, Toronto, Canada) at 6 μL/min. Mass spectra were obtained scanning from 200 to 2000 Da in negative-ion mode with the electrospray ionization source operating at the following settings: nebulizer gas, 21 kPa, curtain gas, 27 kPa, ion-spray voltage, -4500 V, declustering potential, -55 V, focusing potential, -265 V, declustering potential 2, -15 V. The instrument was calibrated using PPG (Applied Biosystems). Collision induced dissociation tandem mass spectrometry (MS/MS) was performed with a collision energy of -50.0 V (laboratory frame of reference) and N2 as the collision gas. Data acquisition, analysis and elemental composition calculations were performed using the Analyst QS 1.1 software. Exact masses of lipid species and product ions were obtained using CS Chem Draw Pro, version 12.0.
Normal phase liquid chromatography mass spectrometry was performed as described previously [1].
2.6. 15N-labeling of E. coli
Cells were grown overnight in LB medium at 37 °C and then diluted into M9 minimal media supplemented with 15NH4Cl to an A600 of 0.01 [13]. The culture was grown at 37 °C, shaking at 225 rpm until the A600 was about 1.0. Cells were harvested and extracted as described above.
2.7. Extractions in the presence of phosgene
A synthetic PE(18:0/20:4) (100 μg) was extracted in the presence or absence of 0.05 % phosgene. Briefly, PE(18:0/20:4) (10 mg/mL in CHCl3) was mixed with 0.5 ml CHCl3, 1 mL CH3OH, 0.4 mL 1X PBS, and 5 μL of toluene or 20% phosgene in toluene and incubated at room temperature for 10 minutes. To each tube 0.5 mL CHCl3 and 0.5 mL 1X PBS was added to generate a two-phase Bligh-Dyer extraction mixture. The tube was centrifuged to resolve the phases and the upper phase discarded. The lower phase was dried under nitrogen.
2.8. Exchanging protons using deuterated solvents
A dried lipid extract was re-dissolved in 100 μL of 2:1 CDCl3/CD3OD, v/v, dried under N2. This resuspension/drying cycle was repeated two more times and finally re-dissolved in 100 μL of 2:1 CDCl3/CD3OD, v/v and analyzed using electrospray ionization mass spectrometry.
2.9. Preparation of cell free extracts and membranes
E. coli W3110 (50 mL) was grown in LB medium as described above. The cell pellet was re-suspended in 3 mL of 20 mM Hepes, pH 7.4 and cells lysed in a French Pressure cell at 18000 psi. The extract was centrifuged for 20 minutes at 4 °C and 3000 × g to pellet un-lysed cells. The cell-free extract was transferred to a microcentrifuge tube and stored at -80 °C.
Membranes were prepared by centrifuging the cell free extract at 4 °C, 100000 × g for 1 hour. The resulting membrane pellet was re-suspended by homogenization in 2 mL of 20 mM Hepes pH 7.4 and the membranes pelleted by centrifugation as described above. The final membrane pellet was re-suspended in 1.5 mL of 20 mM Hepes pH 7.4 and stored at -80 °C. The concentration of the extract was determined using the bicinchoninic acid reagent (Thermo Scientific) with bovine serum albumin as the standard.
2.10. In vitro synthesis of N-succinyl phosphatidylethanolamine
Phosphatidylethanolamine (16:0/18:1) was suspended in 20 mM Hepes buffer, pH 7.4 at a final concentration of 2 mM. Reactions containing 20 mM Hepes buffer, pH 7.4, 0.5 mM PE, 0.2% Triton X-100, in the presence or absence of 1 mg/mL cell free extract and in the presence or absence of 2 mM succinate, succinic anhydride, succinyl-CoA, glutaryl-CoA, or malonyl-CoA were incubated at 30 °C for 2 hours. Five μL of the reaction was spotted to a silica gel Thin layer chromatography plate and developed in CHCl3/CH3OH/H2O/CH3COOH (25:15:4:2, v/v/v/v). The lipids were visualized by charring with 10 % sulfuric acid.
3. Results
3.1. Phosphatidylmethanol
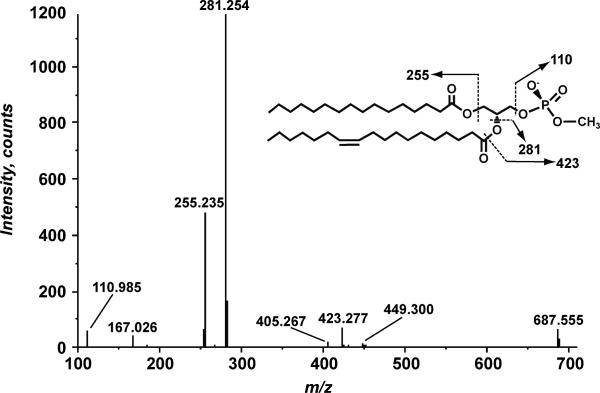
Wild type E. coli lipid extracts were fractionated on a DEAE cellulose column based on charge [1]. A fraction eluting with CHCl3/CH3OH/30 mM NH4Ac (2:3:1, v/v/v) was analyzed by negative-ion electrospray ionization mass spectrometry (ESIMS). The predominant ions between m/z 691 and 773 represent singly charged species [M-H]- of phosphatidylglycerol (PG) as determined by exact mass and MS/MS analysis (Figure 1). In addition several peaks at m/z 631.486, 633.474, 659.502, 673.526, 685.543 and 687.546 were observed (Figure 1 inset). The exact mass of these negative ions is suggestive of phosphatidic acid (PA). However PA is expected to elute from DEAE cellulose with higher concentrations of NH4Ac [1]. In addition, the most prominent ion at m/z 659.502 corresponds by exact mass to PA with 33 cycolpropane fatty acyl chains consistent with the fatty acid composition of E. coli grown to late log phase [14]. Collision-induced dissociation (CID) MS/MS analysis of these peaks revealed fragmentation patterns inconsistent with PA. Figure 2 shows the MS/MS spectrum of another prominent ion in that mass range, at m/z 687.5. While this ion mass may correspond to a PA with 37cyclopropane acyl chains, a fragment ion for a cyclopropane fatty acid is not detected. Instead, product ions corresponding to palmitate (16:0, m/z 255.235) and octadecenoate (18:1, m/z 281.254) are observed. In addition a peak at m/z 110.985 was observed and corresponds by exact mass to methylated phosphate (CH4O4P-, exact mass 110.9853). The ion at m/z 167.026 is 14 atomic mass units (a.m.u.) larger than the common glycerophospholipid fragment ion C3H6O5P-, (exact m/z 152.9953) [15-17] and corresponds to a methylated form of this fragment ion (C4H9O5P-). From these fragment ions we hypothesized that the ion at m/z 687.5 corresponds to phosphatidic acid with a methyl group added to the phosphate (Figure 2), designated phosphatidylmethanol (PM). The other major peaks in the product ion spectra are attributable to losses of acyl chains from the precursor ion. The ions at m/z 423.277 and m/z 449.300 correspond to the loss of the 18:1 and 16:0 acyl chains respectively as ketenes (RCH=CO). The MS/MS analysis of the ions at m/z 631.5, 633.5, 659.5, 673.5 and 687.5 reveal similar fragmentation patterns, especially the presence of the ion at m/z 110.9 ion corresponding to methylated phosphate.
Figure 1.
Negative-ion ESI-MS of E. coli lipids that elute from DEAE cellulose column with 30 mM NH4Ac. The prominent ions at m/z 691.487, 693.489, 705.513, 717.508, 719.462, 733.498, 745.496, 747.497, 759.554, 761.558, and 773.544 correspond to the [M-H]- ions of phosphatidylglycerol with 30:1, 30:0, 31:1cyclopropane (cp), 32:2, 32:1, 33:1cp, 34:2, 34:1, 35:2cp, 35:1cp, 36:2 acyl chains, respectively. The inset shows an expansion of the region from m/z 630 – 700 revealing the unknown ions.
Figure 2.
MS/MS of the negative ion at m/z 687.5. The inset shows the proposed structure of PM and the proposed major product ion fragments.
It has been previously reported that cardiolipin synthase can catalyze the addition of an alcohol via an alcoholysis reaction with cardiolipin [18]. Before pursuing the possible enzymatic formation of PM in E. coli we investigated the possibility that PM may be formed during extraction. Wild-type E. coli cells were extracted in the presence of deuterated methanol and deuterated chloroform. Figure 3 shows the ESI-MS spectrum of total lipids extracted in the presence of non-deuterated solvents (panel A) or deuterated solvents (panel B and C). Lipid extracts were analyzed by ESI-MS after being re-dissolved in non-deuterated (Figure 3, panel A or C) or deuterated solvents (Figure 3, panel B). Because these lipid extracts were not fractionated using anion exchange as above, singly charged [M-H]- PA and doubly-charged [M-2H]2- CL ions isobaric with the PM ions are present. The peak at m/z 659.463, corresponding to PM with 32:1 acyl chains shifts, in part to m/z of 662.482 (panel B). The same result is observed when the lipids extracted in deuterated solvents were re-dissolved in non-deuterated solvents consistent with the formation of PM during extraction and not during ionization or MS analysis. MS/MS of the ion at m/z 662.5 confirms the addition of deuterated methanol to the lipid (Figure 4). The product ion at m/z 110.945 is shifted to 114.003 consistent with the methylated phosphate gaining the methyl group from the solvent during extraction. In addition the fragment ions at m/z 167.012 and 423.254 gain three mass units to m/z 170.029 and 426.269 in the presence of deuterated methanol. These data strongly suggest that PM is being formed during solvent extraction. Extraction in presence of deuterated solvents did not alter ions of the other major glycerophospholipids PE, PG, and CL (data not shown).
Figure 3.
Negative-ion ESI-MS of total E. coli lipids extracted in the presence of nondeuterated and deuterated chloroform and methanol. The region from m/z 656-670 for the lipid extract prepared in non-deuterated (panel A) and deuterated (panel B and C) solvents. The samples were re-dissolved in non-deuterated (panel A and C) or deuterated solvents and analyzed by negative ion ESI-MS. The ions at m/z 660.462 and 662.479 correspond to the [M-H]- ions of PE with 30:1 and 30:0 acyl chains, respectively. The ions at m/z 659.955 and 660.962 correspond to [M-2H]2- CL ions with one 13C and 62:2 and 62:1 acyl chains, respectively. The [M-H]- ion of PM with 32:1 acyl chains (m/z 659.463) shifts to m/z 662.48 when extracted in the presence of deuterated solvents.
Figure 4.
MS/MS of the negative ions at m/z 659.5 and 662.5. Panel A shows the MS/MS of the ion at m/z 659.5 found in lipid extracts prepared in non-deuterated solvents. Panel B shows the MS/MS of the ion at m/z 662.5 found in lipid extracts prepared in deuterated solvents. The fragment ions in boxes are extraction solvent dependent. The inset shows the proposed structure of PM and the proposed major product ion fragments.
3.2. PE-Ethylcarbamate
Lipids were extracted from E. coli lacking the phosphatidylglycerol phosphate synthase (pgsA) gene [10]. As previously reported, ESI-MS confirmed that these cells lack PG and cardiolipin (CL) [2], and the major remaining negative-ions arise from phosphatidylethanolamine (PE). However, we also identified a series of ions [M-H]- with m/z of 760.477, 774.502, 788.494, 800.519, and 814.527 that correspond by exact mass to phosphatidylserine (PS) (Figure 5) with 34:1, 35:1cyclopropane, 36:1, 37:2cyclopropane, and 38:2. PS is an intermediate in the biosynthesis of PE but is not readily detected in wild type E. coli [21]. These ions are also observed in wild-type cells; however they are obscured by the predominant ions [M-H]- of PG.
Figure 5.
Negative-ion ESI-MS of total lipid extracts from E. coli lacking pgsA. The prominent ions at m/z 746.479, 760.477, 74.502, 786.500, 788.494, 800.519, 802.531, and 814.527 correspond to the unknown ions. Also present are ions at m/z 716.478, 742.488, and 744.496, which are the [M-H]- ions of PE with 34:1, 36:2 and 36:1 acyl chains. The ion at m/z 845.629 corresponds to undecaprenyl phosphate [5] by exact mass and MS/MS analysis.
MS/MS of one of these ions, the ion at m/z 760.5, is shown in Figure 6 and is inconsistent with PS that in the negative mode yields a prominent product ion corresponding to the neutral loss of 87 a.m.u [17]. However, product ions indicative of a glycerophospholipid are observed. The ion at m/z 78.951 corresponds to PO3- and the ion at m/z 152.986 corresponds to C3H6O5P- [15]. Acyl chains corresponding to palmitate (16:0) and palmitoleate (16:1) are observed as their carboxylic anions at m/z 255.217 and m/z 253.200, respectively. In addition, the product ion at m/z 524.281 corresponds to the loss of the 16:1 acyl chain as a ketene (RCH=C=O).
Figure 6.
MS/MS of the negative ion at m/z 760.5 found in lipid extracts from E. coli lacking pgsA. The inset shows the proposed structure of PE-EC and the proposed major product ion fragments.
A neutral loss of 46 a.m.u. leads to the formation of the product ion at m/z 673.481, which is interpreted as a 16:0, 18:1 phosphatidic acid anion (C37H70O8P-, exact mass 673.4814) and is consistent with a diacylated glycerophospholipid. The 216 mass units loss, consistently seen in the MS/MS spectra of all of the unknown [M-H]- ions at m/z 861.506, 875.521, 887.525, and 915.548 (data not shown), could correspond to the loss of a part of the head group of a novel diacylated glycerophospholipid.
A neutral loss of 46 a.m.u. leads to the formation of the product ion at m/z 714.489, which is interpreted as loss of ethanol (Figure 6). The product ions at m/z 478.254 and 460.220 correspond to loss of ethanol and the 16:1 acyl chain as a ketene (RCH=C=O) or a fatty acid (RCOOH), respectively.
Several ions in the low mass region are derived from the head group of this lipid. The product ion at m/z 268.040 corresponds to the loss of both acyl chains from the precursor ion, one as a ketene and one as a fatty acid. The product ion at m/z 222.009 is ethanol loss from m/z 268.040. As indicated in Figure 6, the product ion at m/z 212.017 corresponds to the head group and is observed in MS/MS spectra of all of the unknown ions. The ions at m/z 194.008 and m/z 165.978 correspond to the loss of water and ethanol, respectively, from the ion at m/z 212.017.
Several lines of evidence supported a novel modification to the free amine of PE. Continuous labeling of cells in M9 minimal media supplemented with 15NH4Cl shifts these unknown ions one mass unit consistent with the presence of one nitrogen in the molecule. These lipids were retained by DEAE cellulose and eluted with CHCl3/CH3OH/15 mM NH4Ac (2:3:1, v/v/v) consistent with a modification to the free amine of PE. Finally, the ions contained only one exchangeable proton as seen by a one mass unit shift after exchange into deuterated solvents (data not shown).
Taken together we believe that the amine of PE is modified by the addition of - CO2CH2CH3 to form an ethyl carbamate. We propose a new PE derivative which we designate phosphatidylethanolamine ethylcarbamate (PE-EC).
To confirm the structure proposed above a synthetic PE-EC containing two 18:1 acyl chains was obtained. The fragment ions obtained from the synthetic standard and the endogenous PE-EC are very similar (data not shown), strongly supporting the structure shown in Figure 4 and 5.
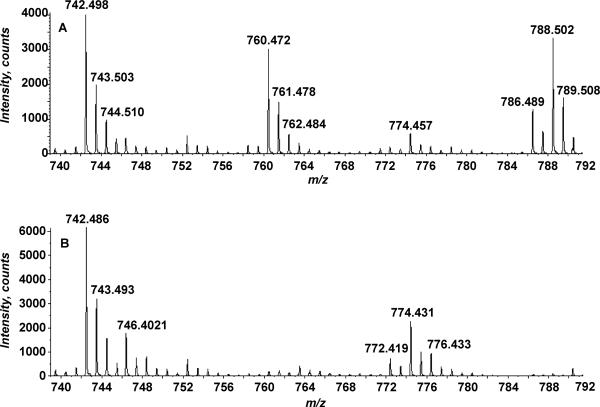
Cone et al. indicated that the formation of ethyl carbamates could occur on amine containing drugs during chloroform extraction [20]. During normal phase liquid chromatography mass spectrometry (LC-MS), PE-EC elutes at ~5 minutes as compared to PE which elutes at ~14.5 minutes (Figure 7), strongly suggesting that PE-EC was formed during extraction. To further confirm this, we next extracted cells using a modified Bligh-Dyer extraction in which methylene chloride (less likely to decompose to phosgene than chloroform) replaced the chloroform. ESI-MS of the methylene chloride extracted samples as well as the chloroform-extracted samples are shown in Figure 8. The PE-EC was not detected in samples that were extracted with methylene chloride indicating that the PE-EC was being formed during extraction in the presence of chloroform.
Figure 7.
Normal phase LC-MS of total lipid extracts from wild-type E. coli. The lipids were extracted using a standard Bligh-Dyer (2:2:1.8, CHCl3/CH3OH/PBS, v/v/v) and analyzed by normal phase LC-MS [1]. Panel A shows the extracted ion current for the 34:1 PE-EC at m/z 788.5. Panle B shows the extracted ion current for 34:1 PE at m/z 716.5.
Figure 8.
Negative-ion ESI-MS of total lipid extracts from E. coli lacking pgsA prepared with CHCl3 or CH2Cl2. The lipids in Panel A were extracted using a standard Bligh-Dyer (2:2:1.8, CHCl3/CH3OH/PBS, v/v/v). The lipids in Panel B were extracted using a Bligh-Dyer extraction in which methylene chloride replaced the chloroform (2:2:1.8, CH2Cl2/CH3OH/PBS, v/v/v).
We tested whether this process was indeed mediated by phosgene. A 18:0, 20:4 synthetic PE standard (exact mass of [M-H]- is 766.5392) was extracted in the presence or absence of added phosgene and then analyzed by ESI-MS. Extraction of PE leads to the formation of a [M-H]- ion at m/z 824.550. This lipid corresponds to PE-MC as determined by exact mass measurement and MS/MS analysis (Figure 9 Panel C). A neutral loss of 32 a.m.u. leads to the formation of the product ion at m/z 792.517, which is interpreted as loss of methanol (Figure 9, panel C). This loss is analogous to the 46 a.m.u. neutral loss of ethanol as occurs with PE-EC (Figure 6). The product ion at m/z 198.018 corresponds to the head group. The ions at m/z 194.08 and m/z 165.978 correspond to the loss of water and methanol, respectively, from the ion at m/z 198.018.
Figure 9.
Effect of phosgene addition on extraction of PE. PE(18:0/20:4) was extracted in the absence (Panel A) and the presence (Panel B) of phosgene. Panel C shows the MS/MS spectrum of the major product at m/z 824.5 formed upon extraction with phosgene to be PE-MC as shown in the inset.
The levels of PE-MC appear to be nearly equal to the levels of PE. However the modification of the amine of PE to form PE-MC likely leads to increased ionization efficiency as compared to PE. When phosgene is present the levels of PE-MC increase (Figure 9 panel A versus panel B), indicating that phosgene is likely involved in the formation of the ethyl and methyl carbamates of PE we have detected. It is hypothesized that the relative levels of PE-MC and PE-EC may be dependent on the chloroform used and whether ethanol is used as a stabilizing agent.
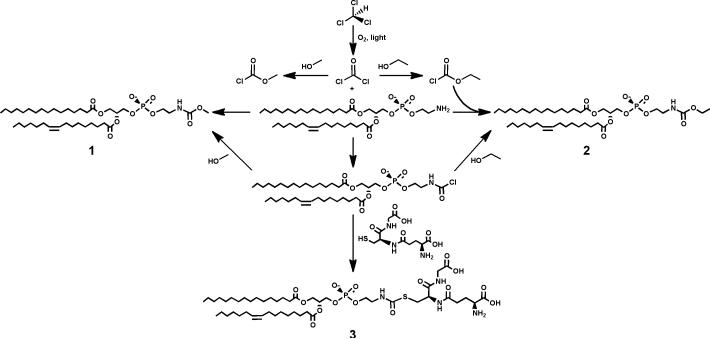
The proposed mechanism for the formation of the ethyl- and methyl-carbamates of PE is shown in Figure 10 (compounds 1 and 2). A second novel minor lipid was detected in the upper phase of a neutral Bligh-Dyer extraction. When this neutral upper phase (U1) is acidified and partitioned against a pre-equilibrated acidic lower phase highly acidic lipids partition to the lower phase. In this acidified lower phase (L2) we detected a new lipid in which glutathione is attached to PE via a carbamate linkage (Figure 10, compound 3, data not shown). Like PE-EC, PE-MC and PE-glutathione are not detected in lipid extracts prepared in the presence of methylene chloride instead of chloroform (data not shown).
Figure 10.
Proposed mechanism for the phosgene dependent formation of PE-EC, PE-MC, and PE-glutathione. Compound 1: PE-MC, compound 2: PE-EC, and compound 3: PE-glutathione.
3.3 N-succinyl phosphatidylethanolamine
Wild type E. coli were extracted to generate an acidic lower phase L2 (described above) [5]. This acidified lower phase was analyzed using ESI-MS (Figure 11). A series of [M-H]- ions with m/z of 786.488, 788.506, 802.526, 814.528, and 816.538 were observed which did not correspond to known lipids. MS/MS of the ions showed a fragmentation pattern consistent with a glycerophospholipid with two acyl chains. Figure 12, panel A shows the MS/MS spectrum of the ion at m/z 788.5. Carboxylic anions of fatty acids, 16:1 and 16:0 are prominent (m/z 253.213 and 255.230, respectively). In addition, the ion at m/z 152.991 corresponds to C3H6O5P-, a diagnostic ion for glycerophospholipids [17]. A neutral loss of 100 a.m.u. yields a product ion (m/z 688.491) with the mass expected for PE with 32:1 acyl chains. The fragment ion at m/z 645.452 corresponds to the loss of the head group. The fragment ions at m/z 532.258 and 534.280 correspond to the loss of the 16:0 and 16:1 acyl chains as a fatty acid (RCOO-). All of the unknown [M-H]- ions yielded similar fragmentation patterns, particularly the neutral loss of 100 a.m.u. to yield a product ion with the mass of PE acylated with the prominent fatty acids product ions. The MS/MS result allowed us to propose a metabolite of PE in which the amine is modified with succinate (Figure 12, panel A).
Figure 11.
Negative-ion ESI-MS of L2 E. coli lipid extracts. The ions at m/z 786.488, 788.506, 802.526, 814.528 and 816.538 correspond to unknown ions.
Figure 12.
MS/MS of the [M-H]- ion at m/z 788.5 and a synthetic N-succinyl PE [M-H]- ion at m/z 842.6. The insets show the proposed structure of N-succinyl PE and the proposed major product ion fragments.
Synthetic carboxyacyl derivatives of phosphatidylethanolamine (PE) are commercially available and have been used to efficiently couple proteins to liposomes [21,22]. To confirm our proposed structure, synthetic N-succinyl PE was obtained (Avanti Polar Lipids, Alabaster, AL) and the MS/MS of the synthetic standard compared to the MS/MS spectra of the unknown ions. As shown in Figure 12, panel B the spectra show similar fragmentation patterns with the characteristic loss of 100 a.m.u. from the precursor ion and the formation of the cognate PE. These results strongly suggest that the structure of the lipids observed is N-succinyl PE.
We attempted to establish an in vitro assay for the formation of N-succinyl PE. We hypothesized that the amine of PE could be modified in a reaction similar to the acylation reactions of other lipids [23]. In those instances, an acyl chain is activated via covalent linkage to CoA or ACP and serves as the acyl donor to either a hydroxyl or an amine [24-26]. We tested to see if succinate could be transferred from succinyl CoA to PE in an enzyme dependent manner. 14C-PE and succinyl CoA were combined in the presence and absence of cell-free extract or membranes derived from wild type E. coli W3110 and incubated for 1 hour. Display of the reaction products using thin layer chromatography revealed the formation of a more hydrophilic product in the absence of added protein (Figure 13, lane 2, marked with *). Interestingly, in the presence of protein, either cell free extracts or membranes, the product was decreased compared to in the absence of protein (Figure 13, lane 2 compared to lane 4 and lane 6). To establish that the product formed in the absence of protein is indeed N-succinyl PE, synthetic, unlabeled 16:0/18:1-PE (exact mass [M-H]- is 716.5236) was reacted with succinyl CoA, the lipid isolated by Bligh-Dyer extraction and analyzed using ESI-MS. An ion 100 a.m.u. larger than the 16:0/18:1 PE was observed (data not shown). MS/MS of the ion at m/z 816.467 revealed a fragmentation pattern consistent with the addition of succinate to 16:0/18:1 PE (data not shown). These results strongly indicate that the formation of N-succinyl PE occurs in an aqueous mixture via reaction of PE with N-succinyl CoA and in the absence of protein.
Figure 13.
Formation of N-succinyl PE from succinyl CoA and PE in the absence of protein. 14C-PE was incubated for 1 hour at 30 °C in the presence or absence of succinyl CoA, wild-type E. coli cell-free extracts or washed membranes. The asterisk indicates the N-succinyl-14C-PE product. This product co-migrates with N-succinyl PE synthetic standard.
The reaction of PE with succinyl CoA is specific. Succinic anhydride does not add to PE under these aqueous reaction conditions. Furthermore addition of CoA does not stimulate the formation of N-succinyl CoA in the presence of succinic anhydride (data not shown). This eliminates the possibility of residual succinic anhydride and/or CoA, which are used to synthesize succinyl CoA [27] and may be present in low levels in commercial preparations of succinyl-CoA, being responsible for the formation of N-succinyl PE. In addition, glutaryl, malonyl, acetoacetyl, and butyryl CoA did not react with PE. Lauryl-CoA was also non-reactive (Figure 14 and data not shown).
Figure 14.
N-succinyl PE is formed from succinyl CoA and PE but not other CoA esters or succinic anhydride. PE (0.5 mM) was incubated for 2 hours at 30 °C in a reaction containing 20 mM Hepes pH 7.4, 0.2 % Triton X-100, and 2 mM of a CoA thioester as indicated; Lane 1, no CoA thioester added, Lane 2, succinyl-CoA, Lane 3, succinic anhydride, Lane 4, glutaryl-CoA, Lane 5, malonyl-CoA. Lane 6 is N-succinyl PE synthetic standard.
In theory, the formation of N-succinyl PE could occur in vivo. Estimations of the in vivo concentrations of succinyl CoA are ~ 4 μM [28] and the free amine of the PE would be readily available as it composes approximately 38% of the total lipid of the inner leaflet of the inner membrane [29]. Alternatively, the N-succinyl PE could be formed during the first step of the lipid extraction. To distinguish between these possibilities, cells were extracted in the presence of excess lysine. If N-succinyl PE was formed during the extraction then the lysine would compete with the PE for reaction with the free succinyl CoA in the extract. If the N-succinyl PE is formed in the cell, the presence of the lysine should have no effect on the levels of the lipid. E. coli cells were extracted with in the presence or absence of 10 mM lysine and the lipid extract analyzed with ESI-MS. When lysine was present during extraction N-succinyl PE is absent from the lipid extract (data not shown). This strongly suggests that the formation of N-succinyl PE is occurring during the extraction and is not an in vivo metabolite of the cell.
The data in Figure 13 show that in the presence of cell extracts or membranes the formation of N-succinyl PE is reduced compared to the formation in the absence of added protein. This is suggestive of a specific enzyme that removes the succinate group from N-succinyl PE. However attempts to enzymatically de-succinylate N-succinyl PE were unsuccessful. Perhaps the high concentration of free amines, from proteins for example, prevents the formation of N-succinyl PE in vivo.
Figure 15 shows a proposed mechanism for the formation of N-succinyl PE. This mechanism is based on the mechanism proposed by Walsh et al for the formation of succinyl phosphate [30]. The free carboxyl group of succinyl-CoA can attack the carbonyl carbon to form a tetrahedral intermediate. The amine of PE can then add to the remaining carbonyl with concurrent loss of the CoA group. This mechanism also explains the results shown in Figure 14. The succinate is able to form a relatively stable six-membered ring intermediate. In contrast cyclization of malonyl-CoA would require the formation of a less stable five-membered ring. Glutaryl-CoA would yield a seven membered ring. Perhaps the additional methylene group in glutaryl-CoA leads to increased flexibility decreasing the likelihood of the formation of the cyclized intermediate.
Figure 15.
Proposed mechanism for the formation of N-succinyl PE.
4. Discussion
Highly sensitive ESI-MS analysis of lipid mixtures reveals the remarkable complexity of this class of biomolecules. In addition, minor lipid species are also detectable and potentially represent new and interesting lipid metabolites whose biosynthesis and function needs to be determined in order to understand fully the biochemistry of even simple organisms such as bacteria. However, as our work has shown some of the minor lipid species detected by ESI-MS are artifacts of extraction in the presence of organic solvents.
We have identified several minor lipid species present in lipid extracts yet formed during the extraction process. The methyl group of PM comes from methanol of the extraction mixture [37] and does not appear to be enzyme dependent unlike the formation of phosphatidylmannitol [18], which depends on cardiolipin synthase. It may result from chemical methanolysis of cardiolipin or another glycerophospholipid; however this was not explicitly tested [38]. An additional possibility is that PM is formed by E. coli phospholipase D [39] that remains active during initial stages of the lipid extraction procedure.
Of particular interest is the reactivity of the amine of PE. PE constitutes approximately 75% of the total glycerophospholipids of the E. coli cell [32]. So far we have identified four amine modifications to PE, ethyl and methyl carbamate modifications, N-succinylation and glutathione addition as a thiocarbamate. While the levels of these lipids are low compared to the levels of the major glycerophospholipids, it highlights the need for caution when using mass spectrometry to identify new lipid metabolites. In all cases one could hypothesize biosynthetic pathways that are reasonable based on known metabolic pathways. It was only with thorough careful extraction controls that these lipids were identified as non-enzymatically formed artifacts.
It is not without precedent for modifications to metabolites to occur during ESIMS [40]. The multiple evidence presented in this paper, however, points to the formation of these non-enzymatically derived lipids during extraction. First, the four a.m.u. shift observed for PM when the extraction is performed in the presence of deuterated solvents persists when the extract is analyzed by ESI-MS after being re-dissolved in nondeuterated solvents. If PM was formed during ESI-MS, the solvent used to re-dissolve the lipid extract, would dictate the results. Second, PE-EC elutes separately from PE during DEAE-cellulose and normal phase chromatography. If these lipids were formed from PE during ESI-MS they would co-elute with PE. Third, PE-glutathione and N-succinyl PE are detected in the acidic lower phase L2 which does not contain PE therefore, it is unlikely for them to be formed from PE during ESI-MS. Whether formed during extraction or during ESI-MS, these lipid species are not formed in the cell. This work highlights the need to couple metabolite identification with careful biochemistry to determine the in vivo substrates and enzymes required for their biosynthesis.
N-succinyl PE formation, quite interestingly, is neither enzyme dependent nor extraction dependent. Indeed its formation occurs efficiently under aqueous conditions. Its formation appears to occur as the cells are solubilized during single-phase extraction. N-succinyl PE formation can be inhibited by addition of high concentrations of free amines in the extraction mixture. This suggests that despite the high concentrations of both succinyl CoA and PE in cells that N-succinyl PE is not formed in vivo perhaps due to different subcellular locations of the two substrates. In fact our data suggest that high concentrations of proteins as would be found in the cell may in fact protect against the formation of N-succinyl PE in vivo.
Mass spectrometric analysis of lipid extracts is sure to lead to the identification of new lipid metabolites with important functions in the cell. These discoveries will need to be coupled with careful biochemistry to identify genuine in vivo substrates and biosynthetic enzymes to establish their relevance.
Highlights.
Extraction of lipids using solvents can generate artifacts
Mass spectrometry identifies lipids formed non-enzymatically during extraction
The amine of phosphatidylethanolamine is particularly reactive during chlorofom extraction
ACKNOWLEDGMENTS
This research was supported by the Large Scale Collaborative Grant GM-069338 to CRHR, which supports the LIPID MAPS mass spectrometry facility at Duke University, and by a Research Corporation for the Advancement of Science Cottrell College Science Award to TAG.
Abbreviations
- DEAE
diethylaminoethyl
- ESI-MS
electrospray ionization mass spectrometry
- LC-MS
liquid chromatography mass spectrometry
- MS/MS
tandem mass spectrometry
- CID
collision induced dissociation
- LB
Luria broth
- PBS
phosphate buffered saline
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PA
phosphatidic acid
- cp
cyclopropane
- CL
cardiolipin
- PM
phosphatidylmethanol
- PE-EC
phosphatidylethanolamine-ethyl carbamate
- PE-MC
phosphatidylethanolamine-methyl carbamate
- N-succinyl PE
N-succinyl phosphatidylethanolamine
- N-acyl PE
N-acyl phosphatidylethanolamine
- a.m.u.
atomic mass unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garrett TA, Raetz CRH, Richardson T, Kordestani R, Son JD, Rose RL. Identification of phosphatidylserylglutamate: A Novel Minor Lipid in Escherichia coli. J. Lipid Res. 2009;50:1589–1599. doi: 10.1194/jlr.M800549-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mileykovskaya E, Ryan AC, Mo X, Lin C-, Khalaf KK, Dowhan W, Garrett TA. Phosphatidic acid and N-acyl phosphatidylethanolamine form membrane domains in Escherichia coli mutant lacking cardiolipin and phosphatidylglycerol. J Biol Chem. 2009;284:2990–3000. doi: 10.1074/jbc.M805189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston NC, Aygun-Sunar S, Guan Z, Ribeiro AA, Daldal F, Raetz CR, Goldfine H. A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J. Lipid Res. 2010;51:1953–1961. doi: 10.1194/jlr.M004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan Z, Li S, Smith DC, Shaw WA, Raetz CR. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry. 2007;46:14500–14513. doi: 10.1021/bi701907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan Z, Breazeale SD, Raetz CRH. Extraction and identification by mass spectrometry of undecaprenyl diphosphate-MurNAc-pentapeptide-GlcNAc from Escherichia coli. Analytical Biochemistry. 2005;345:336–339. doi: 10.1016/j.ab.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010 doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Gross RW. Shotgun lipidomics: Multidimensional MS analysis of cellular lipidomes. Expert Review of Proteomics. 2005;2:253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 8.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 9.Folch J, Lees M, Sloane Stanely GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 10.Kikuchi S, Shibuya I, Matsumoto K. Viability of an Escherichia coli pgsA null mutant lacking detectable phosphatidylglycerol and cardiolipin. J. Bacteriol. 2000;182:371–376. doi: 10.1128/jb.182.2.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JR. Experiments in Molecular Genetics. Anonymous Cold Spring Harbor Laboratory; 1972. p. 433. [Google Scholar]
- 12.Kanjilal-Kolar S, Basu SS, Kanipes MI, Guan Z, Garrett TA, Raetz CR. Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyltransferases. J Biol Chem. 2006;281:12865–12878. doi: 10.1074/jbc.M513864200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radika K, Raetz CR. Purification and properties of lipid A disaccharide synthase of Escherichia coli. J Biol Chem. 1988;263:14859–67. [PubMed] [Google Scholar]
- 14.Wang A, Cronan Jr JE. The growth phase-dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an rpoS[katF]-dependent promoter plus enzyme instability. Molecular Microbiology. 1994;11:1009–1017. doi: 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu F, Turk J. Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization: Structural characterization and the fragmentation processes. Journal of the American Society for Mass Spectrometry. 2005;16:1510–1522. doi: 10.1016/j.jasms.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Hsu F, Turk J. Charge-remote and charge-driven fragmentation processes in diacyl glycerophosphoethanolamine upon low-energy collisional activation: A mechanistic proposal. Journal of the American Society for Mass Spectrometry. 2000;11:892–899. doi: 10.1016/S1044-0305(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 17.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya I, Yamagoe S, Miyazaki C. Biosynthesis of novel acidic phospholipid analogs in Escherichia coli. J. Bacteriol. 1985;161:473–477. doi: 10.1128/jb.161.2.473-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeChavigny A, Heacock PN, Dowhan W. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 1991;266:5323–5332. [PubMed] [Google Scholar]
- 20.Cone EJ, Buchwald WF, Darwin WD. Analytical controls in drug metabolic studies. II. Artifact formation during chloroform extraction of drugs and metabolites with amine substituents. Drug Metab. Disposition. 1982;10:561–567. [PubMed] [Google Scholar]
- 21.Ng K, Zhao L, Meyer JD, Rittmann-Grauer L, Manning MC. Use of circular dichroism spectroscopy in determining the conformation of a monoclonal antibody prior to its incorporation in an immunoliposome. J. Pharm. Biomed. Anal. 1997;16:507–513. doi: 10.1016/s0731-7085(97)00101-5. [DOI] [PubMed] [Google Scholar]
- 22.Kung VT, Redemann CT. Synthesis of carboxyacyl derivatives of phosphatidylethanolamine and use as an efficient method for conjugation of protein to liposomes. BBA - Biomembranes. 1986;862:435–439. doi: 10.1016/0005-2736(86)90247-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Rock CO. Acyltransferases in bacterial glycerophospholipid synthesis. J. Lipid Res. 2008;49:1867–1874. doi: 10.1194/jlr.R800005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Rock CO. Acyltransferases in bacterial glycerophospholipid synthesis. J. Lipid Res. 2008;49:1867–1874. doi: 10.1194/jlr.R800005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon EJ, Shemin D. The preparation of S-succinyl coenzyme A. J. Am. Chem. Soc. 1953;75:2520. [Google Scholar]
- 28.Jackowski S, Rock CO. Consequences of reduced intracellular coenzyme A content in Escherichia coli. J. Bacteriol. 1986;166:866–871. doi: 10.1128/jb.166.3.866-871.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla SD, Green C, Turner JM. Phosphatidylethanolamine distribution and fluidity in outer and inner membranes of the Gram-negative bacterium Erwinia carotovora. Biochem. J. 1980;188:131–135. doi: 10.1042/bj1880131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh CT, Jr., Hildebrand JG, Spector LB. Succinyl phosphate. Its nonenzymatic hydrolysis and reaction with coenzyme A. J. Biol. Chem. 1970;245:5699–5708. [PubMed] [Google Scholar]
- 31.Rawyler AJ, Braendle RA. N-Acylphosphatidylethanolamine accumulation in potato cells upon energy shortage caused by anoxia or respiratory inhibitors. Plant Physiol. 2001;127:240–251. doi: 10.1104/pp.127.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkel O, Schmid PC, Paltauf F, Schmid HHO. Presence and potential signaling function of N-acylethanolamines and their phospholipid precursors in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1734:215–219. doi: 10.1016/j.bbalip.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Gillum MP, Zhang D, Zhang X, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI. N-acylphosphatidylethanolamine, a Gut-Derived Circulating Factor Induced by Fat Ingestion, Inhibits Food Intake. Cell. 2008;135:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raetz CRH, Newman KF. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J. Biol. Chem. 1978;253:3882–3887. [PubMed] [Google Scholar]
- 35.Kilaru A, Isaac G, Tamura P, Baxter D, Duncan SR, Venables BJ, Welti R, Koulen P, Chapman KD. Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids. 2010;45:863–875. doi: 10.1007/s11745-010-3457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilaru A, Blancaflor EB, Venables BJ, Tripathy S, Mysore KS, Chapman KD. The N-acylethanolamine-mediated regulatory pathway in plants. Chem Biodivers. 2007;4:1933–1955. doi: 10.1002/cbdv.200790161. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y-H, Guan Z, Zhao J, Raetz CRH. Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli. J. Biol. Chem. 2011;286:5506–5518. doi: 10.1074/jbc.M110.199265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichihara K, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 51:635–40. doi: 10.1194/jlr.D001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole R, Proulx P. Phospholipase D activity of gram-negative bacteria. J. Bacteriol. 1975;124:1148–1152. doi: 10.1128/jb.124.3.1148-1152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan Z, Liesch JM. Solvation of acylium fragment ions in electrospray ionization quadrupole ion trap and Fourier transform ion cyclotron resonance mass spectrometry. J. Mass Spectrom. 36:264–276. doi: 10.1002/jms.124. [DOI] [PubMed] [Google Scholar]